Mobilized peripheral blood progenitor cells (PBPC) are a potential target for the retrovirus-mediated transfer of cytostatic drug-resistance genes. We analyzed nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse-repopulating CD34+ PBPC from patients with cancer after retroviral transduction in various cytokine combinations with the hybrid vector SF-MDR, which is based on the Friend mink cell focus-forming/murine embryonic stem-cell virus and carries the human multidrug resistance 1 (MDR1) gene. Five to 13 weeks after transplantation of CD34+ PBPC into NOD/SCID mice (n = 84), a cell dose-dependent multilineage engraftment of human leukocytes up to an average of 33% was observed. The SF-MDR provirus was detected in the bone marrow (BM) and in its granulocyte fractions in 96% and 72%, respectively, of chimeric NOD/SCID mice. SF-MDR provirus integration assessed by quantitative real-time polymerase chain reaction (PCR) was optimal in the presence of Flt-3 ligand/thrombopoietin/stem-cell factor, resulting in a 6-fold (24% ± 5% [mean ± SE]) higher average proportion of gene-marked human cells in NOD/SCID mice than that achieved with IL-3 alone (P < .01). A population of clearly rhodamine-123dull human myeloid progeny cells could be isolated from BM samples from chimeric NOD/SCID mice. On the basis of PCR and rhodamine-123 efflux data, up to 18% ± 4% of transduced cells were calculated to express the transgene. Our data suggest that the NOD/SCID model provides a valid assay for estimating the gene-transfer efficiency to repopulating human PBPC that may be achievable in clinical autologous transplantation. P-glycoprotein expression sufficient to prevent marrow aplasia in vivo may be obtained with this SF-MDR vector and an optimized transduction protocol.
Mobilized peripheral blood progenitor cells (PBPC) are an attractive target for gene-therapy applications in the treatment of malignant diseases because they can be collected in ample quantities from peripheral blood (PB) after mobilization by cytokines or cytokine-supported chemotherapy.1 PBPC transplantation has enabled administration of higher doses of cytotoxic treatment to patients with cancer, resulting in higher remission rates for some diseases.1,2 The transfer of cytostatic drug-resistance genes into hematopoietic stem cells (HSC) and hematopoietic progenitor cells (HPC) is expected to extend this concept by protecting the bone marrow (BM) of patients with cancer from myelotoxicity, the main adverse and often dose-limiting effect of most cytostatic drugs. This may enable further dose escalation.3-5 The human multidrug resistance 1 (MDR1) gene is a candidate gene encoding the membrane-located drug-efflux pump P-glycoprotein. P-glycoprotein confers resistance to a wide array of cytostatic agents, such as paclitaxel and etoposide.6-8 In mice, transplantation of BM cells from MDR1 transgenic animals, as well as transplantation of retrovirally transduced primary HPC, resulted in chemoprotection in vivo.9 10
Testing the concept of MDR1-mediated chemoprotection in large animals or in human gene-therapy trials has been hampered by a low number of reconstituting vector-marked cells and inefficient expression of the transgene in vivo, despite high levels of gene transfer into HPC and long-term culture-initiating cells (LTCIC) in vitro.11-17 Because long-term repopulating human hematopoietic cells are mostly quiescent, murine retroviral vectors do not integrate easily.18,19 Commonly used vectors based on murine leukemia virus (MLV) are expressed poorly in HSC and their differentiated myeloid progeny.20 21 Thus, there is a need for optimization of transduction conditions and vector design.
For analyses of human repopulating stem cells, small-animal xenotransplantation models have been developed in immunodeficient mice by several groups in the past 10 years (see Greiner at al22for a review). These models are now considered to be surrogate assays for repopulating stem cells in a clinical transplantation setting.23-29 Nonobese diabetic/LtSz-severe combined immunodeficient/scid (NOD/SCID) mice have multiple defects in innate immunity,30 including a largely reduced natural killer-cell activity and a 2-fold reduced BM cellularity that possibly provides more stem-cell niches for human cells. These mice have been shown to be superior to SCID mice (CB-17scid/scid) both in terms of the frequency of engraftment in the mice and the level of human cell engraftment.30-32 The xenotransplantation of human hematopoietic cells from different hematopoietic cell sources into NOD/SCID mice has provided the basis to define and quantify a novel population of cells, termed SCID-repopulating cells (SRC). SRC are capable of extensive proliferation and multilineage differentiation in vivo.31 33-36
In contrast to LTCIC, SRC are not found in the CD34+/38+ cell fraction.31,37 The engraftment potential of SRC is markedly reduced after ex vivo culture.31,36 Moreover, Gothot et al38 recently provided direct evidence that CD34+ PBPC SRC are predominantly in G0 phase and further showed that G0-G1 transition stimulated by cytokines, which are necessary for integration of murine retroviral vectors, can significantly reduce the engraftment potential of SRC. Gene transfer to SRC has generally been inefficient,31 reflecting the low levels of vector-marked cells observed in large-animal models and pilot gene-therapy trials. Similar findings have been reported for retrovirally transduced CD34+/38− cells from human adult BM transplanted into beige/nude/X-linked (BNX) immunodeficient mice.39 Thus, these in vivo readout systems may be relevant surrogate assays for the human pluripotent stem cell and provide a valuable tool for side-by-side comparisons of human gene-therapy protocols in advance of clinical studies.
Only a few in vitro40-43 and in vivo16,17studies have reported on the efficiencies of gene transfer and recovery of retrovirally transduced primitive hematopoietic cells from mobilized blood. We used CD34+ PBPC from patients with cancer, cells that may have already had a reduced proliferative capacity because of previous cytotoxic chemotherapy. These cells were transduced with the retroviral SF-MDR vector.44,45 Retroviral transduction occurred in the presence of various cytokines with or without addition of cell-free stroma-conditioned medium (SCM).40,46 47 We analyzed the engraftment, transgene integration, and expression in SRC. A high gene-transfer rate and unambiguous expression of theMDR1 transgene were found.
Materials and methods
Selection of CD34+ cells
Samples of mobilized PB apheresis cells obtained in the recovery phase after chemotherapy supported by granulocyte colony-stimulating factor (G-CSF) (Filgrastim, Amgen, Thousand Oaks, CA) were obtained from 2 patients with multiple myeloma, 3 patients with non-Hodgkin's lymphoma, 3 patients with breast cancer, 1 patient with liposarcoma and, after G-CSF alone, from 1 allogeneic donor, with informed consent. The patients with cancer had received a median of 3 chemotherapy cycles (range, 1 to 8) and a median of 1 chemotherapy regimen (range, 1 to 4). Three patients had radiotherapy before mobilization. This study was approved by an ethics committee at the Medical Faculty of the University of Heidelberg. Mononuclear cells (MNC) were collected from apheresis samples by Ficoll density centrifugation (Biochrom KG, Berlin, Germany). CD34+ PBPC were recovered by positive immunoselection by using the MACS CD34 Multisort Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The selected fractions contained a median of 94% CD34+ cells (range, 48%-99%).
SCM
Confluent layers of the preadipocyte cell line FBMD-1, derived from murine BM stroma (provided by R. E. Ploemacher), were grown at 33°C under 10% carbon dioxide.48 The cells were cultured in Dulbecco's minimum essential medium (MEM) supplemented with 5% fetal-calf serum (FCS), 5% horse serum (Stem Cell Technologies, Vancouver, BC, Canada), 3.5 mmol/L HEPES, 2 mmol/L glutamine, 1% penicillin/streptomycin (Gibco, Eggenstein, Germany), 10−5 mol/L hydrocortisone-21-hemisuccinate, 0.2 mmol/L alanine, 0.3 mmol/L asparagine, 0.5 mmol/L asparagine acid, 0.5 mmol/L cysteine, 0.4 mmol/L glutamine acid, 0.3 mmol/L proline, 0.01 mmol/L vitamin B12, 0.1 mmol/L biotin, 10−4 mol/L sodium selenite (Sigma, Deisenhofen, Germany), 0.8 mmol/L sodium pyruvate, and 10−4 mol/L β-mercaptoethanol (all Serva, Heidelberg, Germany, unless otherwise mentioned). After the layers were confluent, the medium was removed and fresh medium was added and conditioned for 10 days. The SCM was harvested and nonadherent cells were removed by centrifugation and stored at −70°C.
Retroviral vector
The SF-MDR vector is based on the Friend mink cell focus-forming/murine embryonic stem-cell virus (FMEV). It contains the human MDR1 gene under the transcriptional control of the spleen focus-forming virus long-terminal repeat (LTR), which has been combined with a permissive leader sequence of the murine embryonic stem-cell virus (MESV) to overcome transcriptional repression of U3-mediated gene expression.44 45
Retroviral Transwell transduction of CD34+ PBPC
Immediately after isolation, 2 × 106 fresh CD34+ selected PBPC were seeded into 6-well plates coated with 20 μg/cm2 of the recombinant fibronectin fragment CH-29649-51 (RetroNectin; Takara Shuzo, Japan). Cells were transduced for 96 hours by Transwell cocultivation with 1 × 105 SF-MDR virus producer cells that were grown above the PBPC in Transwell inserts (high density [0.4 μm pore size], Falcon, Heidelberg, Germany). Titers of cell-free supernatants obtained from the virus producer cells ranged from 1 × 105/mL to 5 × 105/mL. The cells were cultured in α-MEM supplemented with 10% FCS, 2 mmol/L glutamine, and 1% penicillin/streptomycin (referred to as “transduction medium”) with or without 50% SCM. In the different cytokine combinations used, Flt-3 ligand (FL; R&D Systems, Wiesbaden, Germany), stem-cell factor (SCF; R&D Systems), and vascular endothelial growth factor (VEGF; R&D Systems) were added to yield final concentrations of 100 ng/mL each. Thrombopoietin (TPO; R&D Systems) and IL-6 (R&D Systems) were used at concentrations of 20 ng/mL each, and IL-3 (Novartis, Nürnberg, Germany) was used at a concentration of 50 ng/mL. In all experiments, mock transductions were performed over 96 hours at identical cell and growth-factor concentrations. After the 96-hour transduction period, transduced and mock transduced PBPC were harvested, washed 3 times in phosphate-buffered saline (PBS; Gibco) and resuspended in Iscove's modified Dulbecco's medium (IMDM) plus 10% FCS (Stem Cell Technologies) at a concentration of 1 × 106 input of CD34+ PBPC/mL of medium. In experiments 1 to 7, CD34+ PBPC samples were transduced in several separate aliquots in each experiment. The aliquots of each experiment were pooled after transduction for transplantation of cells from 1 patient into a given set of NOD/SCID mice. In experiments 8 to 10, the individual transduction aliquots were transplanted into individual mice (Table 1).
MDR1 gene transfer into lineage-committed progenitor cells and expression in myeloid progeny cells
| Transduction Group . | MDR1-PCR + CFU-GM* (%) . | Rh-123dull Cells† (%) . | Patient Sample No. (=NOD/SCID Mice Set No.) . |
|---|---|---|---|
| Transwell | |||
| IL-3 | 27 ± 2 (n = 3) | 2.9 ± 0.9 (n = 4) | 3, 4, 5, 7 |
| IL-3 + SCM | 20 ± 15 (n = 2) | 3.4 ± 3.3 (n = 2) | 1, 7 |
| IL-3/FL | 23 ± 8 (n = 4) | 3.9 ± 2.1 (n = 6) | 3, 4, 5, 6, 7, 8 |
| IL-3/FL + SCM | 13 ± 4 (n = 3) | 3.2 ± 1.6 (n = 3) | 1, 2, 7 |
| IL-3/IL-6/SCF/FL | 6 | 3.9 ± 0.2 (n = 2) | 8 |
| FL/T/SCF | 45 | 6.2 ± 0.9 (n = 3) | 8 |
| FL/T/V | 25 | 6.2 | 7 |
| Cell-free viral supernatant | |||
| IL-3/IL-6/SCF/FL | NA | 18.7 ± 0.7 (n = 2) | 10 |
| FL/T/SCF | 39 ± 8 (n = 3) | 16.3 ± 2.0 (n = 7) | 9,10 |
| Transduction Group . | MDR1-PCR + CFU-GM* (%) . | Rh-123dull Cells† (%) . | Patient Sample No. (=NOD/SCID Mice Set No.) . |
|---|---|---|---|
| Transwell | |||
| IL-3 | 27 ± 2 (n = 3) | 2.9 ± 0.9 (n = 4) | 3, 4, 5, 7 |
| IL-3 + SCM | 20 ± 15 (n = 2) | 3.4 ± 3.3 (n = 2) | 1, 7 |
| IL-3/FL | 23 ± 8 (n = 4) | 3.9 ± 2.1 (n = 6) | 3, 4, 5, 6, 7, 8 |
| IL-3/FL + SCM | 13 ± 4 (n = 3) | 3.2 ± 1.6 (n = 3) | 1, 2, 7 |
| IL-3/IL-6/SCF/FL | 6 | 3.9 ± 0.2 (n = 2) | 8 |
| FL/T/SCF | 45 | 6.2 ± 0.9 (n = 3) | 8 |
| FL/T/V | 25 | 6.2 | 7 |
| Cell-free viral supernatant | |||
| IL-3/IL-6/SCF/FL | NA | 18.7 ± 0.7 (n = 2) | 10 |
| FL/T/SCF | 39 ± 8 (n = 3) | 16.3 ± 2.0 (n = 7) | 9,10 |
Transwell transductions were done under standard conditions: 96-hour coculture of 2 × 106 CD34+ peripheral blood progenitor cells (PBPC) with 1 × 105 MDR virus producer cells in the presence of the indicated cytokines with or without stroma-conditioned medium (SCM). Transductions with cell-free viral supernatant were done in CH-296–coated wells for 48 hours after 48 hours of prestimulation. Mock transductions were performed in all experiments. In experiments with cells from patient samples 1 to 7, CD34+ PBPC aliquots from the same patient separately transduced under otherwise identical conditions were combined for assaying, whereas each transduced CD34+ PBPC aliquot from patient samples 8 to 10 was assayed individually. No colony-forming units granulocyte-monocyte (CFU-GM) from any mock transduction were found to be positive by polymerase chain reaction (PCR) for the transduced MDR1 gene.
Rh denotes rhodamine; NOD/SCID, nonobese diabetic/severe combined immunodeficient; FL, Flt3 ligand; SCF, stem-cell factor; VEGF, vascular endothelial growth factor; T, thrombopoietin; and NA, not available. Values are mean ± SE unless otherwise indicated.
For each sample, ≥18 CFU-GM colonies were analyzed.
Proportion of Rh-123dull cells in the myelomonocytic progeny of transduced CD34+ PBPC minus background of Rh-123dull cells in the progeny of mock-transduced CD34+ PBPC (range, 0.1%-1.0%).
Generation of retroviral supernatants and supernatant transduction of CD34+ PBPC
Retroviral supernatant was harvested from 90% confluent layers of GPenv+AM12/SF-MDR producer cells after a 16-hour cultivation period in α-MEM supplemented with 10% FCS, 2 mol/L glutamine, and 1% penicillin/streptomycin. It was then filtered (0.45 μm) and kept frozen until use. The viral titer of the supernatants was determined by infecting a known number of A2780 cells with different volumes of viral supernatant. The proportion of cells showing a rhodamine (Rh)-123dull phenotype in the A2780 target cell population was determined 48 hours after infection by using fluorescence-activated cell-separation (FACS) analysis as described below. The titer was calculated by multiplying the percentage of Rh-123dullcells by the number of target cells plated and corrected for the amount of viral supernatant applied. After thawing, viral titers ranged from 1 × 105/mL to 5 × 105/mL. Two million CD34+ cells were prestimulated for 48 hours in 2 mL of transduction medium with IL-3, IL-6, SCF, and FL, or FL (100 ng/mL), SCF (100 ng/mL), and TPO (20 ng/mL). After precultivation, retroviral transduction was done with cell-free viral supernatant twice during 48 hours. The prestimulated CD34+ cells were transferred to dishes coated with CH-296 (20 μg/cm2) that contained 1 mL of virus supernatant supplemented with either IL-3, IL-6, SCF, and FL, or FL, SCF, and TPO. After a 4-hour retroviral infection period, the virus supernatant was replaced with fresh cytokine-supplemented transduction medium. After a total of 96 hours in culture, the cells were harvested as described above.
Colony-forming cell (CFC) assays
Semisolid CFU assays were done after the 96-hour transduction period. CD34+ cells were plated in duplicate at 5 × 102 cells/mL in 1 mL of complete methylcellulose medium (Methocult GF, H4434, Stem Cell Technologies) containing a mixture of recombinant human cytokines (SCF, IL-3, granulocyte-macrophage colony-stimulating factor [GM-CSF], and erythropoietin). After 12 to 14 days of incubation at 37°C, colony-forming units granulocyte-macrophage (CFU-GM) were enumerated. CFU-GM colonies were plucked and analyzed for the presence of the vector-derived MDR1 gene as described below. For detection of human progenitor cells in engrafted NOD/SCID mice, chimeric-mouse BM cells were resuspended in IMDM plus 10% FCS and incubated for 2 to 4 hours at 37°C in tissue culture dishes. The nonadherent cells were plated in duplicate at 5 × 105 cells/mL. BM cells from NOD/SCID mice that did not receive transplants served as controls in every experiment and did not produce CFC under these conditions. To select for MDR1-expressing human CFC, freshly thawed vincristine (Sigma) was added to yield a final concentration of 20 nmol/L.
Liquid culture and Rh-123 efflux assay
Sixty-thousand transduced or mock-transduced CD34+ PBPC cells were cultured for 10 days in a cytokine mixture containing 10 ng/mL each of SCF, IL-1β (IC-Chemikalien, Ismaning, Germany), IL-3, IL-6, G-CSF (Amgen, Thousand Oaks, CA, USA), and GM-CSF (IC-Chemikalien). IMDM containing 15% heat-inactivated FCS was used as the culture medium. Under these conditions, the cultured CD34+ cells differentiated into Rh-123bright CD11b+/CD15+ and CD11b+/CD15− cells, which have been shown to represent precursors and mature cells of the granulomonocytic lineage.52MDR1 gene expression in these cells was determined by their ability to efflux Rh-123, resulting in an Rh-123dull phenotype.43
One million cells obtained after liquid culture of 2 × 106 chimeric-mouse BM cells were washed and resuspended in IMDM containing 5% FCS and Rh-123 (Sigma) at a final concentration of 0.2 μg/mL. After an incubation period of 30 minutes at 37°C, cells were centrifuged, resuspended in IMDM containing 5% FCS, and further incubated for 60 minutes at 37°C to allow the cells to efflux loaded Rh-123. Subsequently, the cells were washed twice and resuspended in 300 μL of staining medium (95% PBS, 4% FCS, and 1% 1 mol/L HEPES). Immediately before flow cytometric measurement, propidium iodide staining (Sigma; final concentration, 1 μg/mL) was done to exclude dead cells from the analysis. Separate control procedures were performed with use of the P-glycoprotein inhibitor cyclosporine (Sandimmun; Novartis, Basel, Switzerland) at a final concentration of 1.5 μmol/L during all incubation steps.
Animals
A breeding colony of NOD/SCID mice was established at the animal laboratory of the German Cancer Research Center (breeding stocks originally from Jackson Laboratories, Bar Harbor, ME). Mice were kept in isolators under pathogen-free conditions. Five to 24 hours before transplantation, 5- to 10-week-old female mice were conditioned by sublethal irradiation with a total dose of 3 Gy. Between 5 × 105 and 4 × 106 human CD34+ PBPC were transplanted intravenously in a volume of 300 μL of IMDM per mouse. Starting on the day of transplantation, animals received 1 to 2 μg of human IL-3 (Novartis) and 2 to 4 μg of human G-CSF (Amgen) 3 times per week subcutaneously. Additionally, all mice were treated with antiasialo GM1 (Wako Chemicals, Neuss, Germany). Immediately before transplantation, the mice received intraperitoneal injections of 250 μL of PBS (Gibco) containing 50 μL of antiasialo GM1; identical treatments were repeated on day 5 and day 11 after transplantation of CD34+ cells.
Analysis of engraftment
Mice were killed by cervical dislocation 5 to 13 weeks after transplantation of human cells. PB was aspirated from the heart. BM cells were flushed from the femurs of each mouse into IMDM, and spleen cells were obtained by homogenization. For lysis of erythrocytes, cells were treated with hemolytic buffer (150 mmol/L ammonium chloride, 12 mmol/L sodium bicarbonate, and 0.1 mmol/L EDTA) immediately after removal. Single-cell suspensions from BM, spleen, and PB were preincubated for 20 minutes at 4°C in staining medium (95% PBS, 4% FCS, and 1% 1 mol/L HEPES) containing 10% unconjugated human IgG (Endobulin; Immuno GmbH, Heidelberg, Germany). Cells (1 × 105-1 × 106 per reaction) were labeled with fluorochrome-conjugated antibodies for 30 minutes at 4°C, then washed and resuspended in 200 μL of staining medium.
All antibodies were from Becton Dickinson unless otherwise noted. Antimouse CD45-fluorescein isothiocyanate (FITC; Sigma) and antihuman CD45-phycoerythrin (PE; Pharmingen, Hamburg, Germany) were used to identify the ratio of human to mouse leukocytes. Immediately before measurement, dead cells were excluded from the analysis by using propidium iodide (Sigma) uptake. Specific subsets of human cells were quantified by a triple-staining method that used antihuman CD45-PE, antimouse CD45-Quantum Red (Sigma), and FITC-conjugated antibodies directed against the following human lineage markers: CD2, CD19, CD33, CD34, and CD38. Alternatively, subsets of human cells were analyzed by gating on human CD45-PerCP+ cells and then assessed by staining with antihuman CD34-FITC/CD38-PE, CD14-FITC/CD33-PE, or CD19-FITC/CD20-PE or stained for CD2-FITC/CD4-PE/CD8-PE. Samples were acquired on a Becton Dickinson FACSCalibur (10 000-20 000 events) and analyzed by using the CellQuest software package. In every experiment, parallel staining and FACS analysis was done on normal human PB cells and on BM, spleen, and PB cells obtained from NOD/SCID mice not given transplants.
Enrichment of human cells from chimeric mice
About 1 × 107 freshly isolated chimeric-mouse BM cells were cultivated overnight in IMDM supplemented with 10% FCS, 2 mmol/L glutamine, and 1% penicillin/streptomycin in the presence of FL/TPO/SCF (10 ng/mL of each cytokine) and subjected to Ficoll density-gradient centrifugation on the next day. Human cells were recovered from chimeric-mouse BM by first labeling the MNC fraction with a combination of paramagnetic microbeads conjugated to monoclonal antimouse antibodies (CD45 and TER119, Miltenyi Biotech) directed against all murine hematopoietic cells and subsequent magnetic cell separation on a depletion column (type AS; Miltenyi Biotech). The unlabeled human cells were recovered in the column flow-through, and specific enrichment of human cells was verified by dual staining of cell aliquots with a combination of PE-conjugated antihuman CD45 and FITC-labeled antimouse CD45 antibodies. The flow-through fraction contained on average 84.5% ± 0.9% human CD45+ cells (n = 5). The average recovery of human cells was 59.3% ± 6.5% (n = 5).
Qualitative polymerase chain reaction (PCR) analysis
CFU-GM grown in semisolid medium were individually plucked and lysed as described.43 Mouse BM cells (1 × 106) were separated by Ficoll density centrifugation (BM-Minificoll), and the interphase (MNC) and the cell-pellet fractions (granulocytes) were individually lysed. Genomic DNAs from mouse BM and spleen cells were isolated from 3-5 × 106 cells by using the QIAamp Blood Kit (Qiagen, Hilden, Germany) with elution of the purified DNAs in a final volume of 200 μL. Nested PCR for the selective detection of the retrovirally transduced MDR1 gene (MDR1 complementary DNA [cDNA]) was done with 10 μL of colony lysates or with 10 μL of each genomic DNA in a total volume of 50 μL by using the Taq PCR Master Mix Kit (Qiagen) supplemented with 20 pmol each of sense and antisense primers. In each PCR, a positive control was set up that used 10 μL of lysates of virus producer cells diluted 10−5-fold in provirus-negative cells (sensitivity reached, 1 transduced cell in 105 negative cells). In both PCR rounds, the sense primers were located in the leader sequence of the SF-MDR retrovirus vector backbone, whereas the antisense primers were complementary to the 5′ end of the MDR1 gene. The first PCR round yielded an 825-base pair (bp) DNA fragment with the sense/antisense primers 5′ CGGATCGCTCACAACCAGTC 3′/5′ ACACCAGCATCATGAGAGGAAGTC 3′. The second PCR round yielded a 565-bp DNA fragment with the sense/antisense primer combination 5′ ACCTTTAACG-TCGGATGGC 3′/5′ CTTCTTTGCTCCTCCATTGC 3′. Amplification conditions were as follows: 95°C for 2.5 minutes, then 30 cycles at 95°C for 45 seconds, 58°C for 30 seconds, and 72°C for 1 minute, followed by extension at 72°C for 10 minutes (PTC-200; MJ Research, Watertown, MA). For assessment of human colonies recovered from the BM of NOD/SCID mice that had transplantation, an internal amplification control was performed on each colony by using primers specific for the human β2-microglobulin gene as described53 (5′ CAGG-TTTACTCACGTCATCCAGC 3′; 5′ TCACATGGTTCACACGGCAGGC 3′; 232-bp product) and primers specific for the β-actin gene of human and mouse origin53 (5′ GTGACGAGG-CCCAGAGCAAGAG 3′; 5′ ACGCAGCTCATTGTAGAAGGTGTGG 3′; 123-bp product). Amplification conditions for β2-microglobulin PCR were as follows: 95°C for 1 minute, then 30 cycles at 95°C for 1 minute, 66°C for 20 seconds, and 72°C for 20 seconds, followed by extension at 72°C for 10 minutes. Amplification conditions for β-actin PCR were identical, except that only 24 cycles of amplification were done. Ten microliters of each PCR product was separated by electrophoresis on a 2% agarose gel and visualized in UV-light after staining with ethidium bromide.
Real-time quantitative PCR
DNA purified by using the QIAamp Blood Kit was digested with 30 units of RNAse A (Sigma, Deisenhofen). Triplicate samples of 5 μL of each DNA were used as templates in a duplex PCR (Kühlcke et al, unpublished data) by using the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems, Weiterstadt, Germany). In brief, primers mdr-f 5′ AGAAAGCGAAGCAGTGGTTCA 3′ and mdr-r 5′ CGAACTGTAGACAAACGATG-AGCTA 3′ amplified a 90-bp fragment from theMDR1 cDNA (reaction 1) that was detected by the FAM-labeled TaqMan probe mdr-p 5′ TGGTCCGACCTTTTCTGGCCTTATCCA 3′. Primers hck-f 5′ TATTAGCACCATCCATAGGAGGCTT 3′ and hck-r 5′ GTTAGGG-AAAGTGGAGCGGAAG 3′ amplified a 80-bp fragment from exon1 of the human hematopoietic cell kinase 1 gene54(reaction 2) that was detected by the VIC-labeled TaqMan probe hck-p 5′ TAACGCGTCCACCAAGGATGCGAA 3′. Amplification conditions were as follows: 50°C for 2.0 minutes, 95°C for 10 minutes, then 45 cycles at 95°C for 15 seconds and 60°C for 60 seconds. For each of the 2 reactions, the PCR cycle number that generated the first fluorescence signal above a threshold (the threshold cycle [CT]) was determined. The difference between the CT of reaction 1 and the CT of reaction 2 (the δCT value) was then used to quantitate the percentage of MDR1-transduced human cells with the help of a standard curve. To obtain a standard curve over 5 log, standards for vector copy number per cell were prepared by sorting variable numbers of MDR1-infected K562 cells that contained a single SF-MDR vector copy into a defined number of noninfected K562 cells. DNA isolated from these cell mixtures and from BM of engrafted mice were analyzed in triplicate in the real-time duplex PCR.
Statistical analysis
The Student t test was used to test for significance in each set of values, assuming equal variance. Mean values ± SE are given unless otherwise stated.
Results
In 8 experiments (8 patient samples), retroviral transduction of CD34+ PBPC was done for 96 hours with or without SCM in the presence of either IL-3 alone, IL-3/FL, IL-3/IL-6/SCF/FL, FL/TPO/SCF, or FL/TPO/VEGF by Transwell cocultivation cultures containing SF-MDR virus producer cells in the insert. We evaluated viral supernatant infection of CD34+ PBPC in 2 additional experiments (2 patient samples) by using the cytokine combinations IL-3/IL-6/SCF/FL or FL/TPO/SCF.
Aliquots of each transduced or mock-transduced CD34+ PBPC sample were plated into methylcellulose-based assays of colony-forming units to assess the efficiency of MDR1 gene transfer into lineage-committed progenitor cells. Other cell aliquots were cultivated for 10 days to determine P-glycoprotein expression in the myelomonocytic progeny of MDR1-transduced PBPC by means of efflux of the fluorescent dye Rh-123. This assay is characterized by a high accuracy and a lower variability than in results of CFC assays plated with cytostatic drugs.43 The remaining transduced and mock-transduced CD34+ PBPC were injected into the tail veins of sublethally irradiated NOD/SCID mice to determine the engraftment capacity of transduced CD34+ PBPC and the levels of gene transfer and expression. Results obtained with Transwell cocultivation are described immediately below. Supernatant data are compared with cocultivation data later.
Gene transfer into clonogenic progenitors
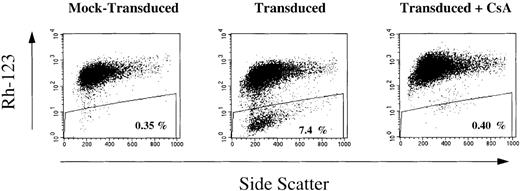
After Transwell transduction, the level of gene transfer into myeloid lineage-committed progenitors ranged from < 5% to 45% (mean, 22%) (Table 1). The proportion of Rh-123dull cells (MDR1-expressing cells) in the myelomonocytic progeny ranged from 0.1% to 7.1% (median, 4.3%). MDR1-transduced PBPC had a median 2-log reduction in the Rh-123 fluorescence intensity compared with mock-transduced control cells (example shown in Figure1). Addition of the P-glycoprotein inhibitor cyclosporine blocked the Rh-123 efflux from transduced cells (Figure 1), confirming that the Rh-123dull events were due to MDR1 gene expression.
MDR1 gene expression in the myelomonocytic progeny of CD34+ peripheral blood progenitor cells (PBPC) after a 96-hour Transwell transduction period in fibronectin-coated wells.
Aliquots of MDR1-transduced and mock-transduced CD34+ PBPC samples were cultured for 10 days in liquid medium containing a myeloid differentiation-inducing cytokine mixture. Rh-123 efflux in the progeny of MDR1-transduced and mock-transduced CD34+ PBPC was determined by fluorescence-activated cell separation (FACS) analysis without or with inclusion of the P-glycoprotein inhibitor cyclosporine (CsA).
MDR1 gene expression in the myelomonocytic progeny of CD34+ peripheral blood progenitor cells (PBPC) after a 96-hour Transwell transduction period in fibronectin-coated wells.
Aliquots of MDR1-transduced and mock-transduced CD34+ PBPC samples were cultured for 10 days in liquid medium containing a myeloid differentiation-inducing cytokine mixture. Rh-123 efflux in the progeny of MDR1-transduced and mock-transduced CD34+ PBPC was determined by fluorescence-activated cell separation (FACS) analysis without or with inclusion of the P-glycoprotein inhibitor cyclosporine (CsA).
Human-cell engraftment in NOD/SCID mice
The repopulating ability of CD34+ PBPC transduced by Transwellcocultivation was analyzed 5 to 13 weeks after transplantation in NOD/SCID mice. Cells recovered from the BM, spleen, and PB were analyzed for the presence of human CD45+ leukocytes. Mice with < 0.1% human CD45+ cells were considered to have nonengraftment.
The average percentage of human CD45+ cells was not statistically different in mice given transplants of 2 × 106transduced CD34+ cells and those given mock-transduced cells (values for both femurs, 18% ± 3% [n = 36] compared with 15% ± 5% [n = 8]; P = .7). There was also no difference in the absolute number of human CD45+ cells (3.0 ± 0.6 × 106 cells [n = 36] compared with 2.5 ± 1.0 × 106 cells [n = 8];P = .7) in these two groups. These data indicate that Transwell cocultivation with murine SF-MDR virus producer cells neither impaired the repopulating capacity of human PBPC cells nor induced a myeloproliferative state. A cell dose-dependent long-term engraftment of human leukocytes in NOD/SCID mice was apparent (Figure2). The findings with respect to the proportions of human leukocytes detected in the spleen and PB at the different graft sizes paralleled the BM results. There was no clear-cut relation between the proportion of human cells and time to analysis (data not shown). However, the individual groups may have been too small to show such a difference.
Cell dose-dependent engraftment of CD34+ PBPC in NOD/SCID mice (n = 63) after injection of Transwell-transduced or mock-transduced cells under various culture conditions.
The number of transplanted cells reflects the input CD34+ cell number (ie, after transduction, the cells were not recounted before xenotransplantation). The proportions of human CD45+ leukocytes in bone marrow ( ), spleen (□), and peripheral blood (▪) determined by FACS are shown as mean ± SE values. In each experiment, normal human PB and mice not given transplants were analyzed as controls. Comparison of human-cell engraftment between mice injected with 5 × 105 CD34+ cells after retroviral transduction in the presence of IL-3 or IL-3/FL yieldedP = .01 (**). The 3 denotes IL-3; 6, IL-6; FL, Flt3-ligand; T, thrombopoietin; S, stem-cell factor; and V, vascular epithelial growth factor.
), spleen (□), and peripheral blood (▪) determined by FACS are shown as mean ± SE values. In each experiment, normal human PB and mice not given transplants were analyzed as controls. Comparison of human-cell engraftment between mice injected with 5 × 105 CD34+ cells after retroviral transduction in the presence of IL-3 or IL-3/FL yieldedP = .01 (**). The 3 denotes IL-3; 6, IL-6; FL, Flt3-ligand; T, thrombopoietin; S, stem-cell factor; and V, vascular epithelial growth factor.
Cell dose-dependent engraftment of CD34+ PBPC in NOD/SCID mice (n = 63) after injection of Transwell-transduced or mock-transduced cells under various culture conditions.
The number of transplanted cells reflects the input CD34+ cell number (ie, after transduction, the cells were not recounted before xenotransplantation). The proportions of human CD45+ leukocytes in bone marrow ( ), spleen (□), and peripheral blood (▪) determined by FACS are shown as mean ± SE values. In each experiment, normal human PB and mice not given transplants were analyzed as controls. Comparison of human-cell engraftment between mice injected with 5 × 105 CD34+ cells after retroviral transduction in the presence of IL-3 or IL-3/FL yieldedP = .01 (**). The 3 denotes IL-3; 6, IL-6; FL, Flt3-ligand; T, thrombopoietin; S, stem-cell factor; and V, vascular epithelial growth factor.
), spleen (□), and peripheral blood (▪) determined by FACS are shown as mean ± SE values. In each experiment, normal human PB and mice not given transplants were analyzed as controls. Comparison of human-cell engraftment between mice injected with 5 × 105 CD34+ cells after retroviral transduction in the presence of IL-3 or IL-3/FL yieldedP = .01 (**). The 3 denotes IL-3; 6, IL-6; FL, Flt3-ligand; T, thrombopoietin; S, stem-cell factor; and V, vascular epithelial growth factor.
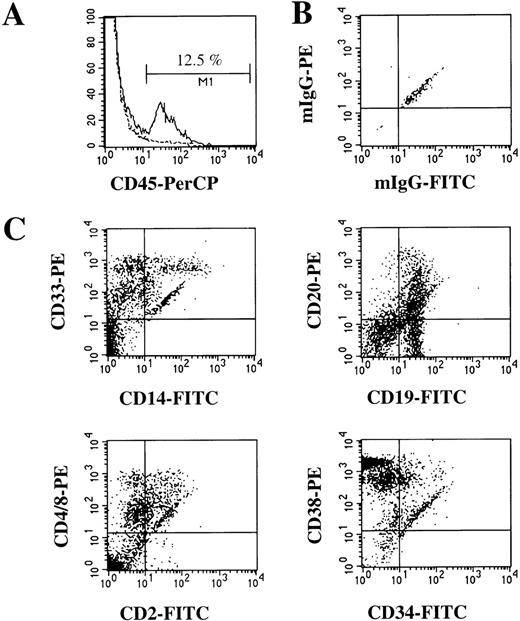
Human-cell engraftment was similar in the different cytokine groups (Figure 2). The addition of SCM during the transduction did not result in higher levels of human cells (data not shown). The multilineage engraftment was similar in MDR1-transduced and mock-transduced CD34+ PBPC and in the different cytokine groups. Human multilineage hematopoiesis in the BM of mice (Figure 3) consisted of myeloid maturation stages (CD33+, 47% ± 5%), monocytes (CD14+, 14% ± 4%), B lymphocytes (CD19+, 15% ± 3%), T lymphocytes and natural killer cells (CD2+, 13% ± 2%) and primitive progenitors (CD34+, 8% ± 2%).
Human multilineage engraftment in the bone marrow (BM) of chimeric NOD/SCID mice.
(A) Histogram of expression of human panleukocyte marker CD45 in BM cells of a mouse given a transplant of human CD34+ PBPC (solid line) and a control mouse (no transplant) (dotted line). (B) Isotype control for nonspecific IgG staining of CD45+ cells shown in (A). (C) Further analysis of human CD45+ cells for the presence of myeloid and monocytic cells (CD33 and CD14), B cells (CD19 and CD20), T cells (CD2 and CD4/8), and immature progenitor cells (CD34 and CD38).
Human multilineage engraftment in the bone marrow (BM) of chimeric NOD/SCID mice.
(A) Histogram of expression of human panleukocyte marker CD45 in BM cells of a mouse given a transplant of human CD34+ PBPC (solid line) and a control mouse (no transplant) (dotted line). (B) Isotype control for nonspecific IgG staining of CD45+ cells shown in (A). (C) Further analysis of human CD45+ cells for the presence of myeloid and monocytic cells (CD33 and CD14), B cells (CD19 and CD20), T cells (CD2 and CD4/8), and immature progenitor cells (CD34 and CD38).
Detection of SF-MDR–marked human cells in BM and spleen of chimeric NOD/SCID mice
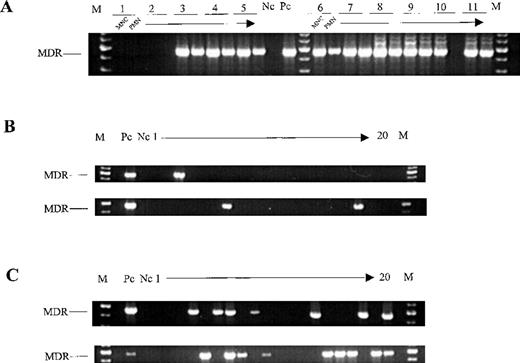
A qualitative nested PCR allowed detection of proviral DNA in all whole BM samples (n = 29) isolated from long-term engrafted NOD/SCID mice given ≥ 1 × 106 CD34+ PBPC per graft (Table2). Human cells containing provirus were detected in the spleen in 68% of the animals (n = 22; Table 2). Additionally, BM cell aliquots from 31 mice were separated by Ficoll density-gradient centrifugation into MNC and polymorphonuclear cells or granulocytes (example shown in Figure4A). Because granulocytes have a short lifespan, detection of SF-MDR provirus DNA in the granulocyte cell fraction 5 to 13 weeks after transplantation indicates that repopulating cells with high proliferative potential are gene marked.55 In 68% of the mice, the SF-MDR provirus was detected in the BM MNC fraction and the BM granulocyte fraction, whereas 26% of the animals had PCR-positive MNC with a PCR-negative granulocyte fraction. The BM in this 26% may have harboredMDR1-transduced repopulating progenitor cells with a lower proliferative potential. Two engrafted mice in which only the BM MNC and granulocyte fractions but not genomic DNA from unseparated BM were analyzed did not have gene-marked cells in either the MNC or the granulocyte fraction. An SF-MDR proviral signal in the granulocyte fraction alone was not observed.
Detection of SF-MDR provirus in chimeric NOD/SCID mice into which MDR1-transduced human CD34+ PBPC were transplanted
| Transduction Group . | NOD/ SCID Set No* . | Human CD45 Cells in BM (%) . | No. of MDR1-PCR+ Mice with SF-MDR/No. Mice Assessed . | |||
|---|---|---|---|---|---|---|
| BM . | Spleen . | BM MNC . | BM PMN . | |||
| Transwell | ||||||
| IL-3 | 3, 4 | 2 ± 1 | NA | NA | 3/4 | 3/4 |
| IL-3 | 5, 7 | 33 ± 11 | 5/5 | 2/2 | 2/2 | 2/2 |
| IL-3 + SCM | 1, 7 | 30 ± 12 | 3/3 | 3/3 | 4/4 | 3/4 |
| IL-3/FL | 3, 4 | 12 ± 6 | NA | NA | 4/4 | 3/4 |
| IL-3/FL | 5, 6, 7, 8 | 15 ± 5 | 12/12 | 4/8 | 8/8 | 4/8 |
| IL-3/FL + SCM | 1, 2, 7 | 23 ± 4 | 1/1 | 0/1 | 3/4 | 2/2 |
| IL-3/IL-6/SCF/FL | 8 | 12 ± 1 | 2/2 | 2/2 | 2/2 | 1/2 |
| FL/T/SCF | 8 | 8 ± 3 | 3/3 | 3/3 | 3/3 | 3/3 |
| FL/T/V | 7 | 1 ± 0.3 | 3/3 | 1/3 | NA | NA |
| Cell-free viral supernatant | ||||||
| IL-3/IL-6/SCF/FL | 10 | 7 ± 1 | 2/2 | NA | 2/2 | 2/2 |
| FL/T/SCF | 10 | 10 ± 3 | 3/3 | NA | 3/3 | 3/3 |
| FL/T/SCF | 9 | 33 ± 7 | 4/4 | NA | NA | NA |
| Transduction Group . | NOD/ SCID Set No* . | Human CD45 Cells in BM (%) . | No. of MDR1-PCR+ Mice with SF-MDR/No. Mice Assessed . | |||
|---|---|---|---|---|---|---|
| BM . | Spleen . | BM MNC . | BM PMN . | |||
| Transwell | ||||||
| IL-3 | 3, 4 | 2 ± 1 | NA | NA | 3/4 | 3/4 |
| IL-3 | 5, 7 | 33 ± 11 | 5/5 | 2/2 | 2/2 | 2/2 |
| IL-3 + SCM | 1, 7 | 30 ± 12 | 3/3 | 3/3 | 4/4 | 3/4 |
| IL-3/FL | 3, 4 | 12 ± 6 | NA | NA | 4/4 | 3/4 |
| IL-3/FL | 5, 6, 7, 8 | 15 ± 5 | 12/12 | 4/8 | 8/8 | 4/8 |
| IL-3/FL + SCM | 1, 2, 7 | 23 ± 4 | 1/1 | 0/1 | 3/4 | 2/2 |
| IL-3/IL-6/SCF/FL | 8 | 12 ± 1 | 2/2 | 2/2 | 2/2 | 1/2 |
| FL/T/SCF | 8 | 8 ± 3 | 3/3 | 3/3 | 3/3 | 3/3 |
| FL/T/V | 7 | 1 ± 0.3 | 3/3 | 1/3 | NA | NA |
| Cell-free viral supernatant | ||||||
| IL-3/IL-6/SCF/FL | 10 | 7 ± 1 | 2/2 | NA | 2/2 | 2/2 |
| FL/T/SCF | 10 | 10 ± 3 | 3/3 | NA | 3/3 | 3/3 |
| FL/T/SCF | 9 | 33 ± 7 | 4/4 | NA | NA | NA |
Bone marrow (BM) and spleen cells were recovered from chimeric NOD/SCID mice 5 to 13 weeks after transplantation. The proportion of human CD45+ cells was determined by fluorescence-activated cell separation. Qualitative PCR for the presence of the SF-MDR provirus was done on either genomic DNA purified from BM and spleen or on mononuclear cells (MNC) or polymorphonuclear (PMN) cells isolated by Ficoll density-gradient separation of whole BM. In all experiments, BM cells or genomic DNA from NOD/SCID mice not given transplanted cells and from control mice that had mock-transduced CD34+ PBPC transplantation was concurrently analyzed to verify the specificity of the PCR reactions for the provirus.
Plus-minus values are mean ± SE.
CD34+ PBPC transplanted per mouse: 1 × 106 for set 3 and 4; 2 × 106 for sets 1, 2, 5, 6, 7, 8, and 10; and 4 × 106 for set 9.
Detection of SF-MDR provirus in cells obtained from chimeric NOD/SCID mice.
(A) PCR analysis of the mononuclear cell (MNC) fraction and the granulocyte (polymorphonuclear; PMN) fractions of BM recovered from 11 mice (NOD/SCID set 7) engrafted with mock-transduced (1 and 2) orMDR1-transduced (3-11) CD34+ PBPC. (B) Detection of the SF-MDR proviral genome in individual colonies derived from 2 chimeric mice (NOD/SCID sets 2 and 8) engrafted with Transwell-transduced CD34+ PBPC. (C) Detection of the SF-MDR proviral genome in individual colonies derived from 2 chimeric mice (NOD/SCID set 10) engrafted with supernatant-transduced CD34+ PBPC. M denotes molecular-weight size marker; Nc, no-template PCR control; and Pc, SF-MDR producer cells diluted 10−5-fold in provirus-negative HL-60 cells.
Detection of SF-MDR provirus in cells obtained from chimeric NOD/SCID mice.
(A) PCR analysis of the mononuclear cell (MNC) fraction and the granulocyte (polymorphonuclear; PMN) fractions of BM recovered from 11 mice (NOD/SCID set 7) engrafted with mock-transduced (1 and 2) orMDR1-transduced (3-11) CD34+ PBPC. (B) Detection of the SF-MDR proviral genome in individual colonies derived from 2 chimeric mice (NOD/SCID sets 2 and 8) engrafted with Transwell-transduced CD34+ PBPC. (C) Detection of the SF-MDR proviral genome in individual colonies derived from 2 chimeric mice (NOD/SCID set 10) engrafted with supernatant-transduced CD34+ PBPC. M denotes molecular-weight size marker; Nc, no-template PCR control; and Pc, SF-MDR producer cells diluted 10−5-fold in provirus-negative HL-60 cells.
Quantification of in vivo gene-marked human cells
Because the nested PCR analysis we used allowed us to determine only whether the engrafted mice contained levels of gene-marked human cells above our detection limit (> .001%), we developed a real-time duplex PCR assay for quantification of vector-marked human cells in genomic DNA from whole BM of engrafted mice. This assay allowed continuous monitoring of 2 amplification products simultaneously during the PCR—one amplicon from the vector-derived MDR1 cDNA and one from the human hematopoietic cell kinase (HCK) gene54—for normalization of the variable content of human DNA (internal standard) in each BM sample.
The proportions of gene-marked human cells in the BM of 29 engrafted NOD/SCID mice are shown in Table 3. Five to 13 weeks after transplantation, gene-marked cells were detected within the human-cell population at average levels ranging from 0.9% to 30%. Six animals (21%) had MDR1 gene-transfer percentages of between 10% and 30% of engrafted human cells. A respectiveMDR1-engraftment proportion ranged from ≥ 1% to < 10% in 11 animals (38%) and from ≥ 0.1% to < 1% in 10 animals (34%).MDR1 marking of < 0.1% of engrafted human cells occurred in only 2 animals (7%). Substantially higher average proportions of vector-marked human cells were detected in the BM of mice given transplants of CD34+ PBPC transduced in the presence of IL-3/IL-6/SCF/FL or FL/TPO/SCF. Mice in the IL-3/IL-6/SCF/FL group had 2.9-fold (P = .03), 4.4-fold (P = .01), and 13.5-fold (P = .003) more vector-marked human cells, respectively, than those in the IL-3 group with or without SCM, the IL-3/FL group, or the FL/TPO/VEGF group. Similarly, engrafted mice in the FL/TPO/SCF group had on average 3.9-fold (P = .02), 5.9-fold (P = .003), and 18.3-fold (P = .08) higher proportions of gene-marked human cells. There were no significant differences between mice in the IL-3/IL-6/SCF/FL group and those in the FL/TPO/SCF group (P = .7).
MDR1 provirus–marked human cells detected in BM of chimeric mice by quantitative real-time PCR and by end point PCR on single colonies
| Transduction Group . | NOD/SCID Set No. . | MDR1 Provirus-Marked Human Cells in BM (%) . | MDR1-PCR+ Human ColonyForming Cells (%) . |
|---|---|---|---|
| Transwell | |||
| IL-3 | 3, 4 | NA | No DNA |
| IL-3 | 5, 7 | 4 ± 2 | NA |
| IL-3 + SCM | 1, 7 | 5 ± 1 | 5 |
| IL-3/FL | 3, 4 | NA | No DNA |
| IL-3/FL | 5, 6, 7, 8 | 3 ± 1 | NA |
| IL-3/FL + SCM | 1, 2, 7 | 20 | 2 ± 2 |
| IL-3/IL-6/SCF/FL | 8 | 12 ± 2 | NA |
| FL/T/SCF | 8 | 17 ± 7 | 10 |
| FL/T/V | 7 | 1 ± 1 | NA |
| Cell-free viral supernatant | |||
| IL-3/IL-6/SCF/FL | 10 | 11 ± 3 | 23 ± 3 |
| FL/T/SCF | 10 | 38 ± 12 | 32 ± 9 |
| FL/T/SCF | 9 | 20 ± 6 | 16 ± 6 |
| Transduction Group . | NOD/SCID Set No. . | MDR1 Provirus-Marked Human Cells in BM (%) . | MDR1-PCR+ Human ColonyForming Cells (%) . |
|---|---|---|---|
| Transwell | |||
| IL-3 | 3, 4 | NA | No DNA |
| IL-3 | 5, 7 | 4 ± 2 | NA |
| IL-3 + SCM | 1, 7 | 5 ± 1 | 5 |
| IL-3/FL | 3, 4 | NA | No DNA |
| IL-3/FL | 5, 6, 7, 8 | 3 ± 1 | NA |
| IL-3/FL + SCM | 1, 2, 7 | 20 | 2 ± 2 |
| IL-3/IL-6/SCF/FL | 8 | 12 ± 2 | NA |
| FL/T/SCF | 8 | 17 ± 7 | 10 |
| FL/T/V | 7 | 1 ± 1 | NA |
| Cell-free viral supernatant | |||
| IL-3/IL-6/SCF/FL | 10 | 11 ± 3 | 23 ± 3 |
| FL/T/SCF | 10 | 38 ± 12 | 32 ± 9 |
| FL/T/SCF | 9 | 20 ± 6 | 16 ± 6 |
Real-time PCR was performed on genomic BM DNA to determine the percentage of provirus-marked human cells. In all experiments, genomic DNA from NOD/SCID mice not given transplanted cells and from control mice that had mock-transduced CD34+ PBPC transplantation was concurrently analyzed to verify the specificity of the PCR reactions for the provirus. BM cells from chimeric mice were plated in semisolid colony assays specific for human progenitors. Individual human-cell colonies derived from cultures under nonselective conditions were plucked and subjected to a nested PCR analysis of SF-MDR proviral DNA.
Values are mean ± SE unless otherwise indicated.
Recovery of transduced and drug-resistant human CFC from chimeric NOD/SCID mice
Unselected human clonogenic progenitor cells recovered from the BM of NOD/SCID mice given transplants were analyzed with a provirus-specific PCR. Human colonies with amplifiable DNA were obtained from 5 of 13 chimeric-mouse BM samples that had Transwell transduction and analysis (Table 3). Three of the 5 BM samples contained provirus-marked colonies (examples shown in Figure 4B). This suggests MDR1 gene-transfer rates to repopulating human progenitor cells of 5% and 10%, respectively, which is comparable to the proportion of gene-marked human cells detected in genomic DNA from the respective whole BM samples. The other 8 analyzed chimeric-mouse BM samples yielded colonies that were negative in PCR amplification controls that used primers specific for either the human β2-microglobulin gene or the actin gene of human and mouse origin. Of 40 chimeric-mouse BM samples that contained human cells capable of forming colonies in human-specific CFC assays under nonselective conditions, 4 BM samples (data not shown) gave rise to human-cell colonies resistant to a concentration of 20 nmol/L of vincristine (6-fold higher than the median inhibitory concentration [IC50]). No colonies of human vincristine-resistant cells were obtained from chimeric-mouse BM samples derived from control mice given mock-transduced CD34+ cells.
Retroviral transduction on CH-296 with cell-free viral supernatant
We analyzed viral supernatant infection of CD34+ PBPC by using the cytokine combinations IL-3/IL-6/SCF/FL and FL/TPO/SCF because they provided efficient gene transfer into long-term repopulating cells in our Transwell cocultivation protocol. Results in the supernatant transduction group are shown in Tables 1 to 3 (patient samples 9 and 10). A notable finding was that after transduction in FL/TPO/SCF, the proportion of Rh-123dull cells in the myeloid progeny of supernatant-transduced CD34+ PBPC compared with Transwell cocultivation-transduced CD34+ PBPC was increased 2.6-fold (P = .01; Table 1). The gene-transfer rate into clonogenic myeloid progenitors (Table 1), the level of human-cell engraftment (Table 2), the proportion of vector-marked human cells (Table 3), and the proportion of vector-marked human-cell colonies (Table 3; examples shown in Figure 4C) were similar in the supernatant and Transwell transduction groups. These findings suggest that our Transwell cocultivation data may be transferable to the more clinically relevant supernatant-transduction setting.
Analysis of gene expression in chimeric mice
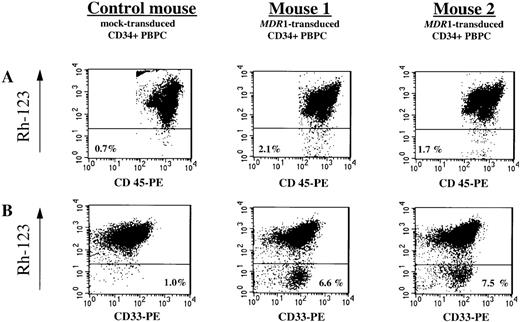
The proportion of human Rh-123dull(MDR1-expressing) cells in human CD45+ leukocytes in unseparated whole BM of chimeric NOD/SCID mice was assessed by flow cytometry (Figure 5A). In 23 of 36 analyzed engrafted mice that had received CD34+ PBPC transduced by either Transwell cocultivation or viral supernatant infection, Rh-123dull cells in the human-cell population were detectable at low levels; the range was from 0.2% to 3.1% above the level of human CD45+/Rh-123dull cells detected in the control mice given mock-transduced CD34+ cells. A paired analysis comparing the average proportion of human CD45+/Rh-123dullcells (0.5% ± 0.1%; n = 28) with the average proportion of vector-marked human cells (10.4% ± 2.6%; n = 28) revealed that, on average, 5% of the vector-marked human cells expressed theMDR1 transgene.
Flow cytometry analysis of MDR1 gene expression in chimeric mice.
(A) Five weeks after transplantation of MDR1-transduced and mock-transduced CD34+ PBPC samples (NOD/SCID set 10), chimeric-mouse BM cells were obtained and analyzed for the presence of human leukocytes (CD45+) and MDR1 expression (Rh-123 efflux) by FACS after staining with rhodamine-123 (Rh-123), phycoerythrin (PE)-conjugated antihuman CD45 antibody and propidium iodide (PI). The proportions of live-gated viable human CD45+ cells expressing theMDR1 gene (Rh-123dull cells) that were detectable in 2 representative mice injected with transduced CD34+ PBPC and in a control mouse into which mock-transduced CD34+ PBPC was transplanted are shown. (B) MDR1 expression in the in vitro myeloid progeny of human cells recovered from the corresponding chimeric mice (upper panel). Human cells selectively enriched from freshly isolated chimeric-mouse BM cells by immunomagnetic depletion of the mouse BM cells were subjected to a 10-day liquid culture in the presence of a myeloid differentiation-inducing cytokine mixture and subsequently analyzed by FACS after staining with Rh-123, PE-conjugated antihuman CD33 antibody, and PI. The proportions of human myeloid (CD33+) Rh-123dull cells are indicated in the right corner of each plot.
Flow cytometry analysis of MDR1 gene expression in chimeric mice.
(A) Five weeks after transplantation of MDR1-transduced and mock-transduced CD34+ PBPC samples (NOD/SCID set 10), chimeric-mouse BM cells were obtained and analyzed for the presence of human leukocytes (CD45+) and MDR1 expression (Rh-123 efflux) by FACS after staining with rhodamine-123 (Rh-123), phycoerythrin (PE)-conjugated antihuman CD45 antibody and propidium iodide (PI). The proportions of live-gated viable human CD45+ cells expressing theMDR1 gene (Rh-123dull cells) that were detectable in 2 representative mice injected with transduced CD34+ PBPC and in a control mouse into which mock-transduced CD34+ PBPC was transplanted are shown. (B) MDR1 expression in the in vitro myeloid progeny of human cells recovered from the corresponding chimeric mice (upper panel). Human cells selectively enriched from freshly isolated chimeric-mouse BM cells by immunomagnetic depletion of the mouse BM cells were subjected to a 10-day liquid culture in the presence of a myeloid differentiation-inducing cytokine mixture and subsequently analyzed by FACS after staining with Rh-123, PE-conjugated antihuman CD33 antibody, and PI. The proportions of human myeloid (CD33+) Rh-123dull cells are indicated in the right corner of each plot.
The Rh-123 efflux assay with freshly isolated BM cells may have underestimated the proportion of vector-marked human cells expressing the MDR1 transgene, since transducedMDR1-expressing CD34+ cells may be masked by their nontransduced human-cell counterparts, which already display moderate levels of endogenous P-glycoprotein expression.6,56 57 To test this hypothesis, we cultured cells with a myeloid differentiation-inducing cytokine mixture that contained SCF, IL-1βbL IL-3, IL-6, GM-CSF, and G-CSF. Human CD45+ cells were enriched by immunomagnetic depletion of the mouse BM cells from BM samples from 5 chimeric animals (NOD/SCID set 10) that had received either MDR1-transduced (n = 4) or mock-transduced (n = 1) CD34+ PBPC. We then cultured 1 × 105 enriched human BM cells for 10 days to determine MDR1 expression in the human myelomonocytic progeny. Dual staining of the cells with Rh-123 and an antihuman CD33 antibody (Figure 5B) showed 2- to 6.5-fold higher levels of up to 6.5% human CD33/Rh-123dull cells.MDR1-expressing cells became more clearly detectable. In side-by-side comparisons, the proportion of MDR1-expressing human cells to MDR1-transduced human cells increased from 6% ± 2% (n = 4) to 18% ± 4% (n = 4; P = .03) after liquid culture.
Discussion
In this study, we showed engraftment in NOD/SCID mice ofMDR1-transduced and P-glycoprotein–expressing PBPC from patients with cancer. We achieved gene-transfer efficiencies of up to 55% in clonogenic progenitors, values similar to those reported by other groups,58-60 either by Transwell cocultivation of CD34+ PBPC with vector-producing cells or by adding viral supernatant. To analyze the engraftment capacity of transduced CD34+ PBPC and to document gene transfer and expression in human cells with in vivo repopulating potential, sublethally irradiated NOD/SCID mice were given transplants of 0.5 × 106 to 4.0 × 106 input CD34+ cells. A cell dose-dependent multilineage engraftment of up to an average of 33% human leukocytes (Table 2 and Figure 2) was observed. Differences in BM cellularity and in the proportions of human CD45+ or CD33+/CD45+ leukocytes, respectively, were not observed in NOD/SCID mice given transplants ofMDR1-transduced or mock-transduced CD34+ PBPC. These data suggest that the SF-MDR vector is not toxic to SRC and does not induce a myeloproliferative state under our culture conditions and observation period of 5 to 13 weeks.61
What factors were responsible for the high level of human-cell engraftment in NOD/SCID mice? The data presented here are consistent with levels of human-cell engraftment reported by Hogan et al62 for freshly isolated CD34+ PBPC from patients with cancer that were transplanted into NOD/SCID mice in comparable graft sizes and with similar NOD/SCID mouse-conditioning procedures, including intraperitoneal injections of antiasialo GM1 antibodies and administration of cytokines. In contrast to these results, significantly lower levels of human-cell engraftment in NOD/SCID mice were reported by van der Loo et al63 with similar graft sizes of fresh CD34+ PBPC. Factors such as the radiation dose62or the treatment of mice with antiasialo GM-163 may account for these differences. In paired comparisons, Hogan et al62 showed that the average engraftment of human CD34+ PBPCs was independent of supplementation with human cytokines, as was previously shown for cord blood CB CD34+ cells.64,65 In contrast, 2 other groups reported that cotransplantation of an irradiated human stromal cell line secreting a number of cytokines (eg, SCF, G-CSF, GM-CSF, and IL-6) or a human IL-3–expressing rat fibroblast cell line into NOD/SCID or SCID mice, respectively, enhanced engraftment of both fresh and cultivated CD34+ PBPC.66 67Because of these contradictory data, we administered recombinant IL-3 and G-CSF to the mice in our study.
Levels of human-cell engraftment in NOD/SCID mice similar to those we observed were reported after retroviral infection over comparably short-term cultivation periods, with use of MNC or CD34-selected cord blood cells (or both),31,68,69 which have a higher engraftment potential in NOD/SCID mice.35 A similar potential of CD34+ PBPC from healthy donors sorted after transduction to engraft human fetal-bone grafts in SCID mice (SCID-human bone assay) has been described.70
We previously observed an approximately 4-fold reduction in the repopulating ability of fresh, noncultured CD34+ PBPC after retroviral transduction in IL-3 alone.32,71 In other studies, SRC derived from cord blood were expanded 2- to 4-fold, but at the expense of a decline of their proliferative potential over time.72,73Others have also found that cytokine-supported short-term retroviral transduction of BM CD34+ cells or CD34+ PBPC in the absence of supporting BM stromal cells resulted in a severe reduction or loss of the ability to efficiently engraft and repopulate for the long term (> 6 months) the BM of immunodeficient BNX mice.46,74Data from the same group75 proved that adhesion to the recombinant fibronectin fragment CH-296 through engagement of VLA-4 and VLA-5 integrins during ex vivo culture was as effective as BM stromal cells in supporting the long-term engraftment potential of human HSC/HPC. This is consistent with the report by Gothot et al38 that uncultured CD34+ PBPC residing in G1phase or unfractionated CD34+ cells after a short-term cultivation (36 hours) in the absence of fibronectin or stroma contact had a severely reduced repopulating capacity in comparison to that of fresh G0 CD34+ cells, independent of the type of cytokines used. Thus, the use of fibronectin-coated culture dishes in conjunction with administration of antiasialo GM1 and human cytokines may have been important in the high level of human-cell engraftment in our study. Interestingly, we observed a high engraftment of vector-marked human cells (Table 3), which suggests a rescuing effect of fibronectin on repopulating cells that traversed the cell cycle. However, fibronectin support in culture does not provide complete maintenance of engraftment capacity, as shown by a 50% reduction in human cord blood CD34+ cell engraftment in NOD/SCID mice after 24-hour culture in the presence of fibronectin and cytokines, including FL (reviewed by Lyman and Jacobsen76).77
We were able to detect integration of the SF-MDR provirus in 96% (48 of 50) of analyzed BM samples (whole BM or Ficoll MNC fraction of BM cells) from chimeric mice, and provirus-marked granulocytes were present in 72% (26 of 36) of mice analyzed (Table 2). The development of a quantitative real-time duplex PCR technique allowed us to monitor the proportion of MDR1-transduced human cells in the BM of chimeric mice. We observed no difference in the level of SF-MDR chimerism between Transwell transduction and viral supernatant transduction in the presence of IL-3/IL-6/SCF/FL or FL/TPO/SCF, respectively. The average proportion of vector-marked human cells in mice ranged from 1% to 38% (Table 3), indicating that these mice were engrafted with one or more transduced SRC. Comparable rates (range, < 5% to 45%) of vector-marked human CFC derived from corresponding individual chimeric-mouse BM samples were found (Table 3), confirming the results obtained with the real-time duplex PCR technique.
Encouraging, clinically relevant gene-transfer rates of 10% to 20% of in vivo repopulating cells present in mobilized PB of 2 different nonhuman primates (rhesus macaques and baboons) have been achieved.78,79 In these 2 studies, both the target cell population (CD34+ PBPC) and the short-term retroviral transduction protocols were similar to those in our study. Therefore, our data are the first to indicate that the NOD/SCID model is a valid assay for measuring gene transfer to repopulating human HSC obtained from mobilized PB and that it is comparable to autologous transplantation in large-animal models. Furthermore, our results support the possibility that vector-marked human cells capable of multilineage hematopoiesis in a murine xenograft may also contribute to in vivo hematopoiesis in humans. This reinforces the relevance of murine in vivo surrogate assays for developing novel vector systems and optimizing clinically applicable protocols for the transduction and ex vivo expansion of primitive human hematopoietic cells. However, direct evidence for this theory can be obtained only by parallel transplantation of transduced CD34+ PBPC into patients and immunodeficient mice. Moderate to high levels of gene transfer to primitive in vivo repopulating human CD34+ cells in cord blood have been observed in studies in NOD/SCID mice.68,69 80 However, no comparable cord blood studies in nonhuman primates or humans have been published to validate these data.
Although we observed no significantly different levels of BM chimerism with human cells for the various cytokine combinations, transduction in the presence of either IL-3/IL-6/SCF/FL or FL/TPO/SCF (supernatant group and Transwell group combined) resulted on average in 3-fold (mean, 12%; n = 4; P < .01) and 6-fold (mean, 24%; n = 10; P < .01) higher proportions of in vivo vector-marked human cells, compared with the mean level of gene-marked human cells observed with IL-3 alone (mean, 4%; n = 5; Table 3). These results suggest that in the presence of IL-3/IL-6/SCF/FL or FL/TPO/SCF, more cells traversed from G0/G1into the S/G2/M phase. In support of this interpretation, Luens et al81 found that only 25% of BM-derived CD34+ cells cultured in FL/TPO/SCF remained undivided until day 4 of culture, compared with a proportion of 88% undivided cells in the presence of IL-3/IL-6/leukemia inhibitory factor, while the engraftment potential (SCID-human bone assay) of the first cell population was retained. Together, these studies show that in vivo repopulating cells can be transduced by recombinant murine retroviral vectors during ex vivo culture periods of 72 hours to 168 hours in the presence of cell-cycle activating cytokines. Incubation with cytokines, in many instances, is associated with some loss of repopulating capacity.
Vector strategies to overcome this limitation have been proposed. Rebel et al77 showed efficient gene transfer into SRC (∼ 25% vector-marked human CFC) by a 1-day cytokine-supported ex vivo culture. The culture used a retroviral MDR1 vector based on a vesicular stomatitis virus G (VSV-G)–pseudotyped MLV. Lentiviral gene-transfer vectors82,83 that are capable of transducing truly quiescent nondividing cells are likely to further improve the functional quality of transduced human HSC/HPC populations. Recently, Miyoshi et al84 reported gene transfer (∼ 30% vector-marked human CFC) to cord blood–derived NOD/SCID repopulating cells mediated by a VSV-G–pseudotyped lentiviral vector within 30 hours of culture in the absence of exogenous cytokines.
A high and sustained level of MDR1 expression in hematopoietic progenitor and differentiated progeny cells is important in providing protection from pancytopenia, specifically from granulocytopenia, a major cause of the morbidity and mortality associated with high-dose chemotherapy. The proportion of MDR1-expressing cells (Rh-123dull cells) in the myeloid in vitro progeny of SF-MDR1–transduced CD34+ PBPC, relative to the proportion of vector-marked CFU-GM progenitor cells, averaged 33% ± 6% before transplantation.
In NOD/SCID mice that received only the human cytokines IL-3 and G-CSF to support engraftment and differentiation of MDR1-transduced human PBPC, MDR1 expression in transduced cells was masked by a significant proportion of naturally Rh-123dull cells (eg, CD34+ cells, 8% ± 2%).6,56,57 When myelomonocytic differentiation of these cells was induced in our cultures, a clearly resolvable Rh-123dull population of 18% ± 4% of transduced cells became apparent (Figure 5B). In human-CFC assays containing vincristine, resistant colonies were observed in only a small number of experiments. This may have been due to the very high concentrations of vincristine (6-fold the IC50) we used. Eckert et al45 reported a plating efficiency of 15% to 20% of SF-MDR1–transduced (PCR-positive) CFC at 3-fold the IC50 of paclitaxel, compared with plating efficiencies of 50% at 1.5-fold the IC50 drug dose. The proportion ofMDR1-expressing cells was probably diminished by aberrant splicing of the human wild-type MDR1 cDNA in transduced human cells that led to variable levels of functional P-glycoprotein or transduction of variable numbers of CD34+ PBPC target cells with nonfunctional, truncated SF-MDR provirus copies. Cryptic splice sites within the wild-type MDR1 gene have been shown to cause aberrant splicing of up to 50% of the vector-derived messenger RNA in human hematopoietic cells and murine virus producer cells.85-87 Finally, transcriptional silencing through down-regulation of long-terminal repeat–mediated MDR1 gene expression in differentiated cells of multiple lineages may have further contributed to the low resistance to vincristine.
The expression rate of transgenes from different vectors and the lineage specificity of the expression needs careful consideration. The average frequencies of G418-resistant human CFC (range, 2%-18%) or the green fluorescent protein (GFP)-expressing human CD45 cells (range, 1%-27%) recovered from the BM of NOD/SCID mice reported by Conneally et al80 and Miyoshi et al84 were higher than the frequency of Rh-123dull cells in our study. Similar differences in the mean proportion of vector-marked human cells relative to the mean proportion of transgene-expressing human cells can be deduced from their data. In a study with a vector based on a murine stem-cell virus,88 an average of 28% of the gene-marked human CFC (9% compared with 32%) were resistant to G418. This indicates that aberrant splicing of the MDR1 gene may result in an approximately 40% reduction of MDR1 expression (18% compared with 28%). In the study with a lentiviral vector by Miyoshi et al,84 82% of the gene-marked human CFC (27% compared with 33%) but only 21% of the human CD45+ cells (7% compared with 33%) expressed the GFP gene. In fact, 20% of the lymphoid CD19+ B cells were positive for GFP, in contrast to only 3% of the monocytic CD14+ cells, indicating the occurrence of transcriptional silencing of the cytomegalovirus promotor in myeloid differentiated human hematopoietic cells. Van Hennik et al69 obtained vector-transduced cord blood CD34+ cells in NOD/SCID mice with SF-EGFP, a vector that is identical to our SF-MDR vector, except for the transgene. They found a median proportion of 23%GFP-expressing human CD45+ cells (range, 2% to 41%) and comparable high transgene expression in differentiated cells of multiple lineages. PCR data were not presented in their study. These data clearly highlight the advantages of FMEV-based retroviral vectors over conventional MLV-based vectors and possibly over current lentiviral vectors for gene delivery to human HSC/HPC and their myeloid progeny, respectively. These data support the idea that aberrant splicing of the human wild-type MDR1 cDNA rather than the vector backbone caused the reduced numbers of Rh-123dullcells compared with vector-transduced cells.
Translated to a clinical setting, our SF-MDR vector could be expected to transduce up to 38% ± 12% of human long-term repopulating cells (Table 3). At an MDR1 expression rate of 18% above background levels, a proportion of 7% transduced progeny cells may be protected from drug-induced apoptosis. This assumption is based on the percentage of Rh-123dull cells we found; in vivo drug selection was not measured. Normal granulocyte counts in the PB are up to 7.7 × 109/L. In an optimal setting, at the calculated P-glycoprotein–expression rate, granulocyte counts of patients given transplants of MDR1-transduced PBPC may not fall below the threshold of 0.5 × 109/L, the hallmark of bone marrow aplasia,1 after MDR1-related chemotherapy.
A novel FMEV-based MDR1 vector containing an optimized 5′ untranslated MESV-leader sequence that may provide a further increase in drug resistance has been developed by Hildinger et al.89Elimination of the cryptic splice sites or inclusion of cis-acting elements like the “hepatitis virus posttranscriptional RNA export element”90 are also likely to further increase the stability of the MDR1 transcription unit, thereby increasing the overall proportion of MDR1-expressing, drug-resistant HSC/HPC. These new MDR1 vectors will be valuable tools for future studies to investigate the advantage of engraftedMDR1-transduced PBPC after repetitive cycles of chemotherapy and will be interesting when compared in an optimal protocol with lentiviral vectors.
In conclusion, we found that potentially clinically relevant efficiencies of gene transfer in repopulating human hematopoietic stem cells are now achievable by using combinations of early-acting cytokines in combination with the recombinant fibronectin fragment CH-296. Our results also emphasize the need for careful optimization of vector design with a focus on gene expression in primitive hematopoietic cells and their differentiated progeny.
Acknowledgments
We are grateful to the continuing support of professors A. D. Ho, A. A. Fauser, and W. Ostertag. We thank James Bender and John P. Fruehauf, both of Irvine, CA, and Fred Koller, San Diego, CA, for critically reviewing the manuscript and for helpful suggestions. We gratefully acknowledge the technical assistance of Bernhard Berkus, Hans Jürgen Engel, and Sigrid Heil and the support of the clinical stem-cell transplantation laboratory and the animal facility team of the German Cancer Research Center. Eike Buss helped in collecting clinical characteristics of the patients. The FBMD-1 cell line was provided by R. E. Ploemacher, Erasmus University, Rotterdam, The Netherlands. IL-3 was provided by Dr Färber, Novartis, Nürnberg, Germany.
Supported in part by grants 10-1018-Ze-I, 10-1294-Ze2, and 10-1063-Ba-I of the Deutsche Krebshilfe/Dr Mildred-Scheel-Stiftung and by the Herbert Daus Fund of the University of Heidelberg.
Reprints:Stefan Fruehauf, Department of Internal Medicine V, University of Heidelberg, Hospitalstr 3, 69115 Heidelberg, Germany; e-mail: stefan_fruehauf@med.uni-heidelberg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal