Thrombopoietin (TPO) deficiency has been proposed as an important etiologic factor for thrombocytopenia in advanced-stage liver disease. To clarify the contributions of platelet production, platelet consumption, coagulation activation, and splenic sequestration to thrombocytopenia in liver disease, we studied TPO serum levels and markers of platelet production, platelet activation, and coagulation activation before and 14 days after orthotopic liver transplantation (OLT) in 18 patients with advanced liver cirrhosis. Thrombocytopenia before transplantation occurred with low-normal serum levels of TPO, normal levels of platelet and coagulation activation markers, and no increase in bone marrow production of platelets. TPO serum levels increased significantly on the first day after OLT, preceding the increase of reticulated platelets by 3 days and peripheral platelets by 5 days. Normalization of the peripheral platelet count occurred in most patients within 14 days of OLT, irrespective of the change in spleen size assessed by computed tomography volumetry. Normalization of platelet counts was not hampered by a certain degree of platelet activation observed during the steepest increase in the peripheral platelet count. Bone marrow production of platelets increased significantly within 2 weeks of transplantation. Low TPO serum levels with low platelet counts and without platelet consumption suggests low TPO production in end-stage liver disease. The rapid increase in TPO serum levels after transplantation induces an increase in the bone marrow production of platelets. Decreased TPO production in the cirrhotic liver is an important etiologic factor for thrombocytopenia in liver disease that is rapidly reversed by transplantation.
Thrombopoietin (TPO), the major regulator of megakaryocyte maturation and platelet production,1 is primarily produced by hepatocytes.2-4 Liver cell mass and peripheral platelet count are directly linked in animals.5In humans, TPO mRNA expression is reduced in cirrhotic livers.6 Reduced platelet production may be the consequence of decreased TPO production in patients with liver disease.7 8
Not only do TPO serum levels reflect TPO production in the liver, they reflect TPO degradation by platelets and megakaryocytes9and platelet turnover.10 Therefore, TPO production in various disease states is not accurately reflected by TPO serum levels. Patients with thrombocytopenia may exhibit markedly elevated and normal TPO serum levels, depending on hepatic TPO production (constant in patients without liver disease)3,11 and platelet turnover. Low or normal platelet turnover yields high TPO serum levels. In contrast, high-turnover states lead to TPO consumption and low serum levels.12 In the absence of information on platelet turnover, TPO levels do not yield conclusive information in liver diseases.6 13-15
After orthotopic liver transplantation (OLT), TPO serum levels increase.6,16 Peripheral platelet counts increase after the elevation of TPO levels with a lag of several days.14 These data suggest reduced TPO production as a major factor for thrombocytopenia in liver disease and restored normal TPO production as the main reason for the rapid reversal of thrombocytopenia after successful OLT. On the other hand, platelet activation and platelet consumption, well documented during graft reperfusion and in the early period after it in patients undergoing OLT,17-19 may also cause thrombocytopenia in advanced-stage cirrhosis.20 To study these variables, reticulated platelets, prothrombin split products F1 + 2, and plasma levels of platelet α-granule proteins, β-thromboglobulin, and platelet factor-4 were measured as specific markers for bone marrow platelet production,21,22 of thrombin generation,23 and of platelet activation,24 respectively, in patients with thrombocytopenia with end-stage liver disease before and after OLT.
Materials and methods
Patients
Eighteen patients awaiting OLT with stable advanced-stage cirrhosis of the liver (Child stages B and C) and platelet counts below 150 000/μL were prospectively studied. Excluded were patients with recent episodes of gastrointestinal bleeding, with antiplatelet antibodies against platelet glycoprotein (GP) Ib/IX and GP IIb/IIIa, determined as described,25 with clinically overt consumption coagulopathy, or with sepsis before OLT (patient characteristics, Table 1). OLT was carried out according to standard operating techniques without the use of femoral–subclavian bypass and without splenectomy. Aprotinin (Trasylol; Bayer, Leverkusen, Germany; median, 1 × 106 U; range, 0.5-2 × 106U) was given routinely during surgery. A cell-saver was used in 9 patients (median volume, 1300 mL; range, 700-2500 mL). Heparin therapy was initiated at the end of surgery and was administered for 2 to 4 days before switching to low-molecular-weight heparin, which was given for the rest of the study period. Immunosuppression after OLT consisted either of cyclosporine (Sandimmune Neoral; Novartis AG, Basel, Switzerland) 10 mg/kg or FK506 (Prograf; Fujisawa Limited, Killorglin, Ireland) 0.15 mg/kg starting 6 hours after OLT. With each regimen, dexamethasone 40 mg was administered at the end of surgery and steroids were gradually tapered in subsequent days, but all patients received at least 15 mg prednisolone at day 14 after OLT. Prophylactic antibiotics (amoxicillin 2 g/clavulanic acid 200 mg) were administered to all patients at the initiation of surgery. Other antibiotics were administered if needed.
Patient characteristics, packed erythrocyte and single-donor platelet unit transfusions, infections, rejection episodes, and extracorporeal therapy
| Patient . | Age (y) . | Sex . | Cause of Cirrhosis . | Child Stage . | Immunosuppression . | Erythrocyte Units During OLT . | Erythrocyte Units After OLT . | Single Donor Platelet Units During OLT . | Single Donor Platelet Units After OLT . | Infections . | Rejection Episodes . | Extracorporeal Therapy (HD) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | Alcoholic | C | Cyclosporine | 14 | — | — | — | — | — | — |
| 2 | 44 | M | Wilson disease | C | Cyclosporine | 11 | — | — | — | — | — | — |
| 3 | 49 | M | Hepatitis C | B | Cyclosporine | 5 | 2 | 1 | 1 | fever day 6 | — | — |
| 4 | 48 | F | Hepatitis B | C | Cyclosporine | 8 | 2 | — | 1 | fever day 7 | — | — |
| 5 | 56 | F | Alcoholic | C | Cyclosporine | 6 | 2 | 1 | — | — | — | — |
| 6 | 61 | M | Hepatitis C, HCC | B | Cyclosporine | 13 | 6 | — | — | fever day 5 | — | — |
| 7 | 68 | F | Hepatitis C | C | FK506 | 10 | 6 | 1 | — | infected venous line day 10 | — | — |
| 8 | 52 | M | Alcoholic | B | Cyclosporine | 12 | 7 | — | — | — | — | — |
| 9 | 42 | M | Alcoholic | C | Cyclosporine | 6 | 6 | — | — | T-drain infection day 9 | — | — |
| 10 | 47 | M | Wilson disease | C | FK506 | 10 | 3 | 1 | — | — | — | 1 × HD |
| 11 | 49 | F | PBC | B | FK506 | 6 | 4 | — | — | T-drain infection day 13 | — | 3 × HD |
| 12 | 30 | M | Hepatitis B | B | Cyclosporine | 6 | 1 | 1 | — | — | — | |
| 13 | 52 | F | Alcoholic | B | Cyclosporine | 6 | — | — | — | — | — | — |
| 14 | 57 | M | Alcoholic, HCC | B | Cyclosporine | 23 | 12 | 1 | — | fever day 11 | — | 5 × HD |
| 15 | 30 | F | Autoimmune hepatitis | C | Cyclosporine | 6 | 4 | — | — | urinary tract infection day 7 | day 7 | — |
| 16 | 53 | M | HCC, hemochromatosis | B | FK506 | 14 | 4 | — | — | infected ascites day 0 | day 12 | — |
| 17 | 42 | M | Alcoholic | C | FK506 | 7 | 1 | — | — | — | — | — |
| 18 | 50 | M | Alcoholic | B | FK506 | 30 | 2 | 4 | 6 | Candida infection day 5 | — | — |
| Patient . | Age (y) . | Sex . | Cause of Cirrhosis . | Child Stage . | Immunosuppression . | Erythrocyte Units During OLT . | Erythrocyte Units After OLT . | Single Donor Platelet Units During OLT . | Single Donor Platelet Units After OLT . | Infections . | Rejection Episodes . | Extracorporeal Therapy (HD) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | F | Alcoholic | C | Cyclosporine | 14 | — | — | — | — | — | — |
| 2 | 44 | M | Wilson disease | C | Cyclosporine | 11 | — | — | — | — | — | — |
| 3 | 49 | M | Hepatitis C | B | Cyclosporine | 5 | 2 | 1 | 1 | fever day 6 | — | — |
| 4 | 48 | F | Hepatitis B | C | Cyclosporine | 8 | 2 | — | 1 | fever day 7 | — | — |
| 5 | 56 | F | Alcoholic | C | Cyclosporine | 6 | 2 | 1 | — | — | — | — |
| 6 | 61 | M | Hepatitis C, HCC | B | Cyclosporine | 13 | 6 | — | — | fever day 5 | — | — |
| 7 | 68 | F | Hepatitis C | C | FK506 | 10 | 6 | 1 | — | infected venous line day 10 | — | — |
| 8 | 52 | M | Alcoholic | B | Cyclosporine | 12 | 7 | — | — | — | — | — |
| 9 | 42 | M | Alcoholic | C | Cyclosporine | 6 | 6 | — | — | T-drain infection day 9 | — | — |
| 10 | 47 | M | Wilson disease | C | FK506 | 10 | 3 | 1 | — | — | — | 1 × HD |
| 11 | 49 | F | PBC | B | FK506 | 6 | 4 | — | — | T-drain infection day 13 | — | 3 × HD |
| 12 | 30 | M | Hepatitis B | B | Cyclosporine | 6 | 1 | 1 | — | — | — | |
| 13 | 52 | F | Alcoholic | B | Cyclosporine | 6 | — | — | — | — | — | — |
| 14 | 57 | M | Alcoholic, HCC | B | Cyclosporine | 23 | 12 | 1 | — | fever day 11 | — | 5 × HD |
| 15 | 30 | F | Autoimmune hepatitis | C | Cyclosporine | 6 | 4 | — | — | urinary tract infection day 7 | day 7 | — |
| 16 | 53 | M | HCC, hemochromatosis | B | FK506 | 14 | 4 | — | — | infected ascites day 0 | day 12 | — |
| 17 | 42 | M | Alcoholic | C | FK506 | 7 | 1 | — | — | — | — | — |
| 18 | 50 | M | Alcoholic | B | FK506 | 30 | 2 | 4 | 6 | Candida infection day 5 | — | — |
HD, hemodialysis.
Informed, written consent was obtained from each patient before entry to the study, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
Prothrombin time and single factor analysis
Prothrombin time (PT) was measured as a percentage of a reference sample (Normotest; Baxter Immuno AG, Vienna, Austria; normal range, 75% to 120%) as described.26
Thrombopoietin–enzyme-linked immunosorbent assay
Thrombopoietin-levels in serum samples were measured using the Quantikine human TPO–enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, UK) with a lower limit of detection of 20 pg/mL. The assay was performed according to the manufacturer's suggestions. The recovery of spiked TPO in human serum was 93% to 109% at 50 to 500 pg/mL. The intra-assay coefficient of variation for a sample at 90 pg/mL was 10.3% (21 repetitions). The inter-assay coefficients of variation at 90 pg/mL, 195 pg/mL, and 710 pg/mL were 10%, 9.9%, and 6%, respectively (10 independent runs). TPO serum levels below 100 pg/ml are considered normal in healthy persons.12 28
β-Thromboglobulin and platelet factor-4
Blood collected in CTAD tubes (Boehringer Mannheim, Vienna, Austria) was immediately cooled. After 15 minutes on ice, blood was centrifuged at 3000g and 4°C for 30 minutes. One milliliter of the middle part of the supernatant was collected carefully without disturbing the separated phases. β-Thromboglobulin (TG) and platelet factor-4 (PF-4) were assayed using the ASSERACHROM β-TG and PF-4 ELISA kits (Boehringer Mannheim).
Reticulated platelets
Reticulated platelets (RPs) were determined as described.29 Briefly, 10 μL platelet-rich plasma was added to 500 μL undiluted thiazolorange reagent (ReticCOUNT; Becton Dickinson, San Jose, CA). Platelets were also stained with a saturation concentration of anti-CD41 (Phycoerythrin-conjugated; Immunotech, Marseille, France) to serve as a gate control. Samples were incubated for 30 minutes at room temperature in the dark, and acquisition on a flow cytometer (FACScan; Becton Dickinson) was begun immediately thereafter. Thirty thousand CD41+ events were acquired. The cutoff between dimly stained normal platelets and the more brightly stained RPs was defined by analysis of 44 healthy subjects in a previous study.29The flow cytometer was calibrated daily with 2-μm beads (DNA QC particles, Vial C; Becton Dickinson) for fluorescence and light scatter.
Markers of coagulation activation and fibrinolysis
Blood for the determination of coagulation activation and fibrinolysis markers was collected in tubes containing 0.11 mol/L sodium citrate. Prothrombin fragments F1 + 2 and thrombin/antithrombin III complex were assayed using the Enzygnost F1 + 2 micro ELISA and the Enzygnost TAT micro ELISA (both Behring Diagnostics, Marburg, Germany), respectively. Fibrin degradation products were quantified by the ASSERACHROM D-dimer ELISA kit (Boehringer Mannheim).
Heparin-induced platelet antibodies
Antibodies against PF4 complex known to cause heparin-associated thrombocytopenia type 2 (HAT II) were tested for using the GTI-H A T45 antigen-enzyme immunoassay (Genetic Testing Institute, Brookfield, WI). The assay was performed according to the manufacturer's suggestions in the presence and absence of heparin.
Estimation of spleen size by computed tomography volumetry
Spleen size was estimated in each patient by computed tomography (CT) volumetry with a Tomoscan SR 7000 or AV-Expander (Philips Medical Systems, Eindhoven, The Netherlands) before OLT and 10 to 14 days after OLT as described.30 Data were acquired in a spiral acquisition mode with 1-second rotation time. Scan parameters were 120 kV, 250 mA. A 5-mm slice collimation, a 10-mm table feed per rotation, and a 2-mm reconstruction increment were used. The acquired axial data were transferred to a Philips Easy Vision workstation (software version 4.0; Philips Medical Systems). A region of interest was drawn for each axial slice that exactly outlined the margins of the spleen. From the overlapping axial data set, the computer calculated the total volume included in the regions of interest. Normal spleens have a median volume of 214 cm3 (range, 107-314 cm3).31
Statistical analysis
Statistical analysis was performed using STATISTICA (Statsoft, Hamburg, Germany) for Windows v. 5.1 (Microsoft, Redmond, CA). The Friedman analysis of variance (ANOVA) by ranks was used for all repeated measurement parameters to evaluate differences between serial measurements. If significant differences were found in the Friedman ANOVA by ranks, the Wilcoxon matched pairs test was used for comparisons within patients between pre-OLT values and individual days after OLT. Differences between patients were calculated using the Mann-Whitney U test. Correlations were calculated using the Spearman rank correlation test.
Results
Peripheral platelet count and thrombopoietin plasma levels
Peripheral platelet counts were decreased in all patients before OLT (Table 2). Platelet counts further decreased in all patients during OLT, and they remained lower than they were before OLT for the next 6 days (P < .02), after which they started to rise (Figure 1A). From day 9 on, platelet counts were above pre-OLT values (P < .01). Platelet counts increased in all patients and reached normal values (>150 000/μL) in 14 of 18 patients within 14 days of OLT, irrespective of the immunosuppression used (cyclosporine versus FK506:P = .24). Peripheral platelet counts remained normal at repeated determinations during the 12 months after OLT. All 4 patients whose peripheral platelet count did not rise to 150 000/μL within 14 days of OLT (median, 108 000; range, 70 000-115 000/μL) had increased platelet counts after 12 months (median, 132 000; range, 102 000-147 000/μL). Mean platelet volume decreased from a median of 10.4 fL (range, 8.8-12.2 fL) before OLT to a median of 10.1 fL (range, 8.4-11.5 fL) at day 14 after OLT (P = .028).
Biochemical values before and 14 days after orthotopic liver transplantation
| Parameter . | Unit . | Before OLT . | Day 14 After OLT . |
|---|---|---|---|
| ALT | U/L | 28 (5-58) | 41 (12-243)* |
| Albumin | mg/dL | 34 (28-43) | 31 (27-38) |
| Bilirubin | mg/dL | 5.8 (1.7-22.4) | 2.8 (0.7-23.5) |
| Creatinine | mg/dL | 1 (0.7-1.5) | 0.9 (0.5-1.7) |
| Blood urea nitrogen | mg/dL | 14.5 (8-30) | 14.5 (6-44) |
| Hemoglobin | mg/dL | 11 (7.5-14.5) | 10 (8.6-11.5) |
| Leukocytes | 1000/μL | 4.5 (2-13.5) | 6.4 (3.1-11.5) |
| Platelets | 1000/μL | 84 (26-112) | 254 (70-398)* |
| TPO | pg/mL | <20 (<20-182) | 59 (<20-639)* |
| RP-absolute | 1000/μL | 0.8 (0.2-3.2) | 1.6 (0.3-3.6)* |
| PT | % | 44 (12-84) | 80 (58-110)* |
| Factor V activity | % | 44 (14-86) | 125 (76-281)* |
| F 1 + 2 | nmol/L | 0.7 (0.3-1.7) | 2.4 (1.3-4.7)* |
| Parameter . | Unit . | Before OLT . | Day 14 After OLT . |
|---|---|---|---|
| ALT | U/L | 28 (5-58) | 41 (12-243)* |
| Albumin | mg/dL | 34 (28-43) | 31 (27-38) |
| Bilirubin | mg/dL | 5.8 (1.7-22.4) | 2.8 (0.7-23.5) |
| Creatinine | mg/dL | 1 (0.7-1.5) | 0.9 (0.5-1.7) |
| Blood urea nitrogen | mg/dL | 14.5 (8-30) | 14.5 (6-44) |
| Hemoglobin | mg/dL | 11 (7.5-14.5) | 10 (8.6-11.5) |
| Leukocytes | 1000/μL | 4.5 (2-13.5) | 6.4 (3.1-11.5) |
| Platelets | 1000/μL | 84 (26-112) | 254 (70-398)* |
| TPO | pg/mL | <20 (<20-182) | 59 (<20-639)* |
| RP-absolute | 1000/μL | 0.8 (0.2-3.2) | 1.6 (0.3-3.6)* |
| PT | % | 44 (12-84) | 80 (58-110)* |
| Factor V activity | % | 44 (14-86) | 125 (76-281)* |
| F 1 + 2 | nmol/L | 0.7 (0.3-1.7) | 2.4 (1.3-4.7)* |
Values are given as median and range.
Significantly different at P < .05 from pretreatment values (Wilcoxon matched pairs test).
ALT, alanine aminotransferase; PT, prothrombin time; RP, reticulated platelet; TPO, thrombopoietin.
Peripheral platelet count and thrombopoietin plasma levels.
(A) Peripheral platelet counts after OLT. Line denotes lower limit of normal. (B) TPO serum levels after OLT. Line denotes upper limit of normal (100 pg/mL). (C) Reticulated platelets after OLT. Lines denote upper and lower limits of normal. Values are given as median, 25th to 75th percentile, and range. Pre, pre-OLT values.
Peripheral platelet count and thrombopoietin plasma levels.
(A) Peripheral platelet counts after OLT. Line denotes lower limit of normal. (B) TPO serum levels after OLT. Line denotes upper limit of normal (100 pg/mL). (C) Reticulated platelets after OLT. Lines denote upper and lower limits of normal. Values are given as median, 25th to 75th percentile, and range. Pre, pre-OLT values.
Before OLT, TPO serum levels were in the lower normal range (median, below the limit of detection of 20 pg/mL). Only 2 of 18 patients had moderately elevated TPO levels (132 and 182 pg/mL). They rose above the upper limit of normal at day 1 after OLT, peaked from days 4 to 6, and declined from day 8. By day 14, TPO serum levels in 14 of 18 (78%) patients were in the normal range (Figure 1B) and were higher than they were before OLT (P < .05; Figure 1B).
No correlation could be found between nadir peripheral platelet count after OLT (R = −0.07; P = .77) or percentage drop in peripheral platelet count after OLT (R = 0.34; P = .16) and peak TPO serum levels after OLT.
Reticulated platelets as markers for bone marrow production of platelets
Before OLT, RPs as markers of bone marrow platelet production were in the normal range (0.5% to 1.5%) in 11 patients and were elevated in 7 patients. On the first day after OLT, RPs started to increase. This increase under stable peripheral platelet counts became significant between days 4 and 6 after OLT (Figure 1C). With increasing peripheral platelet counts after day 6, the relative number of RPs started to decline, and, on day 14 after OLT, the bone marrow production of platelets relative to the peripheral platelet count was in the normal range once again. Even though the relative RP count was significantly lower at day 14 after OLT than before OLT (Wilcoxon matched pairs test: P = .02), the absolute RP number 14 days after OLT was approximately twice as high as it was before OLT (median, 791 RP/μL versus 1633 RP/μL; P = .04).
Individual peripheral platelet counts correlated positively with the absolute RP number. This correlation was best when RPs of the preceding day were matched to peripheral platelet counts of the following day (Spearman rank correlation test: R = 0.58; P < .0001).
Total blood count and biochemical parameters
Hemoglobin and leukocyte levels did not differ 14 days after OLT from what they were before OLT (Table 2), but every patient was administered several packs of erythrocytes during OLT. Liver synthetic function improved after OLT; both PT and the factor V activity (P = .0002; Table 2) increased within 14 days of OLT. Less sensitive parameters of liver synthetic (serum albumin) or excretory (bilirubin) function did not change between levels before and after OLT. Serum alanine aminotransferase (ALT) levels increased after OLT without interfering with liver synthetic function, and, except in 3 patients who required hemodialysis, kidney function remained stable throughout the study (Tables 1 and 2).
Parameters of platelet and coagulation activation
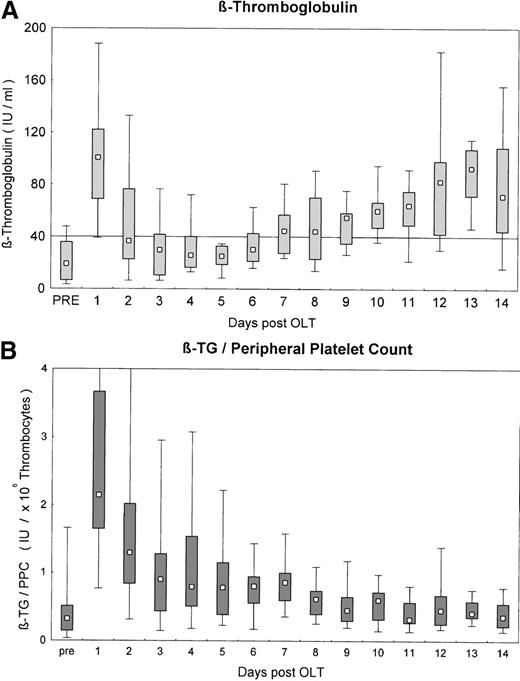
Markers of platelet activation platelet factor-4 (PF-4) and β-thromboglobulin (β-TG) were in the normal range before OLT. After reperfusion of the graft, markers of platelet activation sharply increased. This increase persisted until day 2 (P < .05). Plasma levels of activation markers remained in the normal range from day 3 until day 5 after OLT and started to rise gradually from day 6 on. From day 8 until the end of the study period, platelet activation markers were significantly elevated (P < .05; Figure2A). This was no longer true when β-TG levels were normalized for the peripheral platelet count. β-TG levels were not different before and 14 days after OLT but were significantly elevated from days 1 to 8 (Figure 2B). Markers of platelet activation β-TG and PF-4 did not show any correlation with the peripheral platelet count in the early phase after OLT (days 1 to 5), when platelet counts were stable and low (for β-TG: R = 0.06,P = .6). From day 6 to day 14, when the peripheral platelet count started to increase, a close and highly significant correlation between markers of platelet activation and peripheral platelet count was observed (for β-TG: R = 0.58; P < .000 01). Remarkably, normalized β-TG levels were significantly correlated with serum ALT levels after OLT (R = 0.29; P < .0001).
Parameters of platelet and coagulation activation.
(A) β-Thromboglobulin after OLT. Line denotes upper limit of normal. (B) β-Thromboglobulin after OLT, normalized for the peripheral platelet count. Line denotes upper limit of normal. Values are given as median, 25th to 75th percentile, and range. Pre, pre-OLT values.
Parameters of platelet and coagulation activation.
(A) β-Thromboglobulin after OLT. Line denotes upper limit of normal. (B) β-Thromboglobulin after OLT, normalized for the peripheral platelet count. Line denotes upper limit of normal. Values are given as median, 25th to 75th percentile, and range. Pre, pre-OLT values.
Markers of coagulation activation (TAT, F1 + 2) were in the normal range before OLT in most patients, whereas the less specific marker of fibrinolysis, D-dimer, was already elevated before OLT. At day 1 after OLT, all 3 markers of coagulation activation increased and remained significantly elevated, albeit to a lesser extent, throughout the study period.
Spleen size before and after OLT
Volumetric measurements of the splenic size before and after OLT were available from 14 of the 18 patients studied. Median splenic size before OLT was 751 cm3 (range, 283 to 1510 cm3) and 766 cm3 (range, 219 to 1290 cm3;P = .92) 14 days after OLT. Splenic size decreased in 5 patients (median decrease, 171 cm3; range, 64 to 328 cm3) and increased in 9 patients (median increase, 62 cm3; range, 19 to 232 cm3). There was a weak but significant correlation between spleen size and platelet count before OLT (R = −0.54; P = .047). The change in splenic size after OLT was not significant (P = .92) and was not correlated with the increase in peripheral platelet count (R = −0.27; P = .35).
Discussion
The results of this study confirmed earlier findings that the number of platelets in patients with end-stage cirrhosis increases after OLT, and they extended this observation by studying prospectively such potential confounding variables as platelet activation, platelet consumption, and changes in spleen size.
Before OLT, platelet counts were low and were without compensatory increases in TPO serum levels. Accelerated platelet activation or platelet consumption resulting from DIC32 were not observed; this was in accordance with other studies.33 The rate of platelet production relative to the peripheral platelet count was not elevated, and there was a weak but significant correlation between platelet count and spleen size. Thrombocytopenia in patients with end-stage cirrhosis before OLT was consistent with low platelet production and probably with splenic pooling in some patients.
As expected, OLT caused an acute and profound change in coagulation with hyperfibrinolysis and a drop in peripheral platelet count.17,18,34-36 Accordingly, on day 1 after OLT, platelet counts dropped to levels even lower than those before OLT. Increases in serum markers of platelet (PF-4, β-TG) and coagulation (D-dimer, TAT, F1 + 2) activation suggest that this was partly the result of coagulation and platelet activation. Such decreases can also be induced by liver surgery without transplantation in patients with cirrhosis and in those without it, but the mechanisms have not been studied in detail.37 General anesthesia by itself does not induce a drop in peripheral platelet count or platelet activation.38The early drop in platelet counts may be attributed to dilution caused by multiple blood cell transfusion during surgery (Table 1).
Peripheral platelet counts remained stable during the next 5 days and were not correlated with markers of platelet activation. TPO serum levels increased on the first day after OLT and the increase persisted throughout the entire study period. These findings do not support the hypothesis that platelet consumption continues until day 4 after OLT.39 Increased TPO serum levels were unrelated to decreases in peripheral platelet counts observed on day 1 after OLT40 because neither the nadir platelet count nor the percentage drop in platelet count correlated with peak TPO serum levels after OLT. In contrast, our finding of a parallel increase in the synthesis of liver-derived clotting factors and of TPO levels between days 1 and 4 after OLT supports the concept that TPO is synthesized by the new liver in sufficient quantities.
Increases in RP started by day 4 and in platelet number by day 6 after OLT. This is in good agreement with the time lag between the appearance of RP and the increase in peripheral platelets after the in vivo administration of a recombinant human TPO analogue.41,42The absolute RP number correlated with the peripheral platelet count. This correlation was closest when RP was correlated with the peripheral platelet count of the following day. Evidence shows that the absolute RP number is closely related temporally to the peak peripheral platelet count after the administration of a recombinant TPO-analogue to humans.42 The increase in RP without a pronounced drop in peripheral platelet count on day 1 after OLT39 further supports the relationship of RP to increased thrombopoiesis through the restitution of TPO production. Thus, except for the initial drop after graft reperfusion, the peripheral platelet count after OLT is dependent on the bone marrow production of platelets, which precedes the actual increase in peripheral platelet count by 1 to 2 days.
From day 6 until day 14, peripheral platelet counts rose sharply, and, except in 4 patients, platelet counts became normal within 2 weeks after OLT. This was not caused by surgical trauma because patients without cirrhosis who underwent liver resection regained preoperative platelet counts within 2 weeks but did not have significant increases over baseline values. Patients with cirrhosis who underwent liver resection experienced prolonged thrombocytopenia, and their platelet counts could not return to preoperative levels within 3 months.37 Three of the 4 patients (patients 10, 11, 14; Table 1) whose platelet counts did not return to normal within 14 days of OLT had severe medical problems, including renal failure that required hemodialysis. TPO production does occur in human kidneys in the proximal convoluted tubules,3 but the contribution to total body TPO production in humans is much less than it is in rodents.2 43 The patients in this study all appeared to suffer from postoperative prerenal failure with no signs of renal tubular damage. It seems unlikely that decreased renal TPO production was the reason for the prolonged thrombocytopenia. Patient 4 had primarily an unremarkable postoperative course, a 4-fold increase in platelet count within 2 weeks of OLT, a high platelet production rate (RP, >2000/μL), no signs of platelet consumption, and a continuous low platelet count (123 000/μL) at 12 months after OLT. Patients 4 and 11 showed positivity for antibodies against PF4-complexes responsible for HAT II. These findings may explain the continuous low platelet count to day 14 after OLT. The significance of this finding remains unclear, however, because 2 other patients without thrombocytopenia had these antibodies. Furthermore, HAT II cannot explain the continuing low platelet count long after the cessation of heparin therapy in patient 4.
With increasing platelet counts after OLT, there were also marked increases in β-TG in plasma, which is the release product of platelet α-granules. When normalized for the peripheral platelet count, an increase of β-TG was not detectable. Normalized β-TG levels were only elevated on days 1 and 2 after OLT and to a lesser degree until day 8. Platelet activation has been described during organ reperfusion, which could explain the elevated β-TG levels on days 1 and 2 after OLT.17 An increase in β-TG has also been described after kidney transplantation, when it is not accompanied by thrombocytopenia. This is probably because of the immune-mediated release of α-granule content from platelets during early rejection after kidney transplantation44; this mechanism has not been studied in detail after liver transplantation. The direct correlation between normalized β-TG and serum ALT levels in this study may hint at such a mechanism after OLT. The close and significant correlation between platelet count and β-TG after day 6 could also indicate some minor degree of immune-mediated activation of platelets with the minor release of α-granule proteins. Even though the relative amounts of β-TG were not significantly different from values before OLT, minor increases in β-TG release from each platelet (Figure 2), together with the rapidly increasing platelet counts, could have accounted for the increases in β-TG levels from day 6 to the end of the study period.
Most important, normalized platelet activation was not higher before OLT than it was 14 days after OLT. This, in the absence of direct measurement of platelet turnover, is an indirect indicator that the difference in peripheral platelet counts did not result from reduced platelet destruction after OLT but that it was a function of platelet production rate.
Other evidence supporting the concept of TPO-mediated increases in peripheral platelet counts after OLT is reports of dose-related decreases in mean platelet volume with increasing circulating platelet counts42 after the administration of recombinant TPO to mice, nonhuman primates, and humans. In accordance with these studies, we found a significant decrease in mean platelet volume 14 days after OLT.
Conventionally, thrombocytopenia in cirrhosis is attributed to splenomegaly caused by portal hypertension and subsequent pooling of platelets.45,46 However, a direct correlation between portal pressure and spleen size or platelet count was never demonstrated conclusively.47-49 Normalization of portal pressure with a surgical48 or radiologic shunt14,50,51 does not seem to improve thrombocytopenia. OLT effectively reverses portal hypertension.52 We observed a weak but significant correlation between spleen size and platelet count before OLT. However, in our study, spleen size measured by CT volumetry did not change after OLT. It decreased only in 5 patients, and there was no correlation between change in spleen size and improvement of peripheral platelet count. Spleens of our patients were much larger before and after OLT than spleens of persons without liver disease. Even though spleen size tended to decrease in long-term follow-up after OLT,53 it was not responsible for the restitution of normal peripheral platelet counts in the immediate postoperative period.
Inadequately low hepatic TPO production causing low platelet production by the bone marrow is an important causative factor of thrombocytopenia in advanced cirrhosis of the liver. Experimental5 and clinical data54-56 establish a direct relationship between functional liver mass and peripheral platelet count. Hepatocellular failure seems to outweigh increased platelet consumption and splenomegaly as the primary cause of thrombocytopenia in advanced liver failure, but splenomegaly still seems to play a role. Replacement of the cirrhotic liver with a functional graft reverses thrombocytopenia by increased platelet formation in the bone marrow after the restitution of normal TPO production. Based on these data, it is prudent to speculate that the substitution of recombinant TPO in patients with cirrhosis will alleviate, if not cure, the thrombocytopenia observed in cirrhosis of the liver. Even more important, substitution of recombinant TPO could prevent bleeding complications by reducing the duration and extent of the platelet nadir after OLT.
Acknowledgments
We thank E. Arzberger, E. Hangelmann, and M. Seif for their expert technical assistance.
Supported by the Jubiläumsfonds der Österreichischen Nationalbank (research grant 6689).
Reprints:Markus Peck-Radosavljevic, Department of Gastroenterology and Hepatology, University of Vienna, AKH Wien, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail:markus.peck@akh-wien.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal