The successful prophylactic treatment of hemophilia A by frequent infusions of plasma concentrates or recombinant factor VIII (hFVIII) indicates that gene therapy may be a potential alternative for the treatment of the disease. For efficient delivery and long-term expression of the hFVIII gene, a novel minimal adenovirus (mini-Ad) vector, MiniAdFVIII, has been developed. The vector is devoid of all viral genes and carries the full-length hFVIII cDNA under the control of the human 12.5-kb albumin promoter. The MiniAdFVIII vector was propagated with the assistance of an ancillary vector in 293 cells and was purified by CsCl banding. Sustained expression of hFVIII at physiologic levels (100-800 ng/mL) was achieved in mice after a single intravenous injection of MiniAdFVIII. The expressed hFVIII had a structure identical to that of recombinant hFVIII, as determined by Western blot analysis. The functionality of the protein was confirmed by the restoration of blood coagulation capacity in MiniAdFVIII-treated hemophilic mice, as determined by tail clipping observations. Although antivector or antihuman FVIII antibodies at various levels were detected, long-term expression of the transgene was observed in the mice that did not generate antibodies against the transgene product. The vector DNA persisted in the liver tissues of the mice with long-term expression. No significant histopathologic findings or toxicities were observed to be associated with the vector in the MiniAdFVIII-treated C57BL/6 mice. These results support the further development of MiniAdFVIII for clinical trials toward the treatment of hemophilia A.
Hemophilia A is the most common inherited severe bleeding disorder. The disease is caused by a deficiency in coagulation factor VIII (FVIII), affecting approximately 1 in 10 000 males in the population.1 Based on the residual activity of FVIII in plasma, hemophilia A is categorized as mild, moderate, or severe. Patients with severe cases have less than 1% of normal plasma FVIII activity, resulting in frequent spontaneous hemorrhages in muscles and joints with progressively debilitating effects. Supplements of human FVIII (hFVIII) products such as plasma concentrates or recombinant protein are required to stop episodes of severe bleeding.2Replacement therapy is effective, especially when it is continued as a long-term prophylactic treatment. It has been suggested that frequent dosing of hFVIII protein to maintain low plasma levels of the factor can convert a severe type to a moderate or mild type with significant improvement in the prognosis and in joint complications.3However, the high cost of such a prophylactic practice and the inconvenience of frequent intravenous (IV) injections call for the development of a more cost-effective and less traumatic treatment.
For these reasons, gene therapy represents a potential alternative for treating hemophilia A. Among all ex vivo4-7 and in vivo8-12 studies to date, adenovirus-mediated FVIII gene delivery has proved to be a promising approach. Intravenous administration of adenovirus vectors results in the prominent delivery of the transgene to the liver (Balagué C, unpublished data, 1998).13-15 The liver tropism of the vectors is particularly useful because liver is the natural site of FVIII biosynthesis and production.16,17 Early generation adenoviral (Ad) vectors were used to deliver a B-domain deleted hFVIII cDNA18 and were shown to mediate hFVIII expression at therapeutic levels on systemic administration.8-10,12However, early generations of Ad vector for the treatment of genetic diseases are known to have a limited capacity for carrying heterologous DNA and to induce host immune responses because of the expression of residual viral genes in the vectors.19 20
To overcome these limitations, new adenoviral vector systems with complete elimination of viral coding sequences have recently been developed. These systems have shown greater application,21-25 and they provide a new tool for in vivo gene therapy. In this study, we analyzed the clinical potential of a novel adenovirus vector for delivery of the hFVIII gene. The vector, called MiniAdFVIII, has minimal viral cis-elements (< 1 kb), and it carries a 20-kb expression cassette that contains the full-length hFVIII cDNA coding sequence under the transcriptional control of the human 12.5-kb albumin promoter. MiniAdFVIII was characterized in vitro and assessed in vivo for hFVIII expression, vector DNA status, phenotypic correction, toxicity, and immune responses in mice. Our results indicated that MiniAdFVIII is able to generate the sustained expression of hFVIII at human physiological levels in mice in which anti-hFVIII antibodies did not develop. The vector-treated hemophilic mice were able to stop bleeding in 30 minutes after tail clipping, in contrast to no clotting and no survival in vehicle-treated hemophilic mice. The absence of virus-associated toxicity at effective doses encouraged the development of this vector for clinical trials for the treatment of hemophilia A.
Methods
Propagation and purification of MiniAdFVIII
The construction and generation of MiniAdFVIII and ancillary vectors have been described elsewhere.21 MiniAdFVIII was co-propagated with the ancillary vector in 293 cells. Typically, the cell lysate of a completed infection was used to infect 5 times the number of cells harvested. To purify virus, cells were harvested from fifty 150-mm Petri dishes, subjected to 3 freeze-thaw cycles, and centrifuged. Resultant supernatants were loaded on a CsCl step gradient consisting of 1.25-g/mL and 1.35-g/mL densities and spun for 1 hour at 35 000 rpm, 10°C, in an SW41 rotor. The virus band was collected, and an isopycnic gradient centrifugation (final CsCl density, 1.33 g/mL) was carried out at 30 000 rpm, 10°C, overnight in an NVT65.2 rotor. Two well-defined bands were obtained. The upper one was a MiniAdFVIII, and the lower one was the ancillary vector. Bands were collected and desalted over a PD-10 column (Amersham Pharmacia). The MiniAdFVIII vectors were eluted from the column with phosphate-buffered saline (PBS), and the vector solution was adjusted to 10% glycerol before freezing.
Analysis of purified MiniAdFVIII
After purification, the purity and quantity of MiniAdFVIII were analyzed using the following methods:
Titering.
Viral particles (vp) were determined by absorbance at 260 nm using the conversion factor 1 OD260 = 1012 vp. The number of viral particles was used as a measure of input vector for experimentation.
Restriction analysis.
Viral DNA from 6 × 1010 vp was extracted and digested with PshAI restriction enzyme, which gives distinct DNA fragments for both vectors. The digested DNA was separated on a 0.7% agarose gel containing EtBr, and the specific bands for MiniAdFVIII (9949 bp) and ancillary (8497 bp) vectors were quantified by densitometry.
X-gal staining.
For quantitation of the ancillary vector, which contains the β-gal reporter gene, 106 293 cells/well seeded in 12-well plates were infected with MiniAdFVIII in 0.5 mL medium. The multiplicities of infection used were 1000, 100, and 10 vp/cell. Twenty hours after infection, cells were fixed and stained with X-gal, and positive blue cells were counted.
Replication-competent adenovirus assay.
A supernatant rescue assay for replication-competent adenovirus (RCA) detection was carried out as previously described.26
FVIII protein and functional assays
ELISA.
A double-sandwich enzyme-linked immunosorbent assay (ELISA) was designed to quantify the hFVIII protein in cell culture medium or mouse plasma. In the conditions used, the assay was linear up to 100 ng/mL FVIII. The assay was specific for hFVIII and sensitive down to 1.5 ng/mL. Because the mouse samples were diluted 10-fold for testing, the minimum amount of hFVIII detectable in mouse plasma was 15 ng/mL. Briefly, plates were coated with capture antibodies (ESH-5 and ESH-8; 500 ng each per well; American Diagnostica, Greenwich, CT) in carbonate buffer and incubated for 1 hour at 37°C. Plates were washed with 0.05% Tween 20 in PBS and blocked in 50 mmol/L Tris, pH 7.2, 150 mmol/L NaCl, 0.5% gelatin, and 0.05% Tween 20 for 2 hours at 37°C. Mouse plasma samples were assayed in duplicate at 1:10 dilution in blocking buffer. Purified recombinant FVIII (Hyland; Baxter Healthcare, Glendale, CA), prepared in blocking buffer with 1:10 normal mouse plasma (Harlan, Indianapolis, IN), served as the standard. Samples and standards were incubated for 1 hour at 37°C. Detection antibody (sheep antihuman FVIII, horseradish peroxide [HRP]–conjugated; Cedar Lane, Ontario, Canada) was diluted in blocking buffer and incubated for 1 hour at 37°C. After a final washing step, plates were developed with o-phenylenediamine dihydrochloride peroxidase substrate (Sigma, St. Louis, MO) and the optical density at 490 nm was read using an EL 340 Spectrophotometer (Bio-tech Instruments, Winooski, VT).
Functional assay.
hFVIII activity in cell culture media and mouse plasma samples was detected with a Coatest chromogenic assay (Chromogenix, Molndal, Sweden) modified for microtiter plates. Human plasma (FACT pool reference plasma; George King Biochemicals, Overland Park, KS) was used as a standard, defining 1 U hFVIII as 200 ng/mL and applying the correction factor specified by the manufacturer. Appropriate dilutions of cell culture supernatants and mouse plasma were tested in duplicate, and results were plotted as hFVIII ng/mL.
Specific activity analysis.
The ratio between the functional units and the quantity of the protein was used to calculate the specific activity as U activity/μg protein.
Western blot.
For protein analysis of hFVIII from MiniAdFVIII-treated cultured cells, HepG2 cells were infected with 10 000 vp/cell in medium with 10% fetal calf serum, and the supernatant was harvested 3 days after infection. Immunoprecipitation of hFVIII was carried out with the anti-hFVIII:C monoclonal antibody (Hyland; Baxter Healthcare) coupled to agarose beads. The beads were incubated with culture supernatant overnight at 4°C with constant rotation. Pellets were washed 4 times in 50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 2 mmol/L EDTA, 1% Triton X-100, 0.1% sodium dodecyl sulfate. Proteins were separated by electrophoresis in a 7.5% polyacrylamide gel and transferred to a nitrocellulose membrane. FVIII heavy chain was detected with an HRP-conjugated sheep anti-hFVIII antibody (Cedar Lane). For light chain detection, a biotinylated anti-hFVIII:C antibody (QED Bioscience, San Diego, CA) was used. This was followed by incubation with neutravidin-HRP (Pierce, Rockford, IL). The signal was developed with a chemiluminescence substrate (Enhanced Chemoluminescence; Amersham, Uppsala, Sweden). A Western blot of immunoprecipitated hFVIII was used to analyze plasma samples of MiniAdFVIII-treated mice.
Animal studies
MiniAdFVIII activity studies.
Six- to 8-week-old C57BL/6 and Balb/c mice were purchased from Harlan. Hemophilic mice (exon 16-disrupted FVIII knockout mice) were obtained from the University of Pennsylvania27 and bred in-house. For dose-response studies, groups of 3 mice were injected through tail veins with different doses of MiniAdFVIII. At the designated time points, blood was collected by retro-orbital puncture in tubes containing 0.1 vol of 0.1 mol/L sodium citrate. Cells were removed by centrifugation, and the plasma was tested for hFVIII by ELISA or functional assay.
Phenotypic correction studies.
Groups of 12 mice were injected on day 1 with 2.5 × 1011 vp of MiniAdFVIII or vehicle (PBS), respectively. On day 3, blood was collected by retro-orbital puncture with a glass capillary tube. Tubes were broken at 1-minute intervals to check for clot formation, and the clotting time was recorded. On day 6, a 2-cm section of the tail was clipped from each mouse to measure the bleeding time (time until bleeding stopped) and the volume of blood shed.
Toxicity studies.
Groups of 6 C57BL/6 mice were injected through the tail veins with the selected doses of MiniAdFVIII or with vehicle. Three and 14 days after injection, 3 mice from each group were killed. Blood was collected by cardiac puncture, and serum samples were tested for alanine aminotransferase (ALT) levels (Boehringer Mannheim, Indianapolis, IN). Tissues (liver, spleen, kidney, lung, and heart) were fixed in formalin for 24 hours, paraffin embedded, and processed for histopathology.
Analysis of vector DNA in vivo.
Polymerase chain reaction (PCR) assays with hFVIII-specific primers (Figure 6A) were used for detecting the MiniAdFVIII DNA. Liver tissue (0.25 g per sample) from MiniAdFVIII-treated mice was ground to a powder in a liquid nitrogen–cooled mortar, and DNA was extracted using a Qiagen Tissue Kit (Qiagen, Valencia, CA). Each PCR reaction contained 1 × reaction buffer, 200 μmol/L each deoxynucleotide triphosphate, 2.25 to 4.0 Mg++ (the concentration varied with different primers), 0.4 μmol/L each primer, and 2.5 U Qiagen HotStar Taq polymerase (Qiagen) in a total volume of 50 μL. Amplification was carried out for 1 cycle of 15 minutes at 95°C followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 52°C, and 45 seconds at 72°C, with a final extension of 7 minutes at 72°C. Aliquots of the PCR reactions (15 μL) were analyzed on 1.5% agarose gels. Amplified DNA fragments were semiquantitated by comparing Gelstar-stained band intensities from tissue samples to those for the control plasmid (Gelstar; FMC BioProducts, Rockland, ME). Division of the copies by pg input DNA yielded copies/pg input DNA. These values were normalized to copies/cell equivalent by assuming 6 pg/cell nucleus and adjusting the values accordingly.
Analysis of anti-hFVIII and anti-MiniAdFVIII antibodies.
ELISA assays were used to determine the antibodies against the hFVIII protein and the MiniAdFVIII vector. The assay plates, coated with either purified hFVIII protein or the ancillary vector in carbonate buffer, were blocked with PBS containing 4% fetal bovine serum for 1 hour at room temperature, washed once, and incubated overnight with serially diluted test plasma samples (100 μL/well). After 5 washes, each well was incubated with 100 μL 1:2000 diluted goat antimouse IgG (H + L) conjugated to HRP (Southern Biotechnology Associates, Birmingham, AL) at 37°C for 1 hour. After 5 washes, 100 μL peroxidase substrate solution (o-phenylenediamine) was added to each well. Color development was monitored for 7 minutes, and the reactions were stopped with the addition of 3 N HCl (50 μL/well). The optical density at 490 nm (OD490) was read using an EL340 spectrophotometer (Bio-Tek Instruments, Winooski, VT).
Results
Preparation of purified MiniAdFVIII
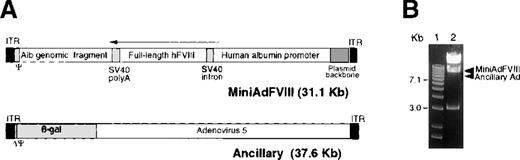
The MiniAdFVIII genome contains a 20-kb hFVIII expression cassette consisting of a 12.5-kb human albumin promoter,28 an SV40 intron, a 7.2-kb full-length hFVIII cDNA, and an SV40 polyadenylation signal (Figure 1A). In addition, a 6.9-kb human albumin genomic fragment was included to increase the viral genome to an optimal packageable size.21,29 The only Ad sequences remaining in the vector are the inverted terminal repeats, the packaging signal, and a 400-bp noncoding fragment of E4. MiniAdFVIII was generated by co-transfecting 293 cells with the plasmid form of MiniAdFVIII and the ancillary virus DNA, as previously described.21 Viruses were co-propagated in 293 cells. MiniAdFVIII was purified and characterized as described in “Methods.” A total viral DNA digestion from a typical purified MiniAdFVIII preparation is shown in Figure 1B. Comparison of the actual digestion pattern with the expected patterns for MiniAdFVIII and ancillary vector provides information on the structural integrity and purity of MiniAdFVIII at the DNA level. In Figure 1B, the absence of the expected band from the ancillary vector indicated the ancillary vector is present at levels below the detection limit for this assay. Because the ancillary vector carries a β-gal reporter, a more sensitive X-gal staining assay was applied to analyze the contamination level of the ancillary vector in the MiniAdFVIII preparation. In a typical lot of purified MiniAdFVIII, the X-gal positively stained cells were observed at a frequency of 1 of 10 000 to 100 000 negatively stained cells when a multiplicity of infection of 10 vp/cell was used (data not shown). Taken together, our data indicate that the purified MiniAdFVIII preparation contains less than 0.1% of the contaminating ancillary vector. The supernatant rescue assay was also applied to determine the level of RCA contamination in preparations of MiniAdFVIII. No RCA was detectable in 109 vp of MiniAdFVIII.
(A) Structure of MiniAdFVIII and ancillary vectors.
MiniAdFVIII is devoid of all viral genes. The presence of the adenovirus ITRs and packaging signal (Ψ) allows the MiniAdFVIII vector to replicate and package efficiently in cells coinfected with the ancillary vector and expressing adenovirus E1 early proteins. (arrow) Direction of transcription. The ancillary vector is an E1-substituted adenovirus with a partially deleted packaging signal. (B) Detection of ancillary vector in MiniAdFVIII preparations. Total viral DNA from 6 × 1010 viral particles was extracted and digested with PshAI. The expected bands for MiniAdFVIII are 17 300, 9949, 3233, and 627 bp; for the ancillary vector, they are 21 382, 8497, and 7770 bp, respectively. The position of the bands for MiniAdFVIII (9949 bp) and ancillary (8497 bp and 7770 bp) vectors are indicated. (1) Molecular weight markers, 1-kb ladder (GIBCO). (2) Viral DNA digested with PshAI restriction enzyme.
(A) Structure of MiniAdFVIII and ancillary vectors.
MiniAdFVIII is devoid of all viral genes. The presence of the adenovirus ITRs and packaging signal (Ψ) allows the MiniAdFVIII vector to replicate and package efficiently in cells coinfected with the ancillary vector and expressing adenovirus E1 early proteins. (arrow) Direction of transcription. The ancillary vector is an E1-substituted adenovirus with a partially deleted packaging signal. (B) Detection of ancillary vector in MiniAdFVIII preparations. Total viral DNA from 6 × 1010 viral particles was extracted and digested with PshAI. The expected bands for MiniAdFVIII are 17 300, 9949, 3233, and 627 bp; for the ancillary vector, they are 21 382, 8497, and 7770 bp, respectively. The position of the bands for MiniAdFVIII (9949 bp) and ancillary (8497 bp and 7770 bp) vectors are indicated. (1) Molecular weight markers, 1-kb ladder (GIBCO). (2) Viral DNA digested with PshAI restriction enzyme.
Characterization of hFVIII produced in vitro
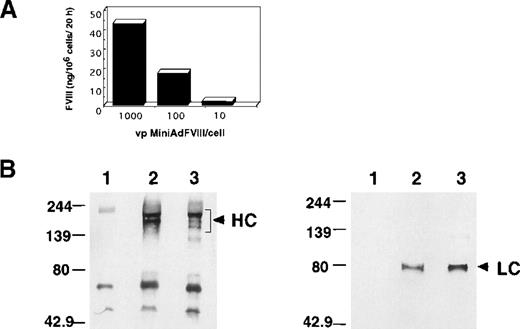
To assess the MiniAdFVIII activity in vitro, 293 cells were infected with the vector at different vp/cell ratios, and the supernatant of the treated cells was analyzed using a Coatest (Chromogenix) chromogenic assay (Figure 2A). The hFVIII activity was detected in the supernatant of the MiniAdFVIII-infected cells in a dose-dependent fashion, indicating that the vector was able to transduce hFVIII and that the hFVIII protein encoded by MiniAdFVIII was biologically active. To analyze further the structure of the hFVIII protein produced by MiniAdFVIII, HepG2 cells were infected with the vector, and the conditioned medium was used for immunoblot analysis (Figure 2B). Detection with a specific antibody to the heavy chain revealed several protein species ranging in size from 100 kd to 200 kd. A unique band of 80 kd was detected with specific antibodies to the light chain. The pattern obtained from infected cells was comparable to that of recombinant FVIII (Hyland-Immuno; Baxter Healthcare). The slight difference in the intensity of the bands of heavy chain (Figure2B; HC) may reflect a difference in posttranslational processing in different cell lines because the recombinant FVIII was produced from Chinese hamster ovary cells. Taken together, the results demonstrate that hFVIII produced in vitro by the MiniAdFVIII-infected cells is biologically active and has the expected protein structure.
Characterization of hFVIII produced in MiniAdFVIII-infected cells.
(A) Production of functional hFVIII secreted into the supernatant of infected 293 cells. (B) Conditioned medium from HepG2 infected cells was used for hFVIII immunoprecipitation with a specific antibody to the heavy chain. Immunodetection was performed on nitrocellulose membranes with specific antibodies to the heavy (left) or light chains (right). (lane 1) Conditioned medium from uninfected cells. (lane 2) Conditioned medium from infected cells. (lane 3) Recombinant FVIII spiked in conditioned medium from uninfected cells. (arrows) FVIII-specific signals. HC, heavy chain; LC, light chain.
Characterization of hFVIII produced in MiniAdFVIII-infected cells.
(A) Production of functional hFVIII secreted into the supernatant of infected 293 cells. (B) Conditioned medium from HepG2 infected cells was used for hFVIII immunoprecipitation with a specific antibody to the heavy chain. Immunodetection was performed on nitrocellulose membranes with specific antibodies to the heavy (left) or light chains (right). (lane 1) Conditioned medium from uninfected cells. (lane 2) Conditioned medium from infected cells. (lane 3) Recombinant FVIII spiked in conditioned medium from uninfected cells. (arrows) FVIII-specific signals. HC, heavy chain; LC, light chain.
Studies of MiniAdFVIII activity in mice
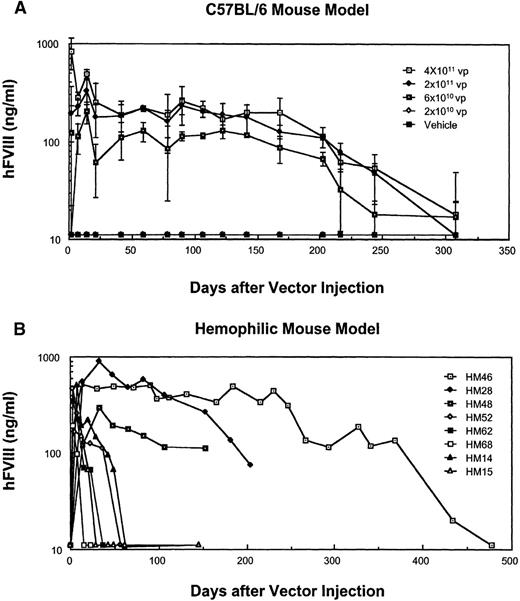
To assess the gene transduction efficiency of MiniAdFVIII and the subsequent hFVIII production in vivo, a dose-response study of the vector was performed in C57BL/6 mice. Groups of 3 mice were injected through the tail veins with different doses of MiniAdFVIII. At various time points, plasma was collected and assayed for hFVIII expression by ELISA (Figure 3A). Doses of 4 × 1011 and 2 × 1011 vp MiniAdFVIII-mediated hFVIII expression in mice at levels above the human physiologic levels (more than 200 ng/ml). Accordingly, 6 × 1010 vp produced relatively lower levels of hFVIII (approximately 100 ng/mL), and at 2 × 1010vp, hFVIII levels were below the assay detection limit. The expression of hFVIII in the mice persisted for 308 days (last time point tested), though a gradual decline in hFVIII expression was observed after postinfection day 160.
Expression of hFVIII in vivo.
(A) Dose-response study in C57BL/6 mice. Three individuals per dose were used, and FVIII expression was measured by ELISA. Mean ± SD is depicted. (B) Expression of hFVIII in hemophilic mice injected with 2 × 1011 vp of MiniAdFVIII, as measured by ELISA. Representative individuals are shown.
Expression of hFVIII in vivo.
(A) Dose-response study in C57BL/6 mice. Three individuals per dose were used, and FVIII expression was measured by ELISA. Mean ± SD is depicted. (B) Expression of hFVIII in hemophilic mice injected with 2 × 1011 vp of MiniAdFVIII, as measured by ELISA. Representative individuals are shown.
A dose-response study in Balb/c mice revealed that the same doses yielded initial levels of hFVIII similar to those in C57BL/6. However, expression levels declined sharply after postinfection day 14 (data not shown).
In another experiment, hemophilic mice27 were injected with a single dose (2 × 1011 vp) of MiniAdFVIII and monitored for the expression of hFVIII by ELISA (Figure 3B) or a Coatest chromogenic assay (data not shown). Even though all the mice were shown to express hFVIII at human physiologic levels after administration, the persistence of hFVIII expression varied greatly among individuals. In 3 of 16 mice injected, hFVIII expression could be detected at all time points, ranging from 5 months through more than 1 year; in the remaining 13, hFVIII levels decreased to undetectable levels 3 to 8 weeks after injection (Figure 3B).
Assessment of MiniAdFVIII immunogenicity in mice
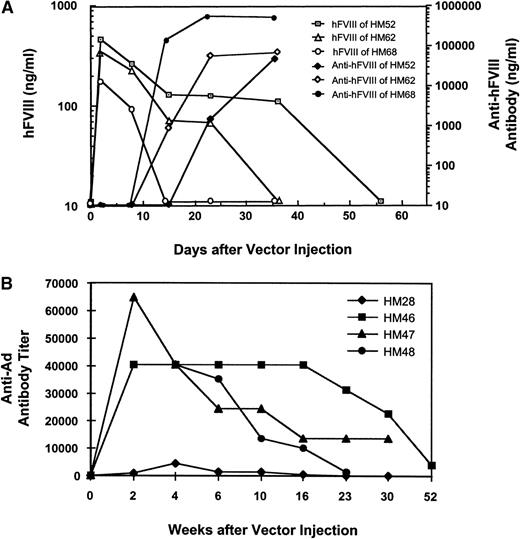
To understand the immunogenicity of MiniAdFVIII and the nature of the variations observed in the duration of hFVIII expression in the MiniAdFVIII-treated mice, anti-hFVIII as well as anti-Ad antibodies were analyzed by ELISA. Those mice with the shortest duration of hFVIII expression had humoral immune responses to the transgene product. In all cases, the rise in antibody titer inversely correlated with the level and duration of hFVIII expression (Figure4A). Administration of MiniAdFVIII induced a stronger and faster humoral immune response in Balb/c mice. In these mice the titers of the anti-hFVIII antibodies rose sharply within 1 week after injection of the vector (data not shown).
Detection of humoral immune responses in hemophilic mice.
(A) Example of anti-hFVIII antibody responses in hemophilic mice 52, 62, and 68 as measured by ELISA. The mice received MiniAdFVIII by tail vein injection at 2 × 1011 vp/mouse. Representative individuals are shown. (B) Anti-Ad antibody responses in hemophilic mice 28, 46, 47, and 48. The mice were given a single injection of MiniAdFVIII (2 × 1011 vp/mouse) and bled at various time points after injection. The anti-Ad IgG titers were measured by ELISA. Endpoint titers are defined as the reciprocal of the last serum dilution giving a half-maximal OD490 reading of 1.5.
Detection of humoral immune responses in hemophilic mice.
(A) Example of anti-hFVIII antibody responses in hemophilic mice 52, 62, and 68 as measured by ELISA. The mice received MiniAdFVIII by tail vein injection at 2 × 1011 vp/mouse. Representative individuals are shown. (B) Anti-Ad antibody responses in hemophilic mice 28, 46, 47, and 48. The mice were given a single injection of MiniAdFVIII (2 × 1011 vp/mouse) and bled at various time points after injection. The anti-Ad IgG titers were measured by ELISA. Endpoint titers are defined as the reciprocal of the last serum dilution giving a half-maximal OD490 reading of 1.5.
Although the C57BL/6 strain was previously shown not to produce anti-hFVIII antibodies on tail vein adenovirus delivery,11a low titer of anti-hFVIII antibodies was detected in the plasma of these mice starting at approximately postinfection day 122. This low titer may be sufficient to account for the observed decline in hFVIII expression (data not shown).
Analyses of the anti-Ad antibodies indicated that all the MiniAdFVIII-treated mice generated humoral immune responses to the adenoviral proteins, regardless of the strain. However, the levels and duration of the antibodies in individual mice varied significantly. In contrast, the presence of anti-Ad antibodies did not affect the persistence of the hFVIII expression, even in the hemophilic mice that had long-term gene expression of hFVIII (Figure 4B). The antiviral antibodies gradually declined to low levels 3 months after injection, as observed in Figure 4B and other data (not shown). Taken together, the in vivo data indicated that in the absence of an antibody response to the transgene product, MiniAdFVIII was able to mediate a long-term expression of hFVIII in mice at human physiologic levels. Humoral immune responses to the MiniAdFVIII vector were detectable but apparently did not affect the level or duration of hFVIII expression in either C57BL/6 or hemophilic mice.
Characterization of hFVIII produced in vivo
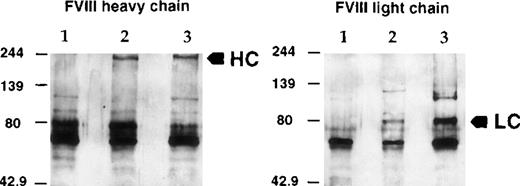
The hFVIII protein secreted into the plasma of injected mice was analyzed by immunoprecipitation and Western blotting (Figure5) using antibodies specific to hFVIII. As in the in vitro assay, both heavy and light chains were detected with molecular masses of 200 kd and 80 kd, respectively, indicating correct posttranscriptional processing of the human protein in the murine host.
Characterization of hFVIII secreted in mouse plasma.
Mouse plasma from a treated C57BL/6 mouse was used for immunoprecipitation of hFVIII and Western blot analysis as in Figure 2. (left) FVIII heavy chain. (right) FVIII light chain. (lane 1) Normal mouse plasma. (lane 2) Mouse plasma from a treated C57BL/6 mouse. (lane 3) Recombinant FVIII spiked in normal mouse plasma. (arrows) FVIII-specific signals. HC, heavy chain; LC, light chain.
Characterization of hFVIII secreted in mouse plasma.
Mouse plasma from a treated C57BL/6 mouse was used for immunoprecipitation of hFVIII and Western blot analysis as in Figure 2. (left) FVIII heavy chain. (right) FVIII light chain. (lane 1) Normal mouse plasma. (lane 2) Mouse plasma from a treated C57BL/6 mouse. (lane 3) Recombinant FVIII spiked in normal mouse plasma. (arrows) FVIII-specific signals. HC, heavy chain; LC, light chain.
To assess the integrity and functional properties of the hFVIII expressed in the mouse model, the hFVIII produced from the vector was compared with the recombinant hFVIII protein in plasma samples of the treated hemophilic mice. Plasma samples were collected either on postinfection days 3 and 6 of MiniAdFVIII (2.5 × 1011 vp/mouse) or at 1.5 hours after injection of the recombinant protein (3.5 or 14 U/mouse). Specific activities of the proteins derived from the 2 sources were calculated by dividing the values derived from the chromogenic assay for FVIII function by those derived by the ELISA assay for FVIII protein. The results, summarized in Table 1, indicated that the specific activities of the transgene product and the recombinant hFVIII in mouse models were comparable. The relatively low activities of the protein detected in the vector-treated mice (both postinfection days 3 and 6) and the high-dose group of recombinant hFVIII-treated mice must be further studied. However, the specific activities of the hFVIII protein generated from the MiniAdFVIII-infected cells in vitro were within the same range as that of recombinant hFVIII at above 4 U/μg protein.
Factor VIII specific activity analysis
| FVIII Source and Dosage . | Hemophilic Mouse . | Sampling Time (PI) . | Coatest Assay (ng/mL) . | ELISA Assay (ng/mL) . | Specific Activity (U/μg) . |
|---|---|---|---|---|---|
| MiniAdFVIII 2.5 × 1011 vp/mouse | 12 | 3 d | 58 ± 39 | 105 ± 45 | 2.8 ± 1.3 |
| MiniAdFVIII 2.5 × 1011 vp/mouse | 12 | 6 d | 53 ± 17 | 106 ± 31 | 2.6 ± 0.6 |
| Recombinant FVIII 3.5 U/mouse | 2 | 1.5 h | 22 ± 11 | 24 ± 13 | 4.5 ± 0.2 |
| Recombinant FVIII 14 U/mouse | 2 | 1.5 h | 92 ± 30 | 149 ± 7 | 3.1 ± 0.8 |
| FVIII Source and Dosage . | Hemophilic Mouse . | Sampling Time (PI) . | Coatest Assay (ng/mL) . | ELISA Assay (ng/mL) . | Specific Activity (U/μg) . |
|---|---|---|---|---|---|
| MiniAdFVIII 2.5 × 1011 vp/mouse | 12 | 3 d | 58 ± 39 | 105 ± 45 | 2.8 ± 1.3 |
| MiniAdFVIII 2.5 × 1011 vp/mouse | 12 | 6 d | 53 ± 17 | 106 ± 31 | 2.6 ± 0.6 |
| Recombinant FVIII 3.5 U/mouse | 2 | 1.5 h | 22 ± 11 | 24 ± 13 | 4.5 ± 0.2 |
| Recombinant FVIII 14 U/mouse | 2 | 1.5 h | 92 ± 30 | 149 ± 7 | 3.1 ± 0.8 |
rFVIII specification: 1400 U/mL and 350 μg/mL, ie, 4 U/μg or 800 ng/μg, assuming 1 U = 200 ng/mL.
Study of MiniAdFVIII efficacy in hemophilic mice
A phenotypic correction study was carried out to evaluate whether the MiniAdFVIII-mediated expression of hFVIII was able to correct the deficient hemostasis in hemophilic mice. Two groups of 12 hemophilic mice were injected with either 2.5 × 1011 vp MiniAdFVIII or vehicle (PBS), respectively. A clotting test assay was performed on day 3, and a tail clipping experiment was carried out on PI day 6 to measure the bleeding time and blood loss. As a control, the same tests were performed in 10 untreated C57BL/6 mice. All the MiniAdFVIII-treated mice expressed the hFVIII (104 ± 26 ng/mL) as measured by a Coatest functional assay. As shown in Table2, clotting time was reduced from 5.3 ± 1.87 minutes in the vehicle-injected mice to 2.58 ± 0.51 minutes in the MiniAdFVIII-treated mice (P < .002). The clotting time in the MiniAdFVIII-treated mice was shorter than that (3.40 ± 0.69 minutes) in C57BL/6 controls. However, this might not have represented better function of hFVIII in mice because the capillary tube method used was not a standard assay.
Correction of hemophilic phenotype by MiniAdFVIII in mouse models
| Group . | Treatment . | hFVIII Expression* (ng/mL) . | Capillary Tube Clotting Test (min) . | Tail Clip Blood Flow Rate (μL/min) . | Tail Clip Bleeding Time (min) . |
|---|---|---|---|---|---|
| Hemophilic mice | 2.5 × 1011 vp | 104 ± 26 | 2.58 ± 0.51 | 8.6 ± 1.07 | 30.7 ± 11.8 |
| Hemophilic mice | Vehicle | ND | 5.30 ± 1.87 | 20.9 ± 4.39 | No clotting |
| C57BL/6 | None | ND | 3.40 ± 0.69 | 4.8 ± 0.86 | 11.2 ± 6.6 |
| Group . | Treatment . | hFVIII Expression* (ng/mL) . | Capillary Tube Clotting Test (min) . | Tail Clip Blood Flow Rate (μL/min) . | Tail Clip Bleeding Time (min) . |
|---|---|---|---|---|---|
| Hemophilic mice | 2.5 × 1011 vp | 104 ± 26 | 2.58 ± 0.51 | 8.6 ± 1.07 | 30.7 ± 11.8 |
| Hemophilic mice | Vehicle | ND | 5.30 ± 1.87 | 20.9 ± 4.39 | No clotting |
| C57BL/6 | None | ND | 3.40 ± 0.69 | 4.8 ± 0.86 | 11.2 ± 6.6 |
Two groups of 12 hemophilic mice received 2.5 × 1011viral particle (vp)/mouse or vehicle, respectively. On PI day 3, a clotting test was performed. On PI day 6, tail clip tests were carried out.
Sampled on PI day 6. ND, no detection.
On tail clipping, bleeding times in the vector-treated hemophilic mouse group were significantly improved (approximately 30 minutes) in contrast to the continuous bleeding with no survival in the vehicle-treated hemophilic mice, and the blood flow rate was reduced from 20.9 ± 4.39 μL/min to 8.6 ± 1.07 μL/min. This compares favorably to the blood flow rate of 4.8 μL/min in C57BL/6 controls. Although the bleeding phenotype was corrected by the hFVIII, in contrast to no clotting and no survival observed in the vehicle-treated control mice, the bleeding time was approximately 3 times that of C57BL/6 as a normal control. The reason behind this phenomenon is to be understood by further studies. Altogether, these results indicate that the hFVIII expressed from MiniAdFVIII in vivo was functional and able to correct the bleeding phenotype of hemophilic mice.
Evaluation of MiniAdFVIII safety in mice
To evaluate the safety of the vector, an acute toxicity study was performed in C57BL/6 mice by the systemic administration of MiniAdFVIII. Three groups of mice were injected with a single dose of the vector at 3 × 1011 vp, 1 × 1011 vp, or 2.5 × 1010 vp per mouse. Tissue samples of heart, lung, liver, spleen, and kidney were collected on PI days 3 and 14 for histopathology analysis. A summary of the analysis is shown in Table3. No microscopic changes were observed in the heart or lung samples of the mice. Other minimal changes (scoring 1) in the liver and kidneys of both groups were regarded as unrelated to the administration of MiniAdFVIII. A mild-grade extramedullary hematopoiesis characterized by the presence of clusters of proliferating cells in the spleen was found in all the injected mice, irrespective of the vector dose used.
Summary of histopathology findings in mouse toxicity studies
| Test Group . | 3 Days postinjection . | 14 Days postinjection . | ||||||
|---|---|---|---|---|---|---|---|---|
| A . | B . | C . | D . | A . | B . | C . | D . | |
| Heart | N | N | N | N | N | N | N | N |
| Lung | N | N | N | N | N | N | N | N |
| Liver | ||||||||
| Inflammation/necrosis | 0.25 | 0.17 | 0.08 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| Spleen | ||||||||
| Extramedullary hematopoiesis | 2.0 | 2.0 | 1.67 | N | 2.0 | 2.0 | 0.33 | N |
| Apoptosis (white pulp) | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 0.42 | N |
| Kidneys | ||||||||
| Degeneration/necrosis | 0.75 | 0.5 | 1.33 | 0.63 | 0.88 | 0.58 | 0.67 | N |
| Inflammation | 1.00 | N | N | N | N | N | 0.08 | N |
| Test Group . | 3 Days postinjection . | 14 Days postinjection . | ||||||
|---|---|---|---|---|---|---|---|---|
| A . | B . | C . | D . | A . | B . | C . | D . | |
| Heart | N | N | N | N | N | N | N | N |
| Lung | N | N | N | N | N | N | N | N |
| Liver | ||||||||
| Inflammation/necrosis | 0.25 | 0.17 | 0.08 | 0.25 | 0.5 | 0.25 | 0.25 | 0.25 |
| Spleen | ||||||||
| Extramedullary hematopoiesis | 2.0 | 2.0 | 1.67 | N | 2.0 | 2.0 | 0.33 | N |
| Apoptosis (white pulp) | 0.5 | 0.5 | 0.5 | 0.25 | 0.5 | 0.5 | 0.42 | N |
| Kidneys | ||||||||
| Degeneration/necrosis | 0.75 | 0.5 | 1.33 | 0.63 | 0.88 | 0.58 | 0.67 | N |
| Inflammation | 1.00 | N | N | N | N | N | 0.08 | N |
Dosages: group A (3 × 1011vp), group B (1 × 1011vp), group C (2.5 × 1011vp), and group D (vehicle). Six mice per group were injected through the tail vein with the indicated doses. Tissue sampling: three mice from each group were killed on PI days 3 and 14, and tissues were processed for histopathology evaluation. Severity grading: 1, minimal; 2, mild; 3, moderate; 4, marked. Grades were determined by the average number for all animals examined and a correction factor for lesion appearance (focal, multifocal, or locally extensive). N, no change.
Levels of serum ALT, a specific liver injury marker, were determined and compared among the test animals. No dose-dependent ALT increase was detected at any time point, indicating that injection of the highest dose did not cause significant liver injury (data not shown). Other markers of liver injury were studied, such as hepatocyte apoptosis and expression of proliferating cell nuclear antigen (PCNA), which reflects the proliferating status of the liver. In both analyses, liver sections for the highest dose group revealed similar numbers of apoptotic and proliferating cells as were found in the control group for both time points (data not shown).
Biodistribution and status of MiniAdFVIII DNA in vivo
PCR analysis was used to detect biodistribution of the vector after the systemic administration of MiniAdFVIII. The vector DNA was detectable in the tissue samples from liver, spleen, lungs, kidneys, and heart of the treated mice (data not shown), with the majority detected in the liver, as previously reported (Balagué C, unpublished data, 1998).13-15 No signal was detected in gonadal (testis) tissue; the limit of sensitivity of the PCR assay was 1 copy per 1 μg tissue.
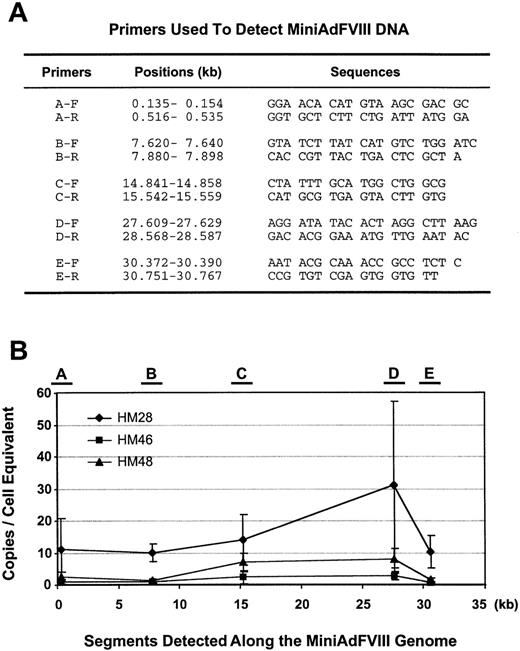
The sustained expression of hFVIII indicates the presence and activity of the vector DNA in treated animals. To analyze the DNA status of MiniAdFVIII, a set of 5 pairs of PCR primers was designed to detect different segments along the MiniAdFVIII genome (Figure6A). The 3 treated mice with long-term transgene expression (HM48, HM28, and HM46) were killed at the last time points indicated in Figure 3B, and liver DNA was extracted for the PCR assays. Specific PCR products of the predicted sizes were obtained, and the copy numbers of the representative fragments were quantified on a cell-equivalent basis. As shown in Figure 6B, though the copy numbers varied between individual mice, an approximately equal number of vector copies/cell were detected within each mouse, regardless of the fragment tested. Thus, the complete vector genome persisted in the transduced cells without apparent loss or amplification of any specific portion. In contrast, no specific products were detected for either of the 2 control mice (data not shown). These results showed that the MiniAdFVIII vector was primarily distributed in the liver through intravenous administration, and the vector DNA probably stayed in an episomal form in the mouse liver.
PCR detection of the MiniAdFVIII vector in vivo.
(A) Primers used to detect junctions between critical fragments in the MiniAdFVIII genome. Five pairs of primers (F = forward; R = reverse) and their sequences are listed. The positions of the primers are marked as the nucleotide length (kb) along the MiniAdFVIII genome. (B) Copies of the vector DNA per cell equivalent were determined at 5 positions along the length of the MiniAdFVIII genome. Locations of the primers are mapped at the top of the panel, and the position of detected segments along the vector genome are indicated in kb on the X-axis.
PCR detection of the MiniAdFVIII vector in vivo.
(A) Primers used to detect junctions between critical fragments in the MiniAdFVIII genome. Five pairs of primers (F = forward; R = reverse) and their sequences are listed. The positions of the primers are marked as the nucleotide length (kb) along the MiniAdFVIII genome. (B) Copies of the vector DNA per cell equivalent were determined at 5 positions along the length of the MiniAdFVIII genome. Locations of the primers are mapped at the top of the panel, and the position of detected segments along the vector genome are indicated in kb on the X-axis.
Discussion
This article describes the successful in vivo gene transfer of a cDNA that encodes a full-length hFVIII polypeptide using a minimal Ad vector. The vector, MiniAdFVIII, is completely devoid of adenoviral coding sequences and carries a 20-kb expression cassette consisting of the full-length hFVIII coding sequence flanked by a human albumin promoter and genomic sequences. MiniAdFVIII is co-propagated with a complementing ancillary vector in an AdE1-expressing cell line.21 The presence of a partially deleted packaging signal in the ancillary vector allows the preferential packaging of MiniAdFVIII, which in turn results in high titers of the vector.
We show that by optimizing the CsCl gradient for MiniAdFVIII purification, highly purified vector with less than 0.1% ancillary Ad contamination was obtained, as assessed by total viral DNA analysis and X-gal staining. We have previously described a dynamic fluctuation in the titers of MiniAdFVIII and ancillary Ad during co-propagation.21 Despite this, we were able to amplify both vectors to form a high-titer research viral bank with an approximate 1:1 ratio of MiniAdFVIII to ancillary vector. It is also of note that MiniAdFVIII was stable in vitro because we did not observe any vector DNA rearrangements after passaging and amplification of the vector.
The characteristics of the hFVIII protein encoded by MiniAdFVIII were studied in vitro and in vivo in mice. In both cases, the hFVIII protein produced from the vector was structurally identical to the recombinant FVIII protein by Western blot analysis. It was also shown to have a specific activity comparable to that of the recombinant protein in vitro and in vivo. The hFVIII expressed from MiniAdFVIII was biologically active and was able to correct the bleeding phenotype of hemophilic mice. Furthermore, the hFVIII expression was persistent in mice lacking anti-hFVIII antibody responses. One such mouse sustained expression for more than 1 year.
The heterogeneity in the magnitude of the anti-hFVIII humoral responses among individual mice in the hemophilic mice colony has not been reported by others. Although the absence of an anti-hFVIII humoral response has been reported in C57BL/6, we detected low titers of antibodies to hFVIII in this strain. The reasons for these discrepancies must be further studied. The observed immune responses demonstrate the need for an animal model tolerized to hFVIII to evaluate further the potential gene therapy vectors for the treatment of hemophilia A.
Another observation with regard to the immune responses to MiniAdFVIII is the development of anti-Ad antibodies in all the vector-treated mice. Unlike the effect of anti-transgene product antibodies, anti-Ad antibodies did not affect the persistence of the hFVIII expression from MiniAdFVIII. The decline in titer of anti-Ad antibody 3 months after injection may allow for readministration of the vector.
The fact that hFVIII expression persisted in the mice that exhibited no detectable anti-hFVIII antibodies suggests that MiniAdFVIII viral DNA remained stable in the transduced cells. This is supported by PCR analysis on the vector DNA segments in liver tissue of mice with long-term hFVIII expression. It was found that the vector genome appeared to be intact, possibly in an episomal form. This observation supports a recent report30 that DNA from minimal and first-generation Ad vectors remained intact in muscle tissue.
Recent studies31 describe the need for the ancillary vector-encoded adenovirus preterminal protein for mini-viral DNA stabilization. Although we cannot rule out the involvement of minimal quantities of the ancillary vector in the stabilization of the MiniAdFVIII genome, the larger size of MiniAdFVIII and the presence of long stretches of human genomic DNA may account for its persistence. Other studies32-34 with mini-Ad vector preparations containing minimal amounts of ancillary vector contamination have also shown long-term transgene expression.
Sustained expression of full-length hFVIII at human physiologic levels in mice by using a minimal Ad vector is significant. In contrast, no detectable expression of full-length hFVIII cDNA delivered by an E1, E4, or E2A-deleted Ad vector was observed, as recently reported.35 Our successful results may be attributable to the advantages of using mini-Ad vector and the 12.5-kb human albumin promoter. It has been suggested30 that the lack of expression from the early-generation Ad vector in the absence of an immune response to the transgene product results from the inhibition of transgene expression. The large payload capacity of the mini-Ad vector provides the ability to accommodate natural genomic fragments for transgene expression; large promoters can be applied, and elements for vector DNA retention, such as normal DNA replicative elements, can be included.
Toxicity associated with the systemic administration of MiniAdFVIII was evaluated at doses up to 3 × 1011 vp per mouse. As previously described, tail vein injection of Ad vectors resulted in marked liver infection, with spleen and kidneys less prominently infected.13-16 In earlier reports, 20,22,36 mice injected through the tail vein with E1-deleted adenovirus showed a biphasic response, characterized by a mild neutrophilic infiltration in the liver and increased serum ALT levels during the first 3 days after injection and a second marked serum ALT peak and mononuclear cell infiltration by postinfection day 14. Both phases have been attributed to the leaky viral gene expression in these vectors,19,20,37 which leads to host immune responses to the infected cells. Our results, including histopathology evaluation, measurement of ALT serum levels, and analysis of hepatocyte apoptosis/proliferation, indicate that the administration of MiniAdFVIII did not induce detectable liver injury. The low toxicity from intravenous administration of mini-Ad vectors containing different transgenes in C57BL/6 mice has also been reported by others.22,33,34 The mechanisms accounting for this decreased toxicity are just being unraveled. A recent study20 has shown that the viral genes in the first-generation Ad vectors play a critical role in the elicitation of an NF-kB-induced innate immune response against infected hepatocytes, whereas this response is absent on the administration of mini-Ad vectors. Hitherto, the generation of specific cellular immune responses against mini-Ad-infected cells has not been reported, though our data and that of others on long-term transgene expression imply the absence or the insignificant contribution of a cellular immune response directed against the transduced cells.
On the other hand, our toxicity study detected an increased extramedullary hematopoiesis in the spleen on tail vein injection of MiniAdFVIII. This mild pathologic finding suggests that further study is needed to follow up beyond the time point of postinfection day 14. The fact that the treated mice showed no sign of distress argues against any major side effect of MiniAdFVIII administration.
Because of its DNA delivery capability, high-level expression of functional full-length hFVIII, in vitro and in vivo stability, and minimal toxicity, MiniAdFVIII represents a promising potential therapeutic approach for hemophilia A. Our results support the further development of this product for clinical application.
Acknowledgments
We thank the CRTS department at Baxter Healthcare Corporation (Round Lake, IL) for excellent technical assistance and expertise in the evaluation of the histopathology data, with special thanks to Ray Ortiz (CRTS) for his diligence in tissue processing and immunostaining. We also thank the Animal Care staff for help with animal injections and blood collections, Patrice Tremble for valuable advice, and Dr William Raschke for critical reading of the manuscript.
Reprints:Wei-Wei Zhang, GenStar Therapeutics Corporation, 10835 Altman Row, Suite 150, San Diego, CA 92121; e-mail:wzhang@genstartherapeutics.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal