Apart from congenital human cytomegalovirus (HCMV) infection, manifest HCMV disease occurs primarily in immunocompromised patients. In allogeneic bone marrow transplantation, HCMV is frequently associated with graft failure and cytopenias involving all hematopoietic lineages, but thrombocytopenia is the most commonly reported hematologic complication. The authors hypothesized that megakaryocytes (MK) may be a specific target for HCMV. Although the susceptibility of immature hematopoietic progenitors cells to HCMV has been established, a productive viral life cycle has only been linked to myelomonocytic maturation. The authors investigated whether HCMV can also infect MK and impair their function. They demonstrated that HCMV did not affect the thrombopoietin (TPO)-driven proliferation of CD34+ cells until MK maturation occurred. MK challenged with HCMV showed a 50% more rapid loss of viability than mock-infected cells. MK and their early precursors were clearly shown to be susceptible to HCMV in vitro, as evidenced by the presence of HCMV in magnetic column-purified CD42+ MK and 2-color fluorescent staining with antibodies directed against CD42a and HCMV pp65 antigen. These findings were confirmed by the infection of MK with a laboratory strain of HCMV containing the β-galactosidase (β-gal) gene. Using chromogenic β-gal substrates, HCMV was detected during MK differentiation of infected CD34+ cells and after infection of fully differentiated MK. Production of infectious virus was observed in cultures infected MK, suggesting that HCMV can complete its life cycle. These results demonstrate that MK are susceptible to HCMV infection and that direct infection of these cells in vivo may contribute to the thrombocytopenia observed in patients infected with HCMV.
Several hematologic complications have been attributed to human cytomegalovirus (HCMV) in immunocompromised hosts, including delayed engraftment and cytopenia. Although mild neutropenia and thrombocytopenia can occur after HCMV infection, frank HCMV-related aplasia has not been described. However, in patients undergoing bone marrow (BM) transplantation, HCMV infection, or simply the presence of HCMV antibodies, has been implicated in delayed or failed engraftment.1 Thrombocytopenia is one of the most commonly reported hematologic sequelae of HCMV infection. It occurs in patients undergoing transplantation procedures,2 but it also develops in immunocompetent persons.3,4 Although delayed platelet recovery after BM transplantation has been frequently reported,5-7 some authors describe the protracted recovery of granulocytes but not platelets.8 The role of HCMV in delayed engraftment has been illustrated in patients with HCMV viremia who have undergone transplantation and have shown more rapid platelet and neutrophil recovery after ganciclovir therapy.9Thrombocytopenia and MK inclusions have also been described in congenital HCMV infection.10
The pathophysiology of thrombocytopenia occurring in conjunction with HCMV has not been completely explained. Several in vitro studies, including those from our laboratory, have demonstrated that the challenge of BM CD34+ cells with HCMV results in the inhibition of their proliferative function.11-16 Results of these studies vary in the degree of the inhibition and specificity of suppressive effects of HCMV,11,16 but almost uniformly, myeloid colony formation is more affected by HCMV than erythroid series.11,12,14 The impaired proliferative capacity of hematopoietic progenitor cells may be related to several factors. BM stromal cells can be targets for HCMV, and stromal infection may negatively affect the function of hematopoietic stem cells and progenitor cell compartments.11,16-19 Theoretically, the inhibition of hematopoietic colony formation could result from a lytic, persistent, latent, or abortive infection of hematopoietic cells. Most of the in vitro studies suggest that early hematopoietic progenitors are susceptible to infection with HCMV,12-15,16,20 which persists in the cultures initiated by CD34+ cells. Despite the inhibition of proliferation, a limited number of CD34+cells challenged with HCMV can produce a significant number of infected cells.13 Immediate/early (IE), early (E), and late (L) transcripts are found in HCMV-infected CD34+ cells by polymerase chain reaction (PCR), and IE genes are found by in situ hybridization.14,21,15 Terminal differentiation appears to be required for a productive HCMV life cycle.20,22 In particular, monocytic, myeloid, or dendritic maturation is required for HCMV gene transcription.12,13,23 24
Lytic HCMV infection of hematopoietic progenitor and stem cells has not been clearly demonstrated. Although it is likely that hematopoietic cells either harbor HCMV in a latent form or support chronic persistent infection, cells with HCMV may also be targets of an immune attack that leads to their destruction. The best evidence for indirect T-cell–mediated inhibition of hematopoiesis during HCMV infection has been provided by experiments showing that the addition of T cells to HCMV-infected BM progenitor cells results in the suppression of colony formation, whereas the depletion of T cells was associated with the abrogation of this effect.25
Direct cytotoxicity of HCMV to hematopoietic progenitor cells, HCMV-related impairment of stromal function, or immune-mediated indirect destruction of infected hematopoietic cells can all be factors in the development of thrombocytopenia in some patients infected with HCMV. Studies on the effects of HCMV on megakaryocytopoiesis and on the mechanisms of HCMV-related thrombocytopenia have been confounded by the inability to obtain sufficient numbers of purified cells. Recently, the introduction of TPO has facilitated the in vitro maintenance and generation of MK from BM progenitor cells. In combination with other hematopoietic growth factors, TPO has been shown to support the significant expansion of BM progenitor cells and the production of MK.26-28
We have hypothesized that the direct infection of megakaryocytic cells with HCMV can lead to the impaired megakaryocytopoiesis. Therefore, we have studied whether MK and their precursor cells are susceptible to infection with HCMV and whether HCMV can impair their function.
Materials and methods
Viral stocks
CMV strains were propagated in human foreskin fibroblasts as previously described.29 A low-passage clinical isolate, Smith13 (stock titer 1 × 108 plaque-forming units [PFU]/mL) and a recombinant strain (Towne/Lox2)13 (titer of 7 × 107 PFU/mL) were used. Towne/Lox2strain was derived from the laboratory strain Towne; it contains the LacZ gene of Escherichia coli regulated by the major IE HCMV promoter.13 Mock-infected cells were incubated with supernatants from virus-infected stocks after infectious virus was pelleted by ultracentrifugation (Sorvall Ultra-80, Wilmington, DE; Bucket TH-641, 38 503 RCF, 15 000 rpm, 4°C, for 60 minutes) or heat-inactivated 70°C water bath for 30 minutes.
Separation of CD34+ cells
Heparinized BM was obtained by aspiration of the posterior iliac crest of healthy volunteers, after they gave informed consent, according to guidelines established by the Institutional Review Boards for Human Research at the University of Nevada and the Department of Veterans Affairs Medical Centers (Reno, NV). The BM was diluted 1:3 with serum free-Iscove modified Dulbecco's medium (IMDM; Gibco Laboratories, Grand Island, NY). Density gradient centrifugation was performed over Ficoll Histopaque (Sigma Diagnostics, St. Louis, MO) in a 1:2 ratio at 1500 rpm for 30 minutes. The mononuclear layer was removed and washed with serum-free IMDM. The CD34+ cells were positively selected by a Macs CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Auburn, CA), an indirect magnetic labeling system, according to manufacturer's instructions. CD34+ cell purity was measured after staining with anti-CD34 monoclonal antibodies (mAb; Becton Dickinson, San Jose, CA) by FACScan flow cytometer (Becton Dickson). On average, the purity ranged from 70% to 90%.
CD34+ cell culture
Cells were cultured in IMDM without phenol red (Gibco Laboratories), 10% fetal bovine serum (Gibco Laboratories), and 10 U/mL30TPO (PeproTech, Rocky Hill, NJ). In preliminary experiments, 50 ng/mL interleukin-3 (Il-3; Genzyme, Pittsburgh, PA), 200 ng/ml stem cell factor (SCF; R&D Systems, Minneapolis, MN), and 50 ng/mL recombinant human Flt-2/Flk-3 (Flt-2/3; R&D Systems) were added. The media was changed at 3, 6, 9, and 12 days.
Infection of CD34+ cells and megakaryocytes
Cells were subjected to centrifugal inoculation (1500 rpm for 30 minutes) with HCMV at a multiplicity of infection (MOI) of 10 infectious units per cell (or an equivalent volume of control supernatant).
Megakaryocytic cell culture and expansion
Peak megakaryocytic concentration was determined by flow cytometric analysis. Cells were removed periodically from cultures and labeled with CD42a (mAb fluorescein isothiocyanate (FITC)-conjugated IgG1; Becton Dickinson) and CD34 (Becton Dickinson). Absolute cell numbers were counted on Glasstic Slides (Hycor Biomedical, Irvine, CA), and viabilities were measured with Trypan blue (Gibco Laboratories). At the time of peak megakaryocytic concentrations, cultured cells were labeled with anti-CD42 mouse IgG1 (Becton Dickson) or FITC-labeled anti-CD42a IgG1antibody (Becton Dickinson) and subjected to magnetic cell sorting with anti-mouse IgG1-conjugated colloidal superparamagnetic microbeads (Miltenyi Biotec). After selection, purity and depletion results were determined by flow cytometry (usual purity, 35%–45%). Purified mature megakaryocytes were then infected with HCMV at an MOI of 10 as described above. For infection experiments of megakaryocyte precursors, CD34+ cells were infected with HCMV, and at peak CD42+ cell concentration, magnetic cell separation was performed as described above.
Detection of HCMV protein
Cultured cells were added to slides and fixed on ice in acetone (Sigma Diagnostics): methanol (Fisher Scientific, Fair Lawn, NJ), (9:1) for 10 minutes. Antibodies included mouse anti-HCMV I/E antigen mAb IgG2a (Chemicon International, Temecula, CA), mouse anti-HCMV pp65 antigen mAb IgG2a (Chemicon International), biotinylated mouse anti-CMV I/E antigen mAb, biotinylated mouse anti-CMV pp65 antigen mAb, FITC-conjugated CD42a anti-CD42 mouse IgG1 (Becton Dickinson), PE-labeled rat anti-mouse IgG2a+b (Becton Dickson), and PE-labeled streptavidin (Becton Dickinson). The primary mAb, anti-HCMV, was diluted 1:100 with PBS, 10% fetal bovine serum was added as a blocking agent, and the slides were incubated for 1 hour at 37°C. Fluorescent antibodies were diluted 1:5 and applied to a PBS-washed slide for 30 minutes at 37°C. Slides were counterstained with DAPI/Antifade (Oncor, Gaithersburg, MD). Positive controls included human foreskin fibroblasts infected with HCMV stained with anti-HCMV mAb. Negative controls included mock-infected cells (heat-inactivated virus and supernatants from which virus was removed by ultracentrifugation), and uninfected cells were stained with anti-HCMV mAb. In all experiments, isotype-matched mAb controls were used. Stained cells were examined under a fluorescent microscope. Staining for CD42 antigen produced green fluorescence, whereas HCMV-infected cells appeared red after they were stained with mouse anti-HCMV pp65 or IE mAb developed with isotype-specific, PE-conjugated anti IgG2a+b. When biotinylated anti-IE or pp65 mAb were used, streptavidin-PE was applied as secondary reagent. Counterstaining with DAPI produced blue nuclear staining.
Detection of LacZ expression
The lacZ gene expression in the cells infected with the laboratory strain Towne/Lox2 was detected using a DetectaGene Green CMFDG lacZ Gene Expression Kit (Molecular Probes, Eugene, OR). The Towne/Lox2 virus contains the E coli lacZ gene regulated by HCMV IE promoter. Cells were stained with this chromogenic-gal reagent and PE-conjugated CD42a mAb (Pharmingen, San Diego, CA). Propidium iodide was used to detect nonviable cells. Negative controls included Towne/Lox2-infected cells, Smith-infected cells treated with and without the CMFDG reagent, and PE-conjugated IgG1(Becton Dickinson).
HCMV-DNA polymerase chain reaction
HCMV DNA was detected using PCR. Total genomic DNA (500 ng) was used for PCR analysis. Each PCR reaction mixture (50μL) consisted of 1 × PCR buffer, 1 mmol/L MgCl2, 200 mmol/L each deoxynucleoside triphosphate, 2.5 U Ampli Taq DNA polymerase (all PCR reagents were purchased from Perkin Elmer, Norwalk, CT), and 100 ng/mL each of the 2 amplification primers. The HCMV primers 5′TTA AGG CAG CGG CAG AAG AAG A3′ and 5′TCG GGC CTA AAC ACA TGA GAA ATA3′) amplified an HCMV-specific, 404-bp fragment. Each cycle consisted of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 45 seconds. Thirty-five amplification cycles were performed, and this was followed by an extension at 72°C for 10 minutes (Gene Amp PCR System 9700, Perkin-Elmer, Branchburg, NJ). Final amplification products were run on a 2% agarose gel containing ethidium bromide and visualized with ultraviolet light.
Detection of infectious virus
Plaque assays were conducted on human foreskin fibroblast cells. A standard plaque-forming assay was used to measure the titers.30 Supernatants from infected CD34+cells and CD42+ cells were removed from culture, and serial dilutions were plated on human foreskin fibroblast cells. A 0.3% agarose overlay was placed on the cells to allow plaque development. After a 2-week incubation period, plaques were stained with methylene blue and counted.
Results
Expansion of megakaryocytes in vitro
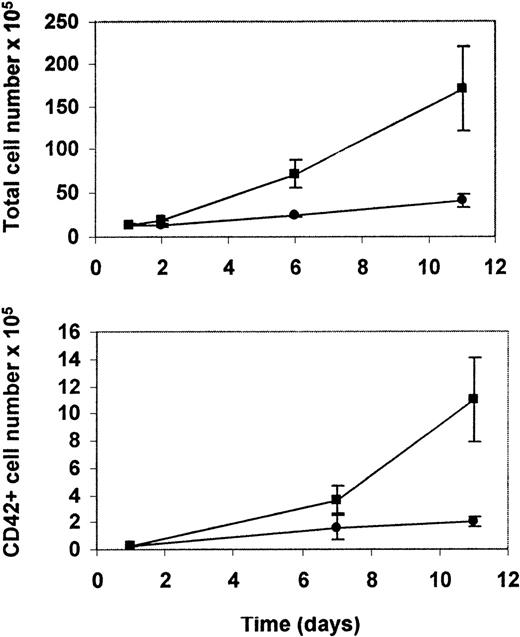
We began our study by determining the conditions for the generation of a large number of MK. Previous reports showed that TPO used alone supported an efficient expansion of MK.30-34 In preliminary experiments, we determined that the addition of SCF, Flt2/3 ligand, and IL-3 to TPO-stimulated cultures potentiates the effects of TPO on cell proliferation but did not substantially increase the percentage and total number of MK. This effect was probably caused by the simultaneous expansion of other cell lineages (data not shown). Therefore, for subsequent experiments, BM-derived CD34+ cells were cultured only with TPO. Megakaryocytic development was serially assessed for a 3-week period by morphology and by flow cytometric analysis of CD42a antigen expression. Although TPO resulted in a lesser expansion of the total cell number, flow cytometric analysis of these cells showed a significant relative and total increase in the number of megakaryocytes (Figure 1). After 8 to 12 days the percentage of MK rose from an initial 1% to 2% to a maximum of 15% by day 14 (Table 1), when the percentage of MK peaked. From this time there was a steady decrease in the number of CD42+ cells in the cultures.
Generation of megakaryocytes in cultures of CD34+ cells infected with HCMV.
Purified CD34+ cells were either HCMV infected or mock infected and were cultured in the presence of thrombopoietin. Upper panel: Total increase in cell number per culture of uninfected (▪) and infected (•) cultures. Lower panel: Total number of megakaryocytic cells, based on the expression of CD42a antigen specific for megakaryocytic lineage in uninfected (▪) and infected (•) cultures.
Generation of megakaryocytes in cultures of CD34+ cells infected with HCMV.
Purified CD34+ cells were either HCMV infected or mock infected and were cultured in the presence of thrombopoietin. Upper panel: Total increase in cell number per culture of uninfected (▪) and infected (•) cultures. Lower panel: Total number of megakaryocytic cells, based on the expression of CD42a antigen specific for megakaryocytic lineage in uninfected (▪) and infected (•) cultures.
Effects of HCMV on the TPO-driven expansion of CD34+ cells and the production of megakaryocytes
| . | Start of Culture (Day 1) . | 14 Days (Peak of Cellular Expansion) . | |
|---|---|---|---|
| Control . | HCMV . | ||
| % of CD42a+ cells | 1.3 ± 0.3% | 14.8 ± 7% | 11.9 ± 1% |
| Total number of CD42a+ cells | 0.17 ± 0.1 × 105 | 11 ± 0.5 × 105 | 2.8 ± 0.8 × 105 |
| . | Start of Culture (Day 1) . | 14 Days (Peak of Cellular Expansion) . | |
|---|---|---|---|
| Control . | HCMV . | ||
| % of CD42a+ cells | 1.3 ± 0.3% | 14.8 ± 7% | 11.9 ± 1% |
| Total number of CD42a+ cells | 0.17 ± 0.1 × 105 | 11 ± 0.5 × 105 | 2.8 ± 0.8 × 105 |
Summary of 3 experiments, mean ± SEM. Cultures were initiated with 1.0 ± 0.32 × 105 CD34+cells. P < .05, paired Student t test. Not significant compared with control.
Influence of HCMV on the expansion of CD34+ cells grown in the presence of TPO
Using conditions determined in initial experiments, we studied the influence of HCMV infection on the production of MK from CD34+ cells cultured in the presence of TPO. Mock-infected controls included heat-inactivated HCMV or virus-free supernatants from infected cells. We showed that HCMV infection of CD34+cells resulted in a decreased cell number. However, the inhibitory effect of HCMV was more pronounced at later time points, correlating with the peak in megakaryocytic differentiation (Figure 1). Although there was a greater degree of expansion in the uninfected cultures of CD34+ cells, the percentages of CD42+ cells were similar in HCMV-infected and control cultures (Table 1).
Susceptibility of megakaryocytic precursor cells to HCMV
In the previous experiments, we infected CD34+ cells with HCMV. Once MK appeared in culture, HCMV decreased in viability and growth rate (Table 2). Therefore, we designed additional experiments to study HCMV during CD34+cell differentiation into MK.
Effects of HCMV on the survival of megakaryocytes in culture
| . | Time After Infection (Hours) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 48 . | 72 . | 96 . | |||||
| No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | |
| Control | 9.9 ± 3 | 100 ± 0 | 7.2 ± 0.6 | 86 ± 7.6 | 5.2 ± 0.2 | 80 ± 8.7 | 6.6 ± 4.4 | 81 ± 7.4 |
| HCMV | 9.9 ± 3 | 97 ± 1 | 5.2 ± 0.2 | 53 ± 14 | ND | 57 ± 13 | 3.2 ± 0.2 | 31 ± 8.4 |
| . | Time After Infection (Hours) . | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 . | 48 . | 72 . | 96 . | |||||
| No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | No. Cells (×105) . | Viability (%) . | |
| Control | 9.9 ± 3 | 100 ± 0 | 7.2 ± 0.6 | 86 ± 7.6 | 5.2 ± 0.2 | 80 ± 8.7 | 6.6 ± 4.4 | 81 ± 7.4 |
| HCMV | 9.9 ± 3 | 97 ± 1 | 5.2 ± 0.2 | 53 ± 14 | ND | 57 ± 13 | 3.2 ± 0.2 | 31 ± 8.4 |
Summaries of 3 experiments, mean ± SEM. 0-time point was obtained immediately after infection. P < .05; paired Student t test. Compared with control. ND, not determined.
After infection, cells were tested for the presence of HCMV DNA by PCR. HCMV DNA was present in the cultured cells up to 20 days after infection (data not shown). Mock-infected cultures did not contain HCMV-specific PCR products. At the end of the culture period, immunohistochemistry showed a significant percentage (70% to 80%) of cells displaying HCMV pp65 protein (data not shown) in infected cultures. Although the persistence of HCMV DNA and the expression of HCMV pp65 protein indicated that HCMV infected some portion of cells in the cultures, HCMV could have selectively infected only nonmegakaryocytic cells. Therefore, additional experiments were needed to demonstrate stringently that MK cells are susceptible to HCMV infection in vitro.
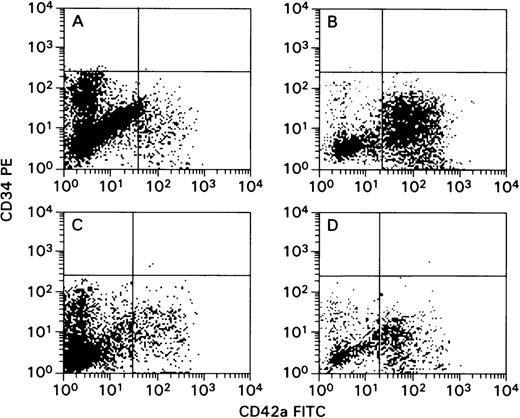
Several methods were used to confirm that MK were infected with HCMV, including a dual-fluorescence staining method. This method allows the simultaneous detection of the megakaryocytic marker and the HCMV protein in the same cell. MK were purified from bulk CD34+cell cultures at peak CD42+ cell concentration (Figure 1). Cells were labeled with CD42a IgG1 mAb (FITC-conjugated and pure), and they were separated using antimouse IgG1-conjugated beads. Wright-Giemsa and fluorescent staining with FITC-conjugated CD42a mAb demonstrated a high degree of enrichment (Figure 2A and 2B). Flow cytometric analysis of cells before and after sorting confirmed this finding (Figure 3). Staining with anti-HCMV pp65 IgG2a mAb and PE-conjugated antimouse IgG2a showed that HCMV was present in infected cultures. Using a dual-fluorescence technique for CD42a antigen and pp65, we directly confirmed that HCMV was present in the megakaryocytic cells (Figure 2C). The negative controls did not display any significant cross-reactivity between isotypes and showed only marginal background staining (Figure 2D). The pp65 and IE proteins (Figures 2E and 2F, respectively) were also detected in infected, and subsequently magnetic bead-purified, CD42+ cells using a biotinylated anti-HCMV mAb and streptavidin-PE. An unconjugated antibody was used to eliminate the possibility of nonspecific binding between FITC and streptavidin (Figure 2E). All these detection procedures yielded similar results, demonstrating that cells of the megakaryocytic lineage are infected with HCMV and express HCMV proteins. Uninfected controls were negative for HCMV-specific fluorescence (not shown).
HCMV infection of megakaryocytes generated in cultures of CD34+ cells challenged with HCMV.
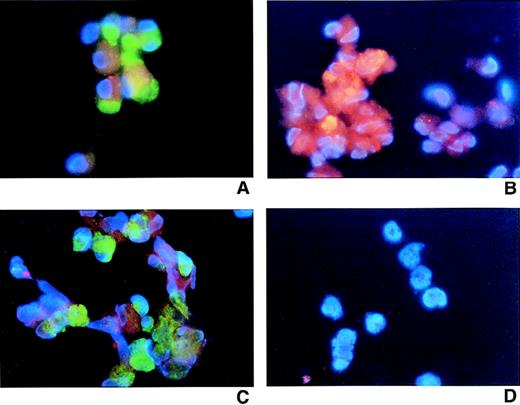
Before initiation of the culture, HCMV was used to infect CD34+ cells that subsequently were allowed to mature to megakaryocytes in the presence of thrombopoietin (TPO). At the end of the culture, HCMV-infected and mock-infected cells (derived from TPO-supplemented cultures) were purified using magnetic beads based on the expression of CD42a antigen after 12 days of culture. Cells were counterstained with DAPI blue nuclear staining and photographed at ×600 magnification. (A) Mock-infected cells stained with fluorescein isothiocyanate (FITC)-conjugated CD42a. (B) Mock-infected cells stained with hematoxylin. (C) HCMV-infected cells stained with CD42a-FITC. (D) Mock-infected cells stained with isotype control antibodies (see “Material and Methods”). (E) HCMV-infected cells stained with biotinylated anti-HCMV pp65 and streptavidin-PE. (F) HCMV-infected cells stained with CD42a-FITC, biotinylated anti-IE mAb, and streptavidin-PE.
HCMV infection of megakaryocytes generated in cultures of CD34+ cells challenged with HCMV.
Before initiation of the culture, HCMV was used to infect CD34+ cells that subsequently were allowed to mature to megakaryocytes in the presence of thrombopoietin (TPO). At the end of the culture, HCMV-infected and mock-infected cells (derived from TPO-supplemented cultures) were purified using magnetic beads based on the expression of CD42a antigen after 12 days of culture. Cells were counterstained with DAPI blue nuclear staining and photographed at ×600 magnification. (A) Mock-infected cells stained with fluorescein isothiocyanate (FITC)-conjugated CD42a. (B) Mock-infected cells stained with hematoxylin. (C) HCMV-infected cells stained with CD42a-FITC. (D) Mock-infected cells stained with isotype control antibodies (see “Material and Methods”). (E) HCMV-infected cells stained with biotinylated anti-HCMV pp65 and streptavidin-PE. (F) HCMV-infected cells stained with CD42a-FITC, biotinylated anti-IE mAb, and streptavidin-PE.
Flow cytometric analysis of magnetic bead-purified CD42a+ cells derived from CD34+ cell cultures supplemented with thrombopoietin.
Cultured cells were harvested from TPO-supplemented cultures of CD34+ cells using magnetic beads based on peak expression of CD42a antigen after 12 days of culture. Gates were set based on the isotypic controls. Log PE fluorescence activity (CD34) versus log fluorescein isothiocyanate activity (CD42a). (A, C) Cells before CD42a-enrichment step. (B, D) Cells after enrichment step. (A, B) Cells derived from uninfected cultures. (C, D) Cells derived from infected cultures.
Flow cytometric analysis of magnetic bead-purified CD42a+ cells derived from CD34+ cell cultures supplemented with thrombopoietin.
Cultured cells were harvested from TPO-supplemented cultures of CD34+ cells using magnetic beads based on peak expression of CD42a antigen after 12 days of culture. Gates were set based on the isotypic controls. Log PE fluorescence activity (CD34) versus log fluorescein isothiocyanate activity (CD42a). (A, C) Cells before CD42a-enrichment step. (B, D) Cells after enrichment step. (A, B) Cells derived from uninfected cultures. (C, D) Cells derived from infected cultures.
When the HCMV-infected cells were counted on the slide, we found that on average 47% ± 4.9% of cells reacting with CD42a antibody (FITC) were also positive for HCMV staining (PE). Only a few contaminating CD42a− cells (10% ± 5%) were also found to contain HCMV proteins.
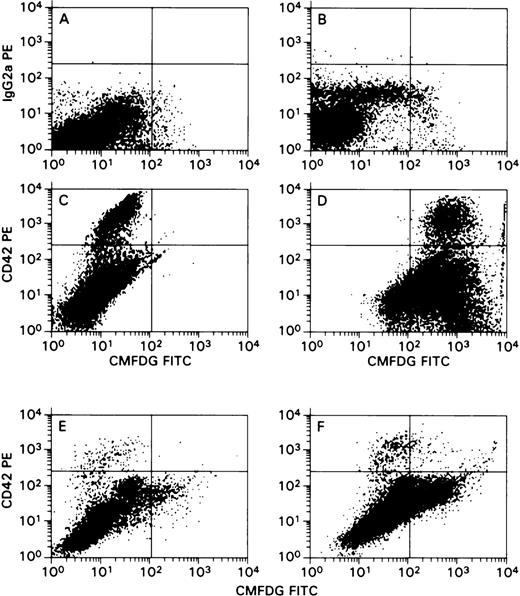
Another set of experiments was designed to confirm the above-described results. In these experiments, a laboratory-derived HCMV isolate, Towne/Lox2 carrying β-gal gene, driven by the HCMV IE promoter, was used to visualize HCMV in infected cells. CD34+ cells were infected with Towne/Lox2, and megakaryocytic cell differentiation and expansion was facilitated by the addition of TPO. Flow cytometric analysis of β-gal expression clearly demonstrated the presence of HCMV in megakaryocytic cells expressing CD42 antigen (Figure 4).
Flow cytometric analysis of β-galactosidase expression in cells derived from thrombopoietin-stimulated CD34+cell cultures after infection with Towne/Lox2 strain of HCMV.
Cells cultured in the presence of TPO were directly subjected to flow cytometric analysis without magnetic bead purification step (as in Figure 3). In the side-versus-forward scatter histograms, gates (not shown) were set to exclude nonviable cells to decrease the background staining associated with dead cells. Log PE fluorescence intensity (CD42a or IgG2 for control) versus log FITC fluorescence intensity (FDG-FITC). (A-D) Staining results of HCMV infection of megakaryocytes generated in cultures of CD34+ cells challenged with HCMV on day 0. (A) Mock-infected cells, PE-conjugated IgG2 versus FDG-FITC. (B) HCMV-infected cells, PE-conjugated IgG2 versus FDG-FITC. (C) Mock-infected cells, PE-conjugated CD42a versus FDG-FITC. (D) HCMV-infected cells, PE-conjugated CD42a versus FDG-FITC. (E, F) Cultures challenged with HCMV after megakaryocytes appeared in the cultures. Numbers of CD42a+ cells were lower than those shown in panels C and D because of the higher number of nonviable cells excluded by gating. This effect was markedly pronounced in HCMV-infected cultures. (E) Mock-infected cells, PE-conjugated CD42a versus FDG-FITC. (F) HCMV-infected cells, PE-conjugated CD42a versus FDG-FITC.
Flow cytometric analysis of β-galactosidase expression in cells derived from thrombopoietin-stimulated CD34+cell cultures after infection with Towne/Lox2 strain of HCMV.
Cells cultured in the presence of TPO were directly subjected to flow cytometric analysis without magnetic bead purification step (as in Figure 3). In the side-versus-forward scatter histograms, gates (not shown) were set to exclude nonviable cells to decrease the background staining associated with dead cells. Log PE fluorescence intensity (CD42a or IgG2 for control) versus log FITC fluorescence intensity (FDG-FITC). (A-D) Staining results of HCMV infection of megakaryocytes generated in cultures of CD34+ cells challenged with HCMV on day 0. (A) Mock-infected cells, PE-conjugated IgG2 versus FDG-FITC. (B) HCMV-infected cells, PE-conjugated IgG2 versus FDG-FITC. (C) Mock-infected cells, PE-conjugated CD42a versus FDG-FITC. (D) HCMV-infected cells, PE-conjugated CD42a versus FDG-FITC. (E, F) Cultures challenged with HCMV after megakaryocytes appeared in the cultures. Numbers of CD42a+ cells were lower than those shown in panels C and D because of the higher number of nonviable cells excluded by gating. This effect was markedly pronounced in HCMV-infected cultures. (E) Mock-infected cells, PE-conjugated CD42a versus FDG-FITC. (F) HCMV-infected cells, PE-conjugated CD42a versus FDG-FITC.
Infection of differentiated megakaryocytes with HCMV
In the previous set of experiments, we showed that MK produced from HCMV-infected CD34+ cells can support viral life cycles. The next experiments were designed to determine whether MK (similar to immature CD34+ cells) are susceptible to infection with HCMV. MK cells were generated in cultures of CD34+ cells supplemented with TPO and enriched using magnetic beads (Figure 3B). Separated CD42+ cells were then infected with HCMV at an MOI of 10. After 4 days of culture, cells were harvested and examined for the presence of HCMV. HCMV infection decreased the viability and proliferation of mature MK during the 4-day culture period (Table 2). Using the staining procedures described in the previous set of experiments, we demonstrated that HCMV IE and pp65 protein were present in MK (Figures 5A, 5B, and 5C). Different HCMV detection methods and stringent negative controls produced consistent results (Figure 5D).
Infection of mature megakaryocytes with HCMV.
Cultured cells were purified from thrombopoietin-supplemented cultures of CD34+ cells using magnetic beads based on the expression of CD42a antigen after 12 days of culture. Cells were counter-stained with DAPI and photographed at ×600 magnification. (A) HCMV-infected cells. CD42a-FITC versus biotinylated HCMV anti-IE and streptavidin PE. (B) HCMV-infected cells: murine HCMV anti-pp65 IgG2 mAb and antimouse IgG-PE. (C) HCMV-infected cells: CD42a-FITC versus biotinylated HCMV anti-IE and streptavidin PE. (D) HCMV-infected cells: isotype controls.
Infection of mature megakaryocytes with HCMV.
Cultured cells were purified from thrombopoietin-supplemented cultures of CD34+ cells using magnetic beads based on the expression of CD42a antigen after 12 days of culture. Cells were counter-stained with DAPI and photographed at ×600 magnification. (A) HCMV-infected cells. CD42a-FITC versus biotinylated HCMV anti-IE and streptavidin PE. (B) HCMV-infected cells: murine HCMV anti-pp65 IgG2 mAb and antimouse IgG-PE. (C) HCMV-infected cells: CD42a-FITC versus biotinylated HCMV anti-IE and streptavidin PE. (D) HCMV-infected cells: isotype controls.
HCMV life cycle in megakaryocytes
The expression of the pp65 matrix HCMV protein suggested that these cells might support a productive rather than a latent mode of infection. Therefore, we investigated whether infectious virus was produced in cultures of mature MK challenged with HCMV. Supernatants were collected immediately, 24 hours, 48 hours, and 96 hours after infection. Plaque assays performed at early time points after the infection of CD34+ cells showed decreasing titers of HCMV consistent with the disappearance of residual virus remaining in the cultures after inoculation (data not shown). However, when fully differentiated MK were challenged with HCMV, an increase in the HCMV titer in the supernatants was observed: after 24 hours of infection, supernatants contained 4 × 105 PFU/mL, and after 48 hours this number increased to 6 × 105 PFU/mL. Four days after infection, we were able to harvest 21 × 105 PFU/mL. Similar results were found by repeat analysis. Although MK did not appear to be highly permissive, the findings suggested that the infected cells were actively producing virus. It is likely that the steady decrease in the viability and cell number of the mature MK after HCMV challenge reflected a lytic mode of infection (Table 2).
Discussion
Previous reports have demonstrated that immature hematopoietic cells can be infected with HCMV.12-15,16,20 These and other studies have shown that the completion of the viral life cycle is dependent on myeloid differentiation12 13 and that only mature myeloid and monocytic cells can support productive HCMV infection. We have speculated that HCMV can also infect and persist in mature megakaryocytic cells. Based on this hypothesis we investigated the susceptibility of MK and their precursors to HCMV infection in vitro and the effects of HCMV on the function of these cells.
We have found that HCMV-infected CD34+ progenitors and stem cells can survive the initial infection and differentiate into mature MK cells. On the differentiation of megakaryocytic precursors, HCMV can complete its life cycle as shown by the presence of pp65 antigen, the expression of HCMV IE promoter-driven β-gal transduced by recombinant HCMV strain, and the release of infectious virus. Using a similar approach we have also shown that differentiated MK can be direct targets of HCMV infection. HCMV infection of immature CD34+progenitor cells did not result in a significant inhibition of proliferation, but, when differentiation occurred, HCMV exerted a suppressive effect on the MK that resulted in a drop in cell viability and slower expansion. Similar effects were observed when mature MK were challenged with HCMV. These results are in agreement with previous studies in which HCMV was capable of infecting CD34+progenitor cells, but differentiation was required for the completion of the viral life cycle.20,21 The mechanism of HCMV latency in immature cells is unclear. Although peculiar reverse-HCMV transcripts have been reported in HCMV myeloid progenitor cells,23 clear latency-associated HCMV proteins, as in the case of Epstein-Barr virus, have not been identified. We speculate that cellular differentiation-specific signaling elements are required for completion of the HCMV life cycle.
HCMV proteins and DNA have been demonstrated in purified MK. To assure a specificity of results, the staining procedures were preformed with carefully matched isotypic controls and a variety of specific secondary antibodies. The percentage of cells showing positive HCMV staining was higher than the proportion of CD42+ cells in the cultures. However, to exclude the possibility that HCMV staining was not caused by the contaminating cells contained in MK preparations, we also applied the dual-fluorescence staining technique. These experiments clearly showed the expression of HCMV IE and pp65 matrix proteins in cells recognized as MK by their characteristic morphology and CD42a expression. This observation was further confirmed using the Towne/Lox2 strain of HCMV containing the β-gal gene.
The HCMV pp65 matrix protein is usually expressed early in infection and indicates the completion of the HCMV life cycle. The presence of pp65 protein in MK suggests a productive infection in these cells. The results of the plaque assay support the observation that HCMV can productively infect MK. However, the infection had low productivity, and low titers of HCMV were recovered. This finding was similar to those also described for myeloid cells and macrophages.12 13 Cellular factors associated with cell maturation appear to be necessary for the productive HCMV life cycle. These factors also seem to be lineage specific, showing that erythroid cells did not support the HCMV infection.
In our study, we used several methods of HCMV detection in MK cells. HCMV DNA was detected in cultures using PCR, providing the basis for the further analysis of HCMV gene expression. Infectious HCMV was isolated from purified MK in increasing titers, suggesting that the virus replicated in the cultures. We applied an immunohistochemical-staining procedure for the detection of the expression of HCMV proteins and the analysis of its life cycle in infected MK cells, because this technique allowed for the direct detection of the virus in relevant cell types. Although reverse transcription–PCR, used for the detection of HCMV mRNA, is a very sensitive method of virus detection, the relative impurity of the cell population used in our experiments precluded its application for determining whether HCMV can infect MK. Therefore, in our study we did not rely on this technique.
The effects of HCMV on the proliferation and survival of MK and their precursors are compatible with previous results1 35 showing HCMV-mediated inhibition of colony formation by CD34+ cells and a relative lack of effects on the proliferation of immature CD34+ cells in suspension cultures. To exclude the effects of cytokines contained in the virus stock that may have negatively influenced the culture results, virus-depleted supernatants were used for mock infection. Furthermore, heat-inactivated virus supernatants were tested to exclude the possibility that the observed effects of HCMV were not caused by virus-encoded proteins rather than those native to the virus.
In addition to the direct inhibition of proliferation and the cytotoxic effects on MK, other in vivo factors may contribute to the decreased viability of infected MK and the decreased production of platelets. These factors include the killing of MK expressing HCMV proteins by specific T cells and cytokine-mediated toxicity. Thus, clinically manifest thrombocytopenia may be a result of several pathophysiologic pathways.
E.D.Z. supported by NIH grants #HL52955 and #HL49042, and S.S.J. and J.P.M., by NIH grant #HL63470.
Reprints:Jaroslaw P. Maciejewski, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Building 10, Room 7C103, Bethesda, MD 20892; e-mail:renosaurus@aol.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal