The ets-family transcription factor PU.1 is required for the proper development of both myeloid and lymphoid progenitors. We used PU.1-deficient animals to examine the role of PU.1 during dendritic cell development. PU.1−/−animals produce lymphoid-derived dendritic cells (DC): low-density class II major histocompatibility complex [MHC-II+] CD11c+ CD8+DEC-205+. But they lack myeloid-derived DC: low-density MHC-II+ CD11c+ CD8−DEC-205−. PU.1−/− embryos also lack progenitors capable of differentiating into myeloid DC in response to granulocyte-macrophage colony-stimulating factor plus interleukin-4. The appearance of lymphoid DC in developing PU.1−/−thymus was initially delayed, but this population recovered to wild type (WT) levels upon organ culture of isolated thymic lobes. PU.1−/−lymphoid DC were functionally equivalent to WT DC for stimulating T-cell proliferation in mixed lymphocyte reactions. These results demonstrate that PU.1 is required for the development of myeloid DC but not lymphoid DC.
Considerable progress has been made recently in our understanding of the developmental relationships within the dendritic cell family. All dendritic cells (DC) are derived from the hematopoietic stem cell (HSC),1 but they subsequently diverge into at least 2 distinct cell lineages. One lineage of DC has 2 subpopulations, Langerhans cells of the skin and monocyte/macrophage-related DC found in most tissues, that are derived from a common myeloid precursor.2 The primary role of myeloid DC is “professional” antigen presentation, which is essential for T-cell activation and the initiation of an immune response.1 Myeloid DC can be cultured in response to myeloid growth factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).3 Recently, a second lineage of DC of thymic origin has been described. This second lineage is derived from a lymphoid-restricted progenitor. Lymphoid DC are found in the thymus, spleen, and lymph nodes. Within the thymus, lymphoid DC are thought to mediate negative selection and subsequent destruction of self-reactive T cells. Lymphoid DC can also activate T cells, but they induce a restricted T-cell cytokine profile and apoptosis.4,5Dendritic cell lineages can be separated by differences in physical properties and cell-surface phenotypes. The majority of DC are low-density cells that express high levels of class II major histocompatibility complex (MHC-II+) and the integrin CD11c. In addition, myeloid DC in situ are CD11b+DEC-205−CD8α−, whereas lymphoid DC are CD11b− DEC-205+CD8α+.6
Gene targeting experiments have demonstrated the importance of transcription factors during hematopoietic development.7 To date, 2 transcription factors, Ikaros and RelB, have been shown to affect dendritic cell development.8,9 Ikaros is a zinc-finger DNA-binding protein whose expression is restricted to early hematopoietic and lymphoid-derived cells.10 Two strains of Ikaros mutant mice have been generated: a dominant negative mutation (IkarosDN)11 and a null mutation (Ikaros−/−).12 IkarosDNmice have severe defects in B and T cells but not in erythroid or myeloid development, whereas Ikaros−/−mice retain limited T-cell development after birth. IkarosDNmice lack all DC, but Ikaros−/−mice still produce reduced numbers of lymphoid DC.8 RelB is a member of the NF-κB/Rel family, whose expression is restricted to lymphoid tissues.13 RelB−/−mice lack medullary epithelium and have a defective thymic medullary structure.14,15 In addition, RelB is required for the development of myeloid (CD8α−), but not lymphoid (CD8α+) DC.9 These studies demonstrate that transcription factors have differential roles in the development of myeloid and lymphoid DC.
The transcription factor PU.1, a member of the ets-family of DNA-binding proteins, is only expressed in hematopoietic cells.16,17 PU.1 is expressed in early hematopoietic progenitors including the circulating monocytic progenitors of myeloid DC17 and in early CD44+ thymic progenitors, which are likely precursors of both T cells and lymphoid DC in the thymus.18 Targeted mutagenesis of thePU.1 locus causes a late-gestational embryonic19 or early neonatal lethality.20Severe defects in both lymphoid and myeloid development are seen during fetal development, characterized by the absence of B-lymphoid and macrophage progenitors.19 Normal numbers of erythroid and megakaryocyte progenitors as well as reduced numbers of T-lymphocyte and aberrant neutrophil progenitors are present in the mutants.18,20-22 Adoptive transfer of fetal liver cells and the generation of embryonic stem–derived (ES-derived) chimeric mice, established that the PU.1 mutation is cell intrinsic and cannot be rescued by a wild type (WT) microenvironment.21
We examined the role of PU.1 in dendritic cell development and found that PU.1−/−animals produce functional CD8α+ lymphoid DC but lack CD8α−myeloid DC. PU.1−/−embryos are also missing any progenitors that are capable of differentiating into DC in response to GM-CSF plus IL-4. The appearance of lymphoid DC in developing PU.1−/−thymus is initially delayed, but this population recovers to WT levels upon organ culture of isolated thymic lobes. These results demonstrate that PU.1 is required for the development of myeloid DC but not lymphoid DC.
Materials and methods
Antibodies
We used purified biotin fluorescein isothiocyanate– or phycoerythrin–conjugated monoclonal antibodies (biotin-, FITC- or PE-conjugated mAbs) from several manufacturers (brand names in parentheses): GR-1 (RB6-8C5), CD11b (M1/70), CD8α (53-6.7), heat stable antigen (HSA) (30-F1), CD3 (7A2), CD4 (L3T4), B220 (RA3-6Bc), TER-119, isotype controls, and avidin-FITC/PE (PharMingen, San Diego, CA); purified CD11c (N418; Serotec, Oxford, England); F4/80 (CalTag, CA); and DEC-205 (NLDC-145; Bioscience, King of Prussia, PA). We also used FITC-conjugated goat-αrat and goat-αhamster F(Ab)2 (Southern Biotechnology, Birmingham, AL) and blocking sera (Sigma, St. Louis, MO).
Mouse strains
Wild type PU.1 embryos (PU.1+/+ orPU.1+/−) and mutant embryos (PU.1−/−) were generated and genotyped as previously described.19 Rapid genotyping was accomplished by flow cytometry, and single-cell suspensions of the fetal liver were prepared by standard methodology. Cells were stained with FITC-conjugated antibodies αGR-1 and αCD11b, and an FACS scan (Becton Dickinson, San Jose, CA) was used to distinguish the WT embryos (PU.1+/+ and PU.1+/−) from PU.1−/−embryos. Propidium iodide uptake and scattergating for size were used to exclude dead cells from analysis. All putative genotypes assigned by FACS analysis were confirmed by Southern blotting.
Dendritic cell isolation and culture
With minor modifications, DC were isolated by the method of Vremec et al.23 Briefly, E16.5 embryos were genotyped by FACS (see below), and WT or PU.1−/−embryos were pooled. Single-cell suspensions were prepared with collagenase and ethylenediaminetetraacetic acid (EDTA). Low-density cells were isolated by density gradient centrifugation in Nycodenz (Nycomed Pharma, Westbury, NY) media (308 mmol/kg H20). DC are greatly enriched in the low-density population of cells. For myeloid dendritic cell culture, the low-density fraction was plated 2 × 105 cells/well in 24-well tissue culture plates in RPMI 1640 media (25 mmol/L HEPES, pH 7.2; 10-4mol/L β-mercaptoethanol; and 10% fetal calf serum [FCS]) supplemented with GM-CSF (15 ng/mL) and IL-4 (5 ng/mL). Low-density cells were cultured for 3 days, fixed with acetone, and stained for dendritic cell–specific markers CD11c or DEC-205. For FACS analysis, low-density cells were further fractionated into lineage-depleted (lin−) MHC-II+ cells by magnetic bead separation.
Low-density cells were stained with a cocktail of mAbs (CD3, Thy1.2, Gr-1, F4/80, B220, and TER-119) and depleted of non-DC by magnetic bead separation (Miltyni Biotechnology, Auburn, CA). Lin−cells were then stained with biotin-conjugated αMHC-II+. MHC-II+ cells were positively selected with avidin-conjugated magnetic beads. Aliquots of the MHC-II+cells were stained with avidin-FITC to check for purity. If the population contained less than 95% of the MHC-II+expression, another round of positive selection was performed. Low-density MHC-II+ (greater than 95%) cells were used for further FACS analysis of dendritic cell subsets. To prevent nonspecific antibody uptake, purified dendritic cell populations were stained, in the presence of 20% blocking serum, with either purified α-CD11c plus FITC-goat-αhamster F(Ab)2; PE-αCD8α; purified αDEC-205 then FITC-goat-αrat F(Ab)2; or isotype controls. Propidium iodide exclusion and scattergating for size were used to exclude dead cells from analysis. For the mixed leukocyte reactions (MLRs), MHC-II could not be used for purification purposes since it is required for T-cell stimulation. Therefore, the isolation protocol was modified to use αCD11c-conjugated magnetic beads (Milteyni Biotech, Auburn, CA) to select DC from the low-density cell populations.
Mixed leukocyte reactions for dendritic cell function
Varying numbers of enriched DC from either WT or PU.1−/−embryos were incubated with 20 000 purified T cells from Balb/c mice. The T cells were purified from the spleen using an enrichment column (#MTCC-5; R&D Systems, Minneapolis, MN) per the manufacturer's instructions. Cells were cultured in 96-well plates with RPMI media, 10% serum for 3 days. We added 37 × 103 Bq (1 μCi)3H-thymidine to each well, and the culture continued for 24 hours. Cells were harvested and counted (Wallac, Gaithersburg, MD). T cells or DC alone served as the background proliferation controls. Each assay test was performed a total of 3 times in triplicate.
Fetal thymic organ culture
Fetal thymic organ cultures were performed as follows. Dissected thymic lobes were cultured in RPMI (GibcoBRL Life Technologies, Grand Island, NY) plus 10% FCS (Cansera, Ontario, Canada), glutamine (GibcoBRL), and gentamycin. They were then suspended on rafts comprising nucleopore filters (Costar, Cambridge, MA) supported by gelfoam (Upjohn, Kalamazoo, MI). Culture medium was partially replaced every 3 days for a total of 10 days.
Histology
Whole E14.5 and E16.5 embryos or cultured thymic lobes were embedded, and 10-μmol/L frozen sections were prepared (Cell Morphology Core, Institute for Human Gene Therapy, University of Pennsylvania, Philadelphia, PA). Immunohistochemistry was performed with staining (Vecta-stain; Vector Labs, Burlingame, CA) and the appropriate purified primary antibody according to manufacturer's protocols. For visualization purposes, we used alkaline phosphatase–conjugated secondary antibodies with a substrate (Vector Black, Vector Labs). To allow quantification using an image analysis system (Leica, Deerfield, IL), counterstain was not used. To determine relative staining, equivalent WT and PU.1−/−sections were stained in concert and digitized (Leica Photomicroscopy system, Leica). Relative staining intensities were sampled, and background was subtracted using image analysis software (Leica and NIH Image). An average of 4 sections were analyzed for each WT and PU.1−/−stain. The staining of WT sections was taken as 100% for each marker analyzed. Relative staining percentages were then calculated for the PU.1−/−sections and assigned to the nearest 5%. Staining for HSA in the fetal thymus was equivalent between WT and PU.1−/−embryos and served as a positive control.
Results
Histology and culture of myeloid dendritic cell progenitors
Previous studies of PU.1−/−mice have demonstrated severe myeloid defects. PU.1 is expressed at high levels in the myeloid lineage and is absolutely required for monocyte/macrophage differentiation.19,20 Very low levels of myeloid progenitors do exist in PU.1−/−animals, however, and these progenitors can differentiate into aberrant neutrophil-like cells in vitro22 or postnatally.20 To investigate the effects of the PU.1 mutation on myeloid DC in tissues, E16.5 embryos were sectioned and stained for DC CD11b or CD11c expression. No positive staining was seen for CD11b in PU.1−/−embryo sections (data not shown). The thymus was positive for the CD11c dendritic cell (Figure1) and negative elsewhere in the embryo, except for background staining in the gut due to endogenous alkaline phosphatase activity (data not shown). These staining patterns suggested that myeloid DC, in addition to monocytic cells, were absent in PU.1−/−mutants. Given the rarity of DC in whole animals, we decided to concentrate and expand myeloid DC by culture from WT and PU.1−/−embryos.
Myeloid dendritic cell culture.
Low-density cells (ρ = 1.077 g/cm3) were isolated from WT and PU.1−/−E16.5 embryos. The cells were cultured (2 × 105 cells/well) in media containing GM-CSF and IL-4 for 3 days. Cells were then fixed and stained for the DC markers CD11c or DEC-205. (A) Photomicrograph of CD11c stained DC from WT or PU.1−/−embryos. (B) Stained cells were counted, and the results were tabulated as DC per 105low-density cells plated in the original culture. Results are given in mean ± SEM (standard error of the mean) per number of embryos tested (n).
Myeloid dendritic cell culture.
Low-density cells (ρ = 1.077 g/cm3) were isolated from WT and PU.1−/−E16.5 embryos. The cells were cultured (2 × 105 cells/well) in media containing GM-CSF and IL-4 for 3 days. Cells were then fixed and stained for the DC markers CD11c or DEC-205. (A) Photomicrograph of CD11c stained DC from WT or PU.1−/−embryos. (B) Stained cells were counted, and the results were tabulated as DC per 105low-density cells plated in the original culture. Results are given in mean ± SEM (standard error of the mean) per number of embryos tested (n).
Several investigators have shown that myeloid DC can be cultured in the presence of GM-CSF and IL-4.3 In addition, PU.1−/−embryos still express GM-CSFR, which suggests that GM-CSF responsive hematopoietic progenitors may still be present.24Therefore, DC were enriched from whole E16.5 WT and PU.1−/−embryos via collagenase treatment and density gradient centrifugation (as described earlier). The enriched cell populations were then cultured for 3 days in media containing GM-CSF and IL-4. In these culture conditions, myeloid DC express CD11c and up-regulate expression of DEC-205. Cultured cells were fixed and immunohistochemically stained for CD11c or DEC-205 expression. Figure1A shows the characteristic staining observed for CD11c, a myeloid DC with clear dendritic processes. The results of triplicate cultures were quantified and expressed as the number of myeloid DC per 105 cells plated in the original culture (Figure 1B). WT cultures contained numerous easily distinguishable DC that were positive for CD11c or DEC-205. PU.1−/−cultures, however, exhibited only background levels of staining for these markers.
Histology of thymic dendritic cells
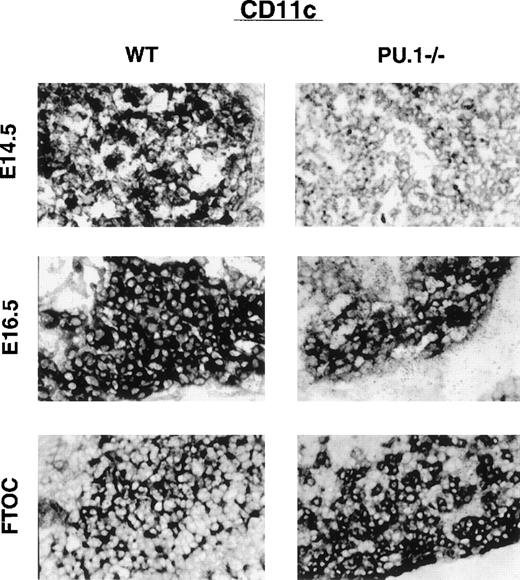
Thymic DC are thought to be derived from a separate lymphoid-related progenitor than myeloid DC.25 Both myeloid and lymphoid DC express the integrin CD11c and high levels of MHC-II. The majority of lymphoid DC, however, express DEC-205 in situ (without the need for culture) and the lymphoid-specific marker CD8α. To examine lymphoid DC in the thymus, frozen sections were prepared from WT or PU.1−/−E14.5 and E16.5 embryos as well as from cultured thymic lobes. Staining for CD11c, DEC-205, or HSA expression was done in concert to allow the assessment of relative staining intensity between sections. To facilitate their quantification, the sections were not counterstained. Figure2 shows the staining pattern of equivalent sections of WT or PU.1−/−thymus stained for CD11c cells. Initial expression of CD11c is delayed in PU.1−/−embryos, as evidenced by the low-level staining of E14.5 thymus. By E16.5 staining, CD11c+ cells in PU.1−/−thymus recovered to near WT levels. When thymic lobes are placed into organ culture for 10 days, the staining intensity for WT and PU.1−/−lobes becomes equivalent. A nearly identical pattern is observed for DEC-205 expression (Figure3). The WT staining pattern for both CD11c and DEC-205 expression was mainly restricted to the subcapsular cortex of the developing thymus in the E14.5 and E16.5 sections. The staining patterns of PU.1−/−thymus were less organized due to their general hypocellularity.
Thymic lobes stained for CD11c expression.
WT or PU.1−/−embryos or cultured thymic lobes were fixed, and cryosections were prepared. Individual sections were stained with αCD11c antibodies followed by an APC secondary antibody. A substrate kit (Vector Black, Vector) was used for visualization. All staining was done in concert to allow relative staining intensities to be compared. No counterstain was used. E14.5 and E16.5 indicate the gestational age of the embryo. FTOC indicates E16.5 thymic lobes that have been placed in organ culture for an additional 10 days prior to sectioning.
Thymic lobes stained for CD11c expression.
WT or PU.1−/−embryos or cultured thymic lobes were fixed, and cryosections were prepared. Individual sections were stained with αCD11c antibodies followed by an APC secondary antibody. A substrate kit (Vector Black, Vector) was used for visualization. All staining was done in concert to allow relative staining intensities to be compared. No counterstain was used. E14.5 and E16.5 indicate the gestational age of the embryo. FTOC indicates E16.5 thymic lobes that have been placed in organ culture for an additional 10 days prior to sectioning.
Thymic lobes stained for DEC-205 expression.
WT or PU.1−/−embryos or cultured thymic lobes were fixed, and cryosections were prepared. Individual sections were stained with αDEC-205 antibodies (Figure 2). E14.5 and E16.5 indicate the gestational age of the embryo. FTOC indicates E16.5 thymic lobes that have been placed in organ culture for an additional 10 days prior to sectioning.
Thymic lobes stained for DEC-205 expression.
WT or PU.1−/−embryos or cultured thymic lobes were fixed, and cryosections were prepared. Individual sections were stained with αDEC-205 antibodies (Figure 2). E14.5 and E16.5 indicate the gestational age of the embryo. FTOC indicates E16.5 thymic lobes that have been placed in organ culture for an additional 10 days prior to sectioning.
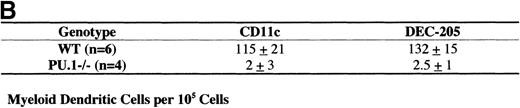
Individual sections from an average of 4 embryos were digitized (Leica Photomicroscopy system, Leica) and analyzed for staining intensity with image analysis software. Background staining from muscle was subtracted, and WT sections were assigned a value of 100%. Figure4 depicts the relative staining intensity of the PU.1−/−sections. HSA expression in the thymus was equivalent between WT and PU.1−/−embryos. At the E14.5 staining, PU.1−/−embryos had fewer than 10% of WT levels for both CD11c+ and DEC-205+ cells. By the E16.5 section, staining for both markers was approximately 70% of WT levels. Individual thymic lobes were also cultured for 10 days, then sectioned and stained. Staining for the lymphoid DC markers CD11c and DEC-205 was equivalent for both genotypes after culture. These staining patterns suggest that lymphoid DC development was initially delayed in PU.1−/−embryos, but then recovered to WT levels. However, both DEC-205 and CD11c are expressed on cells other than DC, which could complicate interpretation of the staining patterns. Therefore, we performed a more stringent flow cytometric analysis for DC subsets.
Quantification of dendritic cell staining.
Immunohistochemical staining of cryosections was performed simultaneously in batches for both WT and PU.1−/−embryos or cultured thymic lobes. Sections were stained for HSA, CD11c, or DEC-205 expressions. A minimum of 3 sections (average of 5 sections) from different embryos were digitized (Leica Photomicroscopy system, Leica) for each gestational age and stain. Staining intensity was quantified with analysis software (NIH-Image). Background staining from muscle was subtracted, and WT sections were assigned a value of 100%. Mean values and standard deviations for relative staining intensity are shown to the nearest 5%.
Quantification of dendritic cell staining.
Immunohistochemical staining of cryosections was performed simultaneously in batches for both WT and PU.1−/−embryos or cultured thymic lobes. Sections were stained for HSA, CD11c, or DEC-205 expressions. A minimum of 3 sections (average of 5 sections) from different embryos were digitized (Leica Photomicroscopy system, Leica) for each gestational age and stain. Staining intensity was quantified with analysis software (NIH-Image). Background staining from muscle was subtracted, and WT sections were assigned a value of 100%. Mean values and standard deviations for relative staining intensity are shown to the nearest 5%.
Specific block to myeloid but not lymphoid dendritic cells
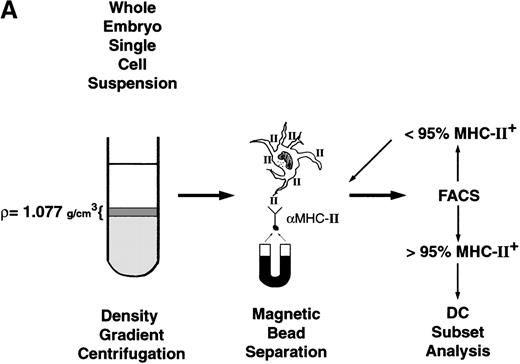
To further characterize dendritic cell populations in PU.1−/−embryos, we directly examined both myeloid and lymphoid DC isolated from whole embryos. All DC are low-density cells that are highly positive for MHC-II+ and CD11c expression. Within this population, lymphoid DC also express CD8α and DEC-205, whereas myeloid DC do not.6 Therefore, the major lymphoid DC population comprises low-density MHC-II+, CD11c+, CD8α+, and DEC-205+ cells, while the major myeloid DC population comprises low-density MHC-II+, CD11c+, CD8α−, and DEC-205−cells. To examine these populations in E16.5 embryos, the enrichment scheme depicted in Figure 5A was followed.
Dendritic cell isolation and flow cytometry analysis for myeloid and lymphoid DC.
(A) Isolation scheme for low-density MHC-II+ cells. Low-density cells are isolated by density gradient centrifugation followed by magnetic bead enrichment for MHC-II+ cells. Cells are subjected to successive rounds of positive selection until more than 95% are MHC II+ cells, as determined by flow cytometry. (B) Low-density MHC-II+ cells (panel 1) are then stained for DC markers CD11c (panel 2), CD8α (panel 3), or DEC-205 (last panels) and analyzed by flow cytometry. Representative staining histograms are presented with isotype controls provided. Dead cells were excluded from analysis by propidium iodide exclusion and scattergating for size.
Dendritic cell isolation and flow cytometry analysis for myeloid and lymphoid DC.
(A) Isolation scheme for low-density MHC-II+ cells. Low-density cells are isolated by density gradient centrifugation followed by magnetic bead enrichment for MHC-II+ cells. Cells are subjected to successive rounds of positive selection until more than 95% are MHC II+ cells, as determined by flow cytometry. (B) Low-density MHC-II+ cells (panel 1) are then stained for DC markers CD11c (panel 2), CD8α (panel 3), or DEC-205 (last panels) and analyzed by flow cytometry. Representative staining histograms are presented with isotype controls provided. Dead cells were excluded from analysis by propidium iodide exclusion and scattergating for size.
Briefly, E16.5 PU.1−/−embryos and WT (PU.1+/+ and PU.1+/−) embryos were pooled, minced, and treated with collagenase to form single-cell suspensions. Low-density cells were then isolated by density gradient centrifugation. With adult lymphoid tissues, such as spleen and thymus, density separation alone results in greater than 100-fold enrichment for DC, and 20%-40% of cells express high-density MHC-II+. For the whole E16.5 embryos, however, less than 2% of the low-density cells were MHC-II+, thus necessitating further enrichment steps. Low-density cells were therefore stained with mAbs to MHC-II+ and positively selected with magnetic beads. Flow cytometry was used to monitor the purification until the cell population was greater than 95% MHC-II+ cells (Figure 5B, first panels). Approximately 3 × 103 low-density MHC-II+ cells were isolated per WT embryo, and 1 × 103 low-density MHC-II+ cells were isolated per PU.1−/−embryo. Purified low-density MHC-II+ cells were then analyzed for either CD11c, CD8α, or DEC-205 expression. Single-color flow cytometric analyses were performed due to the extreme difficulty of isolating the small initial populations and the limitations imposed by the available antibody reagents.
Figure 5B shows the results of flow cytometric analysis for dendritic cell subsets. The first panel shows the MHC-II staining for the input populations. More than 95% of the purified cells were positive for MHC-II. The intensity of MHC-II staining is somewhat reduced due to the presence of avidin-conjugated magnetic beads, which were used for purification. The beads occupy a portion of the available binding sites on the biotinylated antibody, thus reducing the amount of avidin-FITC that can bind. The low-density MHC-II+ cells from both genotypes stained positive for CD11c expression, which indicates that the enriched cells are almost pure DC. WT cells had a bimodal staining profile for both CD8α and DEC-205 expression, and approximately 65% of the DC were negative for both markers, and 35% were positive. Thus WT embryos have both myeloid DC (low-density MHC-II+ CD11c+ CD8α−DEC-205−) and lymphoid DC (low-density MHC-II+ CD11c+ CD8α+DEC-205+). In contrast, PU.1−/−embryos have lymphoid DC (CD8α+ DEC-205+), but lack myeloid DC (CD8α− DEC-205− ).
PU.1−/−lymphoid dendritic cells are functional
To test the ability of PU.1−/−lymphoid DC to present antigen and activate T cells, mixed leukocyte reactions were performed. Various numbers of WT or PU.1−/−DC from a mixed C57BL/6 and 129-SV strain background were cocultured with splenic T cells from Balb/c mice, and the proliferative response was measured. PU.1−/−lymphoid DC demonstrated an equivalent or better ability to stimulate T- cell proliferation when compared to wild type DC (Figure6). Due to limitations in our ability to isolate embryonic DC, peak or saturation levels of T-cell proliferation were not reached, as evidenced by the lack of plateau on the graphs. However, as few as 1000 PU.1−/−DC were able to induce a 10-fold T-cell proliferative response in the mixed leukocyte reaction. This demonstrates that the function of PU.1−/−lymphoid DC is not impaired by the mutation.
Mixed leukocyte cultures to measure dendritic cell function.
Varying numbers of purified DC from WT embryos (▵) or PU.1−/−embryos (□) were cocultured with purified splenic T cells from Balb/c mice. The proliferative response was measured at day 4 by 3H-thymidine incorporation and is presented as total CPM per well. The results represent 3 independent assays done in triplicate, with standard deviations indicated by the error bars.
Mixed leukocyte cultures to measure dendritic cell function.
Varying numbers of purified DC from WT embryos (▵) or PU.1−/−embryos (□) were cocultured with purified splenic T cells from Balb/c mice. The proliferative response was measured at day 4 by 3H-thymidine incorporation and is presented as total CPM per well. The results represent 3 independent assays done in triplicate, with standard deviations indicated by the error bars.
Discussion
Previous studies have shown that transcription factor PU.1 plays a role in the earliest stages of both lymphoid and myeloid development.26 PU.1 appears to be absolutely required in order for myelomonocytic and B-lymphoid progenitors to develop. In addition, PU.1 is required for efficient commitment to and/or differentiation of T lymphocytes. PU.1 is expressed in uncommitted CD44+, CD25− thymocytes, but it is not expressed by the time thymocytes manifest CD4 or CD8 expression.18 Since PU.1 plays a role in both myeloid and lymphoid lineages, we were interested in determining its role in the development of DC.
DC are rare but widely distributed migratory antigen presenting cells (APC) of the immune system.1 The major role associated with DC is the initiation of primary immune responses by T lymphocytes.27 Until recently, DC were known to be derived from the hematopoietic stem cell, but their lineage relationships were unknown. It is now clear, however, that DC can arise from 2 separate hematopoietic lineages and are referred to as myeloid DC or lymphoid DC.28 Most DC are derived from myelomonocytic precursors and express myeloid related markers such as CD11b. These precursors can be matured into myeloid DC in culture in the presence of GM-CSF. At peripheral sites, myeloid DC phagocytose antigens process the antigen and migrate to the lymph nodes, where they mature into cells capable of presenting antigen to T cells in order to initiate an immune response.1 Indeed, myeloid DC are vastly more potent at inducing T-cell responses than any other APC. This fact, coupled with the myeloid dendritic cell migratory nature and APC, makes the cells likely to be the predominate APC in initiating immune responses.
Thymic DC have a different function and origin. Thymic DC are thought to mediate negative selection of developing T lymphocytes. They do so by presenting self-antigens and initiating the death of self-reactive T cells within the thymus. The earliest hematopoietic progenitors found in the thymus have given rise to B cells, T cells, and thymic DC but not myeloid cells.29,30 CD44+ CD25+CD4−CD8−thymic progenitors can give rise to T cells and thymic DC, but not B or myeloid cells, whereas CD25+CD44−CD4−CD8−cells only give rise to T cells.31 Thymic DC are characterized in part by their expression of CD8α. Similar CD8α+ DC are found in the spleen, and they also have negative regulatory effects on T-cell function.4,5 The CD8α+ splenic DC are postulated to arise from the same progenitor as thymic DC,31-34 hence the designation of lymphoid DC used in this paper. PU.1 is expressed in the multipotent thymic progenitors that give rise to lymphoid DC, but PU.1 is down-regulated in committed T-cell progenitors, which suggests that it may play a role in lymphoid dendritic cell development.18
PU.1−/−embryos were sectioned and stained for a variety of markers for both myeloid and lymphoid DC. In any tissue tested, the PU.1−/−embryos did not contain cells that stained for the myeloid-specific marker CD11b (data not shown). This is in accord with previous flow cytometry data.19 Indeed, the lack of CD11b+ cells is used to genotype the embryos. This suggests the absence of all myelomonocytic lineage cells, including myeloid DC, in PU.1−/−embryos. One major caveat, however, is the fact that expression of CD11b is regulated in part by PU.1.35 Thus, the lack of staining may represent only a lack of CD11b expression and not a lack of myeloid cells. To address this concern, we attempted to grow myeloid DC from PU.1−/−embryos via culture in media with GM-CSF and IL-4. While WT embryos yielded numerous CD11c+ and DEC-205+ myeloid DC, myeloid DC could not be cultured from PU.1−/−embryos (Figure 1).
Sections from multiple WT and PU.1−/−embryos were also stained either for CD11c, DEC-205, or HSA expression. Comparison of staining intensities between WT and PU.1−/−embryos suggested a delay in the development of lymphoid DC in the thymus of PU.1−/−embryos (Figures 2-4). At the E14.5 staining, very few stained cells were present in mutant thymus. After culture of thymic lobes, however, both WT and PU.1−/−lobes stained with equal intensity for both lymphoid dendritic cell markers. It still remains to be determined if the delay is due to the direct role of PU.1 in lymphoid dendritic cell differentiation or to a secondary effect from the general hypocellularity of PU.1−/−thymus.
Our next goal was to directly assess which dendritic cell populations are present in PU.1−/− embryos. Given the small size of E16.5 embryos, it was impractical to isolate DC from dissected hematopoietic tissues. Therefore, we took advantage of the high levels of MHC-II+ expressed by all DC to purify them from total low-density cells isolated from whole embryos. It was then possible to analyze low-density MHC-II+ populations by flow cytometry for dendritic cell subsets. The majority of DC found in WT embryos were myeloid DC, MHC-II+ CD11c+CD8α− DEC-205−cells.6WT embryos also contained a smaller population of MHC-II+CD11c+ CD8α+ DEC-205+ lymphoid DC (Figure 5). In contrast, PU.1−/−embryos only contained MHC-II+ CD11c+ CD8α+DEC-205+ lymphoid DC. Myeloid DC were clearly absent from the low-density MHC-II+ population of PU.1−/−cells (Figure 5). This result is supported by the dendritic cell culture results and histology and the fewer numbers of low-density MHC-II+ cells isolated from the mutants (1 × 103 versus 3 × 103 per embryo). Previous transplantation and ES cell chimera experiments have demonstrated that the hematopoietic defects seen in PU.1−/−animals are cell intrinsic.21 The functional proliferative assays demonstrate that PU.1−/−lymphoid DC present antigen and stimulated T-cell activation as well as, if not better than, wild type DC. Therefore, PU.1 is required for myeloid dendritic cell development but not for lymphoid dendritic cell development.
The dendritic cell phenotype of PU.1−/−mice is similar to that seen with RelB−/−miceand Ikaros−/−mice. All 3 mutations lack CD8α− myeloid DC. For both RelB and Ikaros mutants, myeloid development is robust, and RelB−/−mice actually exhibit myeloid hyperplasia. Why both mutations lack myeloid DC remains uncertain. In the case of PU.1, however, the lack of myeloid DC may be directly linked to effects on myeloid progenitors. PU.1 is required for the expression of a host of myeloid-specific genes,36 some of which may be crucial for myeloid dendritic cell development. Another common feature of all 3 mutations is the retention of some T-cell development potential, and all mutations are capable of producing CD8α+ lymphoid DC. PU.1−/−embryos have exceedingly hypocellular thymuses seeded with early hematopoietic progenitors (CD44+, HSABright, c-kitint, Thy1−, CD25−, Sca1−, CD4−, and CD8−). Corresponding progenitors with respect to number and cell-surface phenotype are found in WT fetal thymus.18 Development of lymphoid DC in PU.1−/−thymus is delayed to a much lesser extent than development in T cells (2-fold down at E16.5 versus greater than 100-fold down). This suggests that PU.1 may play a more direct role in the T-cell lineage. An equally attractive alternative is that lymphoid DC must develop within the thymus in order to induce efficient T-cell commitment and/or development. PU.1–deficient mice may provide the experimental means to dissect the requirements for lymphoid dendritic cell development and their function during T-cell differentiation.
Acknowledgments
The authors would like to thank Ken Shortman (WEHI, Melbourne, Australia) for a detailed isolation protocol for DC. We also thank Robert Fisher (University of Pennsylvania) for a critical review of the manuscript.
Supported by grants PO1 DK52558 (L.M.S. and E.W.S.), CA72769, and HL58716 (E.W.S.) from the National Institutes of Health, Bethesda, MD. E.W.S. is a Leukemia Society of America Scholar.
Reprints:Edward W. Scott, Institute for Human Gene Therapy, Biomedical Research Building II, Room 513, 421 Curie Boulevard, University of Pennsylvania, Philadelphia, PA 19104-6140; e-mail:scotte@mail.med.upenn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal