Kaposi's sarcoma-associated herpesvirus (KSHV) encodes 3 genes that are homologous to cellular chemokines. vMIP-III, the product of open reading frame K4.1, is the most distantly related to human chemokines and has yet to be characterized. We have examined the interaction of vMIP-III with chemokine receptors, its expression in KS lesions, and its in ovo angiogenic properties. We show expression of vMIP-III in KS lesions and demonstrate the stimulation of angiogenesis by this chemokine, like vMIP-I and vMIP-II, in the chick chorioallantoic membrane assay. vMIP-III does not block human immunodeficiency virus entry through the coreceptors CCR3, CCR5, or CXCR4. However, vMIP-III is an agonist for the cellular chemokine receptor CCR4. CCR4 is expressed by TH2-type T cells. Consistent with this, vMIP-III preferentially chemoattracts this cell type. Because of these biologic properties and because it is expressed in KS lesions, vMIP-III may play an important role in the pathobiology of KS.
Kaposi sarcoma (KS) is the most common tumor associated with human immunodeficiency virus (HIV) infection.1KS-associated herpesvirus (KSHV, also known as human herpesvirus 8) was originally identified in KS lesions and is considered to be the etiologic agent of KS.1,2 KSHV has also been found in patients with primary effusion lymphoma and multicentric Castleman disease, suggesting that it may play an important role in these lymphoproliferative diseases.3 4
The KSHV genome is comparable to that of other gamma herpesviruses, such as Epstein-Barr virus and herpesvirus Saimiri (HSV).5These viruses encode numerous genes with similarity to human sequences. An interesting feature of KSHV is that it encodes 3 novel chemokine genes located within the long unique coding region of its genome.5-7 Two of these genes, vMIP-I and vMIP-II, share extensive (60%) sequence identity, whereas vMIP-III, the product of KSHV open reading frame K4.1, is more distantly related, sharing homologies to vMIP-I and vMIP-II of approximately 37%.
Previous studies have shown that vMIP-I and vMIP-II interact with cellular chemokine receptors and stimulate angiogenesis.6-8These studies demonstrated the ability of vMIP-I to bind CCR5 and inhibit HIV infection. Additionally, vMIP-II was shown to bind CCR3 and CCR8 as an agonist, block HIV infection through CCR3, and bind as an antagonist on a wide variety of chemokine receptors.6 7 The third KSHV-encoded chemokine, vMIP-III, has yet to be characterized. Although vMIP-I and vMIP-II share significant sequence similarity with human chemokines (43% and 52%, respectively, with MIP-1α), vMIP-III is more distantly related. The closest related human chemokines are TARC and eotaxin, which share 35% and 38% homology, respectively.
To gain a better understanding of the functional properties of vMIP-III, we have expressed this chemokine in mammalian cells and purified it to near homogeneity. In this study, we have determined the receptor binding and chemotactic activation profile for vMIP-III. In addition, we have investigated the expression of vMIP-III in KS lesions along with its role in angiogenesis and HIV infection.
Materials and methods
Cloning, expression and purification of vMIP-III
The coding region of vMIP-III was isolated from a genomic clone of KSHV by polymerase chain reaction and cloned into the mammalian expression vector pDEF10.9 Stable clones of Chinese hamster ovary (CHO) cells expressing high levels of vMIP-III were obtained as described previously.9
Initial purification of vMIP-III was performed from CHO cell culture supernatants by heparin sulfate affinity chromatography using a CL-6B column (Pharmacia, Uppsala, Sweden). The column was washed with 0.2 mol/L NaCl in 20 mmol/L Tris, pH 7.5, and vMIP-III was eluted with 0.6 mol/L NaCl in 20 mmol/L Tris, pH 7.5. The eluted material was separated on an 18% SDS-PAGE gel and electroblotted onto polyvinylidene fluoride (PVDF) membrane (Novex, San Diego, CA). Doublet bands of 9 to 10 kd, corresponding to the predicted migration of vMIP-III, were individually excised from the membrane, and NH2-terminal sequence was determined by Edman degradation using an ABI 473A protein sequencer (Applied Biosystems, Foster City, CA).
Large-scale purification of vMIP-III was performed by cation-exchange chromatography using an S-5 column (Sartorius, Edgewood, NY). The column was washed with 0.2 mol/L NaCl in 20 mmol/L Tris, pH 7.5, and eluted with 0.8 mol/L NaCl in 20 mmol/L Tris, pH 7.5. The elution peak and flanking fractions were analyzed by SDS-PAGE and detected by Coomassie staining.
Mass spectrometric analysis
vMIP-III purified by cation-exchange chromatography was diluted 1:2 with 5 mmol/L octylglucoside in 20 mmol/L Tris, pH 6.8, and mixed 1:2 with calibration mixture #3 in Sinapinic acid matrix (PerSeptive Biosystems, Framingham, MA) according to the manufacturer's instructions. One microliter of the sample-calibration mixture was spotted on the sample plate and allowed to air dry. Matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) was performed using a Voyager Elite mass spectrometer (PerSeptive Biosystems). The system was operated in the linear-delayed extraction mode with 337-nm nitrogen laser (Laser Sciences, Newton, MA). Bovine insulin (M = 5733.59) and Escherichia coli thioredoxin (M = 11 673.48) were used as size standards.
Generation of polyclonal antibodies to vMIP-III
vMIP-III was chemically synthesized by Gryphon Sciences (San Francisco, CA) using t-butyl-oxycarbonyl chemistries on a peptide synthesizer (430A; Applied Biosystems, Foster City, CA). This material could not be refolded and was used as an immunogen to generate polyclonal rabbit antibodies to vMIP-III.10
Western blotting
For Western blotting, samples were electroblotted to PVDF membranes (Novex) using 300 mA constant current for 30 minutes at room temperature. Blots were blocked in tris-buffered saline containing 0.1% tween 20 (TBS-Tw20)/1% bovine serum albumin (BSA) and probed with anti–vMIP-III rabbit polyclonal antisera (1:5000). After 3 washes with TBS-Tw20, goat antirabbit antibodies conjugated to horseradish peroxidase (Transduction Laboratories, Lexington, KY) were added (1:5000) and incubated at room temperature for 30 minutes. Blots were washed 3 times with TBS-Tw20 and detected by autoradiography using electro-chemiluminescence (Renaissance ECL; NEN Life Science Products, Boston, MA).
Kaposi sarcoma lesion preparation
Late-stage KS nodule whole-cell lysates were prepared from paraffin-embedded samples. Twenty-five sections of 25-μm thickness were solubilized in 4 × Laemmeli buffer. Viscous samples were sheared with a 26-gauge needle and boiled, and equivalent amounts of protein were loaded on an SDS-PAGE gel and electroblotted to PVDF for vMIP-III detection.
Cell culture
THP-1 cells (ATCC, Rockville, MD) and the pre-B lymphoid cell line L1.2 (kindly provided by Irv Weissman, Stanford, CA) were grown in RPMI–10% fetal calf serum. L1.2 cells transfected with CCR3 and CCR4 (kindly provided by Osamu Yoshi), and CCR5 were grown in RPMI–10% FCS and 500 μg/mL G418. U87/CD4 cells expressing CCR3, CCR5, or CXCR4 (kindly provided by Dan Littman) were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum.
Angiogenesis assay
The angiogenic activity of vMIP-III was tested in the chicken chorioallantoic membrane assay as described previously.7
HIV infection assay
The effects of vMIP-III on HIV infection were determined in infectivity assays as described previously.11 Briefly, U87/CD4 cells bearing the appropriate chemokine receptor were pretreated with chemokine for 30 minutes before exposure to a dual-tropic primary HIV strain 2028 at approximately 1000 focus-forming U/mL. After 3 hours, cells were washed and incubated for 4 days before fixing and immunostaining for p24 antigen as described previously.12
Receptor binding assays
Sodium iodide 125-I–labeled chemokines (MCP-1, MCP-4, MDC, and MIP-1α) were purchased from Amersham–Pharmacia Biotech (Piscataway, NJ). Receptor-ligand interactions were studied by using whole-cell binding.13 Briefly, cells were washed once in phosphate-buffered saline and resuspended in binding buffer (50 mmol/L HEPES, pH 7.5, 1 mmol/L CaCl2, 5 mmol/L MgCl2, 0.5% BSA, and 0.05% azide). Binding reactions were performed in 96-well, round-bottom tissue culture plates (Costar, Corning, NY) for 90 minutes at room temperature. Each reaction consisted of 5 × 105 cells, 0.1 nmol/L radiolabeled chemokine, and various concentrations of unlabeled chemokine (for competition binding) in a total volume of 200 μL. Samples were then transferred to 96-well, glass-fiber filter plates (Millipore, Bedford, MA), which were precoated with 0.5% polyethylenimine, washed twice with binding buffer containing 0.5 mol/L NaCl, and counted on a β counter (Wallac, Gaithersburg, MD). Nonspecific binding was defined as binding that could not be displaced by a 500-fold molar excess of unlabeled ligand. Data are presented as percentage specific binding, calculated by 100 × ([S-B])/[T-B]), where S is the binding of the radiolabeled ligand, B is nonspecific binding, and T is the total binding in the absence of competitors.
Chemotaxis assays
For chemotaxis assays, 1 × 106 cells were resuspended in 0.1 m RPMI 1640 medium containing 0.5% BSA and loaded into the upper chamber of a transwell chemotaxis chamber (0.3-μm pore size; Costar, Corning, NY). Chemokines were added to the lower wells in a volume of 0.6 mL. After 4 hours at 37°C, cells in the lower chamber were collected and counted by flow cytometry (FACScan, Becton Dickinson, NJ). Data are expressed as the number of cells that migrate through the filter ± SEM or percentage input of cells. Cells that migrated in the absence of chemokine served as a baseline negative control.
Generation of TH1 and TH2 cell lines
Short-term TH1 and TH2 cell lines were derived from the peripheral blood of normal healthy donors as previously described.14Briefly, peripheral blood mononuclear cells were isolated on Histopaque gradients (Sigma, St. Louis, MO). Naive (CD4+, CD45RA+) T cells were obtained by negative selection using a cocktail of monoclonal antibodies to remove monocytes (anti-CD14), B-cells (anti-CD19, anti-CD20), CD8 T cells (anti-CD8), natural killer cells (anti-CD56), and memory CD4 T-cells (anti-CD45RO). All antibodies were obtained from Pharmingen (San Diego, CA). Negative selection was performed using goat antimouse antibodies coupled to magnetic beads (BioMag; PerSeptive Biosystems), according to the manufacturer's instructions. To generate polarized TH1 cell lines, CD4+/CD45RA+ T-cells (2 × 105/mL) were cultured for 4 days in the presence of 2 ng/mL IL-12, 200 ng/mL anti–IL-4, and 2.5 μg/mL phytohaemagglutinin PHA (Sigma). To obtain TH2 lines, T cells from the same donor were cultured for 4 days in the presence of 10 ng/mL IL-4, 2 μg/mL anti–IL-12, and 2.5 μg/mL PHA (cytokines and anticytokine reagents were obtained from R&D Systems, Minneapolis, MN). After polarization, cells were expanded by culture in 100 U/mL IL-2 (Boehringer Mannheim, Indianapolis, IN) for 10 days. TH1/TH2 polarization was confirmed by assaying for interferon (IFN)-γ and IL-4, respectively, in phorbol myristate acetate/ionomycin-treated cultures or by intracellular staining for IFN-γ and IL-4.15
Results
Expression and purification of recombinant vMIP-III
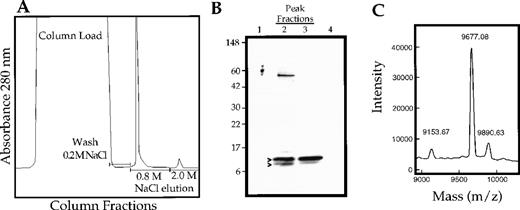
vMIP-III was amplified from the KSHV genome and engineered for expression in mammalian cells. The expression plasmid was stably expressed in CHO cells, and recombinant vMIP-III was secreted into the culture media. vMIP-III was purified to near homogeneity by cation exchange chromatography of culture supernatants and elution with 0.8 mol/L NaCl (Figure 1A). SDS-PAGE analysis of the peak fractions from this purification step revealed that vMIP-III ran as a doublet with a mass between 9 and 10 kd. Amino-terminal sequence analysis confirmed that both bands in the doublet corresponded to vMIP-III beginning at amino acid 27, which is consistent with proteolytic processing of the predicted 26-amino acid signal peptide.
Purification and characterization of vMIP-III expressed in CHO cells.
(A) vMIP-III was purified from culture supernatants of stably transfected CHO cells by cation exchange chromatography. Column washes were performed at 0.2 mol/L NaCl/20 mmol/L Tris, pH 7.2, and vMIP-III was eluted at 0.8 mol/L NaCl/20 mmol/L Tris. (B) SDS-PAGE analysis followed by Coomassie blue staining of the peak fractions shown in A. (C) vMIP-III was subjected to MALDI-MS. The 9677.08 and 9153.67 peaks correspond to full-length vMIP-III and vMIP-III, which have been truncated at the carboxy terminus, respectively.
Purification and characterization of vMIP-III expressed in CHO cells.
(A) vMIP-III was purified from culture supernatants of stably transfected CHO cells by cation exchange chromatography. Column washes were performed at 0.2 mol/L NaCl/20 mmol/L Tris, pH 7.2, and vMIP-III was eluted at 0.8 mol/L NaCl/20 mmol/L Tris. (B) SDS-PAGE analysis followed by Coomassie blue staining of the peak fractions shown in A. (C) vMIP-III was subjected to MALDI-MS. The 9677.08 and 9153.67 peaks correspond to full-length vMIP-III and vMIP-III, which have been truncated at the carboxy terminus, respectively.
Analysis of the purified material by mass spectrometry identified a major protein species at 9677.67 d, which is within 1 mass unit of the predicted molecular weight for mature vMIP-III, and a minor species at 9153.67 d (Figure 1C). The smaller mass species presumably corresponds to the faster migrating band on SDS-PAGE and has a molecular weight consistent with that of vMIP-III after the removal of 4 amino acids at the carboxy terminus. The 9890.63 d species is a matrix adduct. Minor carboxy terminal processing has also been reported for vMIP-II expressed in mammalian cells.6 Additionally, MALDI-MS indicates that the CHO-expressed material is not posttranslationally modified because the observed mass for vMIP-III is identical to the predicted mass of vMIP-III.
Expression of vMIP-III in KS lesions
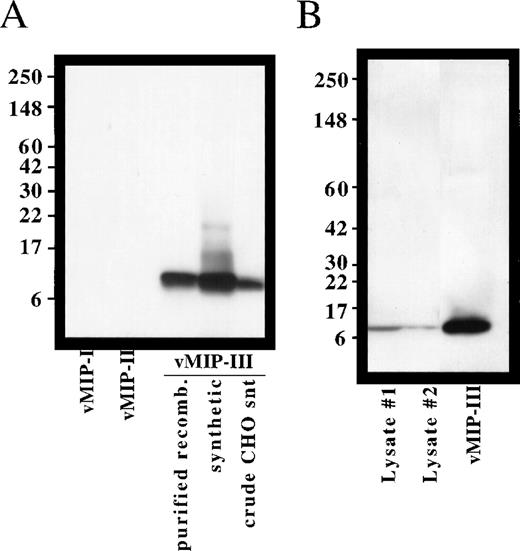
To determine whether vMIP-III protein is expressed in KS lesions, we examined advanced-stage KS nodule lysates by immunoblotting. Polyclonal antisera were prepared in rabbits by immunization with synthetic vMIP-III. This antisera was specific for vMIP-III, as shown by the lack of cross-reactivity with vMIP-I or vMIP-II (Figure2A) or with a panel of human chemokines (data not shown). As shown in Figure 2B, a band that comigrates with recombinant vMIP-III could be detected in 2 KS nodule samples isolated from the same patient. Preimmune sera did not detect these bands (data not shown).
Specificity of vMIP-III antibodies and expression of vMIP-III in KS lesions.
(A) Polyclonal antibodies were generated in rabbits and examined for their ability to recognize synthetic or mammalian cell-derived vMIP-III by Western blotting. With the exception of the crude supernatant, 100 ng each sample was loaded. Synthetic vMIP-I and vMIP-II served as negative controls. (B) Expression of vMIP-III in lesions from advanced-stage KS was determined by Western blotting. Blots probed with preimmune sera did not detect these bands.
Specificity of vMIP-III antibodies and expression of vMIP-III in KS lesions.
(A) Polyclonal antibodies were generated in rabbits and examined for their ability to recognize synthetic or mammalian cell-derived vMIP-III by Western blotting. With the exception of the crude supernatant, 100 ng each sample was loaded. Synthetic vMIP-I and vMIP-II served as negative controls. (B) Expression of vMIP-III in lesions from advanced-stage KS was determined by Western blotting. Blots probed with preimmune sera did not detect these bands.
Induction of angiogenesis by vMIP-III
After our finding that vMIP-I and vMIP-II have angiogenic activities,7 vMIP-III was tested for its ability to stimulate angiogenesis in the chicken chorioallantoic membrane assay. As shown in Table 1, vMIP-III stimulated significant angiogenesis in this assay. One microgram vMIP-III (the highest dose tested) stimulated an angiogenic response that was similar to that observed for the positive control, basic fibroblast growth factor (bFGF).
vMIP-III is angiogenic in the chick embryo chorioallantoic membrane assay
| Sample* . | Dose (μg) . | Number of embryos . | Angiogenic response† . | Score‡ . | Percentage maximum score . | ||
|---|---|---|---|---|---|---|---|
| + . | +/− . | − . | |||||
| vMIP-III | 1.00 | 8 | 3 | 3 | 2 | 4.5 | 56.3 |
| 0.50 | 7 | 0 | 3 | 3 | 1.5 | 21.4 | |
| bFGF | 0.50 | 8 | 5 | 1 | 2 | 5.5 | 68.8 |
| 0.25 | 8 | 2 | 3 | 3 | 3.5 | 43.8 | |
| BSA | 2.00 | 8 | 1 | 1 | 6 | 1.5 | 18.8 |
| H2O | — | 10 | 0 | 0 | 10 | 0.0 | 0.0 |
| Sample* . | Dose (μg) . | Number of embryos . | Angiogenic response† . | Score‡ . | Percentage maximum score . | ||
|---|---|---|---|---|---|---|---|
| + . | +/− . | − . | |||||
| vMIP-III | 1.00 | 8 | 3 | 3 | 2 | 4.5 | 56.3 |
| 0.50 | 7 | 0 | 3 | 3 | 1.5 | 21.4 | |
| bFGF | 0.50 | 8 | 5 | 1 | 2 | 5.5 | 68.8 |
| 0.25 | 8 | 2 | 3 | 3 | 3.5 | 43.8 | |
| BSA | 2.00 | 8 | 1 | 1 | 6 | 1.5 | 18.8 |
| H2O | — | 10 | 0 | 0 | 10 | 0.0 | 0.0 |
Angiogenic induction was performed by methylcellulose disk containing recombinant vMIP-III, bFGF as a positive control. Disks containing BSA, vehicle alone (water) were used as negative controls.
Angiogenic responses were scored by 3 investigators: (+) unanimously positive; (+/−) split or unclear judgment; (−) unanimously negative.
Scores of 1, 0.5, and 0 were counted for (+), (+/−), and (−), respectively. The percentage maximum score is the division of the scores by the number of embryos.
Inhibition of HIV infection
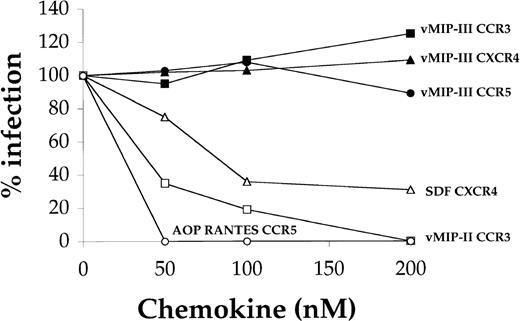
Although vMIP-I and vMIP-II have been shown to inhibit HIV infection in assays using chemokine coreceptors CCR5 and CCR3, respectively,6,7 16 at concentrations as high as 200 nmol/L, vMIP-III did not inhibit infection through these coreceptors or through the CXC chemokine receptor CXCR4 (Figure3).
Effect of vMIP-III on HIV infection.
U87/CD4 cells bearing the relevant chemokine receptor were pretreated with chemokine for 30 minutes before exposure to a dual-tropic primary HIV strain at approximately 1000 focus-forming U/mL. After 3 hours, cells were washed and incubated for 4 days before fixing and immunostaining for p24 antigen.
Effect of vMIP-III on HIV infection.
U87/CD4 cells bearing the relevant chemokine receptor were pretreated with chemokine for 30 minutes before exposure to a dual-tropic primary HIV strain at approximately 1000 focus-forming U/mL. After 3 hours, cells were washed and incubated for 4 days before fixing and immunostaining for p24 antigen.
Chemokine receptor binding and chemotaxis
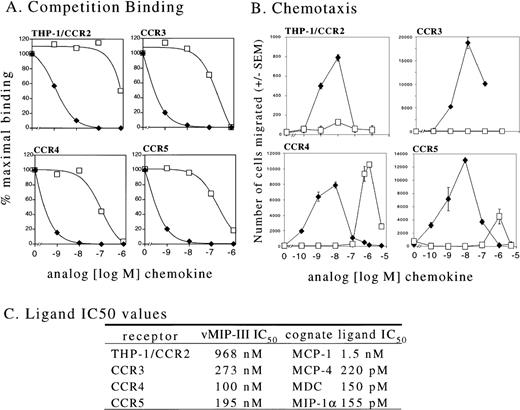
Using competition-binding assays, we next tested the ability of vMIP-III to compete with the natural ligands for the human chemokine receptors CCR2, CCR3, CCR4, and CCR5. Stable transfectants expressing CCR2 were unavailable for this study; therefore, we used THP-1 cells to assess vMIP-III in competition with 125I-labeled MCP-1. THP-1 cells naturally express CCR2,17 and MCP-1 does not interact with other known chemokine receptors.18 Stable transfectants of the mouse pre-B cell line L1.2 were used for CCR3, CCR4, and CCR5 binding. vMIP-III was able to compete for binding to each of these receptors (Figure 4A); however, the IC50 values, which ranged from 100 nmol/L for CCR4 to 968 nmol/L for CCR2 (Figure 4C), were significantly greater than those observed for their natural ligands.
Interaction of vMIP-III with chemokine receptors.
(A) Radioligand competition-binding studies were performed on THP-1 cells (which naturally express CCR2), and L1.2 cells were stably transfected with CCR3, CCR4, or CCR5. The radioligand forms of the natural ligands for these receptors were as follows: MCP-1 (THP-1/CCR2), MCP-4 (CCR3), MDC (CCR4), and MIP-1α (CCR5). Competition was performed using either the unlabeled natural ligand (⧫) or vMIP-III (□). (B) The cell lines used for binding studies were used in chemotaxis assays with either the natural ligands for these receptors; MCP-1 (CCR2), eotaxin (CCR3), MDC (CCR4), RANTES (CCR5), (⧫) or vMIP-III (□). (C) IC50 values from A are summarized.
Interaction of vMIP-III with chemokine receptors.
(A) Radioligand competition-binding studies were performed on THP-1 cells (which naturally express CCR2), and L1.2 cells were stably transfected with CCR3, CCR4, or CCR5. The radioligand forms of the natural ligands for these receptors were as follows: MCP-1 (THP-1/CCR2), MCP-4 (CCR3), MDC (CCR4), and MIP-1α (CCR5). Competition was performed using either the unlabeled natural ligand (⧫) or vMIP-III (□). (B) The cell lines used for binding studies were used in chemotaxis assays with either the natural ligands for these receptors; MCP-1 (CCR2), eotaxin (CCR3), MDC (CCR4), RANTES (CCR5), (⧫) or vMIP-III (□). (C) IC50 values from A are summarized.
To determine whether vMIP-III could act as an agonist for any of these receptors, chemotaxis assays were performed on the cells used for competition binding. vMIP-III at concentrations up to 1 μmol/L did not show any agonist activity toward THP-1 or L1.2/CCR3 cells (Figure4B). The respective ligands for these receptors, MCP-1 and eotaxin, elicited the characteristic bimodal chemotaxis curve with the maximum number of cells migrating at approximately 10 nmol/L chemokine (Figure 4B). vMIP-III displayed weak agonist activity toward L1.2/CCR5 with less than 30% maximal migration of that seen by RANTES, a natural agonist of CCR5. In contrast to the other chemokine receptors, L1.2/CCR4 cells migrated dramatically to vMIP-III. The peak response was observed from 500 nmol/L to 1000 nmol/L (Figure 4B), which is approximately 1.5 to 2 logs greater than that observed for the natural ligand MDC (10 nmol/L).
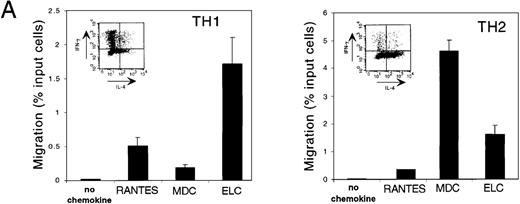
Chemotaxis of primary TH1/TH2 cells to vMIP-III
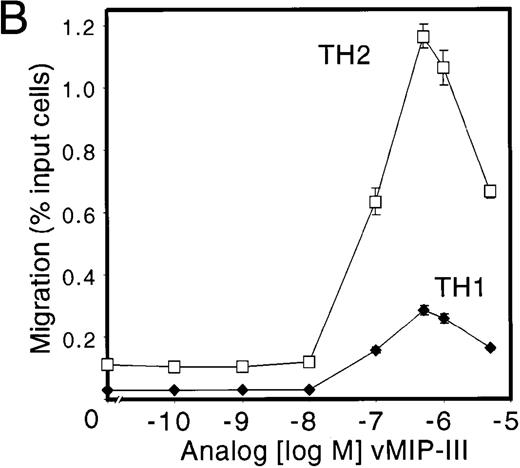
TH1 and TH2 cells have recently been shown to express chemokine receptors differentially.19,20 In particular, TH1 cells express CCR5 and CXCR3, whereas TH2 cells express CCR3 and CCR4. Both populations express CCR7. The CCR4 ligands TARC and MDC have been shown to induce the migration of TH2 cells selectively14; therefore, using the same methods for the generation of TH1 and TH2 lines, vMIP-III was tested for similar activity. Short-term TH1 and TH2 lines were established, and their T-cell polarization was confirmed by intracellular staining of IL-4 and IFN-γ using flow cytometry.15 To demonstrate that T-cell lines prepared in this manner expressed the reported set of chemokine receptors, their chemotactic profiles to TH1- and TH2-specific chemokines were examined (Figure 5A). Using chemokines at concentrations that induced maximal chemotaxis, TH1 cells migrated to the CCR5 ligand RANTES, whereas the response to the CCR4 ligand, MDC, was markedly weaker (Figure 5A, TH1). Conversely, TH2 cells showed a robust chemotactic response to MDC, whereas the response to RANTES was substantially less (Figure 5A, TH2). ELC, a ligand for CCR7, elicited responses in both cell types, which is consistent with the reported expression profile19 (Figure 5A). Polarization of TH1/TH2 lines from a different donor and generated under the same conditions was confirmed by measuring IFNγ and IL-4 production after activation with calcium ionophore and phorbol ester (data not shown). Consistent with the response of TH2 cells to MDC, vMIP-III, in a dose-dependent manner, preferentially stimulated the chemotaxis of TH2 cells (Figure5B). Similar to the chemotaxis profile for L1.2/CCR4 cells (Figure 4B), vMIP-III elicited peak chemotaxis at 500 to 1000 nmol/L, with approximately 5-fold more TH2 cells migrating in response to vMIP-III when compared to TH1 cells from the same donor.
vMIP-III selectively chemoattracts TH2 cells.
(A) Peripheral blood-derived CD4+ TH1 and TH2 lines were confirmed for their respective lineage designation by intracellular staining for IFN-γ and IL-4 (inset) and tested with lineage-specific chemokines RANTES (TH1), MDC (TH2), and ELC (TH1/2) in chemotaxis assays. Chemokine concentrations were used at concentrations that elicited maximal chemotaxis (10 nmol/L for RANTES and MDC; 5 nmol/L for ELC). (B) TH1 and TH2 lines were examined for their ability to undergo chemotaxis in response to vMIP-III. The chemotactic profile is similar to that for CCR4 transfectants (Figure 4B).
vMIP-III selectively chemoattracts TH2 cells.
(A) Peripheral blood-derived CD4+ TH1 and TH2 lines were confirmed for their respective lineage designation by intracellular staining for IFN-γ and IL-4 (inset) and tested with lineage-specific chemokines RANTES (TH1), MDC (TH2), and ELC (TH1/2) in chemotaxis assays. Chemokine concentrations were used at concentrations that elicited maximal chemotaxis (10 nmol/L for RANTES and MDC; 5 nmol/L for ELC). (B) TH1 and TH2 lines were examined for their ability to undergo chemotaxis in response to vMIP-III. The chemotactic profile is similar to that for CCR4 transfectants (Figure 4B).
Discussion
Although the genome of KSHV encodes 3 chemokine-like genes, only vMIP-I and vMIP-II have previously been characterized.6-8,21 22 This study has shown that the open reading frame K4.1 in the KSHV genome encodes a functional CC chemokine that has agonist properties toward the chemokine receptor CCR4. First, we demonstrated that mammalian cells can secrete and correctly process vMIP-III to generate a biologically active protein (Figure 1). Moreover, by Western blot analysis we were able to show vMIP-III expression in KS lesions (Figure 2), suggesting that in vivo expression by virally infected cells may contribute to the pathobiology of KSHV. The expression of KSHV chemokines has previously only been demonstrated at the mRNA level in the context of KSHV-infected B-cell cavity lymphoma after phorbol ester treatment (vMIP-I,-II)16,23 and in KS lesions (vMIP-1).24
Advanced-stage KS lesions are characterized by pronounced neovascularization of the affected tissue.25 The KSHV-encoded G-protein-coupled receptor, ORF 74, binds a wide variety of chemokines and constitutively induces angiogenesis in a vascular endothelial growth factor (VEGF)-dependent manner.26,27Chemokines of the CXC class have been shown to have angiostatic and angiogenic activity,28 but cellular CC chemokines have not been reported to have such activities. On the contrary, we have previously shown that the KSHV-encoded CC chemokines vMIP-I and vMIP-II induce angiogenesis,7 whereas control CC chemokines RANTES and MIP-1α have no effect. Using the same chick chorioallantoic model of angiogenesis, we showed that vMIP-III is also a stimulator of angiogenesis (Table 1). One microgram vMIP-III stimulated angiogenesis similarly to 0.5 μg bFGF. On a per-mole basis, vMIP-III (9.6 kd) at 1.0 μg proved to be approximately 4-fold less potent than bFGF (17 kd) at 0.5 μg in the stimulation of angiogenesis (Table 1). We used the same scoring system in a previous study to evaluate vMIP-I and vMIP-II in the chicken chorioallantoic membrane assay7; however, direct comparisons of the vMIP potencies cannot be made because of the differences in performance of the positive controls used (VEGF vs bFGF). Nevertheless, each vMIP achieved a maximal angiogenic response at approximately 1 μg. On a per-mole basis, relative to the positive controls used, the vMIPs were approximately 4- to 5-fold less effective in the stimulation of angiogenesis. vMIP-I and vMIP-II were, however, more effective than IL-8, a CXC chemokine known to induce angiogenesis.29 These findings suggest that at least 4 distinct viral gene products can potentially contribute to the generation of new blood vessels in KS.
Recent studies have shown that vMIP-I and vMIP-II can inhibit HIV infection by viruses that use CCR5 or CCR3 (respectively) as coreceptors.7 16 vMIP-III does not block viral entry through CCR3, CCR5, or CXCR4 at the concentrations tested (Figure 3), suggesting that in vivo it is unlikely to directly affect the interaction of HIV with host cells (Figure 3).
KS lesions are noted for prominent leukocyte infiltrate consisting of monocytes, lymphocytes, and mast cells.30 Therefore, rather than examine vMIP-III as a leukocyte antagonist, experiments were designed to determine receptor use followed by assays for agonist activity. Although calcium mobilization has historically been a method of choice for initial measurements of agonist activity, it has been reported that chemokines can elicit chemotaxis without inducing calcium mobilization.31 Therefore, cells expressing chemokine receptors that interact with vMIP-III were examined for agonist properties in chemotaxis assays. Competition-binding experiments indicated vMIP-III has similar affinities for CCR3, CCR4, and CCR5 (Figures 4A, 4C) with IC50 values 2 to 3 logs greater than the natural ligands for these receptors. Nevertheless, only cells expressing CCR4 responded substantially to vMIP-III in chemotaxis assays. THP-1 cells, which express several chemokine receptors (CCR1, CCR2, CXCR4,32,33 and C × 3CR1), showed no chemotaxis toward vMIP-III, suggesting the lack of usefulness of these receptors by this ligand. Although the IC50 values are similar, the observation that cells expressing CCR5 and CCR3 show little to no chemotaxis to vMIP-III is intriguing. Comparatively, vMIP-II binds several chemokine receptors as an antagonist and is reported to be an agonist for CCR3.6,7 34 Similarly, vMIP-III may be optimized by the virus to bind, but not activate, various chemokine receptors.
Consistent with the binding data, peak chemotaxis induced by vMIP-III occurred from 500 to 1000 nmol/L, which is 50 to 100 times less potent than MDC (peak chemotaxis at 10 nmol/L) (Figure 4B). Notwithstanding its low potency, vMIP-III is a slightly more efficacious ligand than MDC because L1.2/CCR4 cells consistently show approximately 30% greater migration to vMIP-III.
Although the binding affinity and potency of vMIP-III/CCR4 interactions may appear impractical in a physiological sense, chemokine–chemokine receptor interactions with similar characteristics exist. By comparison, HCC-1 (hemofiltrate CC chemokine) has recently been identified as a ligand for CCR1, which has an IC50 of 93 nmol/L (vs 1.3 nmol/L for MIP-1α) and stimulates maximal chemotaxis at 100 nmol/L (vs 1 nmol/L for MIP-1α).33 Interestingly, HCC-1 is secreted constitutively in vivo to a level of 90 nmol/L, which is near the IC50 of this CCR1 ligand. Even though we can detect vMIP-III by Western blot in KS lesions, we are unable to quantify the amount of vMIP-III in the lesion or in serum at this time.
T-cell subpopulations differentially express chemokine receptors.19,20 The selective expression of chemokine receptors by functionally distinct populations of effector T cells may explain the polarized cytokine profile (TH1 vs TH2) seen in lymphocytes isolated from different disease settings.35,36 TH1 cells preferentially express CCR5 and CXCR3, whereas TH2 cells express CCR3, CCR4, and CCR8.8,19,20 37 Consistent with its ability to act as an agonist for CCR4-bearing cells, vMIP-III preferentially chemoattracts TH2 cells derived from peripheral blood lymphocytes (Figure 5B). Five times as many TH2 cells as TH1 cells migrated in response to vMIP-III. TH1 and TH2 lines prepared in the same manner responded appropriately to lineage-specific chemokines and to ELC, the ligand for CCR7 that is expressed on both populations (Figure5A). The low level of migration observed by the TH1 population (Figure 5B) may be attributed to the level of purity of the TH1/TH2 lines, coupled with the observed weaker response of L1.2/CCR5 cells to vMIP-III (Figure 4B).
The finding that vMIP-III is a functional ligand for CCR4 and has chemotactic abilities raises the question of the physiologic significance of this viralkine in the pathology of KS. It is well established that TH2 cytokines down-regulate TH1 responses.35,36 The host response to viral infection is mediated principally by TH1 cells,38 and several viruses have evolved mechanisms to limit TH1 responses. For example, the interaction of measles virus with its receptor on macrophages inhibits production of IL-12, a cytokine that is critical for the generation of a TH1 response.39 The closely related γ-herpesvirus Epstein-Barr virus encodes a functional homolog of the cytokine IL-10, which inhibits the production of the TH1 cytokine IFN-γ, whereas a number of pox viruses encode soluble proteins that bind and inhibit the biologic activity of TH1 cytokines.38,40 KSHV itself encodes functional cellular homologs that are known promoters of TH2 immunity, vIL-623 and inhibitors of the TH1 cytokine IFN-γ41 vIRF-1.
The demonstration that vMIP-III is expressed in KS lesions and is a chemoattractant for committed TH2 cells suggests that KSHV may evade the host immune response in part by deviating the immune response from a TH1 to a TH2 microenvironment within the KS lesion. vMIP-I,21 vMIP-II,8 and vMIP-III have been shown to chemoattract TH2 cells preferentially. Consistent with these observations, T cells present in the KS lesion show a predominant TH2 pattern of cytokine secretion.8 The TH2 cytokine IL-4 has been shown to induce monocyte secretion of MDC42; therefore, resident monocytes within the KS lesion may further amplify the recruitment of TH2 cells with the release of endogenous CCR4 ligand.
Interestingly, a similar model of immune deviation involving a CCR4 ligand in states of neoplasia exists in Hodgkin disease. Nodular scleroses of Hodgkin disease are histologically similar to KS lesions,43 with Reed-Sternberg (RS) cells analogous to the KS spindle cells. RS cells have been shown to secrete high quantities of the CCR4 ligand TARC.44 The lymphocytes in close vicinity of RS cells are almost exclusively CD4+ and of the TH2 phenotype. The production of TARC by RS cells is thought to be responsible for this predominant TH2 microenvironment and thus is proposed as a possible mechanism for subverting effective immune responses.43
Taken together, these findings suggest that vMIP-III, with its angiogenic and chemotactic abilities, may play a diverse role in the pathobiology of KS. In particular, vMIP-III, by preferentially recruiting TH2 cells, may aid in the deviation of the immune response to a TH2 microenvironment and thus evade the host immune response. This represents a previously undescribed mechanism of viral persistence that may lead to the development of novel therapeutic strategies for the treatment of KSHVmediated disease.
Acknowledgments
We thank Dr Irv Weissman for supplying us with the L1.2 cells, Dr Osamu Yoshie for the CCR3 and CCR4 transfectants, Dr Hai Le Trong for amino terminal sequence analysis of vMIP-III, Dr Ashok Kumar for performing the MALDI-MS, and Dr Yasu Takeuchi for helpful discussions.
Reprints:David Chantry, ICOS Corporation, 22021 20th Avenue SE, Bothell, WA 98021; e-mail: dchantry@icos.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal