The chemokine stromal cell-derived factor-1 (SDF-1), and its receptor, CXCR-4, have been implicated in the homing and mobilization of human CD34+ cells. We show here that SDF-1 may also be involved in hematopoiesis, promoting the proliferation of human CD34+ cells purified from normal adult peripheral blood (PB). CXCR-4 was expressed on PB CD34+ cells. The amount of CXCR-4 on PB CD34+ cells was 10 times higher when CD34+ cells were purified following overnight incubation. CXCR-4 overexpression was correlated with a primitive PB CD34+ cell subset defined by a CD34high CD38lowCD71lowc-KitlowThy-1+antigenic profile. The functional significance of CXCR-4 expression was ascertained by assessing the promoting effect of SDF-1 on cell cycle, proliferation, and colony formation. SDF-1 alone increased the percentage of CD34+ cells in the S+G2/M phases and sustained their survival. In synergy with cytokines, SDF-1 increased PB CD34+ and CD34highCD38low cell expansion and colony formation. SDF-1 also stimulated the growth of colonies derived from primitive progenitors released from quiescence by anti–TGF-β treatment. Thus, our results shed new light on the potential role of this chemokine in the stem cell engraftment process, which involves migration, adhesion, and proliferation. Furthermore, both adhesion-induced CXCR-4 overexpression and SDF-1 stimulating activity may be of clinical relevance for improving cell therapy settings in stem cell transplantation.
Stromal cell-derived factor-1 (SDF-1) is a member of the chemokine CXC subfamily initially cloned from the murine bone marrow stromal cell lines ST-2 and PA6,1 then purified from supernatant from the murine MS-5 cell line.2 It has been suggested that SDF-1 is involved in immune surveillance because it is a highly effective lympho-monocyte chemoattractant3 and it supports B-cell progenitor proliferation.1,4 The constitutive expression of SDF-1 in various tissues and its highly conserved nucleotide and amino acid sequences5,6 suggest that this molecule may play an important biological role. Gene knockout experiments have confirmed the essential role of SDF-1 in hematopoietic development.4 SDF-1–deficient mice have fewer myeloid progenitors than normal mice in bone marrow, but fetal liver hematopoiesis is unaffected in these mice, suggesting that SDF-1 is involved in the migration of hematopoietic stem cells between embryonic hematopoietic sites.4 Other studies have implicated SDF-1 in cell trafficking, in a model in which hematopoietic progenitor migration and mobilization are caused by a gradient of SDF-1 concentration in the bone marrow microenvironment.7,8 SDF-1 may also be involved in megakaryocytic migration and platelet formation, by increasing the adhesion of mature megakaryocytes to endothelium.9 10
SDF-1 has been identified as the ligand for the leukocyte-derived seven transmembrane domain receptor (LESTR). This G-protein-linked receptor was originally identified as an orphan receptor with a structure very similar to that of interleukin (IL)–8 receptors.11,12Owing to the similarity of its sequence to that of other CXC chemokine receptor genes, LESTR was also named CXCR-4.13 It was also called fusin because it mediates HIV-1-CD4 T lymphocyte cell fusion.14 Several authors have reported CXCR-4 expression on mature blood cells,15 including lymphocytes, monocytes, megakaryocytes, and platelets.9,10 It has also been detected on CD34+ progenitors purified from bone marrow (BM), mobilized peripheral blood, and cord blood.10,13,16In vitro transmigration assays have shown that circulating CD34+ cells from healthy adults are sensitive to SDF-1, but expression of its cognate receptor on these progenitors has not been reported.7 8
All of these observations provide evidence that SDF-1 is critical for mobilizing and homing hematopoietic progenitor cells. However, nothing is known about the role of SDF-1 in progenitor proliferation. With respect to the stem cell engraftment process in which trafficking and proliferation are both involved, we investigated a possible role for SDF-1 in hematopoiesis. We found that CD34+ cells isolated from adult peripheral blood (PB) expressed the CXCR-4 receptor and that SDF-1 increased the proliferation of human circulating CD34+ cells. Both the percentage of CXCR-4+cells and the level of CXCR-4 expression were much higher if PB CD34+ cells were purified following overnight incubation on a plastic support (Inc+). CXCR-4 overexpression was correlated with the presence of a primitive CD34highCD38lowCD71lowc-KitlowThy-1+cell subset in PB. CXCR-4 was functional, as shown by the increase in PB CD34+ and CD34highCD38low cell expansion and colony formation caused by SDF-1, in synergy with cytokines. This stimulatory effect was even stronger with Inc+ CD34+ progenitors released from quiescence by an anti–TGF-β antibody and was correlated with a large percentage of CD34+ cells in the S+G2/M phases. Thus, the SDF-1/CXCR-4 couple seems to have a novel biological function in increasing primitive hematopoiesis.
Materials and methods
Cell sample collection
Buffy coats were obtained from healthy adults during the preparation of transfusion products. Peripheral blood was collected (450 mL) in a plastic bag (MacoPharma, Tourcoing, France) containing 80 mL of preservative-free anticoagulant (citrate-phosphate-dextrose) and centrifuged at 2300g for 20 minutes at 20°C in a cryofuge 8500 (Heraeus Sepathec, Prolabo, Briare le Canal, France).
BM cells were collected from normal donors undergoing hip prothesis surgery. Spongy bone and femoral fluid were removed and rapidly stored in RPMI 1640 (Biological Industries, ATGC Biotechnologies, Noisy le Grand, France) containing heparin (100 U/mL, Roche). BM cells nested in spongy bone were recovered with the use of a Potter grinder and several washes with RPMI 1640.
All samples were obtained, with informed consent, from donors at the Centre de Transfusion Sanguine des Armées Jean-Julliard, the Services d'Hématologie Clinique et de Chirurgie Orthopédique de l'Hôpital d'Instruction des Armées Percy (Clamart, France), and the Service de Chirurgie Orthopédique du CHR d'Aulnay-Sous-Bois (France).
Mononuclear cell preparation
PB and BM cell suspensions were diluted 1:2 in Dulbecco's phosphate-buffered saline (Biological Industries, ATGC Biotechnologies) containing 0.5% human serum albumin (HSA) and 5% citrate (PBS/HSA/ACD). Mononuclear cells were isolated by centrifugation on a Ficoll density gradient (d = 1.077 g/mL; Seromed, ATGC Biotechnologies) at 700g for 30 minutes at 20°C. The mononuclear cell layer was collected, washed twice, and resuspended in PBS/HSA/ACD. Two gentle centrifugation steps (91 g for 7 minutes) were performed to remove platelets from PB samples.
Immunomagnetic purification of CD34+ cells
PB and BM mononuclear cells expressing the CD34 antigen were immunomagnetically selected with the use of the MACS system (Miltenyi Biotech, Tebu) directly after density gradient separation (Inc−) or after an overnight incubation (18 to 20 hours) (Inc+). We incubated 5 × 106cells/mL in Iscove's modified Dulbecco's medium (IMDM) containing 2% HSA in 75 cm2 plastic culture flasks (Falcon, Becton Dickinson, Le Pont de Claix, France) at 37°C in a 5% CO2/95% air atmosphere. Nonadherent cells, collected by washing the flask 3 times with PBS/HSA, or mononuclear cells, recovered directly after Ficoll centrifugation, were incubated for 15 minutes at 4°C with 50 μL of blocking reagent (human IgG) and 50 μL of CD34 antibody (QBEND/10, mouse IGg1) per 108 cells. Cells were washed once in PBS/5mmol/L EDTA/0.5% HSA (PEH) and incubated for 15 minutes at 4°C with colloidal MACS microbeads (50 μL/108 cells) directed against the haptenized QBEND/10. Cells were washed, resuspended, and passed through a cell strainer (Falcon) to remove clumps. The labeled cells were added to a sterile positive separation column (VS+) placed in a magnetic field. CD34+ cells were flushed with PEH outside the column, removed from the magnetic field, and collected by centrifugation (700g). Cell numbers and viability (> 97%) were assessed with a hemacytometer and trypan blue. For both the Inc+ and Inc− procedures, MACS purification produced a 90% to 98% pure CD34+ cell preparation. The Inc− and Inc+CD34+ cells accounted for 0.2% ± 0.05% and 0.18% ± 0.07% respectively of the low-density cells.
Cell sorting
PB Inc+ CD34+ cells were used to sort the CD34highCD38low subpopulation. Cell suspensions were incubated with fluorescein isothiocyanate (FITC)-conjugated mouse antihuman CD38 (T16, Coulter-Immunotech, Marseilles, France) and phycoerythrin (PE)–conjugated mouse antihuman CD34 (HPCA2, Coulter-Immunotech) for 30 minutes at 4°C, and washed twice. Then the CD34highCD38low cells were separated with the use of a Coulter Elite flow cytometer (Coulter Electronics, Margency, France). About 10% of CD34high cells with the lowest CD38 labeling were sorted as CD34highCD38low.
Cycloheximide treatment
PB mononuclear cells were treated with cycloheximide (Sigma, Saint Quentin Fallavier, France), a protein synthesis inhibitor, before the overnight incubation step, as follows. Mononuclear cells (5 × 106 cells/mL) were incubated in IMDM 2% HSA with or without cycloheximide (10 μg/mL), at room temperature for 30 minutes. Cells were then washed twice with PBS/HSA and incubated overnight as described above.
Monoclonal antibodies and secondary reagents
The monoclonal antibodies (mAbs) used for the flow cytometry were peridinin chlorophyll protein (PerCP)–conjugated mouse antihuman CD34 (8G12) and matching IgG1 isotype control purchased from Becton Dickinson (Le Pont de Claix, France). FITC- or PE-conjugated mouse antihuman CD38 (T16), CD71 (transferrin receptor YDJ1.2.2), CD117 (c-Kit, 95C3), phosphotyrosine (6D12) and IgG isotype control were obtained from Coulter-Immunotech. PE-conjugated mouse antihuman CDw90 (Thy-1, 5E10), PE- or biotin-conjugated CXCR-4 (12G5) mAbs, and biotin-conjugated IgG isotype control were purchased from Pharmingen (Becton Dickinson). The secondary reagent for CXCR-4 labeling was a streptavidin-allophycocyanin (APC, Becton Dickinson).
Immunophenotyping
We performed 3- and 4-color flow cytometry assays using a FACScalibur flow cytometer (Becton Dickinson) and CellQuest data acquisition software (Becton Dickinson). The instrument was calibrated with the use of beads (Becton Dickinson) according to the manufacturer's instructions.
Surface antigen labeling.
We carried out a multiparameter analysis of the purified cells for cell surface antigen expression. To minimize nonspecific antibody binding, we incubated cells in a flow cytometry buffer containing PBS/2% HSA/0.5% polyvalent human immunoglobulins. Cells (5 × 104 cells/40 μL PBS/0.5% HSA) were then incubated in 96-well culture plates containing 10 μg/mL (saturating concentration) of directly conjugated or unconjugated mAbs. We used biotin-conjugated anti–CXCR-4 mAb, or if it was combined with intracellular labeling, we used PE-conjugated anti–CXCR-4 mAb. Labeling with biotin-conjugated anti–CXCR-4 mAb was followed by a final incubation step with 10 μL of streptavidin-APC. Incubations were performed on ice for 20 minutes in the dark and were followed by 2 washes with ice-cold PBS/0.5% HSA. Immunostained cells were either kept on ice and immediately analyzed by flow cytometry or fixed in 1% formol PBS and analyzed within a week. The percentage of stained cells was calculated by comparison with each isotype control. In multiple staining, we adjusted compensation using the single-stained cell samples.
Intracellular antigen labeling.
Intracellular CXCR-4 and phosphotyrosine staining were performed after permeabilization. CD34+ cells (5 × 104) were incubated in a permeabilizing solution (OrthoPermeafix, Ortho Diagnostic System, Issy les Moulineaux, France) according to the manufacturer's instructions, before staining by a PE-conjugated anti–CXCR-4 or an FITC-conjugated anti-phosphotyrosine. Before intracellular CXCR-4 staining, cells were incubated in 40 μL PBS/0.5% HSA containing an unconjugated anti–CXCR-4 antibody at saturating concentration for 20 minutes in the dark and were washed twice with ice-cold PBS/0.5% HSA. The percentage of stained cells was calculated by comparison with the isotype control.
For each sample, images from 1 × 104 cells were acquired in listmode. Forward (FS) and side (SS) scattering and 2 to 4 fluorescence signals were stored in listmode data files and analyzed on a computer with WinMDI software (Trotter J; The Scripps Research Institute [TSRI], La Jolla, California). Mean fluorescence intensity (MFI) was expressed in arbitrary units (AU).
Clonogenic cell assay
Erythroid (BFU-E), granulocytic (CFU-G), monocytic (CFU-M), granulo-monocytic (CFU-GM), and multilineage (CFU-Mix)–derived colonies.
Progenitors were assessed in methylcellulose medium with the use of a slightly modified version of the Metcalf technique.17Freshly purified or cultured CD34+ cells (500 cells/mL) and sorted CD34+CD38− cells (1000 cells/mL) were plated on methylcellulose culture medium (Stem α ID, Tebu, Le Perray en Yvelines, France) containing a cocktail of 7 recombinant human (rh) cytokines: IL-3 (0.8 ng/mL), IL-6 (10 ng/mL), IL-11 (2 ng/mL), SCF (3 ng/mL), G-CSF (0.75 ng/mL), GM-CSF (0.75 ng/mL), and Epo (2 U/mL). In some experiments, we replaced the cocktail by similar concentrations of IL-3, GM-CSF, SCF (all cytokines were purchased from R&D Systems), alone or in combination (Stem α, Tebu). Duplicate 35 mm2 tissue culture dishes containing 1 mL of cell suspension were incubated at 37°C in a 4.5% CO2/95.5% air atmosphere. BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-Mix were scored on day 14 with the use of an inverted microscope and standard morphological criteria.18 For erythroid progenitors, 2 differentiation stages were distinguished, based on colony size on day 14. Large bursts containing 16 or more clusters with low hemoglobin content were defined as immature BFU-E, whereas mature BFU-E with a higher hemoglobin content were small bursts containing fewer than 16 clusters.19
Megakaryocyte (MK)–derived colonies.
Progenitors were assessed in collagen matrix with the use of the Easymega kit (Hemeris, Grenoble, France) according to the manufacturer's instructions.20,21 We incubated 1 × 104 CD34+ cells in a 1 mL serum-free Easymega medium supplemented with the following cytokines: 2.5 ng IL-3, 10 ng IL-6, and 50 ng thrombopoietin (TPO) (PeproTech Inc, Tebu). Duplicates of the culture were incubated at 37°C in a humidified atmosphere containing 5% CO2. Colonies were scored with the use of an inverted microscope after 10 to 14 days of culture, according to criteria described elsewhere.21 We distinguished 2 MK differentiation stages on the basis of clone size on day 14. Colonies containing more than 10 cells were defined as BFU-MK, whereas CFU-MK contained fewer than 10 cells. Standard cytology staining (May Grünwald Giemsa) or immunocytochemistry with a CD41 mAb (clone CS3) followed by alkaline phosphatase monoclonal anti–alkaline phosphatase detection was also performed for reliable and accurate identification of megakaryocytic colonies.
The biological effect of SDF-1 on colony formation was evaluated in both culture systems by adding free endotoxin rh SDF-1α at various concentrations (0.01, 0.05, 0.1, 0.5, 5, and 10 ng/mL; R&D Systems, Abingdon, UK) to the semisolid control media, in the presence or absence of various cytokines (individually or in combination). In some experiments, anti–rh SDF-1α monoclonal antibody (5 ng/mL, R&D Systems) was added to the culture medium.
Anti–TGF-β antibody treatment
The effect of SDF-1 on high proliferative potential-quiescent cells was evaluated with the use of the model of Hatzfeld et al.22 CD34+ cells (2 × 105cells/mL) were incubated for 48 hours in a cytokine-free/serum-free liquid medium (Stem α A) with or without a polyclonal anti–TGF-β neutralizing antibody (5 μg/mL) or its IgY isotype control (R&D Systems, Abingdon, UK). Cells were then plated on a semisolid medium, with or without SDF-1α as described above, and colonies were scored on day 18.
Liquid cultures
Freshly purified PB CD34+ cells (1 × 105 cells/mL) were cultured for 72 hours in either a cytokine-free medium (Stem α A, Tebu) or in a medium containing rh IL-3 (0.8 ng/mL), IL-6 (10 ng/mL), IL-11 (2 ng/mL), SCF (3 ng/mL), G-CSF (0.75 ng/mL), GM-CSF (0.75 ng/mL), and Epo (2 U/mL) (Stem α A1). The cells were incubated in the presence or absence (control) of rh SDF-1α (0.05 ng/mL). At various time points (0, 48, and 72 hours), cells were harvested and counted. We assessed the possible priming effect of SDF-1 by treating cells with SDF-1α (0.05 ng/mL) in a cytokine-deprived culture medium (Stem α A) for 24 hours before culture.
Stroma-free long-term liquid cultures
Freshly purified PB Inc+ CD34+(3 × 104 cells/mL) or sorted PB Inc+ CD34highCD38low cells (1 × 103 cells/mL) were cultured in quadruplicates in flat-bottomed 24- or 96-well plates for 5 weeks, in 1 mL or 200 μL, respectively, of a serum-free liquid culture medium (Stem α A) containing a combination of the following cytokines: SCF (50 ng/mL), FLT3-ligand (50 ng/mL; R&D Systems), TPO (10 U/mL; PeproTech, Tebu), added twice a week,23 with or without SDF-1 (0.5 ng/mL). At the onset of the culture, the total number of BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-Mix was determined by a methylcellulose assay. Every week, the wells were semidepopulated by the removal of one half of the culture volume, which was replaced with fresh medium and cytokines. Every 1 or 2 weeks, harvested cells were counted, and suitable aliquots were assayed for BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-Mix and for CD34+ cell numeration. Only viable cells incubated with propidium iodide were analyzed by flow cytometry.
Cell cycle analysis
Cell cycle studies were performed by means of DNA–propidium iodide (PI) binding and analysis with a FACScalibur flow cytometer. CD34+ cells (1 × 105cells/mL) were incubated for 72 hours in cytokine-free liquid medium (Stem α A) with or without SDF-1α (0.05 ng/mL). At each time point (0, 24, 48, and 72 hours), cells were harvested by centrifugation, counted, and prepared for cell cycle analysis. Cells (5 × 104) were suspended in 200 μL of PI diluted 1:50 with permeabilizing solution24 and were incubated in the dark at 4°C for 24 hours before analysis. The cell histogram FL-2 was divided into 3 regions according to cell cycle phase: G0/G1, S, or G2/M. Doublets were eliminated by gating on a peak/area plot of PI fluorescence. The data were analyzed with WinCycle software (Phoenix Flow Systems, San Diego).
Tyrosine phosphorylation analysis
BM and PB Inc+ CD34+ cells (5 × 104 cells/mL) were incubated for 20 hours in a cytokine-free liquid medium (Stem α A) with or without SDF-1α (0.05 ng/mL and 10 ng/mL). The cells were collected by centrifugation and were further stimulated with 10 ng/mL SCF or IL-3 for 5 to 15 minutes at 37°C. The time course of total tyrosine phosphorylation was detected by flow cytometry after intracellular anti-phosphotyrosine labeling (as described above).
Statistical analysis
Data were expressed as means ± standard deviation (SD). The significance of differences between groups was determined by Studentt test for paired samples. A P value of < .05 was considered statistically significant.
Results
Distinct expression of CXCR-4 on normal adult BM and PB CD34+ cells
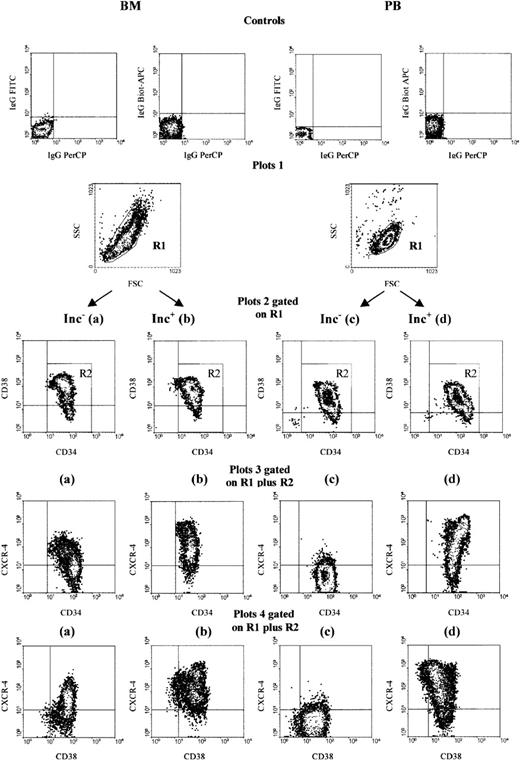
We analyzed CXCR-4 expression on human BM and PB CD34+cells using 3-color cytometry. All samples were analyzed identically. We produced 4 dot plots (Figure 1): the lymphomonocytic cell population was gated (R1) on an FSC versus SSC dot plot excluding dead cells (plot 1); a second dot plot (plot 2), displayed on R1, showed the CD34-PerCP versus CD38-FITC profile. An R2 region was further set around the CD34+ population, excluding CD34neg/low cells. Dot plots 3 and 4, displayed on additive regions R1 plus R2, showed CD34-PerCP versus CXCR-4-APC (plot 3) and CD38-FITC versus CXCR-4-APC (plot 4).
CXCR-4 expression is heterogeneous on PB and BM CD34+ cells and is up-regulated on PB Inc+CD34+ cells.
BM and PB CD34+ cells were purified immediately after density gradient separation (Inc−, plots a and c) or after incubation on a plastic support (Inc+, plots b and d). Cells were stained with CD34-PerCP, CD38-FITC, and CXCR-4-biotin-APC antibodies and analyzed by 3-color cytometry. An R1 region was first drawn by selecting the lymphomononuclear cells and excluding dead cells on an FSC/SSC dot plot (plot 1). A second region R2 corresponding to CD34+ cells was drawn on a second CD34-PerCP/CD38-FITC dot plot gated on R1 (plot 2). Expression of CXCR-4-APC versus CD34-PerCP (plots 3) and of CXCR-4-APC versus CD38-FITC (plot 4) was then assessed on the additive R1 plus R2 gates. The arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (top dot plot). The results shown are for 1 experiment representative of the 5 to 8 performed.
CXCR-4 expression is heterogeneous on PB and BM CD34+ cells and is up-regulated on PB Inc+CD34+ cells.
BM and PB CD34+ cells were purified immediately after density gradient separation (Inc−, plots a and c) or after incubation on a plastic support (Inc+, plots b and d). Cells were stained with CD34-PerCP, CD38-FITC, and CXCR-4-biotin-APC antibodies and analyzed by 3-color cytometry. An R1 region was first drawn by selecting the lymphomononuclear cells and excluding dead cells on an FSC/SSC dot plot (plot 1). A second region R2 corresponding to CD34+ cells was drawn on a second CD34-PerCP/CD38-FITC dot plot gated on R1 (plot 2). Expression of CXCR-4-APC versus CD34-PerCP (plots 3) and of CXCR-4-APC versus CD38-FITC (plot 4) was then assessed on the additive R1 plus R2 gates. The arbitrary quadrants were drawn on the basis of isotype-matched negative control profiles (top dot plot). The results shown are for 1 experiment representative of the 5 to 8 performed.
PB CD34+ cells expressed CXCR-4, but at a lower level than BM CD34+ cells. If PB CD34+ cells were directly purified after gradient separation (Inc−), fewer of them (8.8% ± 3.6%) coexpressed CXCR-4 than was the case for BM Inc− CD34+ cells (56% ± 12.3%; P < .01) (Table 1; Figure 1, plots 3c, 3a). The MFI was also lower in PB than in BM Inc− CD34+ cells (50.2 ± 29.3 AU and 91.2 ± 47.4 AU, respectively; P < .05) (Table 1).
Adult peripheral blood CD34+ cells express CXCR-4
| Source of Cells . | Overnight Incubation (Inc) . | CD34+/CXCR-4+ Cells* . | CD34+/CD38low/ CXCR-4+Cells* . | ||
|---|---|---|---|---|---|
| % of Positive Cells (Mean ± SD) . | Mean Fluorescence Intensity (AU) . | % of Positive Cells (Mean ± SD) . | Mean Fluorescence Intensity (AU) . | ||
| PB (n = 8) | − | 8.8 ± 3.6 | 50.2 ± 29.3 | 6.3 ± 2.9 | 42.3 ± 13.7 |
| + | 73.6 ± 12 | 460 ± 150 | 92.8 ± 4.7 | 605 ± 110 | |
| BM (n = 5) | − | 56 ± 12.3 | 91.2 ± 47.4 | 14.2 ± 8.3 | 48.2 ± 22.5 |
| + | 78.4 ± 17.7 | 338 ± 110 | 88.7 ± 5.3 | 208.2 ± 44.7 | |
| Source of Cells . | Overnight Incubation (Inc) . | CD34+/CXCR-4+ Cells* . | CD34+/CD38low/ CXCR-4+Cells* . | ||
|---|---|---|---|---|---|
| % of Positive Cells (Mean ± SD) . | Mean Fluorescence Intensity (AU) . | % of Positive Cells (Mean ± SD) . | Mean Fluorescence Intensity (AU) . | ||
| PB (n = 8) | − | 8.8 ± 3.6 | 50.2 ± 29.3 | 6.3 ± 2.9 | 42.3 ± 13.7 |
| + | 73.6 ± 12 | 460 ± 150 | 92.8 ± 4.7 | 605 ± 110 | |
| BM (n = 5) | − | 56 ± 12.3 | 91.2 ± 47.4 | 14.2 ± 8.3 | 48.2 ± 22.5 |
| + | 78.4 ± 17.7 | 338 ± 110 | 88.7 ± 5.3 | 208.2 ± 44.7 | |
AU indicates arbitrary units; PB, peripheral blood; BM, bone marrow.
CD34+ cells were purified from PB and BM, labeled, and analyzed for antigen expression with the use of a FACScalibur flow cytometer. Percentage of CD34+ and CD34+CD38low cells coexpressing CXCR-4 was calculated after gating on CD34+ or CD34+ CD38lowcells. Results are based on 5 to 8 independent experiments.
We further characterized the CD34+CXCR-4+subpopulation by analyzing the coexpression of the CD38 antigen, which is rare or absent on early hematopoietic progenitors. We defined the CD34+CD38low subpopulation as the 8% to 10% of CD34+ cells with the lowest level of CD38; the quadrants on plots 2 and 4 of Figure 1 were drawn on the basis of this definition. Regardless of the origin of PB and BM, a small percentage of CD34+CXCR-4+ cells (6.3% ± 2.9% and 14.2% ± 8.3%, respectively) coexpressed low levels of CD38 (42.3 ± 13.7 AU and 48.2 ± 22.5 AU, respectively) (Figure 1, plots 4c, 4a; Table 1).
Up-regulation of CXCR-4 expression on CD34+cells purified after incubation on a plastic surface
The overnight incubation in a plastic flask of PB CD34+cells before purification (Inc+) resulted in a much larger percentage of CXCR-4+ cells and a much higher level of CXCR-4 expression (73.6% ± 12% and 460 ± 150 AU) than was recorded for Inc− cells (8.8% ± 3.6%,P = .0006, and 50.2 ± 29.3 AU, P = .001; n = 8) (Table 1; Figure 1, plots 3d, 3c). In contrast, the percentage of CXCR-4+ cells from BM Inc+ CD34+cells was not significantly different from that for Inc− CD34+ cells (78.4% ± 17.7% and 56% ± 12.3%) (Table 1), but the level of CXCR-4 expression was significantly higher in Inc+ cells (338 ± 110 AU and 91.2 ± 47.4 AU, P = .003, n = 5) (Table 1; Figure 1, plots 3b, 3a).
Surface overexpression of CXCR-4 on PB Inc+CD34+ cells is correlated with a decrease in the CXCR-4 intracellular pool and protein synthesis
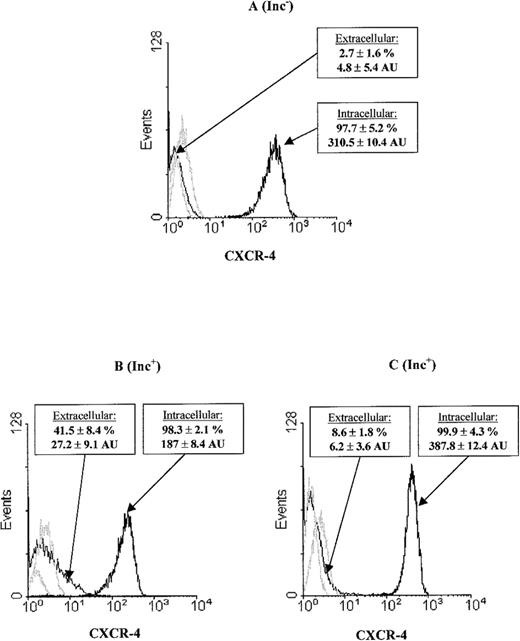
We explored the mechanism of CXCR-4 up-regulation at the PB Inc+ CD34+ cell surface and found that PB CD34+ cells had an intracellular CXCR-4 pool (Figure2). Purification of CD34+ cells after overnight incubation (Figure 2B) resulted in higher surface CXCR-4 levels (27.2 ± 9.1AU) and correspondingly lower levels of intracellular CXCR-4 expression (187 ± 8.4 AU) than were recorded for PB Inc− (4.8 ± 5.4 AU, P = .01, n = 3, and 310 ± 10.4 AU, P < .05, n = 3, respectively) (Figure 2A). We investigated the contribution of protein synthesis to CXCR-4 up-regulation during incubation by treating mononuclear cells with cycloheximide. This resulted in a significant inhibition of the increase in extracellular CXCR-4 (6.2 ± 3.6 AU,P < .01, n = 3) (Figure 2C).
CXCR-4 surface overexpression on PB Inc+CD34+ cells is correlated with a decrease in the CXCR-4 intracellular pool and protein synthesis.
PB CD34+ cells were purified (A) without (Inc−) or (B) with (Inc+) overnight incubation on a plastic support. (C) PB mononuclear cells were treated with cycloheximide before overnight incubation and CD34+purification. We studied CXCR-4 expression at the surface by labeling cells with PE-conjugated anti-CXCR-4 mAbs. CXCR-4 expression within the cell was analyzed after saturation with unconjugated CXCR-4 mAbs, permeabilization, and labeling using PE-conjugated anti–CXCR-4 mAbs. Cytometry analysis was performed on the FSC/SSC gated population as indicated in Figure 1. The histogram represents 8000 to 10 000 events in the total ungated population. The isotyped matched negative controls are shown in the overlay (light lines). Data from at least 3 donors were analyzed, with similar results. Histograms from a typical donor are presented. The mean percentage and MFI of positive cells for CXCR-4 ± SD are shown for each histogram.
CXCR-4 surface overexpression on PB Inc+CD34+ cells is correlated with a decrease in the CXCR-4 intracellular pool and protein synthesis.
PB CD34+ cells were purified (A) without (Inc−) or (B) with (Inc+) overnight incubation on a plastic support. (C) PB mononuclear cells were treated with cycloheximide before overnight incubation and CD34+purification. We studied CXCR-4 expression at the surface by labeling cells with PE-conjugated anti-CXCR-4 mAbs. CXCR-4 expression within the cell was analyzed after saturation with unconjugated CXCR-4 mAbs, permeabilization, and labeling using PE-conjugated anti–CXCR-4 mAbs. Cytometry analysis was performed on the FSC/SSC gated population as indicated in Figure 1. The histogram represents 8000 to 10 000 events in the total ungated population. The isotyped matched negative controls are shown in the overlay (light lines). Data from at least 3 donors were analyzed, with similar results. Histograms from a typical donor are presented. The mean percentage and MFI of positive cells for CXCR-4 ± SD are shown for each histogram.
PB Inc+ CD34+CXCR-4highcells coexpress low levels of Thy-1, CD38, c-Kit, and CD71 antigens
The increase in CXCR-4 expression on PB Inc+CD34+ cells especially affected the CD34+/CD38low subset (Figure 1, plot 4 d) (Table 1). CXCR-4 levels were also much higher on the PB Inc+ CD34+/CD38low cell subset than on PB Inc− cells (605 ± 110 AU and 42.3 ± 13.7 AU, P = .0005, respectively, n = 8) (Table 1). In PB Inc+CD34+ cells, the highest levels of CXCR-4 were detected on the CD38low subpopulation (Figure 1, top left quadrant of plot 4d). In contrast, in BM Inc+ CD34+ cells, the highest levels of CXCR-4 were observed on CD38highcells (Figure 1, top right quadrant of plot 4b).
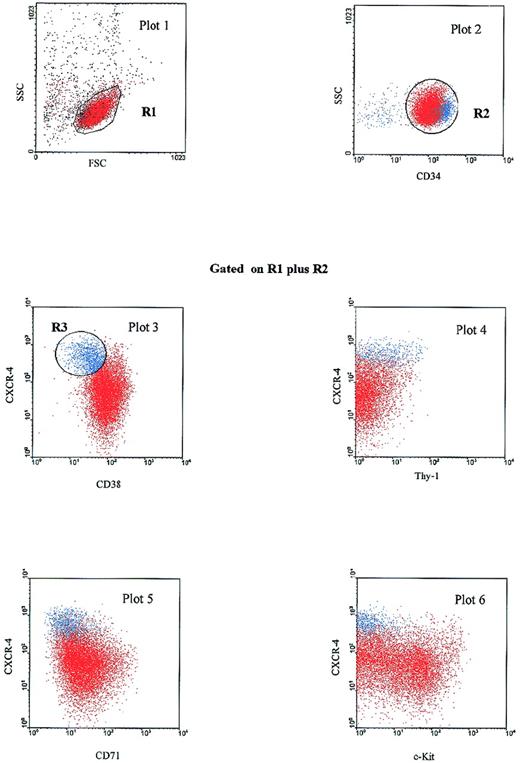
We further analyzed the antigenic profile of the CXCR-4highCD38low subset of PB Inc+CD34+ cells. We carried out phenotypic characterization by studying the coexpression of antigens such as Thy-1,25,26CD71 (transferrin receptor)27 and CD117 (c-Kit),28 low levels of which indicate immaturity. The CD34+CXCR-4highCD38low population defined on the R3 gate (Figure 3) also expressed high levels of CD34 (plot 2), low levels of CD71 and c-Kit (plots 5 and 6, respectively), and various levels of Thy-1 (plot 4). If a reciprocal gate was drawn on the CD34highCXCR-4highThy-1+ subset, this population had a similar phenotypic profile based on CD38, CD71 and c-Kit coexpression (data not shown). Thus, we identified a CXCR-4highCD34high subset in PB Inc+ CD34+ cells exhibiting antigenic characteristics of primitive hematopoietic progenitors, ie, low levels of CD38, c-Kit, CD71 and heterogeneous expression of Thy-1.29
PB Inc+CD34+CXCR-4high cells have a primitive progenitor phenotype.
PB CD34+ cells were purified after incubation on a plastic support (Inc+), stained for CD34-PerCP, CD38-FITC, CD71-PE, c-Kit-PE, or Thy-1-PE and CXCR-4-biotin-APC and analyzed by 4-color cytometry. A first dot plot (FSC versus SSC) was produced to select the lymphomonocytic cell population in a first gate (R1). A second dot plot (CD34-PerCP versus SSC) was displayed on the R1 gate. The CD34+ population was then selected by a second R2 gate. Dot plots 3 to 6, displayed on the additive regions R1 plus R2, show CXCR-4-APC versus CD38-FITC, Thy-1-PE, CD71-PE, and c-Kit-PE expression. On plot 3, a blue gate (R3) was drawn around the cell population coexpressing a high level of CXCR-4 and a low level of CD38. Cells corresponding to the R3 gate (CXCR-4highCD38low subset) are shown in blue on each dot plot. The results shown are from 1 experiment out of the 8 performed.
PB Inc+CD34+CXCR-4high cells have a primitive progenitor phenotype.
PB CD34+ cells were purified after incubation on a plastic support (Inc+), stained for CD34-PerCP, CD38-FITC, CD71-PE, c-Kit-PE, or Thy-1-PE and CXCR-4-biotin-APC and analyzed by 4-color cytometry. A first dot plot (FSC versus SSC) was produced to select the lymphomonocytic cell population in a first gate (R1). A second dot plot (CD34-PerCP versus SSC) was displayed on the R1 gate. The CD34+ population was then selected by a second R2 gate. Dot plots 3 to 6, displayed on the additive regions R1 plus R2, show CXCR-4-APC versus CD38-FITC, Thy-1-PE, CD71-PE, and c-Kit-PE expression. On plot 3, a blue gate (R3) was drawn around the cell population coexpressing a high level of CXCR-4 and a low level of CD38. Cells corresponding to the R3 gate (CXCR-4highCD38low subset) are shown in blue on each dot plot. The results shown are from 1 experiment out of the 8 performed.
SDF-1 increases PB CD34+ colony formation in presence of cytokines
The evidence, in PB, for a primitive CD34+ cell subset expressing high levels of CXCR-4 led us to assess the effects of SDF-1 on hematopoietic progenitor proliferation. We therefore evaluated the effect of SDF-1 on colony formation by CD34+ progenitors purified from normal adult peripheral blood. In all experiments, results are expressed as differences in the percentage of colonies relative to that for the controls (CT).
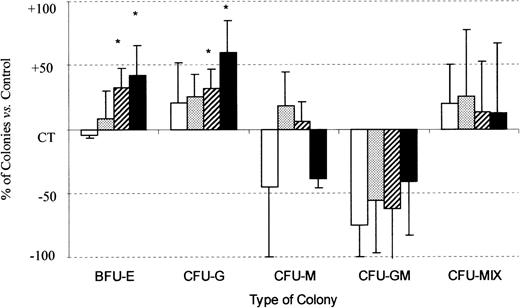
In combination with cytokines, SDF-1 (0.5 ng/mL) gave, respectively, 42% ± 25% and 60% ± 26% more BFU-E and CFU-G colony formation by Inc− CD34+ progenitor cells than was recorded for untreated control cells (P < .05, n = 5) (Figure 4). This stimulatory effect of SDF-1 was dose-dependent. The sizes of BFU-E and CFU-G were not affected by the addition of SDF-1. No significant effect was observed on CFU-M, CFU-GM, and CFU-Mix colony formation (Figure 4). SDF-1 alone had no effect on the growth of PB Inc− CD34+–derived colonies (data not shown).
SDF-1 increases BFU-E and CFU-G colony formation by PB Inc− CD34+ progenitor cells.
PB CD34+ cells were purified without incubation on a plastic support (Inc−) and plated in duplicate at a density of 500 cells/mL on semisolid Stem α ID medium containing IL-3, IL-6, IL-11, SCF, G-CSF, GM-CSF, Epo, and various concentrations of SDF-1. Colonies were scored on day 14, and results are expressed as a percentage of SDF-1 untreated control (CT) cells (mean ± SD). Control colony numbers were 53.7 ± 7 (BFU-E); 15.5 ± 4.2 (CFU-G); 5.4 ± 1.6 (CFU-M); 0.75 ± 1.3 (CFU-GM); and 1.5 ± 0.4 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 16.1% ± 3.6% (n = 5 independent experiments).* indicates significant difference from control values (P < .05); □, SDF-1 (0.01 ng/mL;  , SDF-1 (0.05 ng/mL; ▨, SDF-1 (0.1 ng/mL); ▪, SDF-1 (0.5 ng/mL).
, SDF-1 (0.05 ng/mL; ▨, SDF-1 (0.1 ng/mL); ▪, SDF-1 (0.5 ng/mL).
SDF-1 increases BFU-E and CFU-G colony formation by PB Inc− CD34+ progenitor cells.
PB CD34+ cells were purified without incubation on a plastic support (Inc−) and plated in duplicate at a density of 500 cells/mL on semisolid Stem α ID medium containing IL-3, IL-6, IL-11, SCF, G-CSF, GM-CSF, Epo, and various concentrations of SDF-1. Colonies were scored on day 14, and results are expressed as a percentage of SDF-1 untreated control (CT) cells (mean ± SD). Control colony numbers were 53.7 ± 7 (BFU-E); 15.5 ± 4.2 (CFU-G); 5.4 ± 1.6 (CFU-M); 0.75 ± 1.3 (CFU-GM); and 1.5 ± 0.4 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 16.1% ± 3.6% (n = 5 independent experiments).* indicates significant difference from control values (P < .05); □, SDF-1 (0.01 ng/mL;  , SDF-1 (0.05 ng/mL; ▨, SDF-1 (0.1 ng/mL); ▪, SDF-1 (0.5 ng/mL).
, SDF-1 (0.05 ng/mL; ▨, SDF-1 (0.1 ng/mL); ▪, SDF-1 (0.5 ng/mL).
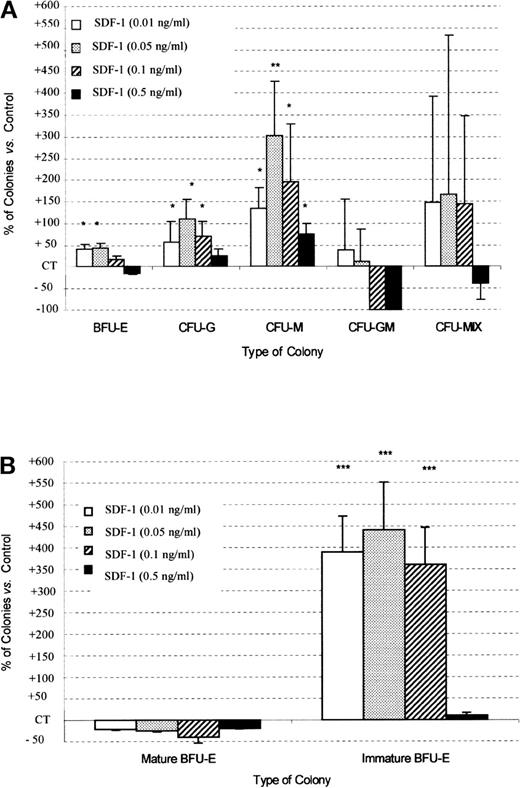
PB Inc+ CD34+ cells (Figure5) treated with SDF-1, in combination with the cocktail of cytokines, produced significantly more colonies than did PB Inc− CD34+ cells (154.4% ± 5.4% and 122.3% ± 14%, respectively, for SDF-1 0.05 ng/mL, P < .05, n = 5). This increase affected mainly the number of CFU-M (301% ± 124% versus 38.2% ± 26.6%, P = .0003, n = 5), CFU-G (110.6% ± 43.8% versus 31.5% ± 17.8%, P = .02, n = 5), and large immature BFU-E (440.4% ± 61.4% versus 15.5% ± 2.3%,P = .000 01, n = 5) (Figure 5A, 5B for Inc+ CD34+ and Figure 4 for Inc− CD34+). Unlike for BFU-E, the sizes of the other types of colonies were not affected. The stimulatory effect of SDF-1 on colony formation by Inc+CD34+ cells, unlike that in Inc−CD34+ cells, was maximal at 0.05 ng/mL, resulting in a bell-shaped curve (Figure 5A).
SDF-1 increases CFU-G, CFU-M, and immature BFU-E colony formation by PB Inc+ CD34+ progenitor cells.
PB CD34+ progenitor cells were purified after incubation on a plastic support (Inc+) and plated in duplicate, at a density of 500 cells/mL, on semisolid Stem α ID medium containing IL-3, IL-6, IL-11, SCF, Epo, G-CSF, GM-CSF, and various concentrations of SDF-1. Colonies were scored on day 14. (A) Results for total BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-Mix are expressed as a percentage of SDF-1 untreated control (CT) cells (mean ± SD). Control colony numbers were 91.7 ± 5.9 (BFU-E); 16 ± 3.1 (CFU-G); 2.8 ± 1.3 (CFU-M); 0.4 ± 0.2 (CFU-GM); and 1.75 ± 1.3 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 23.1% ± 2.1% (n = 5 independent experiments). (B) Results concerning mature and immature BFU-E are expressed as a percentage of untreated control (CT) cells (mean ± SD). BFU-E maturity was determined on the basis of clone size: large bursts containing 16 or more clusters with low levels of hemoglobin were classed as immature BFU-E, whereas small bursts with a higher hemoglobin content, containing fewer than 16 clusters, were defined as mature BFU-E. Control colony numbers for mature BFU-E and immature BFU-E were 77.7 ± 9.2 and 14 ± 3.5, respectively. An asterisk indicates significant difference from control values: * .001 < P < 0.05; ** .0001 < P < .001; *** P < .0001.
SDF-1 increases CFU-G, CFU-M, and immature BFU-E colony formation by PB Inc+ CD34+ progenitor cells.
PB CD34+ progenitor cells were purified after incubation on a plastic support (Inc+) and plated in duplicate, at a density of 500 cells/mL, on semisolid Stem α ID medium containing IL-3, IL-6, IL-11, SCF, Epo, G-CSF, GM-CSF, and various concentrations of SDF-1. Colonies were scored on day 14. (A) Results for total BFU-E, CFU-G, CFU-M, CFU-GM, and CFU-Mix are expressed as a percentage of SDF-1 untreated control (CT) cells (mean ± SD). Control colony numbers were 91.7 ± 5.9 (BFU-E); 16 ± 3.1 (CFU-G); 2.8 ± 1.3 (CFU-M); 0.4 ± 0.2 (CFU-GM); and 1.75 ± 1.3 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 23.1% ± 2.1% (n = 5 independent experiments). (B) Results concerning mature and immature BFU-E are expressed as a percentage of untreated control (CT) cells (mean ± SD). BFU-E maturity was determined on the basis of clone size: large bursts containing 16 or more clusters with low levels of hemoglobin were classed as immature BFU-E, whereas small bursts with a higher hemoglobin content, containing fewer than 16 clusters, were defined as mature BFU-E. Control colony numbers for mature BFU-E and immature BFU-E were 77.7 ± 9.2 and 14 ± 3.5, respectively. An asterisk indicates significant difference from control values: * .001 < P < 0.05; ** .0001 < P < .001; *** P < .0001.
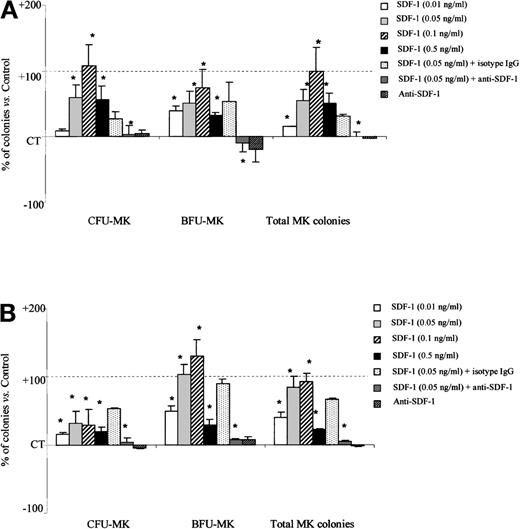
We further assessed the effects of SDF-1 on PB megakaryocytic progenitors. As for the other myeloid progenitors, SDF-1 alone had no effect on MK progenitor–derived colony growth (data not shown). However, in combination with cytokines, SDF-1 (0.1 ng/mL) significantly increased total MK colony formation by PB Inc− (99.8% ± 36.7% more than SDF-1 untreated control cells, P = .003, n = 3) and PB Inc+CD34+ cells (92.1% ± 12% more than untreated control cells, P = .006, n = 3) (Figure6A and 6B). The stimulatory effect of SDF-1 on MK progenitors was dose-dependent and maximal at 0.1 ng/mL. SDF-1 (0.1 ng/mL) treatment of PB Inc+ CD34+ cells gave 130% ± 23.6% more BFU-MK than untreated control cells (P = .006, n = 3) (Figure 6B).
SDF-1 increases the number of megakaryocytic progenitor-derived colonies from PB Inc− and Inc+ CD34+ cells.
PB CD34+ cells were purified (A) without incubation on a plastic support (Inc−) or (B) after overnight incubation (Inc+) and plated in duplicate at a density of 1 × 104 cells/mL in a serum-free Easymega medium containing IL3, IL6, and TPO in the presence or absence of SDF-1 (0.05 ng/mL) or anti–SDF-1 antibody (5 ng/mL) or isotype control (5 ng/mL). Colonies were scored after 10 to 14 days of culture. Two differentiation stages were distinguished on the basis of clone size on day 14. Colonies containing more than 10 cells were classed as BFU-MK, whereas colonies containing fewer than 10 cells were classed as CFU-MK. Results are expressed as mean percentage of SDF-1 untreated control cells (CT) ± SD. Control colony numbers for CFU-MK and BFU-MK from PB Inc− cells were 118.7 ± 43 and 35.6 ± 12.1, and for PB Inc+ cells, 149 ± 27.7; 46.4 ± 15.3, respectively. Control plating efficiency calculated on the total number of MK colonies derived from PB Inc− and PB Inc+ CD34+ cells was 1.5% ± 0.6% and 2% ± 0.75%, respectively (n = 3 independent experiments). * indicates significant difference from control values, P < .05.
SDF-1 increases the number of megakaryocytic progenitor-derived colonies from PB Inc− and Inc+ CD34+ cells.
PB CD34+ cells were purified (A) without incubation on a plastic support (Inc−) or (B) after overnight incubation (Inc+) and plated in duplicate at a density of 1 × 104 cells/mL in a serum-free Easymega medium containing IL3, IL6, and TPO in the presence or absence of SDF-1 (0.05 ng/mL) or anti–SDF-1 antibody (5 ng/mL) or isotype control (5 ng/mL). Colonies were scored after 10 to 14 days of culture. Two differentiation stages were distinguished on the basis of clone size on day 14. Colonies containing more than 10 cells were classed as BFU-MK, whereas colonies containing fewer than 10 cells were classed as CFU-MK. Results are expressed as mean percentage of SDF-1 untreated control cells (CT) ± SD. Control colony numbers for CFU-MK and BFU-MK from PB Inc− cells were 118.7 ± 43 and 35.6 ± 12.1, and for PB Inc+ cells, 149 ± 27.7; 46.4 ± 15.3, respectively. Control plating efficiency calculated on the total number of MK colonies derived from PB Inc− and PB Inc+ CD34+ cells was 1.5% ± 0.6% and 2% ± 0.75%, respectively (n = 3 independent experiments). * indicates significant difference from control values, P < .05.
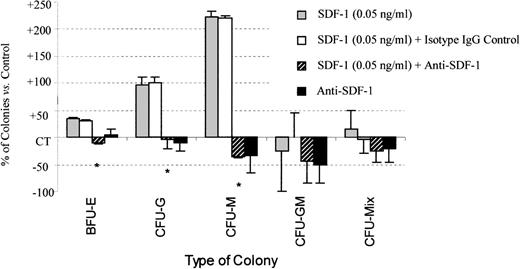
The stimulatory effect of SDF-1 on erythroid, granulo-macrophagic, and megakaryocytic colonies derived from PB CD34+ cells was totally abolished by treatment with neutralizing anti–SDF-1 antibody, demonstrating the specificity of SDF-1 biological activity (Figures 6and 7). Addition of either an anti–SDF-1 antibody alone or its isotype IgG control had no significant effect on colony formation.
Anti–SDF-1 treatment inhibits the stimulatory effect of SDF-1 on colony formation by PB Inc+ CD34+progenitor cells.
PB Inc+ CD34+ cells, purified after incubation on a plastic support, were plated in duplicate at a density of 500 cells/mL on semisolid Stem α ID medium containing IL3, IL6, IL11, SCF, G-CSF, GM-CSF, and Epo, in the presence or absence of SDF-1 (0.05 ng/mL) or anti–SDF-1 antibody (5 ng/mL), or isotype control (5 ng/mL). Colonies were scored on day 14. Results are expressed as mean percentages of SDF-1 untreated control cells (CT) ± SD. Control colony numbers were 90.2 ± 8.6 (BFU-E); 12.2 ± 5.4 (CFU-G); 3.5 ± 1.8 (CFU-M); 0.8 ± 0.3 (CFU-GM); and 1.2 ± 1.2 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 19.3% ± 5.4% (n = 3 independent experiments). * indicates significant difference from control values: 0.001 < P < .05.
Anti–SDF-1 treatment inhibits the stimulatory effect of SDF-1 on colony formation by PB Inc+ CD34+progenitor cells.
PB Inc+ CD34+ cells, purified after incubation on a plastic support, were plated in duplicate at a density of 500 cells/mL on semisolid Stem α ID medium containing IL3, IL6, IL11, SCF, G-CSF, GM-CSF, and Epo, in the presence or absence of SDF-1 (0.05 ng/mL) or anti–SDF-1 antibody (5 ng/mL), or isotype control (5 ng/mL). Colonies were scored on day 14. Results are expressed as mean percentages of SDF-1 untreated control cells (CT) ± SD. Control colony numbers were 90.2 ± 8.6 (BFU-E); 12.2 ± 5.4 (CFU-G); 3.5 ± 1.8 (CFU-M); 0.8 ± 0.3 (CFU-GM); and 1.2 ± 1.2 (CFU-Mix). The control plating efficiency (calculated on the total number of colonies) was 19.3% ± 5.4% (n = 3 independent experiments). * indicates significant difference from control values: 0.001 < P < .05.
We further evaluated the effects of SDF-1 on colony formation from BM CD34+ cells. Addition of various concentrations of SDF-1 (from 0.05 up to 10 ng/mL) did not increase either the number (Table 2) or the size of any type of colony from Inc+ and Inc−CD34+ cells.
SDF-1 does not affect the colony formation by BM Inc− or Inc+ CD34+ cells
| SDF-1 . | BM Inc−CD34+ (No. of Colonies [mean ± SD])* . | BM Inc+ CD34+ (No. of Colonies [mean ± SD])* . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-Mix . | CFU Total . | BFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-Mix . | CFU Total . | |
| 0 | 25 ± 4 | 44.5 ± 1.5 | 20 ± 3 | 23.5 ± 0.5 | 2.5 ± 0.5 | 115.5 ± 3.5 | 35 ± 3 | 66.5 ± 1.5 | 35.5 ± 3.5 | 20.5 ± 0.5 | 2.5 ± 1.5 | 160 ± 1 |
| .05 | 27 ± 2 | 47 ± 2 | 20.5 ± 6.5 | 21.5 ± 5.5 | 2 ± 1 | 104.5 ± 6.5 | 38 ± 5 | 70 ± 2 | 37.5 ± 6.5 | 22.5 ± 1.5 | 2.5 ± 0.5 | 170.5 ± 11 |
| .5 | 23.5 ± 3.5 | 46.5 ± 2.5 | 22.5 ± 7.5 | 17.5 ± 1.5 | 3 ± 1 | 113 ± 2 | 38.5 ± 3.5 | 68 ± 3 | 33 ± 3 | 22 ± 1 | 2.5 ± 1.5 | 164 ± 1 |
| 5 | 22 ± 1 | 45 ± 4 | 24.5 ± 0.5 | 19 ± 6 | 2.5 ± 1.5 | 113 ± 3 | 36 ± 1 | 69.5 ± 1.5 | 36 ± 4 | 23.5 ± 1.5 | 2.5 ± 0.5 | 167.5 ± 6 |
| 10 | 27.5 ± 1.5 | 44.5 ± 2.5 | 23.5 ± 2.5 | 20 ± 4 | 1.5 ± 1.5 | 117 ± 4 | 39.5 ± 1.5 | 67.5 ± 5.5 | 34 ± 1 | 26 ± 1 | 3 ± 1 | 170 ± 8 |
| SDF-1 . | BM Inc−CD34+ (No. of Colonies [mean ± SD])* . | BM Inc+ CD34+ (No. of Colonies [mean ± SD])* . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-Mix . | CFU Total . | BFU-E . | CFU-G . | CFU-M . | CFU-GM . | CFU-Mix . | CFU Total . | |
| 0 | 25 ± 4 | 44.5 ± 1.5 | 20 ± 3 | 23.5 ± 0.5 | 2.5 ± 0.5 | 115.5 ± 3.5 | 35 ± 3 | 66.5 ± 1.5 | 35.5 ± 3.5 | 20.5 ± 0.5 | 2.5 ± 1.5 | 160 ± 1 |
| .05 | 27 ± 2 | 47 ± 2 | 20.5 ± 6.5 | 21.5 ± 5.5 | 2 ± 1 | 104.5 ± 6.5 | 38 ± 5 | 70 ± 2 | 37.5 ± 6.5 | 22.5 ± 1.5 | 2.5 ± 0.5 | 170.5 ± 11 |
| .5 | 23.5 ± 3.5 | 46.5 ± 2.5 | 22.5 ± 7.5 | 17.5 ± 1.5 | 3 ± 1 | 113 ± 2 | 38.5 ± 3.5 | 68 ± 3 | 33 ± 3 | 22 ± 1 | 2.5 ± 1.5 | 164 ± 1 |
| 5 | 22 ± 1 | 45 ± 4 | 24.5 ± 0.5 | 19 ± 6 | 2.5 ± 1.5 | 113 ± 3 | 36 ± 1 | 69.5 ± 1.5 | 36 ± 4 | 23.5 ± 1.5 | 2.5 ± 0.5 | 167.5 ± 6 |
| 10 | 27.5 ± 1.5 | 44.5 ± 2.5 | 23.5 ± 2.5 | 20 ± 4 | 1.5 ± 1.5 | 117 ± 4 | 39.5 ± 1.5 | 67.5 ± 5.5 | 34 ± 1 | 26 ± 1 | 3 ± 1 | 170 ± 8 |
SDF-1 indicates stromal cell-derived factor-1; BM, bone marrow; BFU-E, erythroid; CFU-G, granulocytic; CFU-M, monocytic; CFU-GM, granulo-monocytic; CFU-Mix, multilineage. BM CD34+ cells were purified without (Inc−) or with (Inc+) overnight incubation on a plastic support and plated in duplicate at a density of 500 cells/mL on semisolid Stem α ID medium containing IL-3, IL-6, IL-11, SCF, G-CSF, GM-CSF, Epo, and various concentrations of SDF-1 (from 0 up to 10 ng/mL).
Colonies were scored on day 14, and results are based on 2 independent experiments.
SDF-1 acts in synergy with SCF in promoting colony formation and tyrosine phosphorylation in PB CD34+ cells
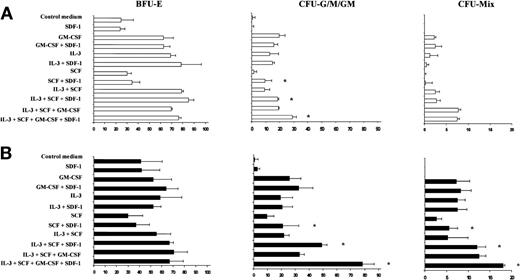
We investigated the capacity of SDF-1 to promote PB CD34+ colony formation in synergy with various cytokines individually or in combination. SDF-1 alone had no effect on PB Inc− or Inc+ colony formation (data not shown). The addition of SCF significantly increased the number of CFU-G, CFU-M, and CFU-GM colonies derived from Inc+CD34+ cells (Figure 8A). This effect is strengthened if IL-3 and GM-CSF are added to SCF. In contrast, addition of IL-3 or GM-CSF alone did not increase the promoting effect of SDF-1 on CFU-G, CFU-M, and CFU-GM colony formation. In these experimental conditions, the promoting effect of SDF-1 on BFU-E was not observed (Figure 8A), suggesting that the cytokine combinations used were ineffective in immature BFU-E formation.
SDF-1 increases CFU-G, CFU-M, CFU-GM, and CFU-Mix colony formation by PB Inc+ CD34+ progenitor cells in synergy with SCF.
PB CD34+ progenitor cells purified after overnight incubation on a plastic support (Inc+) were incubated for 48 hours in serum- and cytokine-free liquid culture medium (Stem α A) (A) without or (B) with anti–TGF-β antibody (5 μg/mL). Cells were harvested, counted, and plated in duplicate, at a density of 500 cells/mL, on semisolid Stem α medium containing Epo (control medium) and various combinations or individual cytokines (GM-CSF, IL-3, SCF, IL-3 + SCF, IL-3 + SCF + GM-CSF); SDF-1 (0.05 ng/mL) was added or not to the culture medium. Results of 2 independent experiments are expressed as the number of colonies scored on day 14. * indicates significant difference from control values: P < .05.
SDF-1 increases CFU-G, CFU-M, CFU-GM, and CFU-Mix colony formation by PB Inc+ CD34+ progenitor cells in synergy with SCF.
PB CD34+ progenitor cells purified after overnight incubation on a plastic support (Inc+) were incubated for 48 hours in serum- and cytokine-free liquid culture medium (Stem α A) (A) without or (B) with anti–TGF-β antibody (5 μg/mL). Cells were harvested, counted, and plated in duplicate, at a density of 500 cells/mL, on semisolid Stem α medium containing Epo (control medium) and various combinations or individual cytokines (GM-CSF, IL-3, SCF, IL-3 + SCF, IL-3 + SCF + GM-CSF); SDF-1 (0.05 ng/mL) was added or not to the culture medium. Results of 2 independent experiments are expressed as the number of colonies scored on day 14. * indicates significant difference from control values: P < .05.
We further analyzed the effects of SDF-1–pretreatment on global tyrosine phosphorylation of intracytoplasmic proteins in response to various cytokines. Pretreatment with SDF-1 markedly increased the SCF-induced tyrosine phosphorylation in PB Inc+CD34+ cells (Figure 9). This phosphorylation was time-dependent and was not detected after incubation with IL-3. In contrast, in BM Inc+CD34+ cells, the SCF signaling was not altered by SDF-1–pretreament even if SDF-1 was used at 10 ng/mL (data not shown).
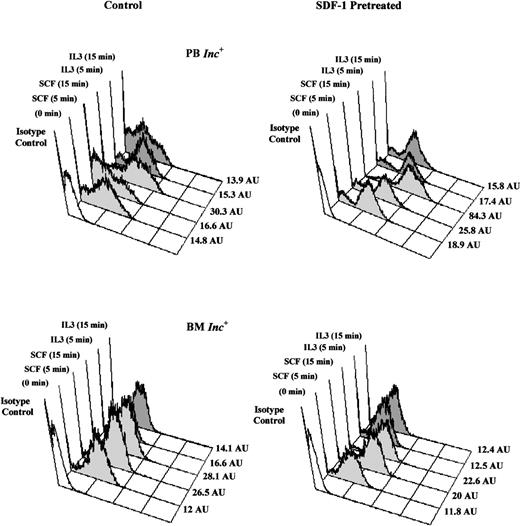
SDF-1 increases SCF-induced tyrosine phosphorylation in PB Inc+ CD34+ cells.
PB and BM CD34+ cells purified after incubation on a plastic support (Inc+) were pretreated without (control) or with SDF-1 (0.05 ng/mL) for 20 hours in serum- and cytokine-free medium (Stem α A) at a density of 5 × 104cells/mL. Cells were harvested, washed by centrifugation, and stimulated, with SCF (10 ng/mL) or IL-3 (10 ng/mL), for 5 to 15 minutes at 37°C. The time course of total tyrosine phosphorylation was detected by flow cytometry after intracellular anti-phosphotyrosine labeling. Histograms from a typical donor are presented. The MFI (AU) of positive cells for phosphotyrosine are shown for each histogram.
SDF-1 increases SCF-induced tyrosine phosphorylation in PB Inc+ CD34+ cells.
PB and BM CD34+ cells purified after incubation on a plastic support (Inc+) were pretreated without (control) or with SDF-1 (0.05 ng/mL) for 20 hours in serum- and cytokine-free medium (Stem α A) at a density of 5 × 104cells/mL. Cells were harvested, washed by centrifugation, and stimulated, with SCF (10 ng/mL) or IL-3 (10 ng/mL), for 5 to 15 minutes at 37°C. The time course of total tyrosine phosphorylation was detected by flow cytometry after intracellular anti-phosphotyrosine labeling. Histograms from a typical donor are presented. The MFI (AU) of positive cells for phosphotyrosine are shown for each histogram.
SDF-1 stimulates PB CD34highCD38low cell expansion and increases colony formation by primitive hematopoietic progenitors released from quiescence by anti–TGF-β antibody treatment
In an effort to investigate the possible effects of SDF-1 on primitive hematopoietic progenitors contained in PB Inc+CD34+ cells, we set up a stroma-free liquid culture containing early acting cytokines according to the in vitro culture system described by Piacibello et al.23 In synergy with TPO, SCF, and FLT3-ligand, SDF-1 increased the expansion of PB CD34+ cells, CD34highCD38low cells, and committed progenitors (CFC). After liquid culture for 5 weeks, the total number of CD34highCD38low and CD34+ cells generated in the presence of SDF-1, was, respectively, 1.5 times (412.5 versus 628 cells) and 2.4 times (486 versus 1161 cells) higher than for the untreated cells (Table3). The output of total CFC (BFU-E, CFU-G, CFU-M, and CFU-GM) generated by SDF-1–treated CD34highCD38low and CD34+ cells after 4 to 5 weeks of culture was 2.5 times (1280 versus 3264 CFC) and 9.7 times (980 versus 9525 CFC) higher than for the SDF-1–untreated control cells (Table 3). In preliminary experiments, with the use of a cocktail of cytokines at low concentrations (0.1 to 1 ng/mL) in addition to TPO and FLT3-ligand, the output of total CFC generated by SDF-1–treated CD34highCD38low after 4 weeks of culture was greatly increased, since it was 10.5 times (912 versus 9570 CFC) higher than for SDF-1–untreated cells. SDF-1 alone had no effect either on CD34+ and CD34highCD38low cell expansion or on CFC generation (Table 3).
SDF-1 stimulates expansion of PB CD34+ and CD34highCD38low cells in stroma-free long-term liquid culture in synergy with cytokines
| Cytokines . | No. of Cells/100 Initial Cells . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonadherent Cells (Mean No. of Nonadherent Cells)3-150 . | CD34+ or CD34high CD38low (Mean No. of Cells)3-150 . | Total CFC (Mean No. of committed progenitors)3-150 . | ||||||||||
| Start . | 3 wk . | 4 wk . | 5 wk . | Start . | 3 wk . | 4 wk . | 5 wk . | Start . | 3 wk . | 4 wk . | 5 wk . | |
| CD34+ | ||||||||||||
| Cytokine-free medium | 100 | 150 | 0 | 0 | 100 | 0 | 0 | 0 | 71.6 | 0 | 0 | 0 |
| SDF-1 | 100 | 143 | 0 | 0 | 100 | 0 | 0 | 0 | 71.6 | 0 | 0 | 0 |
| TPO + SCF + FLT3-L | 100 | 1057 | 2470 | 8470 | 100 | 271.5 | 343 | 486 | 71.6 | 236 | 600 | 980 |
| TPO + SCF + FLT3-L + SDF-1 | 100 | 1590 | 3480 | 113703-151 | 100 | 364 | 9573-151 | 11613-151 | 71.6 | 4413-151 | 26733-151 | 95253-151 |
| CD34highCD38low | ||||||||||||
| Cytokine-free medium | 100 | 50 | 0 | 0 | 100 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 |
| SDF-1 | 100 | 42 | 0 | 0 | 100 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 |
| TPO + SCF + FLT3-L | 100 | 2200 | 5500 | 8250 | 100 | 225.5 | 313.5 | 412.5 | 14.2 | 940 | 1280 | ND |
| TPO + SCF + FLT3-L + SDF-1 | 100 | 2400 | 6700 | 10560 | 100 | 452.83-151 | 5363-151 | 6283-151 | 14.2 | 1150 | 32643-151 | ND |
| Cytokines . | No. of Cells/100 Initial Cells . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonadherent Cells (Mean No. of Nonadherent Cells)3-150 . | CD34+ or CD34high CD38low (Mean No. of Cells)3-150 . | Total CFC (Mean No. of committed progenitors)3-150 . | ||||||||||
| Start . | 3 wk . | 4 wk . | 5 wk . | Start . | 3 wk . | 4 wk . | 5 wk . | Start . | 3 wk . | 4 wk . | 5 wk . | |
| CD34+ | ||||||||||||
| Cytokine-free medium | 100 | 150 | 0 | 0 | 100 | 0 | 0 | 0 | 71.6 | 0 | 0 | 0 |
| SDF-1 | 100 | 143 | 0 | 0 | 100 | 0 | 0 | 0 | 71.6 | 0 | 0 | 0 |
| TPO + SCF + FLT3-L | 100 | 1057 | 2470 | 8470 | 100 | 271.5 | 343 | 486 | 71.6 | 236 | 600 | 980 |
| TPO + SCF + FLT3-L + SDF-1 | 100 | 1590 | 3480 | 113703-151 | 100 | 364 | 9573-151 | 11613-151 | 71.6 | 4413-151 | 26733-151 | 95253-151 |
| CD34highCD38low | ||||||||||||
| Cytokine-free medium | 100 | 50 | 0 | 0 | 100 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 |
| SDF-1 | 100 | 42 | 0 | 0 | 100 | 0 | 0 | 0 | 14.2 | 0 | 0 | 0 |
| TPO + SCF + FLT3-L | 100 | 2200 | 5500 | 8250 | 100 | 225.5 | 313.5 | 412.5 | 14.2 | 940 | 1280 | ND |
| TPO + SCF + FLT3-L + SDF-1 | 100 | 2400 | 6700 | 10560 | 100 | 452.83-151 | 5363-151 | 6283-151 | 14.2 | 1150 | 32643-151 | ND |
SDF-1 indicates stromal cell-derived factor-1; ND, not determined; PB, peripheral blood. Cultures were initiated with 3 × 104 PB Inc+ CD34+ cells or 1 × 103 PB Inc+ CD34highCD38low sorted cells and maintained as described in materials and methods.
Generated, after normalization for each result per 100 cells seeded. Two experiments were performed in quadruplicate.
P < .05 versus SDF-1 untreated control.
We also assessed whether SDF-1 affected primitive progenitors released from quiescence by anti–TGF-β antibody treatment. PB or BM Inc+ CD34+ cells were incubated with an anti– TGF-β antibody or its isotype IgY control for 48 hours in a serum- and cytokine-free liquid culture medium, then plated on methylcellulose.
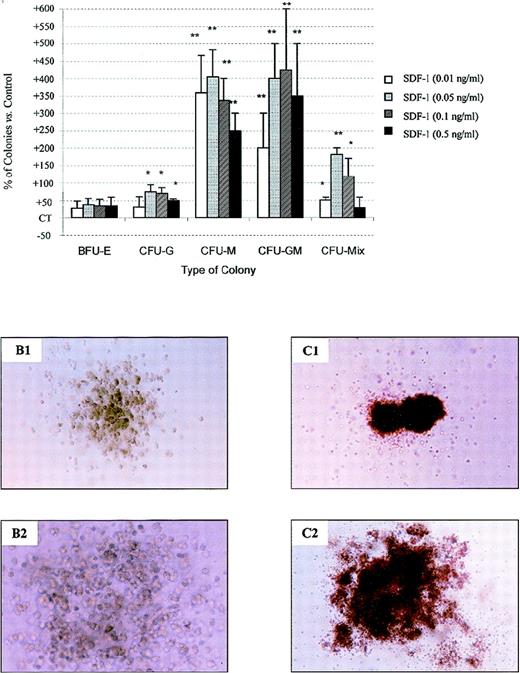
Treatment of PB Inc+ CD34+ cells with an anti–TGF-β antibody in the presence of either SCF (alone or in combination with IL-3 and GM-CSF) (Figure 8B) or a cocktail of cytokines (Figure 10) potentiated the effect of SDF-1 on CFU-GM and CFU-Mix colony formation. The number of such colonies was 425% ± 175% (P = .002, n = 3, for SDF-1 0.01 ng/mL) and 181.2% ± 18.7% (P = .04, n = 3, for SDF-1 0.05 ng/mL) higher than for cells not treated with the anti–TGF-β antibody (Figure10A). Anti–TGF-β antibody treatment also resulted in a greater increase (by 75% ± 19%) in the number of CFU-G (P < .05, n = 3) and in the number of CFU-M (404% ± 79.1%, P = .001, n = 3) caused by SDF-1 (0.05 ng/mL). In the presence of an anti–TGF-β antibody, SDF-1 also increased the CFU-M and CFU-Mix colony sizes (Figure 10B and10C). In these experimental conditions, SDF-1 activity was maximal for concentrations from 0.05 ng/mL to 0.1 ng/mL, and followed a bell-shaped curve (Figure 10A).
SDF-1 stimulates colony formation by PB Inc+ CD34+ progenitor cells released from quiescence by anti–TGF-β antibody treatment.
PB Inc+ CD34+ cells, purified after overnight incubation on a plastic support were incubated for 48 hours in serum-free liquid culture medium without cytokine (Stem α A) containing or not containing anti–TGF-β antibody (5 μg/mL) or its isotype IgY control (5 μg/mL). Cells were harvested, counted, and plated in duplicate at a density of 500 cells/mL on methylcellulose medium containing IL3, IL6, IL11, SCF, G-CSF, GM-CSF, and Epo (Stem α ID) in the presence or absence of SDF-1 at various concentrations. Colonies were scored on day 18. (A) Data are expressed as mean percentages of SDF-1 untreated control cells (CT) ± SD calculated by subtracting the percentage of colonies obtained with control CD34+cells not treated with anti–TGF-β antibody from that for anti–TGF-β-treated CD34+ cells. Control (not treated with SDF-1) colony numbers were 25.4 ± 2.5 (CFU-G); 4.3 ± 1.2 (CFU-M); 1.2 ± 0.6 (CFU-GM); 6.6 ± 1.2 (CFU-Mix); 66 ± 1.6; and 16.6 ± 2 (mature and immature BFU-E, respectively). The control plating efficiency, calculated on the total number of colonies was 37.9% ± 3.3% (n = 3 independent experiments). Photomicrographs of (B) CFU-M and (C) CFU-Mix from anti–TGF-β antibody-treated CD34+ cells incubated (B1 and C1) without or (B2 and C2) with SDF-1, × 40. An asterisk indicates significant differences from control values: * .001 < P < .05; ** .0001 < P < .001; *** P < .0001.
SDF-1 stimulates colony formation by PB Inc+ CD34+ progenitor cells released from quiescence by anti–TGF-β antibody treatment.
PB Inc+ CD34+ cells, purified after overnight incubation on a plastic support were incubated for 48 hours in serum-free liquid culture medium without cytokine (Stem α A) containing or not containing anti–TGF-β antibody (5 μg/mL) or its isotype IgY control (5 μg/mL). Cells were harvested, counted, and plated in duplicate at a density of 500 cells/mL on methylcellulose medium containing IL3, IL6, IL11, SCF, G-CSF, GM-CSF, and Epo (Stem α ID) in the presence or absence of SDF-1 at various concentrations. Colonies were scored on day 18. (A) Data are expressed as mean percentages of SDF-1 untreated control cells (CT) ± SD calculated by subtracting the percentage of colonies obtained with control CD34+cells not treated with anti–TGF-β antibody from that for anti–TGF-β-treated CD34+ cells. Control (not treated with SDF-1) colony numbers were 25.4 ± 2.5 (CFU-G); 4.3 ± 1.2 (CFU-M); 1.2 ± 0.6 (CFU-GM); 6.6 ± 1.2 (CFU-Mix); 66 ± 1.6; and 16.6 ± 2 (mature and immature BFU-E, respectively). The control plating efficiency, calculated on the total number of colonies was 37.9% ± 3.3% (n = 3 independent experiments). Photomicrographs of (B) CFU-M and (C) CFU-Mix from anti–TGF-β antibody-treated CD34+ cells incubated (B1 and C1) without or (B2 and C2) with SDF-1, × 40. An asterisk indicates significant differences from control values: * .001 < P < .05; ** .0001 < P < .001; *** P < .0001.
Treatment with anti–TGF-β antibody had no effect on colony formation from the BM Inc+ CD34+ cells in response to SDF-1 (data not shown). In every experiment, the addition of the isotype IgY control had no significant effect on CD34+colony formation (data not shown).
SDF-1 increases the percentage of PB CD34+ cells in the S-phase of the cell cycle
We investigated the stimulatory effects of SDF-1 on progenitor proliferation by evaluating cell-cycle status and the number of PB CD34+ cells cultured in a liquid medium with or without SDF-1 and cytokines.
In the absence of cytokine, a 48-hour incubation with SDF-1 (0.05 ng/mL) significantly increased the percentage of PB Inc+ CD34+ cells in the S+G2/M phases (33.1% ± 1.6%) in comparison with untreated control cells (12.1% ± 0.6%, P = .004, n = 3) and PB Inc− CD34+ cells (8.6% ± 0.8%,P < .0001, n = 3) (Table 4). SDF-1 alone did not stimulate the proliferation of PB CD34+cells, but did result in a smaller decrease in the PB Inc+CD34+ cell number than for untreated cells (86.1 ± 0.8 × 103 and 78 ± 1.4 × 103, respectively, at 72 hours,P < .05, n = 3) (Table 5). If PB Inc+ CD34+ cells were primed before culture with SDF-1 to stimulate cell-cycling, the effect of SDF-1 on the cell number was even greater (88.8 ± 2.3 × 103 and 62.4 ± 2.9 × 103, respectively, at 72 hours,P < .05, n = 3) (Table 5).
SDF-1 increases the percentage of PB Inc+CD34+ cells in the S + G2/M phases
| Source of Cells . | Incubation Time (h) . | SDF-1 Treatment . | % of Cells in Cell Cycle Phases4-150 (Mean ± SD) . | |
|---|---|---|---|---|
| G0/G1 . | S + G2/M . | |||
| PB Inc−CD34+ | 0 | 98.6 ± 0.8 | 1.4 ± 0.8 | |
| 48 | − | 92.4 ± 0.6 | 7.6 ± 0.6 | |
| + | 90.9 ± 1.3 | 8.6 ± 0.8 | ||
| 72 | − | 89.2 ± 2.6 | 9.4 ± 0.8 | |
| + | 88.5 ± 4.1 | 9.2 ± 0.4 | ||
| PB Inc+ CD34+ | 0 | 98.3 ± 0.4 | 1.7 ± 0.4 | |
| 48 | − | 87.8 ± 0.6 | 12.1 ± 0.6 | |
| + | 66.8 ± 1.6 | 33.1 ± 1.64-151,‡ | ||
| 72 | − | 82.2 ± 1.2 | 16.8 ± 1.7 | |
| + | 69.7 ± 1.8 | 30.1 ± 2.24-151,‡ | ||
| Source of Cells . | Incubation Time (h) . | SDF-1 Treatment . | % of Cells in Cell Cycle Phases4-150 (Mean ± SD) . | |
|---|---|---|---|---|
| G0/G1 . | S + G2/M . | |||
| PB Inc−CD34+ | 0 | 98.6 ± 0.8 | 1.4 ± 0.8 | |
| 48 | − | 92.4 ± 0.6 | 7.6 ± 0.6 | |
| + | 90.9 ± 1.3 | 8.6 ± 0.8 | ||
| 72 | − | 89.2 ± 2.6 | 9.4 ± 0.8 | |
| + | 88.5 ± 4.1 | 9.2 ± 0.4 | ||
| PB Inc+ CD34+ | 0 | 98.3 ± 0.4 | 1.7 ± 0.4 | |
| 48 | − | 87.8 ± 0.6 | 12.1 ± 0.6 | |
| + | 66.8 ± 1.6 | 33.1 ± 1.64-151,‡ | ||
| 72 | − | 82.2 ± 1.2 | 16.8 ± 1.7 | |
| + | 69.7 ± 1.8 | 30.1 ± 2.24-151,‡ | ||
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were cultured (1 × 105 cells/mL) in a serum- and cytokine-free medium with or without (control) SDF-1 (0.05 ng/mL). At various time points, cells were harvested by centrifugation, counted, stained with propidium iodide, and analyzed by flow cytometry.
Based on three independent experiments.
P = .004 versus SDF-1 untreated control cells.
P < .0001 versus Inc− cells.
SDF-1 increases PB Inc+CD34+ cell proliferation in synergy with cytokines
| Source of Cells . | SDF-1 Priming Before Culture . | Culture Time (h) . | SDF-1 Treatment . | Total Cell Number (×103)5-150 . | |
|---|---|---|---|---|---|
| Without Cytokines . | With Cytokines . | ||||
| PB Inc−CD34+ | − | 0 | 100 | ND | |
| 48 | − | 86.6 ± 1.8 | ND | ||
| + | 88 ± 1.8 | ND | |||
| 72 | − | 76 ± 1.4 | ND | ||
| + | 76.7 ± 1.9 | ND | |||
| PB Inc+CD34+ | − | 0 | 100 | 100 | |
| 48 | − | 87.7 ± 1.5 | 122 ± 1.4 | ||
| + | 94.1 ± 0.65-151 | 132.8 ± 8.5 | |||
| 72 | − | 78 ± 1.4 | 136.9 ± 5.1 | ||
| + | 86.1 ± 0.85-151 | 142.9 ± 11.2 | |||
| PB Inc+ CD34+ | + | 0 | 100 | 100 | |
| 48 | − | 76.4 ± 1.9 | 144.5 ± 13.5 | ||
| + | 89 ± 9.255-151 | 185.2 ± 15.75-151,5-152 | |||
| 72 | − | 62.4 ± 2.9 | 200.8 ± 13.95-153 | ||
| + | 88.8 ± 2.35-151 | 288.1 ± 14.75-151,5-155 | |||
| Source of Cells . | SDF-1 Priming Before Culture . | Culture Time (h) . | SDF-1 Treatment . | Total Cell Number (×103)5-150 . | |
|---|---|---|---|---|---|
| Without Cytokines . | With Cytokines . | ||||
| PB Inc−CD34+ | − | 0 | 100 | ND | |
| 48 | − | 86.6 ± 1.8 | ND | ||
| + | 88 ± 1.8 | ND | |||
| 72 | − | 76 ± 1.4 | ND | ||
| + | 76.7 ± 1.9 | ND | |||
| PB Inc+CD34+ | − | 0 | 100 | 100 | |
| 48 | − | 87.7 ± 1.5 | 122 ± 1.4 | ||
| + | 94.1 ± 0.65-151 | 132.8 ± 8.5 | |||
| 72 | − | 78 ± 1.4 | 136.9 ± 5.1 | ||
| + | 86.1 ± 0.85-151 | 142.9 ± 11.2 | |||
| PB Inc+ CD34+ | + | 0 | 100 | 100 | |
| 48 | − | 76.4 ± 1.9 | 144.5 ± 13.5 | ||
| + | 89 ± 9.255-151 | 185.2 ± 15.75-151,5-152 | |||
| 72 | − | 62.4 ± 2.9 | 200.8 ± 13.95-153 | ||
| + | 88.8 ± 2.35-151 | 288.1 ± 14.75-151,5-155 | |||
PB CD34+ cells purified immediately after density gradient separation (Inc−) or after incubation on a plastic support (Inc+) were cultured (1 × 105 cells/mL) in a serum-free medium with or without cytokines. The cells were incubated with or without (control) SDF-1 (0.05 ng/mL). At various time points, cells were harvested and counted. In some experiments, the priming effect of SDF-1 was investigated by prior treatment of the cells with SDF-1 (0.05 ng/mL) in a cytokine-free culture medium (Stem α A) for 24 hours before culture.
Based on 3 independent experiments.
P < .05 versus SDF-1 untreated control.
P = .03.
P = 0.004.
P = .002 versus unprimed cells.
In the presence of cytokines, SDF-1 treatment significantly resulted in more PB Inc+ CD34+ cells if the cells had been previously primed with SDF-1 than if the cells had not been primed (288.1 ± 14.7 × 103 and 142.9 ± 11.2 × 103, respectively, at 72 hours,P = .002, n = 3) (Table 5) or untreated (200.8 ± 13.9 × 103, P < .05, n = 3) (Table5). Priming with SDF-1 for 24 hours itself significantly increased the total number of PB Inc+ CD34+ cells, (200.8 ± 13.9 × 103 versus 136.9 ± 5.1 × 103, for unprimed control cells, at 72 hours,P = .004, n = 3) (Table 5).
Discussion
We present here evidence of a role for the chemokine SDF-1 as an enhancing factor for human circulating CD34+ progenitor proliferation, in synergy with cytokines. Several studies have reported CXCR-4 expression on CD34+ cells purified from BM,10,30 mobilized peripheral blood,13,16,30and cord blood,30,31 but this is the first demonstration of CXCR-4 expression on CD34+ cells purified from adult unmobilized peripheral blood. The percentage of CXCR-4+ cells and receptor levels were both lower for CD34+ cells isolated from PB than for CD34+cells from other sources.10,13,16 The presence of CD34+ cells in the liquid phase (PB, cord blood, or mobilized peripheral blood) rather than in organized tissue (BM), in which CD34+ cells are intimately associated with stroma,7,30,32 may account for such differences. The involvement of the CXCR-4/SDF-1 couple in hematopoietic progenitor migration and homing is now well documented.8 It has been suggested that changes in the concentration gradient of chemoattractants such as SDF-1 and SCF favor the homing of CD34+ progenitors to BM or their peripheralization. According to this model, SDF-1 may inhibit mobilization of BM progenitors.7 The observed differences in CXCR-4 levels in BM and PB CD34+ cells are consistent with this notion. The low level of CXCR-4 expression on PB CD34+ cells is also consistent with their circulating status.
The expression of CXCR-4 at the cell surface was significantly increased by purifying progenitors after incubation on a plastic surface (Inc+). This up-regulation affected mostly PB Inc+ CD34+ cells because CXCR-4 levels were about 10 times higher on these cells. Various factors may be involved in CXCR-4 up-regulation during incubation, including cell-surface adhesion, cell-cell contact between CD34+ and low-density mononuclear cells, growth factor production, and adhesion molecule interactions. Cell-surface adhesion was recently suggested as being involved in the CXCR-4 increase on BM progenitors and PB granulocytes.33,34 The lower level of CXCR-4 expression for PB CD34+ cells purified after incubation in an adhesion-free flask than for PB Inc+ CD34+cells (21.5 ± 2.2% and 73.6 ± 12%, respectively; experiment not described in this paper) is consistent with this notion. The higher level of CXCR-4 expression for CD34+ cells initially purified without adhesion and then incubated on a plastic surface (38.6 ± 8.7%) than for Inc−CD34+ cells (8.8 ± 3.6%) also supports this hypothesis. However, in these experimental conditions, CXCR-4 levels were lower than those of Inc+ CD34+, suggesting that other events, such as interactions between progenitors and mononuclear cells, are also involved in CXCR-4 up-regulation. Lapidot et al35 recently reported that SCF and IL6 can regulate CXCR-4 expression. It is possible that these growth factors are released by mononuclear and/or CD34+ cells during incubation.36 Surface CXCR-4 up-regulation may also involve integrins.35 Our preliminary results showed no differences in VLA-4, VLA-5, and LFA-1 levels in PB CD34+ cells, purified with and without incubation (data not shown); however, we cannot exclude the possibility that they were activated.
We further investigated the mechanisms of CXCR-4 overexpression on PB Inc+ CD34+ cells. The correlation between the increase in surface CXCR-4 and the decrease in the intracellular pool is consistent with possible translocation of the receptor from the cytoplasm to the surface membrane. Cycloheximide treatment indicated that protein synthesis was involved in the up-regulation of surface CXCR-4.
In PB Inc+ CD34+ cells, we identified for the first time a CXCR-4highCD34high subpopulation with the antigenic characteristics of primitive pluripotent progenitors, ie, CD38low, CD71low, c-Kitlow, and Thy-1+. In contrast, in BM, most of the CXCR-4highCD34+ cells were also CD38high. This CXCR-4highCD38highpopulation also coexpressed high levels of CD19 antigen (data not shown), suggesting that these cells constitute a pre-B lymphocytic progenitor subset. The significance of the primitive CD34highCXCR-4highCD38lowCD71lowc-KitlowThy-1+subpopulation detected in PB after incubation is not clear. The higher CD34 expression level of PB Inc+ CD34+ cells than of Inc− cells (44.6 ± 9.5 AU versus 63.4 ± 10.7 AU) (Figure 1, plots 2c, 2d) may facilitate the recovery of a higher proportion of CD34high cells, including this primitive subpopulation.
Some chemokines have different effects on hematopoiesis, depending on progenitor maturity, but most have a more general effect on suppressing progenitor cell proliferation.31,37 We found high levels of CXCR-4 expression on a primitive subset of PB Inc+CD34+ cells. This led us to assess the effect of SDF-1 on hematopoietic progenitor proliferation and differentiation. Our results show for the first time that SDF-1, a known chemotactic factor, also modulates hematopoietic progenitor cell proliferation. SDF-1 increased colony formation by PB CD34+. This effect was significantly greater if CD34+ cells were purified after an incubation step, and it applied to all types of myeloid progenitors. A combination of cytokines is necessary to increase colony formation in the presence of SDF-1, because SDF-1 alone has no activity.38 SCF, alone or in combination with at least IL-3 and GM-CSF, is involved in this synergistic promoting effect. An increase in the SCF-induced tyrosine phosphorylation in response to SDF-1 is consistent with this notion and suggested that SDF-1 enhances downstream signaling of SCF.39
The stimulatory effect of SDF-1 on colony formation was dose-dependent and was totally inhibited by the addition of a neutralizing antibody, demonstrating its specificity. If PB CD34+ cells were incubated on a plastic surface, the SDF-1 concentration effective in colony formation was 1000 times lower than that effective in migration tests.7 Such a difference in dose range is observed for other chemokines acting on migration and hematopoiesis.7,8,37,40 Hematopoietic progenitor proliferation is regulated by short-range factors produced in tiny quantities within the microenvironment.41 The bell-shaped curve is a common feature of chemokine biological activity,42 and its sharpness for low SDF-1 concentrations is consistent with a subtle and accurate hematopoiesis regulation.
Despite high levels of CXCR-4 expression on BM CD34+ cells, SDF-1 (from 0.05 up to 10 ng/mL) had no significant effects on either the number or the size of colonies from BM CD34+ cells even if they were treated by anti–TGF-β antibody. The inability of SDF-1 to increase the cytokine-induced tyrosine phosphorylation in BM CD34+ cells may elucidate why these cells do not respond to SDF-1. These data are also consistent with the results of Gotoh et al32 and Majka et al43 and with the possible lack of correlation between CXCR-4 expression and the functional activity of SDF-1.44It is possible that CXCR-4 desensitization occurs in BM progenitors in an SDF-1–enriched microenvironment; such a regulation process is typical of G-protein–coupled receptors.45 Also, we could not exclude the possibility that such an SDF-1–responsive progenitor subset is present in the BM, but at too low a proportion to be detected by our clonogenic assay.
Our results demonstrating a stimulatory effect of SDF-1 on PB colony formation by erythroid, granulocytic, macrophagic, and megakaryocytic progenitors, suggest that SDF-1 acts on pluripotent progenitors. Such cells, thought to be mainly dormant, are released from quiescence by a neutralizing anti–TGF-β antibody, according to the “high proliferative potential-quiescent cells” model.46 We assessed the effect of SDF-1 on CD34+ cells treated by anti–TGF-β antibody and showed that SDF-1 increased the number of CFU-Mix colonies by up to 180%. SDF-1 also dramatically increased the number of CFU-GM and CFU-M over that in untreated cells (425% ± 175% and 404% ± 79.1%, respectively). This positive effect on both the number and the size of colonies, associated with the evidence for a CD34highCXCR-4highCD38lowCD71lowc-KitlowThy-1+subpopulation, strongly suggests that SDF-1 acts on primitive hematopoietic progenitors. Such a hypothesis is strengthened by our results showing a stimulatory effect of SDF-1 on PB CD34+and CD34highCD38low cell expansion and myeloid progenitors amplification, in stroma-free liquid culture containing SCF, FLT3-ligand, and TPO.
We further evaluated the effect of SDF-1 on the cycle status of PB Inc+ CD34+ cells in serum- and cytokine-free suspension culture. Our results, showing that 98.6% ± 0.8% of PB CD34+ cells were in the G0/G1phases, confirm that human steady-state PB progenitors are cell-cycle dormant.47,48 After culture for 48 hours, the percentage of cells in S+G2/M was higher in PB Inc+CD34+ cells than in Inc− cells, suggesting a relationship between CD34+ cell-cycle status and adhesiveness, consistent with the data of Yamaguchi et al.49 The addition of SDF-1 alone significantly increased the proportion of PB Inc+ CD34+ cells in the S+G2/M phases but had no effect on Inc−cells. The effect on Inc+ cells was not detected before 48 hours of culture, suggesting that most PB Inc+CD34+ cells were in early G0/G1phase. Thus, SDF-1 may interfere with cell cycling by shortening the time required to go through the G1 phase, as has been suggested for other early-acting growth factors.50 Such a decrease in the duration of G1 might increase the relative proportion of responding cells in S-phase, as reported by Roberts et al.47 SDF-1 alone increased the percentage of CD34+ cells in S+G2/M phases. It supported cell survival but did not induce cell proliferation. This is consistent with the role of SDF-1 in preventing cell death, as proposed by Gotoh et al.32 Our SDF-1 priming studies indicate that SDF-1 potentiated the stimulatory effect of cytokines on PB Inc+CD34+ cell proliferation. Thus, SDF-1 may act as a sensitizing factor, rendering progenitors responsive to cytokines.
In conclusion, we report evidence for a primitive PB CD34high cell subset that overexpresses CXCR-4 after overnight incubation. The experimental procedures used, which involved cell-cell interactions, growth factor production, and cytoadhesion, might mimic the physiological processes occurring during progenitor cell engraftment. The presence of such primitive progenitors is consistent with heterogeneity in stem cell engraftment potential depending on CXCR-4 expression, as reported by Peled et al.51 Our data also provide evidence that SDF-1 functions as a promoting factor for circulating progenitor proliferation, as demonstrated by its stimulatory effect on cell cycling, colony formation, and progenitor expansion, in synergy with cytokines. Thus, our results provide new insight into the role of the CXCR-4/SDF-1 couple in hematopoiesis and progenitor cell engraftment, which involve migration, adhesion, and proliferation. Furthermore, our demonstration of adhesion-induced CXCR-4 overexpression associated with SDF-1 stimulatory activity may be of clinical relevance for improving cellular therapy settings for stem cell transplantation.
Acknowledgments
We thank Pr Nedellec, Dr Dishino, and Dr Masse for continuously supplying bone marrow samples. We are indebted to Dr M. C. Martyré and Dr D. Dormont for their critical reading of this article.
Supported by grants from the DRET (Direction des Recherches Etudes et Techniques, Ministère de la Défense, No 957) and the ANRB (Association Nouvelles Recherches Biomédicales).
Submitted April 9, 1999; accepted September 10, 1999.
Reprints:M. C. Le Bousse-Kerdilès, INSERM U268, Hôpital Paul Brousse, 14, Avenue Paul Vaillant Couturier, 94800 Villejuif, France; e-mail: lebousse@infobiogen.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal