When bcl-2 is immunoprecipitated from 32P-labeled cell extracts of all-trans retinoic acid (ATRA)-treated acute myeloblastic leukemia (AML) blasts, a phosphorylated protein of approximately 30 kd is coprecipitated. This protein has been identified as ribosomal protein S3a. The biologic effects of S3a include favoring apoptosis and enhancing the malignant phenotype. We sought to determine whether S3a, like bcl-2, influenced the response of cells to chemotherapeutic drugs and ATRA. Cell lines were studied in which S3a was genetically increased or disrupted; increased S3a was regularly associated with increased plating efficiency and increased sensitivity to either cytosine arabinoside (ara-C) or doxorubicin (DNR). S3a did not affect the sensitivity of cells to paclitaxel. Pulse exposures to either 3HTdR or ara-C showed a greater percentage of clonogenic cells in the S phase of the cell cycle in cells with increased S3a than in controls. Cells with increased S3a responded to ATRA by increased ara-C or DNR sensitivity, whereas cells with reduced S3a protein were either protected by ATRA or not affected. We studied cryopreserved blast cells from patients with AML or chronic myelomonocytic leukemia (CMML). S3a protein levels were heterogeneous in these populations. In 32 cryopreserved blast populations, S3a levels were significantly correlated with both bcl-2 and with cell growth in culture. As in cell lines, high S3a in cryopreserved blasts was associated with ATRA-induced sensitization to ara-C. No significant association was seen between S3a levels and response to treatment.
The sensitivity in culture of the blast cells of acute myeloblastic leukemia (AML) to cytosine arabinoside (ara-C) and doxorubicin (DNR) is affected by the presence of regulators in the medium. Factor-sensitive blasts are protected by IL-3 or granulocyte-macrophage colony-stimulating factor (GM-CSF) but are sensitized by granulocyte colony-stimulating factor (G-CSF).1-3 Blast sensitivity is reduced by hydrocortisone before the administration of ara-C or DNR, and blasts become more sensitive if all-trans retinoic acid (ATRA) is given after exposure to either drug.4-6 Regulators appear to alter drug sensitivity by acting on events after drug-induced injury; these mechanisms regulate the probabilities of cell recovery or apoptosis.7
The bcl-2 family members are important regulators of apoptosis.8,9 In the course of testing the hypothesis that bcl-2 is part of the mechanism(s) by which ATRA affects drug sensitivity,10 we observed that bcl-2 becomes phosphorylated after the exposure of blasts to ATRA in culture.11 As part of the study, control and ATRA-treated blasts were labeled metabolically with32Pi; immunoprecipitates were then examined by radioautography, and Western blots were stained for bcl-2. Phosphorylated bcl-2 was increased compared to controls; however, in the lane with immunoprecipitated bcl-2, another band of phosphorylated protein was observed with a molecular weight of approximately 30 kd. This was identified as the ribosomal protein S3a.
S3a is a highly basic protein; DNA clones from human12 and from rat cells13 have been characterized and shown to encode a highly conserved 29.8-kd protein. The S3a gene was cloned independently in 2 other laboratories: Kho et al14 used revertants after v-fos transformation of Rat-1 cells to isolate a gene that cooperated with v-fos to maintain the malignant phenotype, which they termed the v-fos transformation effector gene (Fte-1). Subsequently, Fte-1 was shown to be identical with S3a.13 Naora et al15 reported that thenbl gene, isolated because of its abundance in a Burkitt lymphoma cDNA library, was part of the regulation of dexamethasone apoptosis; nbl was also found to be identical with S3a.16
With these reports showing the biologic activity of S3a, we asked whether the gene product was associated with the regulation of drug sensitivity. As an established example of the activity of S3a, we obtained Rat-1 cells and 2 sublines, 1302 with enhanced S3a activity and R2,2 with a disrupted S3a gene.17 This system provided evidence that S3a has a role in regulated drug sensitivity. As a link with malignant hemopoiesis, S3a was transfected to human histiocytic line U-937 cells in the sense and antisense orientations. These cells provided confirmation of the effects seen with the rat cell lines. Together both models confirmed S3a's role in cell growth (increased plating efficiency) and progression through the cell cycle.17 A further link with regulated drug sensitivity was provided by the observation that ATRA sensitized cells with high S3a values but not control cells. We then looked at S3a and bcl-2 in human cryopreserved AML and CMML blast cells. Expression of both proteins was heterogeneous, but the 2 protein levels were significantly associated. From the survey, we identified 2 blast populations with high S3a values and 2 blast populations with low values. In agreement with the results obtained in the cell lines, the blasts with high S3a were sensitized to ara-C toxicity by ATRA, whereas those with low values were unaffected by ATRA. We concluded that S3a may be part of the mechanism of regulated drug sensitivity and that its participation may include a relationship with bcl-2.
Material and methods
Isolation and identification of the 30-kd protein
OCI/AML-5 cells were treated with ATRA and labeled in vivo with32Pi, and cell lysates were prepared from the treated cells. Large-scale immunoprecipitation of bcl-2 and related binding proteins was performed with anti Bcl-2 antibody. The immunoprecipitates were separated through sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and the resultant gel was exposed to x-ray films for radioautography to localize the phosphorylated proteins. Subsequently, the gel was stained by metal salt (0.3 mol/L zinc chloride). In addition to phosphorylated Bcl-2, which is a 26-kd protein, a 30-kd phosphorylated protein was coprecipitated with Bcl-2; this was visible by radioautography and on a metal salt-stained gel. The 30-kd protein band was excised from the gels and sent to collaborators at Amgen (Thousand Oaks, CA), who used matrix-assisted laser desorption/ionization–electrospray ionization mass spectroscopy to identify the peptide of the protein. By peptide-mass searching of the EST databases, the 30-kd putative protein coprecipitating with Bcl-2 was identified as the ribosomal protein S3a.18
Transfection of S3a into leukemia cells
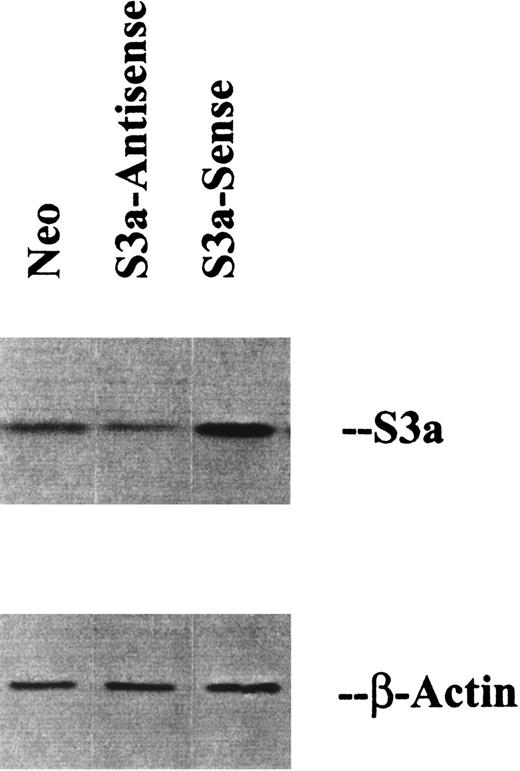
Mammalian expression vector pcDNA3 (Invitrogen, San Diego, CA) was used for constructions of human S3a. Full-length human S3a cDNA was excised from vector pMneo-Fte1(S3a), generously provided by H Zarbl (Fred Hutchinson Cancer Center), by EcoR I digestion.19 The S3a cDNA was then ligated to the pcDNA3 vector. Endonuclease mapping identified constructs in sense and antisense orientations of S3a. U-937 monocytic cell line20 was used for the expression of S3a by gene transfection. Through electroporation with a Bio-Rad gene pulser (Hercules, CA) at 250 V and 950 μFD, we transfected 20 μg purified pcDNA3 plasmid carrying human S3a in sense and antisense orientations to U-937 cells. Forty-eight hours later, cells were placed in growth medium containing G-418 for clone selection. Figure1 shows Western blots of the parental U-937 cells and the sense and antisense transfectants stained for S3a. The data in the figure confirm that increased S3a protein levels followed the transfection of sense-oriented cDNA, whereas transfection of cDNA in the antisense orientation led to decreased protein levels.
Western blots of lysates prepared from U937 cells transfected with vector alone (neo) or with S3a in the sense or antisense orientation.
The blots were also stained for β-actin as a loading control.
Western blots of lysates prepared from U937 cells transfected with vector alone (neo) or with S3a in the sense or antisense orientation.
The blots were also stained for β-actin as a loading control.
Cell lines
H. Zarbl kindly provided Rat-1 cells and 2 sublines prepared in his laboratory. The first, 1302, is a v-Fos-transformed Rat-1 cell line shown to have increased expression of S3a.17 The second, R2,2, was made by infecting 1302 with DNA from a normal human fibroblast cDNA retroviral expression library. A single copy of the retrovirus was inserted in the S3a gene of R2,2, disrupting its function.17 The AML blast cell lines OCI/AML-2 and OCI/AML-5, isolated in this laboratory, were used in metabolic labeling experiments.
Reagents and antibodies
All-trans retinoic acid (Sigma, St Louis, MO) was dissolved in 100% ethanol at a concentration of 10−2 mol/L. At a concentration of 10−2 mol/L, ara-C and DNR were dissolved in phosphate-buffered saline (pH 6.5). The solutions were diluted to the final concentrations in the culture. Monoclonal antibodies against human bcl-2, 6C8, was a gift from S. J. Korsmeyer (St Louis, MO). Goat polyclonal antibodies against S3a (S3a62-120 and S3a67-183) were gifts from J. Stahl (Berlin, Germany).32Pi was obtained from Dupont (Mississauga, Ontario, Canada)
Metabolic labeling
For in vivo metabolic labeling with 32Pi, OCI/AML-5 cells were cultured in α-minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal calf serum (FCS; growth medium) and 10% medium conditioned by 5637 cells (5637-CM). An hour before labeling, the medium was removed and the cells were washed twice with Tris-buffered saline. Cells were resuspended in phosphate-free MEM (Sigma) supplemented with 5% dialyzed FCS at a concentration of 106 cells/mL and were incubated at 37°C for 30 minutes. 32Pi (Dupont) was added to the culture at the final radioactivity of 1 mCi/mL in the presence of 10−7 mol/L ATRA. The cells were incubated at 37°C for another 15 hours. Orthophosphate-labeled cells were washed with Tris-buffered saline and lysed with lysis buffer. Radiolabeled lysate containing 1 mg protein from each sample was immunoprecipitated with the anti-bcl-2 antibody 6C8, and the precipitates were separated by 12.5% SDS-PAGE and subsequently electrotransferred onto polyvinylidene difluoride filters (Dupont, Boston, MA). Filters with radiolabeled bcl-2 and coprecipitated proteins were first exposed to a phosphor imaging screen (Molecular Dynamics, Sunnyvale, CA) for the detection of radioactivity and then were stained with anti-Bcl-2 antibody (bcl-2 124) or anti-S3a antibody (S3a 62-120); this was followed by immunostaining.
Western blot analysis and immunoprecipitation
Western blot analysis was used to measure bcl-2 and S3a proteins. Briefly, cells at the concentration of 5 × 106cells/mL were lysed in lysis buffer (10 mmol/L Tris, pH 7.4, 0.15 mol/L NaCl, 5 mmol/L EDTA, 1 mmol/L phenylmethylsulfonyl fluoride, 2 mg/mL aprotinin, and 1% triton X-100) with freshly added protease inhibitors. Nuclei were removed by centrifugation. Protein (100 μg) from the nuclear-free preparation of each sample was solubilized with SDS-PAGE sample buffer and electrophoresed through 12.5% SDS-polyacrylamide gels. For immunoblotting, the proteins separated by SDS-PAGE were electrotransferred to nitrocellulose filters (Amersham, Oakville, Canada). Filters with the proteins were blocked with phosphate-buffered saline containing 2% nonfat milk and 0.1% Tween-20 (Sigma) and were incubated with primary antibody for 2 hours. Then they were incubated with horseradish peroxidase-labeled secondary antibodies (Amersham) for another 2 hours. After that, they were developed using the echochemiluminescence detection system (Amersham). For immunoprecipitation, the lysates from untreated and treated cells were precleared by adding 2 μg normal hamster IgG and 20 μL 50% (vol/vol) protein G–agarose for 2 hours at 4°C, followed by centrifugation to remove the protein G-agarose beads. Specific antibody against human bcl-2 6C8 was added to the lysate and incubated for 2 hours; this was followed by incubation with 20 μL 50% protein G-agarose for another 2 hours to capture the immunoprecipitates. Immunoprecipitates were then separated by 12.5% SDS-PAGE and electrotransferred to nitrocellulose filters (Amersham). The bcl-2 protein on the filters was detected by the immunostaining method described above. For gradient gels, equivalent amounts of protein (100 μg) from each cell lysate were separated by 10% to 20% gradient SDS-PAGE. After electrophoresis, the proteins were transferred to nitrocellulose filters, and Western blot analysis was carried out as described above.
Analysis of phosphorylation of amino acids
Phospho-amino acid analysis was used to determine which amino acids in S3a protein were phosphorylated in AML cells exposed to ATRA. Cells were incubated overnight in phosphate-free medium with 5% dialyzed FCS and 32Pi (1 mCi/mL) in the presence of ATRA (10-7 mol/L) as described above. The cells were lysed and the lysate precleared and immunoprecipitated with anti-bcl-2 antibody (6C8). Immunoprecipitates separated by SDS-PAGE were cleared again, immunoprecipitated with anti-bcl-2 antibody 6C8, and transferred to a polyvinylidene difluoride membrane. After autoradiography, the radiolabeled coprecipitating 30-kd S3a protein was excised, digested with TPCK-trypsin, lyophilized, and treated with 6 N HCl for 1 hour at 110°C and dried. Residual hydrochloride was removed with water by lyophilization. The phospho-amino acids were then identified by 2-dimensional electrophoresis first in buffer pH 1.9 and then in buffer pH 3.5 on a thin-layer chromatography plate; radioactive amino acids were visualized by autoradiography. These were compared to the position of ninhydrin-stained control phospho-amino acids.
Clonogenic assay
Cells were cultured in suspension in growth medium for various times as determined by the experimental design. U937, transfectants of U937, and the 2 OCI/AML lines were plated in a 96-well plate at a concentration of 103 cells per well with 0.8% methylcellulose with α-MEM and 10% FCS. For the factor-responsive OCI/AML-5 cells, 5637-CM was added at a concentration of 10%. Plates were incubated at 37°C in an atmosphere of 5% CO2 in air. Colonies containing more than 20 cells were counted after 5 to 7 days using an inverted microscope. Clonogenic cell recovery was calculated by multiplying the cell number by plating efficiency in methylcellulose. Drug dose response curves were obtained by exposing cell suspensions to increasing drug concentrations for 24 hours, washing the cells, and plating a constant volume in 4 wells. Clonogenic cell recovery was calculated from the mean of 4 replicate wells; this was plotted on semilogarithmic paper as a function of drug concentration. Each survival curve was repeated at least twice.
Cryopreserved blast cells from patients
Cryopreserved cells from 32 patients with AML were thawed, cultured for 24 hours in growth medium and 10% 5637-CM cells known to contain G-CSF and GM-CSF, and used for cell culture experiments or as sources of lysates to be examined for S3a and bcl-2 proteins.
Statistics
To calculate the slopes (D10 values) of the drug survival curves, simple negative exponential curves were fitted to the average of the replicates by using a log-linear model with variance proportional to the mean. Comparisons between curves were made by forming F-ratios. The numerator of these F-ratios consisted of the decrease in Poisson deviance obtained by including additional parameters, divided by the number of additional parameters. For the denominator of the F-ratios, the overdispersion parameter was estimated by chi-square analysis of the larger model divided by the degrees of freedom.21 The calculations were implemented as a set of Minitab macros (Minitab Inc, University Park, PA). Correlation coefficients and Mann-Whitney U test comparisons were calculated using programs included in Minitab.
Results
Identification of S3a
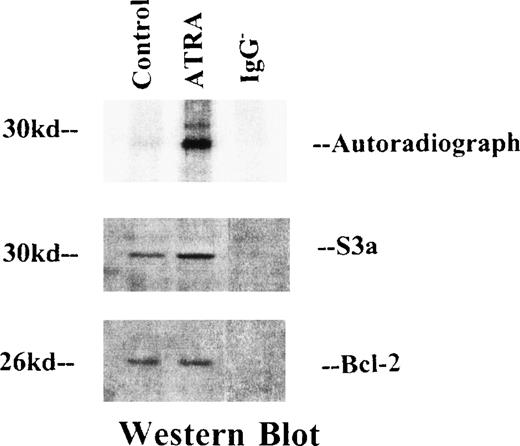
To confirm the identification of the 30-kd protein coprecipitating with phosphorylated bcl-2 as S3a, OCI/AML-5 cells were incubated for 15 hours with 32Pi as a control or with ATRA (10−7 mol/L). Lysates of metabolically labeled control and ATRA-treated cells were immunoprecipitated with anti-bcl-2 antibodies and prepared either for radioautography or Western blot analysis. Results are shown in Figure 2. Radioautographs at the top of the figure show the expected strikingly increased phosphorylation of bcl-2 in the ATRA-treated cells; the phosphorylated 30-kd protein is also evident in the lane for the immunoprecipitate from ATRA-treated cells. Western blots were stained either with anti-bcl-2 (bottom) or anti-S3a (middle). The 30-kd protein stained positively for S3a, whereas the 26-kd protein stained for bcl-2. The presence of S3a in the Western blot of the control lysate from cells not treated with ATRA is evidence that phosphorylation of bcl-2 or S3a may not be required for the binding of these 2 proteins. In other experiments (data not shown), S3a copreciptated with bcl-2 in experiments in which cells were neither labeled metabolically nor treated with ATRA.
OCI/AML-5 cells were labeled with32Pi and immunoprecipitated with anti-bcl-2 antibodies.
Immunoprecipitates were separated by SDS-PAGE electrophoresis. (top) Autoradio-graph of the gel, showing 2 bands of radiolabeled protein. (bottom) Western blots. The blot in the middle was stained for S3a and shows a band at the position of a 30-kd protein. The lower blot was stained with anti–bcl-2; it shows a band representing the 26-kd bcl-2.
OCI/AML-5 cells were labeled with32Pi and immunoprecipitated with anti-bcl-2 antibodies.
Immunoprecipitates were separated by SDS-PAGE electrophoresis. (top) Autoradio-graph of the gel, showing 2 bands of radiolabeled protein. (bottom) Western blots. The blot in the middle was stained for S3a and shows a band at the position of a 30-kd protein. The lower blot was stained with anti–bcl-2; it shows a band representing the 26-kd bcl-2.
Phosphorylation of serine in S3a from ATRA-treated cells
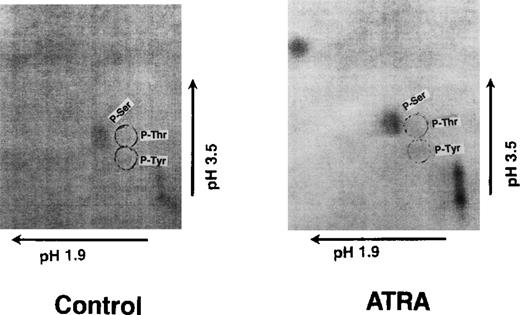
Phosphorylated S3a was excised from the filter illustrated in Figure2, and the phosphorylated amino acid was identified as described in “Materials and Methods.” Figure 3shows the 2-dimensional thin-layer chromatograph with the label detected by autoradiography. The positions of P-serine, P-threonine, and P-tyrosine were detected by ninhydrin staining. It is evident that only phosphorylation of serine was detected.
Thin-layer chromatographs show phosphorylation of S3a on serine after the treatment of OCI/AML-5 cells with ATRA.
See description in text.
Thin-layer chromatographs show phosphorylation of S3a on serine after the treatment of OCI/AML-5 cells with ATRA.
See description in text.
Response to Ara-C, DNR, and paclitaxel
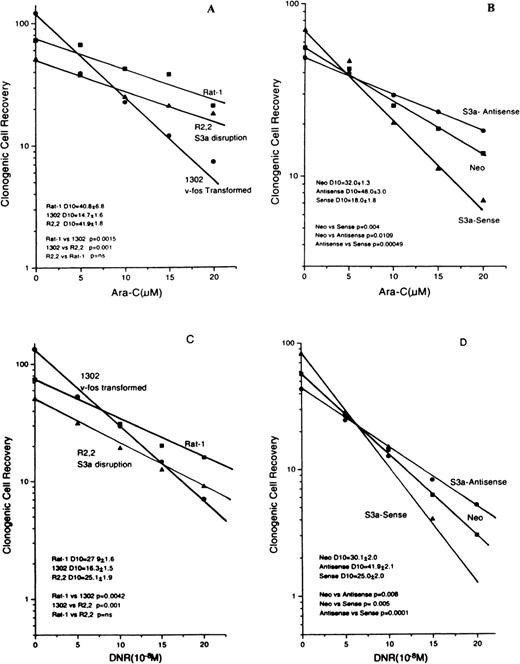
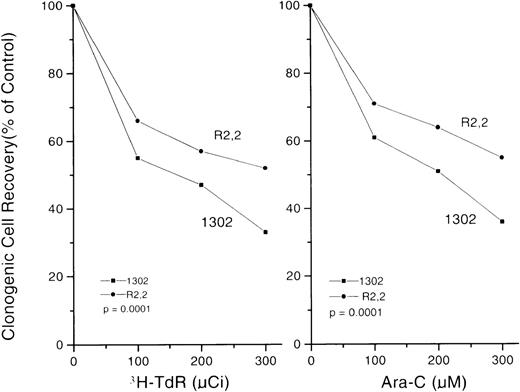
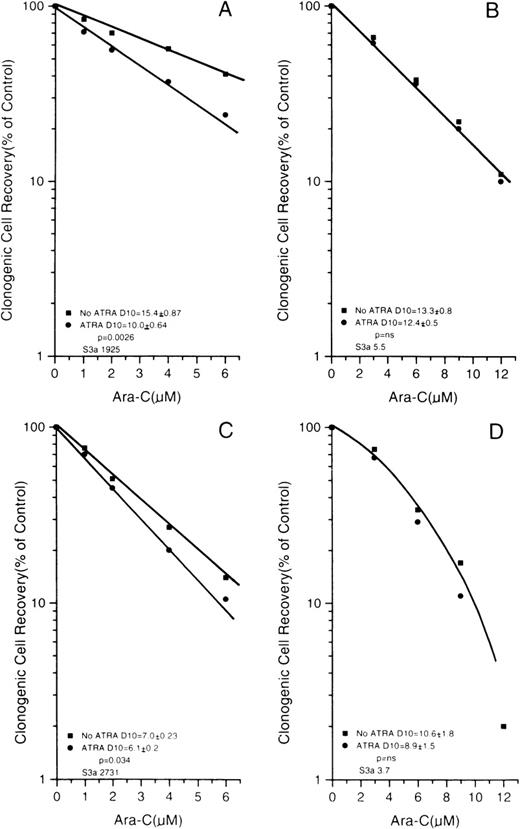
The ara-C and DNR dose-response curves for Rat-1 and its 2 sublines, 1302 and R2,2, are shown in Figures 4 A and 4C; the curves for the monocytic cell line U-937 and its sense or antisense transfectants are in Figures 4B and 4D. The cell lines with altered S3a have common features. First, cells with increased S3a (1302 in the Rat-1 system and sense transfectants of U-937) had higher plating efficiencies in the absence of drug compared to controls with less S3a (parental Rat-1 cells and R2,2 cells in the rat system and U-937 cells transfected only with empty vector). The highly significantly increased clonogenic cell recovery seen for populations with increased S3a indicated an effect of S3a in growth, as was expected from its role in protein synthesis. Second, cells with increased S3a were more sensitive to both ara-C and DNR than their appropriate controls, as seen by a decrease in the drug dose required to reduce survival rates to 10% of untreated levels (D10 values). Thus, the effects of S3a were consistent in both systems and for both ara-C and DNR. In contrast, the slope of paclitaxel dose-response curves of 1302 and R2,2 cells were not significantly different (D10 values 13.5 × 10−7 mol/L paclitaxel ± 1.7 and 15.7 × 10−7 mol/L paclitaxel ± 1.1;P = NS).
Dose-response curves for Rat-1 cells and sublines R2,2 and 1302 exposed to either ara-C (A) or DNR (C).
Dose-response curves for U937 cells transfected with vector only or S3a in the sense or antisense orientations. (B) Cells exposed to ara-C. (D) Cells exposed to DNR. The slopes and the comparisons of slopes are shown for each panel.
Dose-response curves for Rat-1 cells and sublines R2,2 and 1302 exposed to either ara-C (A) or DNR (C).
Dose-response curves for U937 cells transfected with vector only or S3a in the sense or antisense orientations. (B) Cells exposed to ara-C. (D) Cells exposed to DNR. The slopes and the comparisons of slopes are shown for each panel.
Clonogenic cells in DNA synthesis
The highly significantly increased clonogenic cell recovery seen for populations with increased S3a indicated an effect of S3a in growth, as was expected from its role in protein synthesis. We asked whether the effect of S3a on drug sensitivity had a similar basis. Accordingly, we measured dose-response curves of 1302 and R2,2 cells exposed to 1-hour pulses of either high-specific activity 3HTdR or high ara-C concentration. The 3HTdR-pulse experiment yielded dose-response curves that were characterized by an initial fall. Cells in the S phase of the cycle are killed by incorporation of the radioactive thymidine into DNA. With increasing specific activity, no further reduction is seen because cells not in the S phase are not killed.22 A pulse exposure to ara-C at increasing concentrations yields curves similar to those seen for3HTdR because ara-C killing is caused by incorporation of the drug into DNA. Figure 5 contains clonogenic cell survival curves for 1302 and R2,2 cells after a3HTdR pulse (left panel) or an ara-C pulse (right panel). For both agents, the form of the survival curves was similar, showing that the killing of clonogenic cells was caused by incorporation of the lethal agent into DNA. However, in both experiments, the percentage of cells in DNA synthesis, as shown by maximum killing, was greater for the 1302 cells than for the R2,2 cells, indicating that clonogenic cells with intact S3a had a higher percentage of cells in the S phase of the cycle. This result was consistent with cell-cycle modification by S3a as a mechanism for the increased drug sensitivity seen in such cells.
Rat-1 sublines R2,2 and 1302 exposed to 1-hour pulses of increasing activity of 3HTdR (left) or increasing concentrations of ara-C (right). For each condition, the plateau of the dose-response curve was lower for 1302 cells than for R2,2 cells.P values were calculated by comparing the values for the 4 replicates at each exposure point.
Rat-1 sublines R2,2 and 1302 exposed to 1-hour pulses of increasing activity of 3HTdR (left) or increasing concentrations of ara-C (right). For each condition, the plateau of the dose-response curve was lower for 1302 cells than for R2,2 cells.P values were calculated by comparing the values for the 4 replicates at each exposure point.
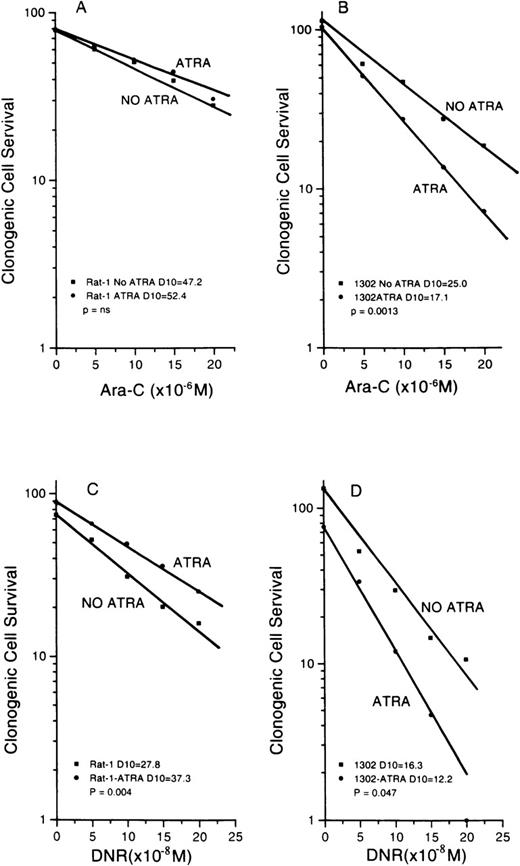
Response to ATRA
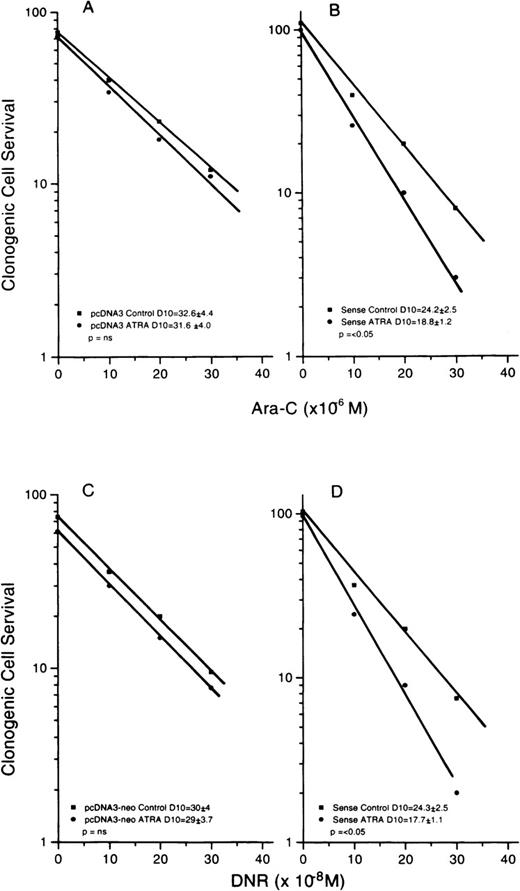
We asked whether the level of S3a altered the cellular response to ATRA, as measured by changes in drug sensitivity. Test cells were exposed to increasing concentrations of either DNR or ara-C for 24 hours, washed, treated with ATRA (10−7 mol/L), washed again, and plated. Clonogenic cell recovery was determined after 6 days of incubation. The ara-C survival curves for Rat-1 and 1302 cells are shown in Figures 6A and 6B; those for DNR are shown in Figures 6C and 6D. Comparing the control survival curves for cells given only drug, the increased initial plating efficiency of 1302 cells compared to Rat-1 cells is seen again. For Rat-1 cells, the addition of ATRA slightly increased resistance to both drugs, though not significantly. In contrast, ATRA significantly sensitized 1302 cells to ara-C and DNR. The curves for R2,2 cells are not shown because these were not significantly different from the Rat-1 curves. A similar pattern was seen for U937 cells and the transfectants. The survival curves for ara-C-treated cells are shown in Figures7A and 7B, and those for DNR are shown in Figures 7C and 7D. Again, sensitization by ATRA was seen only in the cells transfected with S3a in the sense orientation. Survival curves for antisense transfectants were not significantly different from those for vector-only controls and are not shown. The experiments showed that ATRA changed the response to drug only in the cells with intact or increased S3a.
Survival curves of Rat-1 cells and subline 1302 exposed to either ara-C (top panels) or DNR (bottom panels) after 24-hour treatment with ATRA (10−7 mol/L).
For the parental cells, there was no significant change in D10 (A) or the cells became more resistant (C). For 1302 cells, D10 values were decreased significantly after ATRA for cells exposed to ara-C (B) or DNR (D). Results with R2,2 cells were similar to the parental controls and are not shown.
Survival curves of Rat-1 cells and subline 1302 exposed to either ara-C (top panels) or DNR (bottom panels) after 24-hour treatment with ATRA (10−7 mol/L).
For the parental cells, there was no significant change in D10 (A) or the cells became more resistant (C). For 1302 cells, D10 values were decreased significantly after ATRA for cells exposed to ara-C (B) or DNR (D). Results with R2,2 cells were similar to the parental controls and are not shown.
Survival curves for U937 cells transfected with empty vector (neo) or S3a in the sense orientation, exposed to ara-C (top panels) or DNR (bottom panels) with or without previous exposure to ATRA (10−7 mol/L) before drug.
For the parental cells, there was no significant change in D10 (A) or the cells became more resistant (C). For 1302 cells, D10 values were decreased significantly after ATRA for cells exposed to ara-C (B) or DNR (D). S3a transfectants in the sense orientation showed sensitization to ara-C and DNR, whereas controls showed no effect of ATRA. Curves for transfectants in the antisense orientation were similar to those for controls and are not shown.
Survival curves for U937 cells transfected with empty vector (neo) or S3a in the sense orientation, exposed to ara-C (top panels) or DNR (bottom panels) with or without previous exposure to ATRA (10−7 mol/L) before drug.
For the parental cells, there was no significant change in D10 (A) or the cells became more resistant (C). For 1302 cells, D10 values were decreased significantly after ATRA for cells exposed to ara-C (B) or DNR (D). S3a transfectants in the sense orientation showed sensitization to ara-C and DNR, whereas controls showed no effect of ATRA. Curves for transfectants in the antisense orientation were similar to those for controls and are not shown.
Cryopreserved blasts from 32 patients
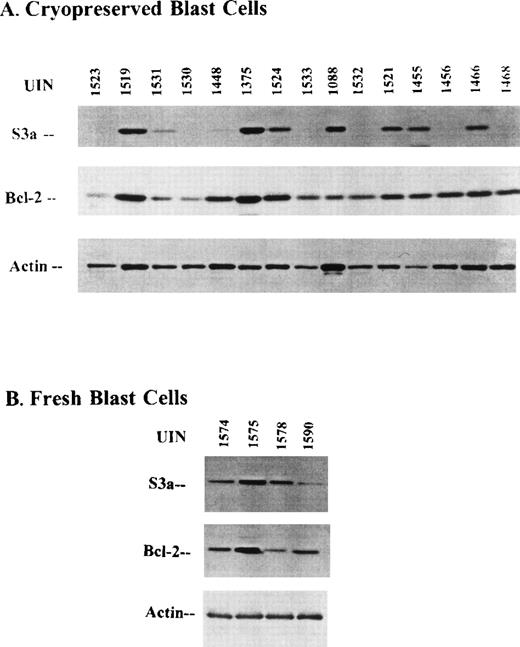
Cryopreserved blast cells were selected from the archives on the basis of availability of adequate numbers of vials. Cells were thawed, washed, and recultured, at a concentration of 106 cells/mL, for 24 hours in the presence of 5637 conditioned medium (5637-CM). Cultures were harvested, and cells of aliquots were lysed; 100μg protein (from approximately 2 × 106 cells) were loaded in each lane for Western blot analysis. Western blots were stained with anti-S3a and anti-bcl-2 antibodies. Patient-to-patient variation was seen for both proteins; it was particularly striking for S3a. Figure 8shows representative Western blots stained for either S3a or bcl-2; they were stained with anti-β-actin as a loading control, and they illustrated the heterogeneity of S3a and bcl-2. Variation is also observed in β-actin; the most extreme examples are for patients with UIN numbers 1088 and 1455. Densitometry was used to quantitate the bands in the Western blots. Data from cryopreserved blasts of 32 patients with AML or CMML are shown in Table1. Because of the variation in the β-actin loading controls, ratios were calculated by dividing the densitometry values for S3a and bcl-2 with the β-actin values. Values for the ratios were highly correlated with densitometry measurements of S3a and bcl-2 (Table 2). Figure 8 also shows Western blots from lysates of blasts freshly obtained from 4 patient with AML stained for S3a, bcl-2, and β-actin. The heterogeneity of S3a is evident in this small sample of blasts that had not been frozen.
Western blots of lysates stained for either bcl-2 or S3a
(A) Cryopreserved blast cells. (B) Freshly obtained blasts.
Western blots of lysates stained for either bcl-2 or S3a
(A) Cryopreserved blast cells. (B) Freshly obtained blasts.
Laboratory and clinical data: 32 blast populations
| UIN . | S3a . | BCL-2 . | Recovery (×105) . | PE . | Sensitivity . | Diagnosis . | Outcome . | Actin . | BLASTS (×10−9/L) . |
|---|---|---|---|---|---|---|---|---|---|
| 1088 | 1443 | 1212 | 7.6 | 14 | 9.30 | AML | F | 2983 | 3.4 |
| 1519 | 1925 | 3252 | 10.0 | 628 | 2.20 | AML | CR | 2219 | 34.0 |
| 1531 | 432 | 1332 | 8.7 | 12 | 6.70 | AML | F | 1439 | 29.7 |
| 1524 | 949 | 2939 | 5.4 | 30 | 77.80 | AML | NT | 1722 | 349.0 |
| 1523 | 1 | 1074 | 6.8 | 2 | >1.00 | CMML | F | 1514 | 10.0 |
| 1521 | 917 | 1978 | 10.0 | 375 | 38.00 | AML | F | 1486 | 220.0 |
| 1533 | 4 | 1499 | 5.1 | 940 | >1.00 | CMML | CR | 904 | 11.5 |
| 1532 | 5 | 1458 | 4.2 | 85 | >1.00 | AML | F | 1020 | 320.0 |
| 1530 | 4 | 1005 | 4.5 | 64 | >1.00 | AML | CR | 1572 | 9.0 |
| 1455 | 788 | 1838 | 9.1 | 116 | 5.70 | AML | F | 765 | 110.0 |
| 1448 | 180 | 2386 | 8.0 | 23 | 6.00 | AML | NT | 2603 | 172.0 |
| 1375 | 2731 | 3262 | 15.5 | 105 | 4.10 | AML | F | 1535 | 70.0 |
| 1468 | 124 | 1945 | 10.3 | 44 | >1.00 | AML | F | 2194 | 54.0 |
| 1456 | 3 | 1809 | 7.9 | 119 | 11.40 | AML | CR | 1795 | 159.0 |
| 1466 | 1058 | 2194 | 8.1 | 44 | 3.00 | AML | NT | 2451 | NA |
| 1331 | 1161 | 2106 | 14.7 | 845 | 2.30 | AML | NT | 1763 | 214.0 |
| 1373 | 2636 | 3704 | 11.1 | 1155 | 2.70 | AML | NT | 1944 | NA |
| 1064 | 27 | 368 | 7.2 | 46 | 20.00 | CMML | F | 2036 | 20.0 |
| 1002 | 2405 | 2222 | 15.0 | 146 | 7.90 | AML | CR | 1837 | 47.2 |
| 993 | 2208 | 2300 | 9.3 | 6 | >1.00 | AML | CR | 1942 | 1.4 |
| 1326 | 2093 | 1890 | 7.4 | 3 | >1.00 | AML | CR | 1993 | 18.6 |
| 1286 | 743 | 2846 | 9.9 | 4 | 17.90 | AML | F | 2049 | 111.0 |
| 1270 | 2266 | 2644 | 10.5 | 484 | 2.50 | AML | CR | 1817 | 9.3 |
| 1219 | 5 | 3326 | 8.8 | 326 | 5.90 | AML | CR | 2016 | 102.0 |
| 1128 | 1775 | 2064 | 12.6 | 605 | 9.60 | AML | CR | 1404 | 27.0 |
| 1179 | 2160 | 1536 | 9.0 | 93 | 2.90 | AML | CR | 1986 | NA |
| 1556 | 784 | 958 | 8.3 | 432 | 4.80 | AML | CR | 1867 | 40.0 |
| 1445 | 3162 | 2094 | 23.0 | 506 | 7.90 | AML | F | 1762 | 43.0 |
| 1440 | 918 | 2317 | 8.4 | 154 | 4.70 | AML | F | 1833 | NA |
| 907 | 1618 | 2835 | 9.3 | 196 | 91.30 | AML | F | 1796 | 88.0 |
| 904 | 2203 | 2002 | 5.8 | 907 | 5.95 | AML | F | 1844 | 7.0 |
| 899 | 3649 | 3719 | 14.7 | 131 | 10.40 | AML | NT | 2076 | 112.0 |
| UIN . | S3a . | BCL-2 . | Recovery (×105) . | PE . | Sensitivity . | Diagnosis . | Outcome . | Actin . | BLASTS (×10−9/L) . |
|---|---|---|---|---|---|---|---|---|---|
| 1088 | 1443 | 1212 | 7.6 | 14 | 9.30 | AML | F | 2983 | 3.4 |
| 1519 | 1925 | 3252 | 10.0 | 628 | 2.20 | AML | CR | 2219 | 34.0 |
| 1531 | 432 | 1332 | 8.7 | 12 | 6.70 | AML | F | 1439 | 29.7 |
| 1524 | 949 | 2939 | 5.4 | 30 | 77.80 | AML | NT | 1722 | 349.0 |
| 1523 | 1 | 1074 | 6.8 | 2 | >1.00 | CMML | F | 1514 | 10.0 |
| 1521 | 917 | 1978 | 10.0 | 375 | 38.00 | AML | F | 1486 | 220.0 |
| 1533 | 4 | 1499 | 5.1 | 940 | >1.00 | CMML | CR | 904 | 11.5 |
| 1532 | 5 | 1458 | 4.2 | 85 | >1.00 | AML | F | 1020 | 320.0 |
| 1530 | 4 | 1005 | 4.5 | 64 | >1.00 | AML | CR | 1572 | 9.0 |
| 1455 | 788 | 1838 | 9.1 | 116 | 5.70 | AML | F | 765 | 110.0 |
| 1448 | 180 | 2386 | 8.0 | 23 | 6.00 | AML | NT | 2603 | 172.0 |
| 1375 | 2731 | 3262 | 15.5 | 105 | 4.10 | AML | F | 1535 | 70.0 |
| 1468 | 124 | 1945 | 10.3 | 44 | >1.00 | AML | F | 2194 | 54.0 |
| 1456 | 3 | 1809 | 7.9 | 119 | 11.40 | AML | CR | 1795 | 159.0 |
| 1466 | 1058 | 2194 | 8.1 | 44 | 3.00 | AML | NT | 2451 | NA |
| 1331 | 1161 | 2106 | 14.7 | 845 | 2.30 | AML | NT | 1763 | 214.0 |
| 1373 | 2636 | 3704 | 11.1 | 1155 | 2.70 | AML | NT | 1944 | NA |
| 1064 | 27 | 368 | 7.2 | 46 | 20.00 | CMML | F | 2036 | 20.0 |
| 1002 | 2405 | 2222 | 15.0 | 146 | 7.90 | AML | CR | 1837 | 47.2 |
| 993 | 2208 | 2300 | 9.3 | 6 | >1.00 | AML | CR | 1942 | 1.4 |
| 1326 | 2093 | 1890 | 7.4 | 3 | >1.00 | AML | CR | 1993 | 18.6 |
| 1286 | 743 | 2846 | 9.9 | 4 | 17.90 | AML | F | 2049 | 111.0 |
| 1270 | 2266 | 2644 | 10.5 | 484 | 2.50 | AML | CR | 1817 | 9.3 |
| 1219 | 5 | 3326 | 8.8 | 326 | 5.90 | AML | CR | 2016 | 102.0 |
| 1128 | 1775 | 2064 | 12.6 | 605 | 9.60 | AML | CR | 1404 | 27.0 |
| 1179 | 2160 | 1536 | 9.0 | 93 | 2.90 | AML | CR | 1986 | NA |
| 1556 | 784 | 958 | 8.3 | 432 | 4.80 | AML | CR | 1867 | 40.0 |
| 1445 | 3162 | 2094 | 23.0 | 506 | 7.90 | AML | F | 1762 | 43.0 |
| 1440 | 918 | 2317 | 8.4 | 154 | 4.70 | AML | F | 1833 | NA |
| 907 | 1618 | 2835 | 9.3 | 196 | 91.30 | AML | F | 1796 | 88.0 |
| 904 | 2203 | 2002 | 5.8 | 907 | 5.95 | AML | F | 1844 | 7.0 |
| 899 | 3649 | 3719 | 14.7 | 131 | 10.40 | AML | NT | 2076 | 112.0 |
UIN, unique identifying number; Recovery, number of clonogenic cells recovered after 24 hours in culture with an input of 106 clonogenic cells; PE, plating efficiency; Sensitivity, ara-C D10 value; F, failure; CR, complete remission; NT, palliative treatment only; NA, not available.
Correlation coefficients
| . | Recovery . | S3a . | bcl-2 . | S3a/actin . |
|---|---|---|---|---|
| S3a | 0.657† | |||
| bcl-2 | 0.384* | 0.467* | ||
| S3a/actin | 0.695‡ | 0.963 | 0.466* | |
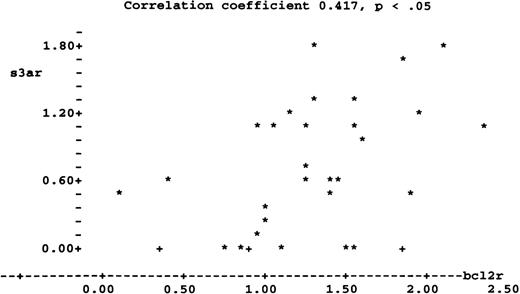
| bcl-2/actin | 0.256 | 0.279 | 0.741‡ | 0.417* |
| . | Recovery . | S3a . | bcl-2 . | S3a/actin . |
|---|---|---|---|---|
| S3a | 0.657† | |||
| bcl-2 | 0.384* | 0.467* | ||
| S3a/actin | 0.695‡ | 0.963 | 0.466* | |
| bcl-2/actin | 0.256 | 0.279 | 0.741‡ | 0.417* |
P < .05.
P < .0001.
P < .00001.
Correlation coefficient 0.963 has a P value too large to calculate.
The remaining blast cells were incubated for another 24 hours in suspension with ara-C at doses of 5, 10, 20, and 40 μmol/L. After that they were washed and plated in methylcellulose; colonies were counted after 4 to 6 days, depending on colony development, to determine the survival of clonogenic cells. This wide range of ara-C concentrations was chosen because of the known patient-to-patient variations in response to the drug.23 Additional assays measured other characteristics of cryopreserved cells. Number of cells harvested after 24 hours in suspension culture was an estimate of the capacity of the cells to survive cryopreservation and to grow; values less than input (106 cells) indicated loss during culture, whereas greater values were evidence of survival, growth, or both; colony counts from blast cells plated in methylcellulose (the control values for the ara-C survival measurements) gave the plating efficiency (PE), a measure of the number of clonogenic stem cells in the population; counts from cells treated with ara-C were used to calculate D10 values, the dose required to reduce survival to 10% of control. The clinical database provided the diagnosis and the number of blasts in the peripheral blood and indicated whether a patient had been treated with intensive chemotherapy (a combination of an anthracycline and high dose ara-C) and whether this treatment resulted in complete remission. Six patients did not receive intense chemotherapy (NT). Data for the blasts from the 32 patients are given in Table 1.
Examination of the laboratory data in Table 1 shows significant correlations between S3a, bcl-2, and recovery of cryopreserved cells after 24 hours in culture. These correlations are given in Table 2; it is evident that the most significant correlation linked S3a to cell recovery, a measure of survival after cryopreservation and growth. The correlation between S3a and bcl-2 was the next most significant; the correlation of bcl-2 with recovery was also significant. The ratios S3a/actin and Bcl-2/actin are plotted in Figure9 to illustrate the correlation between S3a and bcl-2. Values for the 3 patients with CMML are indicated in Figure9 by separate symbols. Recalculating the correlation coefficient after omitting the patients with CMML did not change the significance of the correlation. A significant correlation was seen between S3a/actin ratio and recovery; bcl2/actin ratio was not significantly correlated with recovery (Table 2).
A plot of the ratio S3a/actin (s3ar) on the vertical axis against the ratio bcl-2/actin (bcl2r) on the horizontal axis.
(*) Values from patients with AML. (†) Values from patients with CMML.
A plot of the ratio S3a/actin (s3ar) on the vertical axis against the ratio bcl-2/actin (bcl2r) on the horizontal axis.
(*) Values from patients with AML. (†) Values from patients with CMML.
The clinical data of Table 1 show that 12 of the 26 patients treated with intensive chemotherapy achieved remission. This may reflect the high blast counts of the test population (Table 1). We compared the laboratory values for the 12 responding patients with those of the 14 nonresponders by using the Mann-Whitney U test. No significant association was found between response of the treated patients, their laboratory characteristics (including S3a/actin, Bcl-2/actin ratios), drug sensitivity, and recovery of blasts after culture. The number of blasts in the peripheral blood was not significantly different in responding and nonrespondent patients, nor were blast counts associated with any of the laboratory characteristics of the patients.
Effect of ATRA on the ara-C sensitivity of cryopreserved blasts
Results obtained from experiments with Rat-1 cells and their sublines and U937 transfectants provided evidence of a relationship between S3a levels and response to ATRA. Laboratory data from patients' cryopreserved blast cells permitted the identification of blast populations of known S3a status and with ara-C sensitivity suitable for the construction of the detailed survival curves needed to determine ATRA effects on drug sensitivity. Samples from patients UIN 1219 and UIN 1456 had low S3a values and intermediate ara-C sensitivities. They were matched with patients UIN 1519 and UIN 1375, whose cells had high S3a values and intermediate ara-C sensitivities. Ara-C survival curves were prepared from these populations as controls and after exposure to ATRA (10−7 mol/L) for 24 hours. The data are shown in Figure 10. It is evident that ATRA significantly increased the ara-C sensitivity of cells with high S3a values but did not affect those with low S3a values. These data are similar to those obtained using cell lines.
Ara-C survival curves for blasts from 4 patients recovered after cryopreservation. D10 values were measured for control cells and cells exposed to ATRA (10−7 mol/L) for 24 hours before drug administration.
(A) Patient UIN 1519 and (C) patient UIN 1375 had significant sensitization after ATRA; (B) patient UIN 1219 and (D) patient UIN 1456 had no ATRA effect. Curves are normalized to control values as 100%. (B and D) Survival cures were not significantly different; a single line has been drawn through values for control and ATRA-treated cells. Values used for normalization are (A) 220, 201; (B) 63, 55; (C) 90, 80; (D) 50, 31. Values for cells not exposed to ATRA are followed by values for cells exposed to ATRA.
Ara-C survival curves for blasts from 4 patients recovered after cryopreservation. D10 values were measured for control cells and cells exposed to ATRA (10−7 mol/L) for 24 hours before drug administration.
(A) Patient UIN 1519 and (C) patient UIN 1375 had significant sensitization after ATRA; (B) patient UIN 1219 and (D) patient UIN 1456 had no ATRA effect. Curves are normalized to control values as 100%. (B and D) Survival cures were not significantly different; a single line has been drawn through values for control and ATRA-treated cells. Values used for normalization are (A) 220, 201; (B) 63, 55; (C) 90, 80; (D) 50, 31. Values for cells not exposed to ATRA are followed by values for cells exposed to ATRA.
Discussion
S3A is 1 of approximately 70 proteins associated with RNA in the large and small subunits of the ribosome. It is generally accepted that the primitive ribosome consists only of RNA. There is debate, however, as to whether proteins were developed to improve RNA folding and stability or whether preexisting proteins were adapted to bind with ribosomal RNA.24 Many ribosomal proteins have DNA-binding motifs such as zinc finger25 and helix-turn-helix motifs.26 These motifs may facilitate ribosomal protein binding to RNA; the ribosomal proteins may play roles not directly associated with ribosomal structure. Wool24 has tabulated 31 examples of ribosomal proteins with extraribosomal function. Several of these examples suggest a role for ribosomal proteins in apoptosis. For example, LPL7 has a basic-region-leucine zipper, which may explain the capacity of the gene, transfected into Jurkat cells, to suppress the translation of 2 nuclear proteins. The transfectants also show decreased growth and increased apoptosis after treatment with cyclohexamide.27 Recently, the gene encoding ribosomal protein S19 has been found to be mutated in several unrelated patients with Diamond-Blackfan anemia, indicating a role for this ribosomal protein in erythropoiesis.28
Independent isolations of the gene for the ribosomal protein S3a provided evidence of a function for the protein in maintaining the malignant phenotype of Fos-transformed cells17 and in regulating apoptosis in Burkitt lymphoma cells exposed to dexamethasone.16 The experiments reported in this article support a role for S3a in the cellular response to chemotherapy. Using cell lines with S3a altered either by disruption or transfection, we demonstrated an effect of S3a protein on the survival of clonogenic cells after exposure to either ara-C or DNR. Consistently, an increase in S3a was associated with improved growth in suspension cultures in the absence of drug and with sensitization of the cells to the lethal action of ara-C and DNR. Disruption of the gene or transfection with antisense S3a reversed both effects. The finding of increased growth led us to examine the influence of S3a on the cell cycle. We used measurements of colony formation after pulse exposure to either3HTdR or ara-C to identify clonogenic cells in the S phase of the cycle. The pulse-labeling method was used to measure cells in DNA synthesis because it selects for clonogenic cells, the same population measured in the drug dose-response curves. With both agents, we found increased killing, indicating an increased proportion of S-phase cells in Fos-transformed Rat-1 cells (1302 subline) with increased S3a compared to cells of a subline in which the S3a gene was disrupted. We concluded that this change in the percentage of cells in the S phase may explain, in part, the increased sensitivity of the cells to drugs that act on DNA. We used paclitaxel (Taxol), a drug that acts on microtubules, not DNA, to test this view, and we measured the drug's sensitivity to 1302 cells with the increased S3a and R2,2 cells and a disrupted S3a gene. The slopes of the paclitaxel dose-response curves were not different for these 2 cell lines. The data support the hypothesis that S3a is a regulator of drug sensitivity only when cytotoxicity includes damage to DNA.
We considered S3a as a candidate regulator of drug sensitivity because it is phosphorylated in ATRA-treated cells and coprecipitates with phosphorylated bcl-2. We asked whether S3a had a role in increased drug sensitivity seen after ATRA. We found that cells with increased S3a after either Fos transformation or transfection of the gene in the sense orientation were ATRA sensitized to ara-C or DNR, whereas control cells did not show the ATRA effect. Results obtained with cryopreserved blast cells from 4 selected patients with AML were consistent with the observations in cell lines because ATRA treatment increased the sensitivities to ara-C of cells with high levels of S3a but not for those with low levels of the protein. These observations may provide a basis for the selective action of ATRA, in combination with chemotherapy against tumor cells, rather than normals because S3a is usually increased in malignant cells.17 The data also emphasize the need to use laboratory data indicating responses to ATRA in evaluating clinical trials that include the regulator.
Cryopreserved samples of blast cells from patients with AML were thawed and incubated for 24 hours in growth medium supplemented by growth factors; S3a and bcl-2 were measured in lysates from these cultured cells. The incubation of thawed cells is used regularly in the laboratory to ensure that populations to be used in experiments are healthy. It is possible that the culture procedure altered the expression of the proteins under examination; nonetheless, the blast populations varied greatly in S3a protein as detected in Western blots. We used this heterogeneity to test for associations between S3a and biologic or clinical characteristics of 32 cryopreserved blast populations. A striking correlation was found between S3a levels and the recovery of cryopreserved blast cells after 24 hours in liquid suspension. Because recovery reflects survival and growth of the blast populations, this observation supports the view that S3a promotes growth and it extends that hypothesis to clinical samples from patients with AML. The correlation between S3a and bcl-2 levels may reflect the biologic functions of the 2 proteins, with the antiapoptotic role of bcl-2 complementing the growth-promoting role of S3a. The clinical data for the patients whose cells were in the archive confirmed that the patients had high blast numbers in peripheral blood, the characteristic that led to their selection for cryopreservation. This unfavorable characteristic of the patient population may explain, in part, the low success rate for remission induction. The bias introduced by studying cells only from patients with high blast counts (patients with bad prognoses) may contribute to our failure to find an association between clinical outcomes and levels of S3a or bcl-2. A prospective study of unselected patients receiving intensive chemotherapy might be rewarding. The finding of S3a heterogeneity in a small sample of AML blast cells freshly obtained from patients supports the feasibility of a study.
In summary, the work reported in this article adds S3a, a ribosomal protein, to the players that may regulate the responses of cancer cells to chemotherapy. The role of S3a in growth promotion and the maintenance of the malignant phenotype support the importance of this protein and its role in cell cycle control. S3a is responsive to regulators such as ATRA; the response includes phosphorylation and increase in sensitivity to ara-C and DNR. Manipulation of S3a levels may prove a useful addition to chemotherapy in AML.
Acknowledgments
We thank Dr Salomon Minkin for providing the Minitab macros used in the statistical analyses of survival curves. We also thank Drs C. Richardson and S. D. Patterson of Amgen for the identification of the p30 band as S3a.
Supported by the National Cancer Institute of Canada.
Reprints:E. A. McCulloch, The Ontario Cancer Institute/Princess Margaret Hospital, 610 University Avenue, Toronto, Ontario, M5G 2M9; e-mail: mcculloch@oci.utoronto.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal