Patients (n-987) with a histologically confirmed diagnosis of follicular lymphoma were studied with the aim of developing a prognostic model specifically devised for this type of lymphoma. We collected information on age, sex, Ann Arbor stage, number of extranodal disease sites, bone marrow (BM) involvement, bulky disease, B symptom criteria (fever, night sweats, and weight loss), performance status (PS), serum lactate dehydrogenase (LDH) level, serum albumin level, hemoglobin level, and erythrocyte sedimentation rate (ESR). In the training sample of 429 patients with complete data, multivariate analysis showed that age, sex, number of extranodal sites, B symptoms, serum LDH level, and ESR were factors predictive for overall survival. Using these 6 variables, a prognostic model was devised to identify 3 groups at different risk. The 5- and 10-year survival rate was 90% and 65% for patients at low risk, respectively; 75% and 54% for patients at intermediate risk; and 38% and 11% for those at high risk (log-rank test, 86.62; P < .0001). The model was also predictive (P = .0001) in the validation sample of 265 patients with complete data only for the 6 variables used in the development of the model and even in the group of 210 patients from the validation sample uniformly treated with doxorubicin-containing regimens (P = .0001). The prognostic model appears to be very useful in identifying patients with follicular lymphoma at low, intermediate, or high risk.
Follicular lymphoma is the most frequent subtype of malignant lymphoma in western countries and accounts for approximately 25% of all adult non-Hodgkin's lymphoma.1 Before the advent of chemotherapy, the majority of patients with follicular lymphoma died within 5 years. With current therapy the expected median survival is approximately 8-10 years.2 The management of these patients is one of the most controversial issues in oncology. A variety of treatment approaches, including the use of single alkylating agents, combination chemotherapy with or without doxorubicin, total lymphoid irradiation, and a combination of chemo-radiotherapy, have been applied to these patients. With current treatment options, complete remission rates range from 65% to 85%. Despite these attempts, in the last 2 decades overall survival was not significantly influenced by different therapies.3-7 Sometimes patients over 60 years of age and asymptomatic at diagnosis can defer treatment until signs of disease progression appear, and many patients do not require treatment for a number of years. In randomized trials,8 9 the long-term survival of these patients was similar to the survival of patients treated immediately at time of diagnosis.
Although patients with follicular lymphoma have relatively long median survival times and exhibit dramatic responses to initial therapy, they should be considered affected by a fatal malignancy. Patients tend to relapse over time, their response to salvage therapy is of shorter duration after every relapse, and they eventually die of disease-related causes. Finally, a small but significant group of patients with follicular lymphoma survive a relatively short time. Although it is difficult to identify high-risk and low-risk patients at diagnosis, it would be helpful to select those patients who are suitable for experimental therapy and those patients who should avoid undue therapy-related toxicity. In patients with follicular lymphoma, a variety of prognostic factors have been found including age, stage, tumor burden, bone marrow (BM) involvement, B symptoms, performance status (PS), serum lactate dehydrogenase (LDH) level, anemia, erythrocyte sedimentation rate (ESR), and beta-2 microglobulin.10-14 More recently the International Prognostic Index (IPI)15 and other predictive models have been applied to low-grade lymphomas with conflicting results.16-19
To assess the reproducibility of the IPI and to develop, if possible, a prognostic model specifically devised for follicular lymphoma, the Intergruppo Italiano Linfomi (Italian Lymphoma Intergroup) prompted a collaborative study. Here we present the results of this study performed on 987 patients treated at cooperating centers between 1985 and 1996.
Methods
Eligibility criteria
We considered eligible for this study all patients with a histologically confirmed diagnosis of follicular lymphoma, including follicular large-cell lymphoma, according to updated Kiel classification, who were either enrolled in prospective clinical trials between 1985 and 1996 or treated at participating centers according to specified guidelines. Initial diagnosis was not revised, and thus the grading of follicular lymphoma was not assessed. A total of 1096 patients from 8 single centers and 2 cooperative groups were merged into a preliminary working file. We excluded 109 patients from the study for the following reasons: revised histology, 12 patients; incomplete data, 30 patients; inadequate follow-up, 67 patients. Finally, 987 patients were included in the analysis. We collected information on those parameters that were already known to be of prognostic relevance and were available for almost all patients: age, sex, Ann Arbor stage, number of extranodal disease sites, BM involvement, B symptoms, bulky disease, PS, serum LDH level, serum albumin level, hemoglobin level, and ESR. Separation levels, based on the following, formed the 2 most distinct populations at risk in terms of survival: albumin less than or equal to 30 mmol/L, ESR greater than 30 mm, and hemoglobin less than 120 mmol/L for men and less than 100 mmol/L for women. These levels were used for subsequent analyses. In addition to other extralymphatic tissues, we recorded BM and spleen as sites of extranodal involvement. For the purpose of this study, peripheral blood involvement was not recorded. Bulky disease was defined as a mass with the largest dimension greater than or equal to 10 cm or, for the mediastinum only, as a mass larger than one-third of the chest diameter. According to Ann Arbor criteria, B symptoms were defined as recurrent fever (more than 38°C), night sweats, or the loss of more than 10% of body weight. Serum β2-microglobulin measurement was not routinely assessed. Moreover, due to the retrospective essence of the study, not all variables were available for each patient.
All patients were clinically staged. The extent of the disease was determined by a standardized staging evaluation that included chest and abdomen computed tomography and BM biopsy. Response to treatment was assessed 1 month after the end of induction therapy by performing all examinations necessary to control abnormal findings present at the time of diagnosis.
Complete remission (CR) was defined as the disappearance of all clinical evidence of the disease and the normalization of all laboratory values and radiographs that had been considered abnormal before starting treatment, including a normalization of BM, if initially involved. Moreover, patients who achieved a CR during therapy, but relapsed within 30 days after therapy had been completed, were classified as nonresponders. Partial remission (PR) was defined as a greater than 50% reduction in the largest dimension of each anatomic site of measurable disease for at least 1 month. No response (NR) was defined as a less than 50% regression or stable or progressive disease. All early deaths due to disease progression or treatment-related toxicity were considered as treatment failures, and included in the NR group. All evaluations of clinical stage and response to treatment were based on the original data recorded by local physicians.
Treatment strategies
Patients received a variety of treatment strategies: 75 patients (7.6%) were treated with radiotherapy alone or received no initial treatment according to the watch and wait strategy; 122 patients (12.4%) were treated with a single agent either with or without α-interferon (IFN-α); 128 patients (13.0%) were treated with CVP20 (cyclophosphamide, vincristine, and prednisolone) or CVP-like regimens; 633 patients (64.1%) were treated with CHOP21 (cyclophosphamide, hydroxyldaunomycin, vincristine [Oncovin], and prednisone) or other doxorubicin-containing regimens; and 29 patients (2.9%) were treated with a variety of different approaches.
Statistical analysis
All data were analyzed with the Statistical Package for the Social Sciences (SPSS).22 Differences in patient characteristics, response rates, and treatment failures among groups were analyzed by the Fisher's exact test for contingency tables. Survival, relapse-free survival (RFS), and failure-free survival (FFS) curves were estimated by the method of Kaplan-Meier. The date of therapy initiation was not available in some cases, thus the survival was calculated from the date of diagnosis until death from any cause. However, the mean interval between the date of diagnosis and the start of therapy in patients with complete data was 26 days. RFS was applied only to patients in CR and was calculated from the end of induction therapy to the first evidence of relapse. FFS was calculated for all patients and was measured from the beginning of therapy to the time of disease progression (date of assessment of response for patients with less than PR; date of start of second-line therapy for patients with PR), relapse (for patients in CR), or death. The log-rank test was used to assess the significance of differences in survival for each prognostic factor. Cox proportional hazards regression model was used in multivariate analysis to determine whether the identified risk factors independently influenced survival rate. The limit of significance for all analyses was defined as P = .05. Two-sided tests were used in all calculations.
Results
Analysis of response
Table 1 summarizes the characteristics of 987 patients eligible for the study. With induction therapy, 65% of patients achieved a CR and 24% achieved a PR, with an overall response rate of 89%. Significant correlations were observed between CR rate and the following: age (P = .0073); sex (P = .0025); Ann Arbor stage (P < .0001); B symptoms (P = .0004); number of extranodal sites (P = .0275); performance status (P = .0003); BM involvement (P < .0001); serum LDH (P < .0001); hemoglobin (P < = .0001);and ESR (P = .0067).
Characteristics of patients
| Characteristic . | No. of Cases . | % . |
|---|---|---|
| Age | ||
| No more than 60 years | 599 | 61 |
| Greater than 60 years | 388 | 39 |
| Sex | ||
| Female | 521 | 53 |
| Male | 466 | 47 |
| Ann Arbor stage | ||
| I-II | 250 | 25 |
| III-IV | 737 | 75 |
| No. of extranodal sites | ||
| 0-1 | 933 | 95 |
| At least 2 | 54 | 5 |
| BM involvement (959) | ||
| Absent | 461 | 48 |
| Present | 498 | 52 |
| Bulky disease (937) | ||
| Absent | 819 | 87 |
| Present | 118 | 13 |
| B Symptoms (980) | ||
| Absent | 832 | 85 |
| Present | 148 | 15 |
| Performance status (ECOG) (762) | ||
| 0-1 | 696 | 91 |
| At least 2 | 66 | 9 |
| Serum LDH level (838) | ||
| Normal | 696 | 83 |
| Above upper normal limit | 142 | 17 |
| Serum albumin level (676) | ||
| Greater than 3 g/dL | 646 | 96 |
| No more than 3 g/dL | 30 | 4 |
| Hemoglobin (823) | ||
| At least 12 g/dL (10 g/dL for w) | 757 | 92 |
| Less than 12 g/dL (10 g/dL for w) | 66 | 8 |
| ESR (772) | ||
| Less than 30 mm | 627 | 81 |
| At least 30 mm | 145 | 19 |
| International Prognostic Index (649) | ||
| Low risk | 369 | 57 |
| Low intermediate risk | 196 | 30 |
| High intermediate risk | 65 | 10 |
| High risk | 19 | 3 |
| Characteristic . | No. of Cases . | % . |
|---|---|---|
| Age | ||
| No more than 60 years | 599 | 61 |
| Greater than 60 years | 388 | 39 |
| Sex | ||
| Female | 521 | 53 |
| Male | 466 | 47 |
| Ann Arbor stage | ||
| I-II | 250 | 25 |
| III-IV | 737 | 75 |
| No. of extranodal sites | ||
| 0-1 | 933 | 95 |
| At least 2 | 54 | 5 |
| BM involvement (959) | ||
| Absent | 461 | 48 |
| Present | 498 | 52 |
| Bulky disease (937) | ||
| Absent | 819 | 87 |
| Present | 118 | 13 |
| B Symptoms (980) | ||
| Absent | 832 | 85 |
| Present | 148 | 15 |
| Performance status (ECOG) (762) | ||
| 0-1 | 696 | 91 |
| At least 2 | 66 | 9 |
| Serum LDH level (838) | ||
| Normal | 696 | 83 |
| Above upper normal limit | 142 | 17 |
| Serum albumin level (676) | ||
| Greater than 3 g/dL | 646 | 96 |
| No more than 3 g/dL | 30 | 4 |
| Hemoglobin (823) | ||
| At least 12 g/dL (10 g/dL for w) | 757 | 92 |
| Less than 12 g/dL (10 g/dL for w) | 66 | 8 |
| ESR (772) | ||
| Less than 30 mm | 627 | 81 |
| At least 30 mm | 145 | 19 |
| International Prognostic Index (649) | ||
| Low risk | 369 | 57 |
| Low intermediate risk | 196 | 30 |
| High intermediate risk | 65 | 10 |
| High risk | 19 | 3 |
Analysis of survival
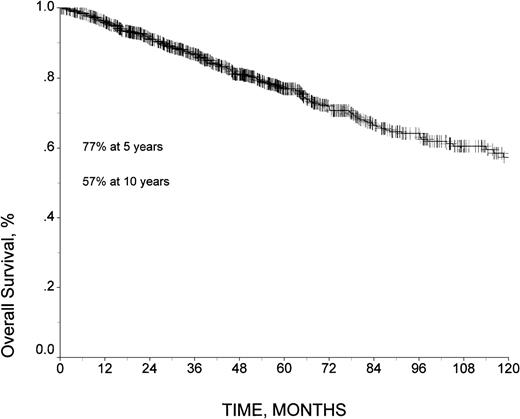
After a median follow-up of 51 months (54 months for patients still alive), 224 patients had died, and 763 were alive. The 5- and 10-year overall survival rates were 77% and 57%, respectively, and a plateau had not been reached at time of analysis (Figure 1). The 5-year and 10-year RFS among patients who achieved CR was 62% and 43%, respectively, and the 5-year and 10-year FFS was 48% and 30%, respectively. The median FFS time was 54 months (95% confidence intervals [CI], 46-62 months).
In univariate analysis of survival, poorer prognosis was associated with the following (Table 2): age greater than 60 years (P < .0001); male sex (P = .0025); advanced stage (P < .0001); the presence of B symptoms (P < .0001); the involvement of more than 1 extranodal site (P = .0275); low performance status (P < .0001); BM involvement (P = .0001); serum LDH level above the upper normal limit (P = .0002), low serum albumin level (P = .0086); low hemoglobin level (P = .0001); and high ESR (P < .0001). The international prognostic index proved to be of highly significant prognostic value (P < .0001).
Survival rates according to patient characteristics
| Characteristic . | 5 Year . | 10 Year . | P Value . |
|---|---|---|---|
| Age | <.0001 | ||
| No more than 60 years | 86 | 69 | |
| Greater than 60 years | 61 | 38 | |
| Sex | .0025 | ||
| Female | 80 | 64 | |
| Male | 72 | 49 | |
| Ann Arbor stage | <.0001 | ||
| I-II | 89 | 84 | |
| III-IV | 73 | 49 | |
| No. of extranodal sites | .0494 | ||
| 0-1 | 77 | 58 | |
| Greater than or equal to 2 | 68 | 47 | |
| BM involvement | .0001 | ||
| Absent | 85 | 72 | |
| Present | 73 | 46 | |
| Bulky disease | .8070 | ||
| Absent | 80 | 59 | |
| Present | 75 | 65 | |
| B Symptoms | <.0001 | ||
| Absent | 80 | 61 | |
| Present | 59 | 40 | |
| Performance status (ECOG) | <.0001 | ||
| 0-1 | 78 | 56 | |
| 2-3 | 54 | 19 | |
| Serum LDH level | .0002 | ||
| Normal | 82 | 60 | |
| Above upper normal limit | 62 | 59 | |
| Serum albumin level | .0086 | ||
| Greater than 3 g/dL | 83 | 65 | |
| No more than 3 g/dL | 64 | 54 | |
| Hemoglobin | .0001 | ||
| At least 12 g/dL (10 g/dL for w) | 83 | 65 | |
| Less than 12 g/dL (10 g/dL for w) | 60 | 53 | |
| ESR | <.0001 | ||
| Less than 30 mm | 85 | 68 | |
| At least 30 mm | 67 | 52 |
| Characteristic . | 5 Year . | 10 Year . | P Value . |
|---|---|---|---|
| Age | <.0001 | ||
| No more than 60 years | 86 | 69 | |
| Greater than 60 years | 61 | 38 | |
| Sex | .0025 | ||
| Female | 80 | 64 | |
| Male | 72 | 49 | |
| Ann Arbor stage | <.0001 | ||
| I-II | 89 | 84 | |
| III-IV | 73 | 49 | |
| No. of extranodal sites | .0494 | ||
| 0-1 | 77 | 58 | |
| Greater than or equal to 2 | 68 | 47 | |
| BM involvement | .0001 | ||
| Absent | 85 | 72 | |
| Present | 73 | 46 | |
| Bulky disease | .8070 | ||
| Absent | 80 | 59 | |
| Present | 75 | 65 | |
| B Symptoms | <.0001 | ||
| Absent | 80 | 61 | |
| Present | 59 | 40 | |
| Performance status (ECOG) | <.0001 | ||
| 0-1 | 78 | 56 | |
| 2-3 | 54 | 19 | |
| Serum LDH level | .0002 | ||
| Normal | 82 | 60 | |
| Above upper normal limit | 62 | 59 | |
| Serum albumin level | .0086 | ||
| Greater than 3 g/dL | 83 | 65 | |
| No more than 3 g/dL | 64 | 54 | |
| Hemoglobin | .0001 | ||
| At least 12 g/dL (10 g/dL for w) | 83 | 65 | |
| Less than 12 g/dL (10 g/dL for w) | 60 | 53 | |
| ESR | <.0001 | ||
| Less than 30 mm | 85 | 68 | |
| At least 30 mm | 67 | 52 |
Training sample
Information on the above-mentioned prognostic factors was not available in all cases: for example, staging information was available for 984 patients, whereas serum albumin levels were available only for 673 patients. Information on all 11 prognostic factors significantly associated with outcome in univariate analysis was complete for 429 patients. These 429 patients were selected as a training sample in which to identify independent factors necessary to construct a prognostic model.
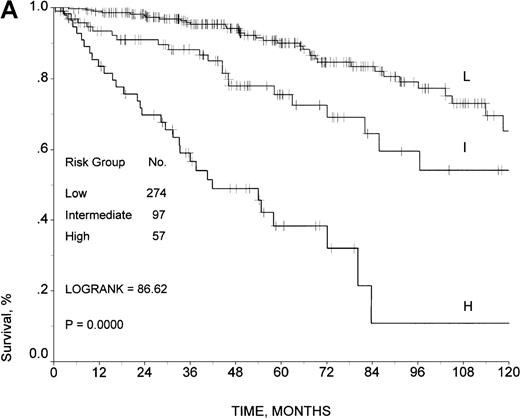
The six characteristics that remained independently significant in the analysis of the training sample were sex, age, B symptoms, number of extranodal sites, serum LDH, and ESR (Table 3). Since the relative risk associated with each of the 6 factors was comparable, we constructed a risk score, simply summing the number of risk factors present in a single patient at time of diagnosis. There were no cases presenting all 6 adverse factors, thus only 6 categories (score 0 through 5) were initially detected (Table 4). Using the same methodology adopted for the construction of the international prognostic index for aggressive non-Hodgkin's lymphoma, risk groups were defined by comparing the relative risk of death in patients with each possible number of presenting risk factors and combining categories with similar relative risk.15 Patients with similar relative risk were subsequently stratified in 3 risk groups: score 0-1, low risk; score 2, intermediate risk; score 3-5, high risk. The survival curves for the 3 risk groups are shown in Figure2A. The 5- and 10-year survival rates were 90% and 65%, respectively, for 274 patients at low risk; 75% and 54% for 97 patients at intermediate risk; and 38% and 11% for 57 patients at high risk (log-rank test, 86.62; P < .0001). The 5-year RFS of patients in CR after initial therapy was 65% for patients at low risk, 63% for patients at intermediate risk, and 51% for patients at high risk (P not significant). The 5-year FFS of patients in CR after initial therapy was 59% for patients at low risk, 50% for patients at intermediate risk, and 23% for patients at high risk (log-rank test, 28.95; P < .0001).
Factors with independent prognostic value of survival in the training sample
| Factor . | Relative Risk of Death . | 95% CI . | P Value . |
|---|---|---|---|
| Age (no more than 60 vs greater than 60) | 2.6 | 1.6-4.1 | <.0001 |
| Sex (F vs M) | 1.8 | 1.2-2.8 | .0079 |
| Extranodal sites (0-1 vs at least 2) | 2.1 | 1.0-4.2 | .0445 |
| Serum LDH (normal vs elevated) | 2.0 | 1.2-3.5 | .0074 |
| B symptoms (absent vs present) | 2.2 | 1.2-4.1 | .0128 |
| ESR (less than 30 vs at least 30) | 2.4 | 1.5-4.1 | .0006 |
| Factor . | Relative Risk of Death . | 95% CI . | P Value . |
|---|---|---|---|
| Age (no more than 60 vs greater than 60) | 2.6 | 1.6-4.1 | <.0001 |
| Sex (F vs M) | 1.8 | 1.2-2.8 | .0079 |
| Extranodal sites (0-1 vs at least 2) | 2.1 | 1.0-4.2 | .0445 |
| Serum LDH (normal vs elevated) | 2.0 | 1.2-3.5 | .0074 |
| B symptoms (absent vs present) | 2.2 | 1.2-4.1 | .0128 |
| ESR (less than 30 vs at least 30) | 2.4 | 1.5-4.1 | .0006 |
Outcome of 429 patients selected in the training sample according to the number of presenting risk factors
| No. of Risk Factors . | No. of Cases . | No. of Deaths . | % Censored . | % 5-Year Survival . |
|---|---|---|---|---|
| 0 | 86 | 5 | 94 | 97 |
| 1 | 188 | 32 | 83 | 87 |
| 2 | 97 | 22 | 77 | 75 |
| 3 | 43 | 20 | 53 | 49 |
| 4 | 11 | 8 | 27 | 14 |
| 5 | 3 | 3 | 0 | 0 |
| No. of Risk Factors . | No. of Cases . | No. of Deaths . | % Censored . | % 5-Year Survival . |
|---|---|---|---|---|
| 0 | 86 | 5 | 94 | 97 |
| 1 | 188 | 32 | 83 | 87 |
| 2 | 97 | 22 | 77 | 75 |
| 3 | 43 | 20 | 53 | 49 |
| 4 | 11 | 8 | 27 | 14 |
| 5 | 3 | 3 | 0 | 0 |
For 5-year survival, log-rank test, 128, 76; P < .0001.
Survival in the training sample.
Survival rates are given according to (A) the prognostic model developed by the Intergruppo Italiano Linfomi and (B) the IPI.
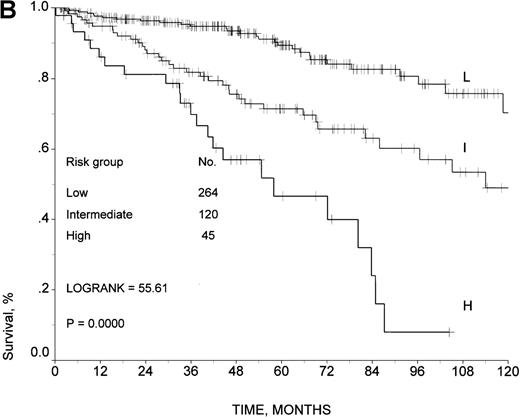
Survival in the training sample.
Survival rates are given according to (A) the prognostic model developed by the Intergruppo Italiano Linfomi and (B) the IPI.
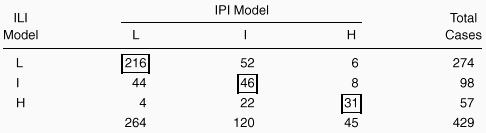
The predictive model developed for aggressive non-Hodgkin's lymphoma was able to stratify the same 429 patients in 3 different risk groups: score 0-1, low risk, 264 patients; score 2, intermediate risk, 120 patients; score 3-5, high risk, 45 patients. The 5- and 10-year survival rates were 89% and 70%, respectively, in the low-risk group; 71% and 49% in the intermediate-risk group; and 47% and 8% in the high-risk group (log-rank test, 55.61; P < .0001) (Figure2B). As shown in Table 5, 293 patients (69%) were allocated in the same risk group using both indexes; 70 patients (16%) with our model were placed into a higher risk group, and 66 patients (15%) with the IPI were placed into a higher risk group. No statistically significant differences in survival were observed between groups of patients placed into higher risk groups with either model (log-rank test, 0.17; P = .57). According to our predictive model, 17 out of 429 patients (4.0%) who were 60 years of age or younger were at high risk, while only 7 patients (1.6%) were at high risk according to the IPI.
Validation sample
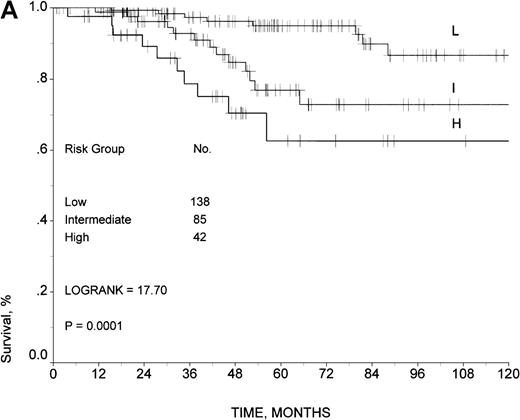
The outcome of 429 patients with complete data was similar to the outcome of the 559 patients with incomplete data (log-rank test, 1.50;P = .2204). As a result, we used the latter group as the source to identify a validation sample for our model. Out of the 559 patients with incomplete data, we found 265 patients with complete data for the 6 variables used in the development of our predictive model, and we selected all of them as a validation sample. The predictive model was also able to stratify this group of patients into 3 risk groups with statistically significant different outcomes (Figure3A). The 5- and 10-year survival rates were 95% and 87%, respectively, in the low-risk group; 77% and 73% in the intermediate-risk group; and 62% and 62% in the high-risk group (log-rank test, 17.7; P = .0001).
Survival in the validation sample.
Survival rates are given according to the prognostic model developed by the ILI for (A) all 265 patients and (B) 210 patients uniformly treated with doxorubicin-containing regimens.
Survival in the validation sample.
Survival rates are given according to the prognostic model developed by the ILI for (A) all 265 patients and (B) 210 patients uniformly treated with doxorubicin-containing regimens.
As in the training sample, no differences in the 5-year RFS were observed among patients at different risks according to our model. However, the 5-year FFS was 60% for low-risk patients, 56% for intermediate-risk patients, and 36% for high-risk patient (log-rank test, 6.02; P = .0494).
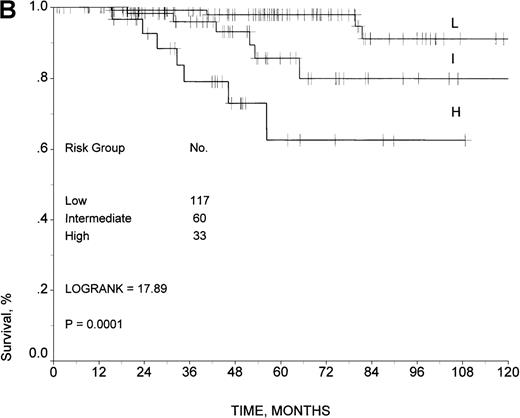
Moreover, the model was also predictive for 210 patients from the validation sample who were uniformly treated with doxorubicin-containing regimens (Figure 3B). The 5- and 10-year survival rates for low-risk patients were 98% and 91%, respectively, and 86% and 79% for intermediate-risk patients. High-risk patients had a 5-year survival of 62% (P = .0001), and it was not possible to assess the survival rate at 10 years because no patient in the high-risk group had a follow-up period beyond 8 years.
Discussion
This report covered a very large series of patients with follicular lymphoma, by far the largest ever studied, and provides definite information on the outcome of patients diagnosed in several institutions between 1985 and 1996 and treated with conventional approaches. As previously shown, the outcome of our study population was characterized by a 10-year overall survival of 57%, RFS of 43%, and FFS of 30% and is comparable to that reported by several other studies.2-6
The aim of this study was to analyze clinical variables in a large number of cases and to explore the possibility of better predicting the outcome of these patients. Among the several endpoints that can be studied in prognostic analyses, we measured the outcome in terms of remission rates, RFS, FFS, and overall survival. However, according to Coiffier et al,13 it is difficult to assess CR in follicular lymphoma because of possible minimal BM involvement. Thus, CR and duration of remission do not seem the most reliable endpoints for studying the prognosis of patients with follicular lymphoma. For this reason, although we analyzed remission rates, RFS, FFS, and overall survival, our prognostic model was based on the analysis of overall survival.
In the literature, many prognostic factors have been shown to predict a poor survival in follicular lymphoma. They include older age, number of extranodal sites, high tumor burden, BM involvement, bulky disease, B symptoms, poor PS, high LDH or β2-microglobulin levels, anemia, and gut involvement.10-14
In order to better predict survival rates, we investigated several pretreatment clinical features that are considered important predictors of outcome, namely those recognized by the IPI. However, we also looked for clinical features different from those considered in the IPI model. These features included albumin level and ESR. Unfortunately we were not able to assess the prognostic role of β2-microglobulin because this information was lacking in most cases.
A few patients had a serum albumin level less than 30 mmol/L, and they did poorly. A low serum albumin level has proved to be a poor prognostic factor in other lymphomas, like Hodgkin's disease.23 In some cases hypoalbuminemia may reflect a deficient caloric intake, intestinal protein loss, or liver damage, but in most instances a linkage with tumor mass itself must be sought.23 BM involvement, present in 52% of our patients, was linked to a poorer survival. Romaguera et al17 found a significant correlation between the degree of BM involvement (more than 20%) and survival. Other studies did not show survival differences between patients with or without BM involvement.24,25 In our series, patients with B symptoms or low hemoglobin level also had shorter survival times, as reported in other studies.16,18Patients with an ESR greater than 30 mm had significantly poorer survival than patients with a normal ESR. This feature has not been previously reported as a poor prognostic factor in follicular lymphoma. However, this observation has already been described in other types of lymphomas26 and can be related to an increased secretion of interleukins or tumor necrosis factor (TNF). Finally, female sex was associated with a better outcome. This fact is not easy to explain, but it has been observed by others.17
When all 11 clinical features that were significant in univariate analysis were considered, a multivariate regression analysis with survival as the endpoint identified 6 key features of independent importance: age, sex, B symptoms, number of extranodal sites, LDH level, and ESR. These 6 independent variables defined a prognostic model with 3 risk groups. Patients with no or 1 unfavorable variable were considered to be at low risk, those with 2 unfavorable variables were at intermediate risk, those with 3 or more unfavorable variables were at high risk. These 3 risk groups had different 5-year survival rates: score 0-1 (low risk), 90%; score 2 (intermediate risk), 75%; and score 3 (high risk), 38%. This model was developed from the 429 patients for whom all 11 prognostic variables were available, and it was validated on an independent cohort of 265 patients who had all 6 variables used in the development of the model available for analysis. Moreover, the model was also predictive in the group of 210 patients from the validation sample who were uniformly treated with doxorubicin-containing regimens.
Before the introduction of the IPI, the design of predictive models was based on some of these features; however, they were not widely accepted, perhaps because they were derived from a limited number of cases and sometimes were difficult to apply.16-18 The IPI, originally devised for aggressive lymphomas, is simple to use but has been applied in follicular lymphomas with contradictory results. Moreover, the IPI has a limited discriminating power because most patients are allocated in the favorable or intermediate risk groups. In a study by Lopez-Guillermo et al19 performed on 125 patients with low-grade lymphoma, the IPI identified 20.8% of the patients at intermediate-high risk and 11.2% at high risk. However, Lopez-Guillermo et al19 reported no differences in the outcomes among patients at low-intermediate or high-intermediate risk, and they were merged into 1 intermediate group. When the IPI was applied in our series of patients, this model was able to discriminate 3 risk groups with statistically different survival rates (Figure 2B). As in other reports, the percentage of patients allocated in the intermediate-high or high-risk group is quite low (13%), and the IPI applied to our population of patients was useful in predicting response and survival, but given the size of the study population, this result is not surprising.
We believe that the prognostic model developed by the Intergruppo Italiano Linfomi and based on the analysis of several hundred patients with follicular lymphoma can be considered a step forward in the study of the prognosis of these patients. In addition to age, number of extranodal sites of involvement, and serum LDH level, variables already recognized as important predictors by the IPI, our study found that the prognosis of patients with follicular lymphoma depends also on the presence of B symptoms or elevated ESR. In patients with follicular lymphoma, these latter 2 parameters probably assume more important prognostic value than the poor performance status itself. Additional advantages of our model over the IPI are the remarkably higher discriminating power among groups (log-rank test, 86.62 versus 55.61 for the IPI) and the ability to identify a higher number of patients less than 60 years old with a poor outcome (4.0% versus 1.6%), which will help determine aggressive or innovative therapeutic approaches.
In recent years new therapeutic approaches have been investigated in follicular lymphoma. These include myeloablative treatment with stem cell rescue, purine analogues, and immunologic therapy with anti-CD20 monoclonal antibodies.27-32 Some therapeutic approaches result in not only complete clinical remission but also molecular remission in most patients. Patients who obtain molecular remission (disappearance of bcl-2 or immunoglobulin gene rearrangements) are likely to have a prolonged survival and RFS, and they may be cured of their lymphoma.33,34 However, t(14;18)–containing cells have been detected in the peripheral blood of patients who have been in remission for a number of years, which indicates that a positive result may not have absolute prognostic significance.35 High-dose therapy followed by hemopoietic stem cell support increases the number of complete remissions, but it is too early to draw any conclusions regarding the effect of this approach on survival. Thus, in follicular lymphoma, a reliable prognostic index would be useful in order to identify patients at different risks of failure and to select those who might benefit from new therapeutic approaches.
The score system proposed in this study identifies 3 risk groups with statistically significant differences in outcome. Patients at low risk had a very long survival and did fairly well with conventional treatment. Patients at intermediate risk had a relatively good 5-year survival but a reduced RFS, with a continuous pattern of relapse and no suggestion of cure. In these patients the above-mentioned new promising approaches can rationally be tested in randomized trials. Finally, patients at high risk fared very poorly, with low CR rates, high incidence of early relapse, and short median survival. This group of patients needs to be treated with innovative approaches early.
In conclusion, our model uses simple clinical characteristics, usually collected at the time of diagnosis. It separates groups of patients with substantially different outcomes. It may be useful to tailor the therapy for the individual patient or to design prospective randomized trials. Combining our model with other prognostic variables, such as β2-microglobulin level or functional and molecular unfavorable factors (p-53, bcl-2, bcl-xL, CDK family, sCD23, TNF, and vascular endothelial growth factor),36-38 may improve the model's discriminating power and identify more precisely those patients with a poor prognosis who are suitable for innovative approaches.
Acknowledgments
We wish to thank Angela Sirotti for assistance in data collection and Monica Bellei for assistance in preparation of the manuscript. We are also grateful to Kathryn Webb for linguistic review of the manuscript.
Participating institutions and principal investigators of the Intergruppo Italiano Linfomi on follicular lymphoma include the following:
Gruppo Italiano Studio Linfomi [Oncologia Medica, Università di Modena (V. Silingardi, M. Federico, V. Clo'); Dipartimento di Emato-Oncologia, Azienda Ospedaliera Bianchi-MelacrinoMorelli, Reggio Calabria (F. Nobile, M. Brugiatelli, V. Callea); Cattedra di Ematologia, Università di Milano (M.T. Maiolo, L. Baldini, M. Colombi); Divisione Medicina Ia, Sezione di Ematologia, Osp. Civile, Piacenza (L. Cavanna, D. Vallisa, R. Bertè); Medicina Interna, Oncologia Medica, Università di Pavia (E. Ascari, P.G. Gobbi, C. Pieresca); Servizio di Ematologia, Arcispedale S.Maria Nuova Reggio Emilia (L. Gugliotta, P. Avanzini, F. Merli); Dipartimento di Ematologia e Oncologia, USL di Pescara (M. Lombardo, F. Angrilli); Divisione di Ematologia, Ospedale A. Pugliese-Ciaccio, Catanzaro (S. Molica, M.G. Kropp); Istituto di Ematologia, Università di Messina (V. Pitini); Divisione di Ematologia, IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo (M. Carotenuto, N. Di Renzo); Clinica Medica Ia, Università di Modena (S. Sacchi, G. Longo); Divisione di Ematologia, Università di Modena (G. Torelli)]; U.O.A. Ematologia, Azienda Ospedaliera San Giovanni Battista, Torino (E. Gallo, U. Vitolo, C. Boccomini,); Istituto di Ematologia e Oncologia Medica L&A Seràgnoli, Università di Bologna (S. Tura, P.L. Zinzani); Divisione di Ematologia, Ospedale Civile Venezia-Mestre (T. Chisesi); Cattedra e Divisione di Ematologia, Università di Firenze (P.L. Rossi Ferrini, G. Bellesi, R. Alterini); Clinica Medica Generale, Policlinico Monteluce, Perugia (F. Grignani, M. Liberati); Dipartimento di Biotecnologie Cellulari ed Ematologia, Università La Sapienza, Roma (F. Mandelli, G. Avvisati, M. Martelli); Ematologia Universitaria Tor Vergata, Roma (A. Perrotti); Dipartimento di Ematologia, Ospedale Santa Maria Goretti, Latina (F. Ciccone, A. Chiericini); Dipartimento di Ematologia, Ospedale San Giacomo, Roma (A. Andriani); Cattedra e Servizio di Ematologia, Azienda Ospedaliera Policlinico, Bari (V. Liso, V. Pavone, A. Guarini); Dipartimento di Scienze Biomediche, Sezione di Ematologia, Università di Ferrara (G.L. Castoldi, A. Cuneo); and Divisione di Ematologia Ia, Ospedale San Martino, Genova (G. Santini, E.E. Damasio).
Supported by grants from the Fondazione Ferrata Storti, Pavia, and Associazione Angela Serra per la Ricerca sul Cancro, Modena, Italia.
Reprints:M. Federico, Oncologia Medica, Università di Modena, Policlinico, via del Pozzo 71, 41100 Modena, Italia; e-mail: federico@unimo.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.