A semiquantitative polymerase chain reaction assay was used to monitor the blood levels of Epstein-Barr virus (EBV)-DNA in 9 patients receiving allogeneic bone marrow transplants (BMT). Four of 5 recipients of HLA-mismatched T-cell–depleted grafts showed a 4- to 5-log increase of EBV-DNA within 1 to 3 months after BMT. Administration of 2 to 4 infusions of 107 EBV-specific cytotoxic T-lymphocytes (CTLs)/m2 starting from the time of maximal virus load resulted in a 2- to 3-log decrease of virus titers in 3 patients. One patient, who received a T-cell culture lacking a major EBV-specific component, progressed to fatal EBV-positive lymphoma. Administration of EBV-CTLs before the onset of the EBV-DNA peak resulted in stabilization of the virus titers within 2 to 3 logs above the normal levels in the fifth patient. A moderate increase of virus titers was also detected in 3 of 4 patients receiving unmanipulated HLA-matched grafts, whereas 1 patient with Wiskott-Aldrich syndrome reached a 5-log increase of EBV-DNA load within 70 days after BMT. Our results suggest that a rapid increase of circulating EBV-DNA occurs in the absence of EBV-specific T-cell precursors or in the presence of congenital immune defects that prevent the reestablishment of virus-specific immunity. Prophylactic administration of EBV-CTLs early after BMT appears to provide the most effective protection against the development of EBV-associated lymphoproliferative disease.
After a mild, self-limiting primary infection that usually occurs during childhood, Epstein-Barr virus (EBV) persists for life in healthy immunocompetent carriers (reviewed in 1). Latently infected B lymphocytes, which can grow in vitro as immortal EBV-carrying lymphoblastoid cell lines (LCLs), are found in the blood and lymphoid organs of the vast majority of adults.2-4These EBV-infected cells are kept in check by a strong specific immunity that can be readily monitored by the reactivation of EBV-specific cytotoxic T lymphocytes (EBV-CTLs) upon in vitro stimulation of lymphocytes from healthy virus carriers with the autologous EBV-transformed LCL.5 In carriers of congenital immune defects and persons who have become immunosuppressed as a consequence of medication or during human immunodeficiency virus infection, EBV-infected B lymphocytes may give rise to lymphoma or leukemia.6 7
Patients receiving immunosuppressive therapy after organ or bone marrow transplantation (BMT) run a particularly high risk of developing EBV-associated posttransplant lymphoproliferative disease (PTLD).8,9 The disease often presents early after transplantation with fever and disseminated adenopathy and may take a rapidly fatal course, not responding to reduced immunosuppression or chemotherapy. Whereas PTLD in organ transplant recipients is of recipient cell origin, donor B cells usually cause the lymphoproliferative disorders occurring after BMT.10 The incidence of PTLD varies depending on the type of transplant.11 Although this complication is observed in less than 1% of recipients of unmanipulated BMT from HLA-compatible related donors, in settings in which mismatched, T-cell–depleted (TCD), or unrelated stem cell grafts are used, PTLD may occur in up to 25% of patients.
The prophylactic and therapeutic aspects of PTLD have been the subject of several recent reviews.12-15 Papadopoulos et al16 were the first to report that infusion of donor lymphocytes induced clinical remission of PTLD in 5 recipients of allogeneic BMT. The therapeutic effect was attributed to the reconstruction of EBV-specific immunity by repletion of CTL precursors present in the lymphocyte infusion. However, the concomitant infusion of alloreactive T cells was associated with a significantly increased risk of serious graft-versus-host disease (GVHD). To avoid this problem, Heslop et al17 and Rooney et al18successfully applied EBV-specific cytotoxic T-cell cultures reactivated in vitro by stimulation of donor lymphocytes. Using genetically marked EBV-CTLs, they demonstrated that the virus-specific effectors persisted in the recipient for at least 18 months and could expand in vitro as well as in vivo in response to renewed challenge with EBV-infected cells.15 Previous studies have shown a correlation between the occurrence of PTLD and the amount of EBV-DNA detected in the peripheral blood of transplant recipients.19-21 Thus, semiquantitative polymerase chain reaction (PCR) techniques have the potential to identify patients at risk of developing EBV-associated lymphomas. However, it is unclear to what extent the levels of EBV-DNA correlate with the presence of subclinical or clinically manifest PTLD, disease outcome, or the levels of EBV-specific immunity in these patients.
The purpose of this study was to examine the correlation between the levels of EBV-DNA in the blood of BMT recipients early after transplantation and (1) the type of transplant received, (2) the presence of underlying immune defects, and (3) the clinical outcome of adoptive EBV-CTL therapy.
Materials and methods
Patients
The characteristics of the patients are summarized in Table1. Two children with acute lymphatic leukemia (ALL, patients 1 and 3), 1 with acute myeloid leukemia (AML, patient 5), 1 with hemophagocytic lymphohistiocytosis (HLH, patient 4), and 1 adult patient with AML (patient 2) received TCD bone marrow grafts from unrelated donors mismatched for 1 MHC class I allele or 1 class II allele. One child with Wiskott-Aldrich syndrome (WAS, patient 6) received an unmanipulated bone marrow graft from an unrelated HLA-A–, -B–, and -DR–compatible donor. One child with severe aplastic anemia (SAA, patient 7) and 1 child with thalassemia (patient 9) received unmanipulated (U) HLA-identical bone marrow from siblings. Patient 8 with ALL received peripheral blood stem cells from an unrelated HLA-compatible donor. Genomic HLA class I and II typing was performed by PCR with allele-specific primers.22 Five to 10 mL of blood was collected from the patients starting from the second week after BMT and every 2 to 4 weeks thereafter. This study was performed under protocols approved by the local human research ethics committee of the Karolinska Institutet, Stockholm, Sweden.
Characteristics of the patients and therapeutic procedures
| Patient No. . | Age . | Sex . | Diagnosis . | HLA I Recipient . | HLA I Donor . | Bone Marrow . | Conditioning . | Immunosuppression . | Day of Engraftment* . | GVHD Grade . | Chimerism† . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B . | T . | |||||||||||
| 1 | 5 | M | Pre-B ALL CR2 | A2,74 B44,B62 DRB1*0401,0701 | A2,29 B44,62 DRB1*0401,0701 | TCD | ATG, Cy FTBI | CsA, Mtx prednisolone | 18 | I | 63 | 63 |
| 2 | 39 | F | AML, CR3 | A1,32 B39,61 DRB1*1501,0404 | A1,32 B39,55 DRB1*1501,0404 | TCD | OKT3, Cy TBI | CsA, Mtx prednisolone | 22 | I | ND | ND |
| 3 | 8 | M | T-cell ALL CR2 | A3,25 B8,57 DRB1*0301,1401 | A2,25 B8,57 DRB1*0301,1401 | TCD | OKT3, Cy FTBI | CsA, Mtx | 22 | 0 | 42 | 104 |
| 4 | 6 | M | HLH | A30,32 B8,27 DRB1*0301,1101 | A32 B8,27 DRB1*0301,1101 | TCD | ATG, OKT3, Bu, Cy, VP16, TLI | CsA, Mtx prednisolone | 12 | 0 | 50 | ‡ |
| 5 | 13 | M | AML, CR1 | A3,25 B7,51 DRB1*1501,0404 | A3,25 B7,51 DRB1*1501,0408 | TCD | OKT3, Cy, FTBI | CsA, Mtx prednisolone | 17 | I-II | 501-153 | 501-153 |
| 6 | 1 | M | WAS | A1,2 B8,18 DRB1*0301 | A1,2 B8,18 DRB1*0301 | U | ATG, Bu, Cy | CsA, Mtx prednisolone | 18 | 0 | 661-154 | 661-154 |
| 7 | 14 | M | SAA | A2,32 B8,44 DRB1*0301,1301 | A2,32 B8,44 DRB1*0301,1301 | U | OKT3, Cy | CsA, Mtx prednisolone | 17 | 0 | ND | ND |
| 8 | 12 | F | ALL, CR3 | A1,2 B7,35 DRB1*1501,1104 | A1,2 B7,35 DRB1*1501,1104 | PBSC | OKT3, Cy, VP16, TBI | CsA, Mtx prednisolone | 11 | I | ND | ND |
| 9 | 7 | F | THAL | A2,32 B55,35 DRB1*1104,1401 | A2,32 B55,35 DRB1*1104,1401 | U | ATG, Bu, Cy | CsA, Mtx | 20 | I | ND | ND |
| Patient No. . | Age . | Sex . | Diagnosis . | HLA I Recipient . | HLA I Donor . | Bone Marrow . | Conditioning . | Immunosuppression . | Day of Engraftment* . | GVHD Grade . | Chimerism† . | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B . | T . | |||||||||||
| 1 | 5 | M | Pre-B ALL CR2 | A2,74 B44,B62 DRB1*0401,0701 | A2,29 B44,62 DRB1*0401,0701 | TCD | ATG, Cy FTBI | CsA, Mtx prednisolone | 18 | I | 63 | 63 |
| 2 | 39 | F | AML, CR3 | A1,32 B39,61 DRB1*1501,0404 | A1,32 B39,55 DRB1*1501,0404 | TCD | OKT3, Cy TBI | CsA, Mtx prednisolone | 22 | I | ND | ND |
| 3 | 8 | M | T-cell ALL CR2 | A3,25 B8,57 DRB1*0301,1401 | A2,25 B8,57 DRB1*0301,1401 | TCD | OKT3, Cy FTBI | CsA, Mtx | 22 | 0 | 42 | 104 |
| 4 | 6 | M | HLH | A30,32 B8,27 DRB1*0301,1101 | A32 B8,27 DRB1*0301,1101 | TCD | ATG, OKT3, Bu, Cy, VP16, TLI | CsA, Mtx prednisolone | 12 | 0 | 50 | ‡ |
| 5 | 13 | M | AML, CR1 | A3,25 B7,51 DRB1*1501,0404 | A3,25 B7,51 DRB1*1501,0408 | TCD | OKT3, Cy, FTBI | CsA, Mtx prednisolone | 17 | I-II | 501-153 | 501-153 |
| 6 | 1 | M | WAS | A1,2 B8,18 DRB1*0301 | A1,2 B8,18 DRB1*0301 | U | ATG, Bu, Cy | CsA, Mtx prednisolone | 18 | 0 | 661-154 | 661-154 |
| 7 | 14 | M | SAA | A2,32 B8,44 DRB1*0301,1301 | A2,32 B8,44 DRB1*0301,1301 | U | OKT3, Cy | CsA, Mtx prednisolone | 17 | 0 | ND | ND |
| 8 | 12 | F | ALL, CR3 | A1,2 B7,35 DRB1*1501,1104 | A1,2 B7,35 DRB1*1501,1104 | PBSC | OKT3, Cy, VP16, TBI | CsA, Mtx prednisolone | 11 | I | ND | ND |
| 9 | 7 | F | THAL | A2,32 B55,35 DRB1*1104,1401 | A2,32 B55,35 DRB1*1104,1401 | U | ATG, Bu, Cy | CsA, Mtx | 20 | I | ND | ND |
GVHD indicates graft-versus-host disease; ALL indicates acute lymphatic leukemia; TCD indicates T-cell[en]depleted bone marrow; ATG indicates antithymocyte globulin; Cy indicates cyclophosphamide; FTBI indicates fractionated total-body irradiation; CsA indicates cyclosporin A; Mtx indicates methotrexate; AML indicates acute myeloid leukemia; TBI indicates total-body irradiation; ND indicates not done; HLH indicates hemophagocytic lymphohistiocytosis; Bu indicates busulfan; VP16 indicates etoposide; TLI indicates total lymph node irradiation (2 Gy for 4 consecutive days); WAS indicates Wiskott-Aldrich syndrome; U indicates unmanipulated bone marrow; SAA indicates severe aplastic anemia; PBSC indicates peripheral blood stem cells; THAL indicates thalassemia.
Days after BMT.
Days after BMT when 100% of the indicated cell subpopulation was found to be of donor origin.
The T cells of the patients were predominantly of recipient origin during the entire period of observation.
Recipient B and T cells were detected again in this patient 2 months later.
The first chimerism test was performed at the indicated time.
Transplant regimens
The preparative regiments varied depending on the clinical protocol. Patients with leukemia received 120 mg/kg of cyclophosphamide over 2 days and 10 Gy of total-body irradiation (TBI). Children receiving partially HLA-mismatched bone marrow grafts were given 14.4 Gy of fractionated TBI.23 The WAS and thalassemia patients received 16 mg/kg busulfan and 200 mg/kg cyclophosphamide. The SAA patient received 200 mg/kg cyclophosphamide.24 All patients received 2 mg·kg−1·d−1 of antithymocyte globulin (Thymoglobuline; Pasteur-Merieux, Lyon, France) over 5 days or 2.5 to 5 mg/d of the murine anti-CD3 monoclonal antibody OKT (Ortho Biotech Inc, Raritan, NJ) for 5 days before BMT.25 T-cell depletion of HLA-mismatched bone marrow grafts was performed using immunomagnetic beads conjugated with CD2-, CD3-, and CD8-specific mouse monoclonal antibodies (Dynal, Oslo, Norway).26 Cyclosporin A (CsA) and a short course of intravenous (IV) methotrexate (15 mg/m2 at day + 1 and 10 mg/m2 at days +3, +6, and +11) were given for GVHD prophylaxis. As a general rule, patients with nonmalignant disorders, regardless of the type of donor, and patients with hematologic disorders receiving unrelated or HLA-mismatched grafts were monitored to achieve blood levels of CsA of 200 to 300 ng/mL during the first month after BMT, followed by maintenance levels of 100 to 200 ng/mL. Patients with hematologic malignancies receiving grafts from HLA-identical siblings were kept at CsA blood levels of 100 ng/mL during the first month, which were thereafter tapered in the absence of GVHD to be discontinued within 3 to 6 months, if possible.27 Acyclovir (500 to 1500 mg·m−2·d−1 IV) was given to patients with herpes simplex virus titers of 10 000 or greater. Preemptive treatment with foscarnet (120 to 180 mg·kg−1·d−1 for 2 to 3 weeks) or gancyclovir (10 mg·kg−1·d−1 for 2 to 3 weeks) was given to patients with signs of cytomegalovirus (CMV) reactivation.28 Details of the supportive care have been published elsewhere.23,24 29 The day of engraftment was defined as the day when the absolute neutrophil count exceeded 0.5 × 109/L.
Chimerism assay
The status of donor/recipient chimerism was analyzed by a semiquantitative PCR-based assay for DNA fragment length polymorphism, as described previously.30
Semiquantitative EBV-specific PCR
The details of the PCR assay were described previously.31 Briefly, peripheral blood mononuclear cells (PBMCs) isolated by centrifugation on Lymphoprep gradients (Nycomed, Oslo, Norway) were resuspended in lysis buffer (25 μL/106cells) containing 10 mmol/L Tris-HCl, pH 9.0, 0.1 mmol/L ethylenediaminetetraacetic acid, 0.5% Nonidet P-40, 0.5% Tween-20, and 400 μg/mL proteinase-K. After incubation for 1 hour at 55°C, the proteinase-K was inactivated by heating for 15 minutes at 95°C. EBV-DNA was amplified with a set of nested primers derived from the EBNA1 coding region of the prototype B95.8 EBV genome. After denaturation at 94°C for 5 minutes, 25 PCR cycles of 1-minute denaturation at 94°C, 1-minute annealing at 55°C, and 1-minute extension at 72°C were run with the following pair of outer primers: EB3, 5′-AAGGAGGGTGGTTTGGAAAG-3′; and EB4, 5′-AGACAATGGACTCCCTTAGC-3′ (nucleotides 109 331-109 350 and 109 608-109 627, respectively). One tenth of the resulting products was further amplified by 30 PCR cycles of 1-minute denaturation at 94°C, 1-minute annealing at 60°C, and 1-minute extension at 72°C using the following pair of inner primers: EB1, 5′-ATCGTGGTCAAGGAGGTTCC-3′ (nucleotides 109 352-109 371); and EB2, 5′-ACTCAATGGTGTAAGACGAC-3′ (nucleotides 109 541-109 560). The PCR products were resolved by electrophoresis in a 1.5% agarose gel containing 0.5 μg/mL ethidium bromide. DNA isolated from the EBV-negative T-cell line HSB2, water, and lysis buffer were included as negative controls in each assay. The DNA equivalents of 50, 10, and 2 cells from the EBV-positive BL line Namalwa, which contains 2 EBV genome copies per cell, were included in each test as positive controls. Calibration tests performed by diluting graded numbers of Namalwa in EBV-negative cells have shown that this PCR method allows detection of 1 EBV genome copy per 106cells.
Determination of EBV load
A simplified method was developed for routine determination of EBV-DNA load in BMT recipients. Serial 5-fold dilutions of DNA extracted from PBMCs were prepared starting from the amount of DNA corresponding to 106 cells, and each sample was analyzed for the presence of EBV-DNA. The last positive dilution and the preceding and following dilutions were retested in 10 independent PCR reactions each. The minimal number of cells containing 1 EBV genome equivalent was calculated from the number of positive reactions in each dilution according to the Poisson distribution and is expressed as “EBV load.” In routine tests, only the initial set of dilutions was analyzed, and the last positive sample was used to estimate the EBV-DNA load. The validity and reproducibility of this simplified method were confirmed on several occasions by complete 10-sample dilution assays.
Generation and characterization of EBV-specific CTLs
After informed consent, an additional 100 mL of blood was drawn from each bone marrow donor at the time of retyping or workup, 3 to 6 weeks before BMT. An EBV-transformed LCL was established by infection of 107 PBMCs with spent supernatant from the EBV producer B95.8 cell line,32 and the remaining PBMCs were cryopreserved for later use. EBV-specific CTLs were generated by stimulation of PBMCs with the autologous LCL, as described previously.33 After 3 consecutive restimulations, the cultures were expanded in interleukin (IL)-2–containing medium (30% MLA144 culture supernatant, 10 U/mL human recombinant IL-2). Restimulation with a mixture of allogeneic phytohemagglutinin (PHA)-activated (10 μg/mL for 1 hour at 37°C) and irradiated (3000 rad) PBMCs and the autologous LCL (9:1 ratio) was used for large-scale expansion. The EBV specificity of the cultures was tested in standard 4-hour 51Cr-release assays using a panel of targets including the autologous LCLs, allogeneic LCLs matched through 1 or several MHC class I alleles, mismatched LCLs, and donor PHA-activated blasts.
CTL infusions
EBV-CTLs were given to the patients when the virus load reached or exceeded an arbitrarily set level of 1 EBV genome in 103cells, or when sufficient numbers of cells became available. In standard protocols, the patients received 4 weekly doses of approximately 107 cells/m2 by IV infusion. The CTL cultures were washed extensively in phosphate-buffered saline before infusion. Tests performed at the time of injections confirmed that the cells were free of bacteria, Mycoplasma contamination, and infectious EBV and did not contain reverse transcriptase activity.
Results
Monitoring of EBV-DNA load
The EBV-DNA load was monitored by semiquantitative PCR in 5 recipients of HLA-mismatched TCD bone marrow grafts and 4 recipients of unmanipulated HLA-compatible bone marrow. All donor-recipient pairs were EBV seropositive as determined by detection of serum IgG antibodies to viral capsid antigen before the initiation of workup procedures (data not shown). Table 1 summarizes the clinical characteristics of the patients. Monitoring was initiated within 2 to 3 weeks after transplantation and was continued at regular intervals for at least 100 days and up to 450 days. Pretransplant levels of EBV-DNA were measured in some cases, depending on the availability of material, and were found to be within the range detected by our PCR assays in PBMCs from healthy EBV carriers (i.e., < 1 EBV genome in 106 cells).31 EBV-DNA titers greater than 1 genome/105 cells were detected in all patients within 3 weeks after BMT, at which time bone marrow engraftment was also confirmed. In 4 patients receiving TCD grafts and 1 WAS patient receiving an unmanipulated graft, the EBV-DNA load showed a rapid increase, reaching 1 genome in 6.4 cells (patients 1 and 6), 1 genome in 32 cells (patients 2 and 4), and 1 genome in 160 cells (patient 3) within 31 to 130 days after grafting (Figures1-5). In the fifth patient receiving a TCD graft, CTL therapy was initiated at day 31 after BMT when the PCR assay detected an EBV-DNA load of 1 genome in 800 cells (Figure 6). In 3 patients receiving unmanipulated grafts (patients 7, 8, and 9), the viral load did not exceed 1 genome in 4000 cells during observation periods ranging between 120 and 336 days after BMT (Figure7). Although recipients of HLA-mismatched grafts received a stronger immunosuppressive regimen, as a rule, there was no obvious correlation between the amount of medication and the magnitude of EBV-DNA load during the posttransplant period. This is illustrated by the comparison of serum CsA levels and EBV-DNA loads in patients 1, 5, and 6. Administration of antiviral therapy either at the time of BMT (patients 2 and 6) or after detection of elevated CMV-DNA and/or EBV-DNA titers (patients 1, 2, and 4) had no apparent effect on the EBV-DNA load.
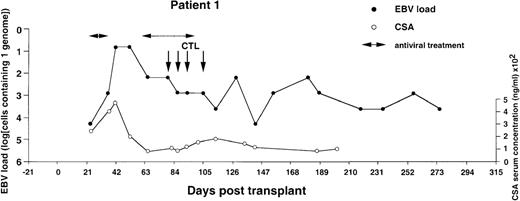
Monitoring of EBV-DNA load and CsA levels in patient 1.
A 5-year-old boy with ALL received a TCD bone marrow graft from a mismatched unrelated donor. The posttransplant course was uncomplicated. A limited acute GVHD of the skin was treated with oral prednisolone. Four infusions of EBV-CTLs were given starting from day +79 after BMT without exacerbation of GVHD. Cyclosporin A was continued during follow-up.
Monitoring of EBV-DNA load and CsA levels in patient 1.
A 5-year-old boy with ALL received a TCD bone marrow graft from a mismatched unrelated donor. The posttransplant course was uncomplicated. A limited acute GVHD of the skin was treated with oral prednisolone. Four infusions of EBV-CTLs were given starting from day +79 after BMT without exacerbation of GVHD. Cyclosporin A was continued during follow-up.
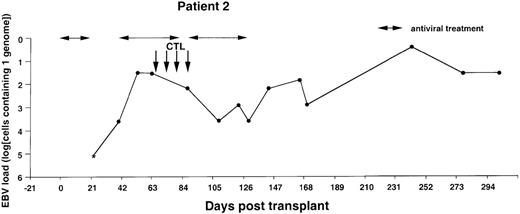
Monitoring of EBV-DNA load in patient 2.
A 39-year-old woman with AML received a TCD bone marrow graft from a mismatched unrelated donor. Grade I GVHD developed after engraftment. Four CTL infusions were given starting from day +67. A booster of TCD marrow was given on day +134 because of threatening rejection. Cyclosporin A was continued during follow-up. *EBV-DNA was not detected in the DNA equivalent of 105 cells.
Monitoring of EBV-DNA load in patient 2.
A 39-year-old woman with AML received a TCD bone marrow graft from a mismatched unrelated donor. Grade I GVHD developed after engraftment. Four CTL infusions were given starting from day +67. A booster of TCD marrow was given on day +134 because of threatening rejection. Cyclosporin A was continued during follow-up. *EBV-DNA was not detected in the DNA equivalent of 105 cells.
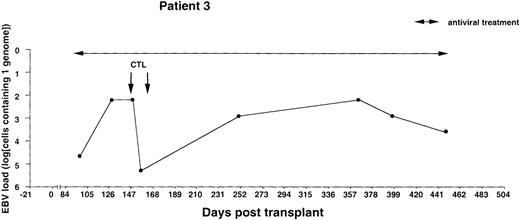
Monitoring of EBV-DNA load in patient 3.
A 10-year-old boy with T-cell ALL received a TCD bone marrow graft from a mismatched unrelated donor. The early posttransplant course was uncomplicated. Intravenous infusions of foscarnet were given during the entire observation period because of detection of CMV-DNA in leukocytes and leukopenia. The majority of circulating T cells were of recipient origin during the first 3 months after BMT, whereas B cells, macrophages, and granulocytes were of donor origin as determined by PCR. Donor T cells were infused on days +62 and +82, and only donor T cells were detected in the circulation 6 months after BMT. Two infusions of 107/m2 EBV-CTLs were given on day +148 and +160. There were no clinical signs of GVHD after T-cell infusions or EBV-CTL treatment. Cyclosporin A was discontinued 9 months after BMT. The T-cell leukemia relapsed 16 months after BMT, with a lethal outcome.
Monitoring of EBV-DNA load in patient 3.
A 10-year-old boy with T-cell ALL received a TCD bone marrow graft from a mismatched unrelated donor. The early posttransplant course was uncomplicated. Intravenous infusions of foscarnet were given during the entire observation period because of detection of CMV-DNA in leukocytes and leukopenia. The majority of circulating T cells were of recipient origin during the first 3 months after BMT, whereas B cells, macrophages, and granulocytes were of donor origin as determined by PCR. Donor T cells were infused on days +62 and +82, and only donor T cells were detected in the circulation 6 months after BMT. Two infusions of 107/m2 EBV-CTLs were given on day +148 and +160. There were no clinical signs of GVHD after T-cell infusions or EBV-CTL treatment. Cyclosporin A was discontinued 9 months after BMT. The T-cell leukemia relapsed 16 months after BMT, with a lethal outcome.
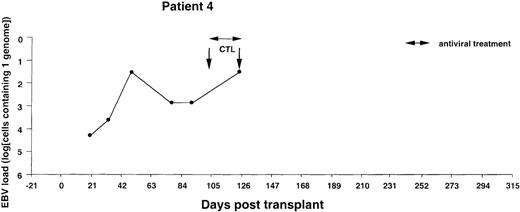
Monitoring of EBV-DNA load in patient 4.
A 5-year-old boy with HLH received a TCD bone marrow graft from a mismatched unrelated donor. The early posttransplant course was uncomplicated, without clinical signs of GVHD. Despite the successful engraftment of BMT, repeated infusions of donor T cells, and a booster of peripheral stem cells, recipient T cells persisted in the blood. Lymphopenia and positive PCR tests for CMV-DNA in the blood prompted preemptive antiviral therapy. EBV-CTLs were infused on days +102 and +125, at which time the patient presented with fever and abdominal pain. A bilateral neck mass developed rapidly, and an excisional biopsy performed on day +140 disclosed a highly malignant EBV-positive NHL with dissemination to the abdomen and central nervous system. The patient died of progressive disease 2 weeks after diagnosis.
Monitoring of EBV-DNA load in patient 4.
A 5-year-old boy with HLH received a TCD bone marrow graft from a mismatched unrelated donor. The early posttransplant course was uncomplicated, without clinical signs of GVHD. Despite the successful engraftment of BMT, repeated infusions of donor T cells, and a booster of peripheral stem cells, recipient T cells persisted in the blood. Lymphopenia and positive PCR tests for CMV-DNA in the blood prompted preemptive antiviral therapy. EBV-CTLs were infused on days +102 and +125, at which time the patient presented with fever and abdominal pain. A bilateral neck mass developed rapidly, and an excisional biopsy performed on day +140 disclosed a highly malignant EBV-positive NHL with dissemination to the abdomen and central nervous system. The patient died of progressive disease 2 weeks after diagnosis.
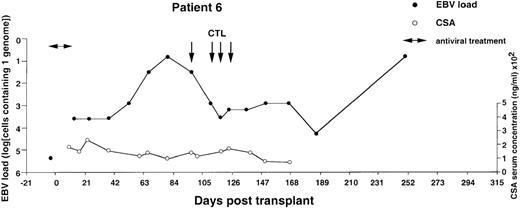
Monitoring of EBV-DNA load and CsA levels in patient 6.
A 1-year-old boy with WAS received an unmanipulated bone marrow graft from an unrelated donor. The early posttransplant course was uncomplicated, with no GVHD. However, a mixed chimerism developed. Half of the B cells, granulocytes, and monocytes were of recipient origin, whereas T cells were exclusively of donor extraction. A moderate reticulocytopenia and mild hemolysis were treated with erythropoietin and steroids. Tapering of CsA was initiated but not completed during follow-up. Four EBV-CTL infusions were started on day +97. The EBV-DNA load determined before BMT is shown by an isolated filled circle.
Monitoring of EBV-DNA load and CsA levels in patient 6.
A 1-year-old boy with WAS received an unmanipulated bone marrow graft from an unrelated donor. The early posttransplant course was uncomplicated, with no GVHD. However, a mixed chimerism developed. Half of the B cells, granulocytes, and monocytes were of recipient origin, whereas T cells were exclusively of donor extraction. A moderate reticulocytopenia and mild hemolysis were treated with erythropoietin and steroids. Tapering of CsA was initiated but not completed during follow-up. Four EBV-CTL infusions were started on day +97. The EBV-DNA load determined before BMT is shown by an isolated filled circle.
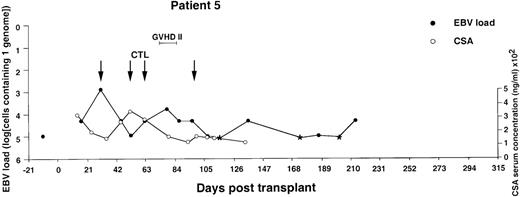
Monitoring of EBV-DNA load and CsA levels in patient 5.
A 13-year-old boy with AML received a TCD bone marrow graft from a mismatched unrelated donor. Grade 2 GVHD was treated with oral prednisolone and psoralen plus ultraviolet A. Four infusions of EBV-CTLs were given prophylactically starting from day +31. The last injection was postponed for 2 weeks because of fever, mild exacerbation of GVHD, and pancytopenia. The EBV-DNA load determined before BMT is shown by an isolated filled circle. *EBV-DNA was not detected in the DNA equivalent of 105cells.
Monitoring of EBV-DNA load and CsA levels in patient 5.
A 13-year-old boy with AML received a TCD bone marrow graft from a mismatched unrelated donor. Grade 2 GVHD was treated with oral prednisolone and psoralen plus ultraviolet A. Four infusions of EBV-CTLs were given prophylactically starting from day +31. The last injection was postponed for 2 weeks because of fever, mild exacerbation of GVHD, and pancytopenia. The EBV-DNA load determined before BMT is shown by an isolated filled circle. *EBV-DNA was not detected in the DNA equivalent of 105cells.
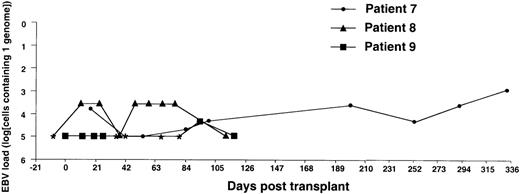
Monitoring of EBV-DNA load in patients 7, 8, and 9.
Patients 7 and 8, a 15-year-old boy with SAA and an 11-year-old girl with ALL, respectively, received unmanipulated bone marrow from HLA-identical unrelated donors. Patient 9, an 8-year-old girl with thalassemia, received unmanipulated marrow from an HLA-matched sibling. The posttransplant course was uncomplicated with no GVHD in all 3 patients. * EBV-DNA was not detected in the DNA equivalent of 105 cells.
Monitoring of EBV-DNA load in patients 7, 8, and 9.
Patients 7 and 8, a 15-year-old boy with SAA and an 11-year-old girl with ALL, respectively, received unmanipulated bone marrow from HLA-identical unrelated donors. Patient 9, an 8-year-old girl with thalassemia, received unmanipulated marrow from an HLA-matched sibling. The posttransplant course was uncomplicated with no GVHD in all 3 patients. * EBV-DNA was not detected in the DNA equivalent of 105 cells.
Generation of EBV-specific CTL lines
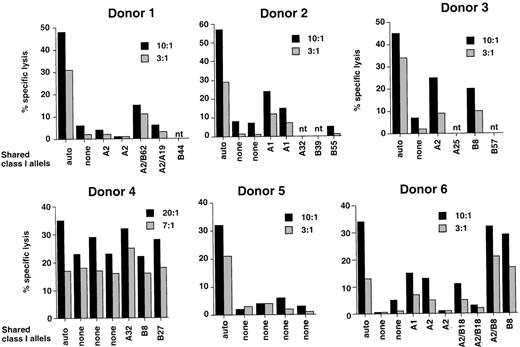
EBV-specific CTL lines were initiated before BMT in 6 patients considered to be at risk of EBV-PTLD because of T-cell depletion of the grafts or congenital immunodeficiency. Donor lymphocytes were stimulated with the autologous B95.8 virus-transformed LCL according to standard protocols developed in our laboratory. After 3 consecutive restimulations, each CTL line was tested against autologous and allogeneic HLA class I–matched or –mismatched LCLs and autologous PHA blasts. Representative experiments illustrating the cytotoxic activity at the time of CTL transfer are shown in Figure8. All CTL lines lysed the autologous LCLs in standard 4-hour 51Cr-release assays (32% to 58% specific lysis at a 10:1 effector-target ratio). In 4 cases (donors 1, 2, 3, and 6), the pattern of cytotoxic activity corresponded to that observed in EBV-specific cultures; that is, virtually no lysis of HLA-mismatched LCLs and autologous or allogeneic PHA blasts (data not shown) and variable levels of killing of LCLs matched through single HLA class I alleles. It was shown previously that the level of lysis against allogeneic HLA-matched LCLs correlates with the immunogenicity of the presented epitopes and with variations in the EBV-specific CTL repertoire of the responder.34 The CTL culture from donor 5 appeared to be EBV specific in that it lysed the autologous LCL but not a panel of HLA-mismatched LCLs, but the class I restriction could not be determined because of lack of appropriate single matched targets. The CTL culture from donor 4 lysed equally well the autologous and allogeneic class I–matched or –mismatched LCLs. This pattern of recognition corresponds to that observed in cultures containing lymphokine activated killing activity and is probably due to failure to achieve a significant enrichment of EBV-specific CTLs.
Reactivity of CTL lines generated from 6 healthy individuals who donated their bone marrow to the patients included in the study.
CTL lines were generated by stimulation of donor PBMCs with the autologous B95.8 virus–transformed LCL. The results of 1 representative 51Cr-release assay performed at the time of transfer are shown for each CTL line.
Reactivity of CTL lines generated from 6 healthy individuals who donated their bone marrow to the patients included in the study.
CTL lines were generated by stimulation of donor PBMCs with the autologous B95.8 virus–transformed LCL. The results of 1 representative 51Cr-release assay performed at the time of transfer are shown for each CTL line.
Effect of CTL therapy on EBV-DNA load
Previous reports have shown that detection of EBV-DNA titers exceeding the normal levels by at least 3 to 4 logs is highly predictive of EBV-PTLD. Therefore, in patients 1, 2, 3, 4, and 6, infusions of donor-derived CTL lines were initiated at the earliest possible time after detection of EBV-DNA titers exceeding an arbitrarily set threshold of 1 genome in 103 cells. Patient 5 received CTL infusions at the earliest possible time after transplantation. The treatment was initiated already at day 31 after BMT. Patients 3 and 4 received only 2 CTL infusions because of shortage of cells or unavailability of the patient, whereas the other patients received all 4 infusions. The weekly interval between infusions was occasionally prolonged, pending availability of sufficient numbers of CTLs. In patient 5, the last infusion was postponed for 2 weeks because of fever, pancytopenia, and a limited reactivation of acute GVHD of the skin, which also coincided with tapering of ongoing prednisolone medication (Figure 6). None of the other patients had clinical symptoms of GVHD in connection with or after the CTL infusions. In patients 1, 2, 3, and 6, the infusions were followed by a 2- to 4-log decrease of EBV-DNA titers within 3 to 4 weeks of the first CTL infusion. In patient 1, the EBV-DNA load remained relatively stable during the subsequent observation period of 273 days. In patients 2, 3, and 6, the initial decrease of EBV-DNA titers was followed by a slow increase in titers, which eventually culminated in new peaks registered at 243, 366, and 248 days after BMT, respectively. A spontaneous decrease of EBV-DNA titers was documented in patients 2 and 3, whereas additional tests could not be performed in patient 6. Patient 5, who received CTL transfer early after BMT, did not reach the high EBV-DNA load observed in other recipients of TCD grafts but remained within values ranging between 1 genome in 4 × 103 to 105cells during the entire observation period of 215 days. The adoptive T-cell transfer had no obvious effect in patient 4, who received a CTL culture lacking a clear EBV-specific component. This patient died shortly after the last CTL infusion from a disseminated EBV-positive non-Hodgkin lymphoma (NHL) (see Figure 4 for details). None of the remaining patients developed PTLD during follow-up. Patient 3 died in a relapse of his T-cell ALL 16 months after transplantation. The other patients were still disease free 471 to 568 days after transplantation.
Discussion
The results of this study support the notion that infusions of EBV-specific CTLs are effective in the prophylaxis of EBV-associated immunoblastic lymphomas in patients receiving TCD HLA-mismatched bone marrow grafts from EBV-seropositive donors. Furthermore, the design of our study, with monitoring of EBV-DNA load in peripheral blood initiated within the first 2 to 3 weeks after BMT, yielded interesting new insights into the early dynamics of EBV reactivation and the correlation between the rise in virus titers and the type of graft and amount of immunosuppressive therapy administered to these patients.
We have shown earlier that our semiquantitative PCR assay, which is based on amplification of a unique region within the EBNA1 coding sequence, has a detection capacity of approximately 1 EBV genome in 106 mononuclear cells.31 At least 106 unfractionated PBMCs are usually required to detect a positive PCR signal in blood from healthy EBV carriers. This finding is well in line with previous reports, by ourselves and by others, that 1 in 105 to 106 purified B cells carries the virus.31 35 Bone marrow engraftment was demonstrated in all our patients within the first 3 weeks after transplantation, and complete chimerism of the B- and T-cell compartments was established in all but 1 patient at the time of detection of peak levels of EBV-DNA (compare Table 1 and Figures 1-6). Independent of the type of graft, all patients showed a 1- to 3-log increase of virus titers within the first 2 to 3 weeks after BMT, corresponding to detection of 1 EBV genome in 105 to 4 × 103 mononuclear cells. Administration of drugs that inhibit herpes virus replication at the time of BMT did not affect the early increase of EBV-DNA load in patients 2 and 6, suggesting that the increase is largely due to proliferation of the EBV-infected cells present in the graft rather than de novo infection after activation of virus production. This is also confirmed by our general failure to detect EBV-DNA in the serum of these patients, although trace amounts were found in some cases concomitantly with detection of peak levels in PBMCs (data not shown).
Despite similar clinical characteristics early after BMT, patients receiving TCD or unmanipulated grafts differed significantly during subsequent follow-up. Three of 4 patients who received unmanipulated marrow maintained constant viral loads ranging between 1 EBV genome in 105 to 4 × 103 cells during observation periods of 115 to 336 days. In contrast, 4 of 5 patients receiving TCD marrow and 1 patient receiving unmanipulated marrow showed a dramatic increase of viral loads, reaching titers between 4 and 5 logs higher than in healthy virus carriers within 40 to 120 days after BMT. Patients receiving HLA-mismatched grafts are subject to a stronger immunosuppressive regimen, including maintenance of high serum levels of CsA for prolonged periods, to prevent GVHD.36 However, there was no direct correlation between variations in the serum levels of CsA and the onset of EBV-DNA peaks (see patients 1, 5, and 6). Thus, the initial increase of EBV-DNA load appears to be due to the depletion of EBV-specific T-cell precursors in the graft rather than to the degree of immunosuppression achieved during the posttransplant period. Indeed, in 4 of the patients receiving EBV-CTLs, the elevated levels of viral DNA decreased significantly within 3 to 4 weeks after the first infusion and remained relatively low for prolonged periods. Subsequent increases of EBV-DNA load were kept in check by resident immune responses that may be reestablished because of the long-term survival of the infused CTLs, as shown by Rooney et al,15 or by de novo activation in the chimeric lymphoid system. It is noteworthy that, although clearly effective, the EBV-CTL infusions did not bring the EBV-DNA load down to the levels of healthy EBV carriers or of patients receiving unmanipulated grafts. Conceivably, the continuous administration of high doses of CsA may have hampered the engraftment of EBV-CTLs in our patients. Alternatively, some of the EBV-carrying cells present in the circulation may have been insensitive to this type of control. This possibility is supported by the observation that administration of EBV-CTLs early after BMT prevented the onset of peak EBV-DNA titers in patient 5 and stabilized the viral load at the levels detected in recipients of unmanipulated bone marrow. Further studies are currently in progress to determine the pattern of latent viral genes expressed in the blood and lymphoid organs of these patients.
Although the presence of EBV-specific precursors is clearly a prerequisite for the recovery of specific immunity, the importance of the host environment is emphasized by the dramatic increase of EBV-DNA titers in patient 6, who received an unmanipulated HLA-matched graft. This patient suffered from Wiskott-Aldrich syndrome, an X-linked immunodeficiency syndrome due to mutation of the gene encoding for the Wiskott-Aldrich syndrome protein (WASP), which has been implicated in receptor signaling and cytoskeletal organization.37Congenital immunodeficiencies are often associated with an increased risk of EBV-associated lymphoproliferative disorders.38 A relatively high incidence of PTLD was reported previously in WAS patients, even after transplantation of HLA-matched marrow.39,40 This suggests that the presence of EBV-specific precursors may not warrant a rapid recovery of immunity. Conceivably, other cell types, such as dendritic cells, which are functionally defective in WAS patients,41 may be required to provide an adequate environment for the reactivation of virus-specific responses. It is intriguing that this putative helper function appears to be provided by the host, suggesting that the relevant cells may have been spared by the bone marrow eradication procedure or that they are not of bone marrow origin.
Only 1 of 6 patients receiving EBV-CTLs developed lymphoma, in contrast to the uniform association between this complication and elevated levels of EBV-DNA reported in previous studies.12,19Patient 4 differed from other patients in that the majority of the circulating T cells were of recipient origin during the entire posttransplant period (Table 1). Furthermore, despite repeated administration of donor-derived CTLs, the EBV-DNA titers remained elevated for 3 months before death due to PTLD. The lack of a strong EBV-specific component in the T-cell culture derived from donor 4 (Figure 8 ) may explain the failure of CTL transfer therapy. Our attempts to improve the specificity of the T-cell culture by restimulation with the autologous LCL were unsuccessful (data not shown). Several reasons could account for this failure, including the introduction of minor variations in the protocol used for CTL activation, such as the early addition of recombinant IL-2 and allogeneic feeders, to prevent cell death and to ensure a timely expansion of the cultures. However, it was shown previously that the EBV-specific response of HLA-B8–positive individuals is often dominated by reactivities against a single B8-restricted epitope derived from the EBV nuclear antigen, EBNA3, FLRGRAYGL (FLR).42 43 The DNA sequence encoding for this peptide is mutated in the B95.8 virus, resulting in LCLs that cannot reactivate FLR-specific CTLs. It is noteworthy that donors 3 and 6 mounted efficient B8-restricted CTL responses upon restimulation of the autologous B95.8-transformed LCLs, suggesting that other B8-restricted epitopes were probably recognized. Alternatively, donor 4 may have been infected with a rare type-B EBV strain, resulting in a weak response to the only partially cross-reactive type A B95.8 virus. We could not test this possibility because of lack of material. It is also possible that the donor-derived CTLs were cleared rapidly by alloresponses induced in the persistent recipient T-cell population.
Although epitope variations and a significant degree of epitope focusing in CTL cultures generated by LCL stimulation may prevent the generation of efficient EBV-specific cultures in some cases, in our experience T-cell lines that would meet the criteria for infusion can be produced in more than 90% of healthy EBV-seropositive donors. A more significant drawback to the clinical implementation of CTL transfer therapy appears to be the time required to generate the CTL lines, which is dependent on the establishment of EBV-transformed LCLs for T-cell stimulation and CTL testing. This point is illustrated by the results obtained with patient 5, in whom the early infusion of EBV-specific CTLs may have prevented the increase of viral load to potentially hazardous levels. The implementation of this protocol requires that lymphocytes from the donor become available 4 to 6 weeks before BMT. It may be possible to simplify and accelerate the production of CTLs by substituting EBV-positive LCLs with dendritic cells that express EBV antigens. Dendritic cells can also induce primary immune responses44 and may allow the selective reactivation of CTL specific for subdominant epitopes derived from the restricted set of viral antigens expressed in Hodgkin's disease and nasopharyngeal carcinoma.
Taken together, our findings suggest that early administration of virus-specific CTL may become a feasible clinical routine for antiviral prophylaxis in patients at high risk to develop EBV-associated cancers. The timely introduction of this therapeutic measure after BMT may allow the concomitant administration of a strong immunosuppressive regimen in patients receiving HLA-mismatched grafts. Conceivably, a similar strategy of passive reconstitution of specific immune responses could also be beneficial in preventing the reactivation of other viral infections, such as CMV, that are potentially harmful to this group of patients.
Acknowledgment
We wish to thank the patients and their families and the personnel at the Departments of Pediatrics, Center of Allogeneic Stem Cell Transplantation, and Outpatient Units at Huddinge Hospital who made this work possible by collecting samples and providing competent and compassionate medical care.
Supported by grants from the Swedish Cancer Society, the Children's Cancer Foundation, the Foundation for Strategic Research, and the Karolinska Institute, Stockholm, Sweden.
A.G. and V.L. contributed equally to this work.
Reprints:Maria G. Masucci, Microbiology and Tumor Biology Center, Karolinska Institute, Box 280, 171 77 Stockholm, Sweden; e-mail: maria.masucci@mtc.ki.se.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal