Mutations in the Wiskott-Aldrich syndrome protein (WASP) have been hypothesized to cause defective actin cytoskeletal function. This resultant dysfunction of the actin cytoskeleton has been implicated in the pathogenesis of Wiskott-Aldrich syndrome (WAS). In contrast, it was found that stimulated actin polymerization is kinetically normal in the hematopoietic lineages affected in WAS. It was also found that the actin cytoskeleton in WAS platelets is capable of producing the hallmark cytoarchitectural features associated with activation. Further analysis revealed accelerated cell death in WAS lymphocytes as evidenced by increased caspase-3 activity. This increased activity resulted in accelerated apoptosis of these cells. CD95 expression was also increased in these cells, suggesting an up-regulation in the FAS pathway in WAS lymphocytes. Additionally, inhibition of actin polymerization in lymphocytes using cytochalasin B did not accelerate apoptosis in these cells. This suggests that the accelerated apoptosis observed in WAS lymphocytes was not secondary to an underlying defect in actin polymerization caused by mutation of the WAS gene. These data indicate that WASP does not play a universal role in signaling actin polymerization, but does play a role in delaying cell death. Therefore, the principal consequence of mutations in theWAS gene is to accelerate lymphocyte apoptosis, potentially through up-regulation of the FAS-mediated cell death pathway. This accelerated apoptosis may ultimately give rise to the clinical manifestations observed in WAS.
Wiskott-Aldrich syndrome (WAS) is an X-linked recessive disorder characterized by recurrent bacterial infection, thrombocytopenia, eczema, and lymphoreticular malignancy. The disease affects most hematopoietic lineages, including platelets, lymphocytes, monocytes, and neutrophils.1,2 The platelets are among the most severely affected cells in that they are abnormally small, and circulating counts can be 10% of normal or lower. Lymphocytes are also notably affected in that the antibody responses to polysaccharide and protein antigens are compromised, and these cells are typically deficient in surface sialoglycoproteins and microvilli. Lymphopenia is evident in patients with WAS by 6 years of age.1 2
The gene responsible for WAS has been cloned and subsequently shown to be allelic with X-linked thrombocytopenia (XLT), a clinically milder disorder.3,4 Analysis of the WAS protein (WASP) structure has revealed several domains, including a GTPase binding domain and domains homologous to actin-regulating proteins. WASP has been shown to bind the small guanosine triphosphate-binding protein Cdc42.5,6,7 Cdc42 is a member of the Ras superfamily of small GTPases and has been implicated as playing a role in signaling cytoskeletal reorganization and specifically in the formation of filopodia.8,9 Furthermore, in vivo overexpression of WASP increased polymerized actin, and this increase was dependent on WASP binding Cdc42.5 In addition, recombinant Cdc42 has been shown to induce actin polymerization in lysates from neutrophils (which express WASP).10
These observations have raised the possibility that the underlying problem in WAS is related to defects in the Cdc42/WASP-mediated signaling pathways that regulate actin polymerization. This hypothesis is consistent with the observation that lymphocytes from patients with WAS exhibit cytoarchitectural defects, most notably the paucity of microvilli. Microvilli are slender surface projections that cover normal blood lymphocytes. Because actin-binding proteins and filaments have been found at the base of these structures, microvillus formation is thought to be dependent on proper microfilament organization.11 The observation that impaired microvillus formation was present in newborns with WAS suggests that it occurs as a direct consequence of mutations in the WAS gene.11However, 2 recent observations are not consistent with this proposed pathogenesis. Deletion of the WASP binding domain from Cdc42 does not impair the ability of cells to produce filopodia.12 In addition, coexpression of WASP and active Cdc42 does not result in filopodia formation.13 One alternative explanation for WASP function may lie in the observation that activated Cdc42 induces apoptosis in Jurkatt T-lymphocytes.14 This suggests that WASP, through its interactions with Cdc42, might play a role in the regulation of cell death. Accelerated lymphocyte death could explain the progressive T-cell lymphopenia observed in patients with WAS.1 2
The purpose of this study was to determine the consequence of a mutated or absent WASP for leukocyte and platelet function in primary cells isolated directly from patients rather than in cell lines expressing mutant WASP. The principle of the model tested was that mutations in WASP impair the ability of hematopoietic cells to polymerize actin in response to relevant stimuli. In healthy persons, platelets and leukocytes are capable of producing an actin polymerization/depolymerization response on activation by a variety of physiologically relevant stimuli.15-19 Therefore, any defect in WASP-dependent signaling to the actin cytoskeleton would be readily identified from detailed comparison of the actin polymerization response in platelets and leukocytes from patients with classic WAS and XLT and from controls. Additionally, we examined apoptosis and caspase-3 activity in lymphocytes isolated from patients with WAS and XLT and from controls.
Patients and methods
Materials
N-formyl-methionyl-leucyl-phenylalanine (FMLP), 9,11-dideoxy-11α, 9α-epoxymethano-prostaglandin F2α (PGF2α, U46 619), and adenosine diphosphate (ADP) were purchased from Sigma Chemical (St. Louis, MO). Phorbol-12-myristate-13-acetate (PMA) was purchased from Calbiochem (LaJolla, CA). Fluorescein isothiocyanate (FITC)–anti-CD3 and phycoerythrin (PE)–anti-CD14 antibodies were purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA). FITC–anti-CD95 and Cy5–anti-CD3 antibodies were purchased from PharMingen (San Diego, CA). Monoclonal anti-CD3 receptor antibody (OKT3) was a generous gift from Dr Richard A. Kroczek (Robert Koch Institut, Berlin, Germany). Monoclonal anti-FCRIII receptor antibody 3G8 was purchased from Medarex (Annandale, NJ). Monoclonal anti-CD18 receptor antibody (IB4) was purchased from Ancell (Bayport, MN). Polyclonal goat antimouse antibody was purchased from Jackson Immunoresearch (West Grove, PA). Apoptosis detection kit was purchased from R&D Systems (Minneapolis, MN). Caspase-3 assay kit was purchased from OncoImmunin (College Park, MD). zVAD-fmk (benzyloxycarbonyl-Val-Ala-Asp(Ome)-fluoromethylketone) was purchased from Enzyme Systems (Livermore, CA).
Patients and controls
Five patients with XLT were studied, 4 of whom had missense mutations in the plekstrin homology domain of WASP (2 siblings-L39P, T48I, V75 M). The fifth had a splice site mutation in intron 6 (nt593 + 5 g→a), resulting in multiple products. Of the 2 patients with WAS, 1 had a single nucleotide deletion in exon 2 (211delT), resulting in a frameshift mutation and stop at amino acid 75; the other had a splice site mutation in intron 3 (nt395-1 g→a), resulting in insertion of intron 3, frameshift, and stop at amino acid 200 of WASP. Patients with XLT were defined as those who exhibited platelet abnormalities (small platelets, thrombocytopenia) but did not have significant immunologic defects. Patients with WAS were defined as those who exhibited significant immune defects (eg, recurrent bacterial infection) in addition to the platelet abnormalities observed in the patients with XLT. One patient with WAS underwent bone marrow transplantation after the initiation of this project and was therefore removed for the remainder of the study. Western blot analysis of cell lysates from B-lymphoblastoid cell lines using a polyclonal anti-WASP antibody revealed decreased amounts of normal-sized WASP in cells from patients with missense mutations (4 with XLT), but it failed to detect WASP expression in the others (1 with XLT and 2 with WAS).20 Peripheral blood leukocytes and platelets were isolated from at least 5 different volunteers to serve as controls for actin polymerization and viability studies. Informed consent was obtained from all patients and volunteers, as prescribed by the Human Studies Review Committee at the University of Washington and the Institutional Review Board at the University of Michigan.
Leukocyte and platelet isolation
Platelets and leukocytes were isolated essentially as previously described.21-23 Briefly, whole blood was partially purified by dextran sedimentation to remove red blood cells. The leukocytes in the partially purified whole blood were separated into 2 bands, 1 consisting primarily of neutrophils and the other of monocytes and lymphocytes, by centrifugation on a 42% to 60% Percoll gradient. The neutrophil band was resuspended for 1 minute in an ammonia buffer to lyse red blood cells. Subsequent to this lysis step, neutrophils were resuspended at a concentration of 1 × 108 cells/mL and stored at 4°C in a modified Gey's buffer (MGB) without calcium (10 mmol/L HEPES, 5 mmol/L KCl, 0.22 mmol/L KH2PO4, 1.1 mmol/L Na2HPO4, 5.5 mmol/L glucose, 0.3 mmol/L MgSO4, 1 mmol/L MgCl2, 147 mmol/L NaCl, pH 7.4).
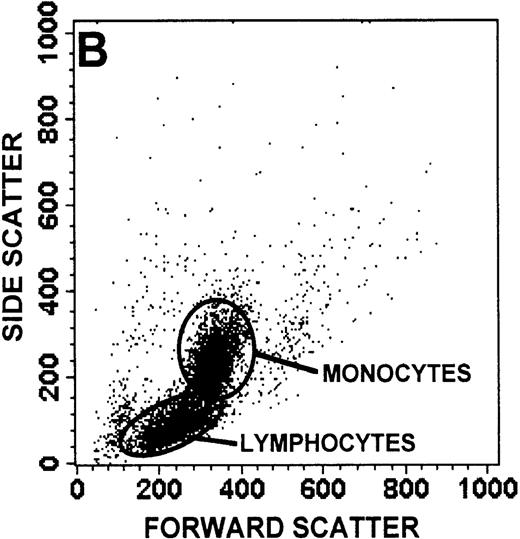
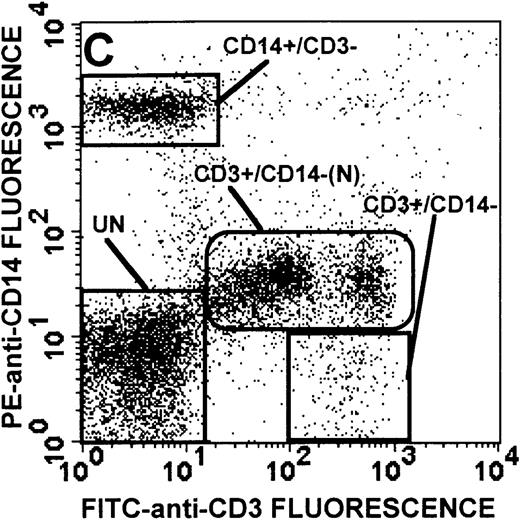
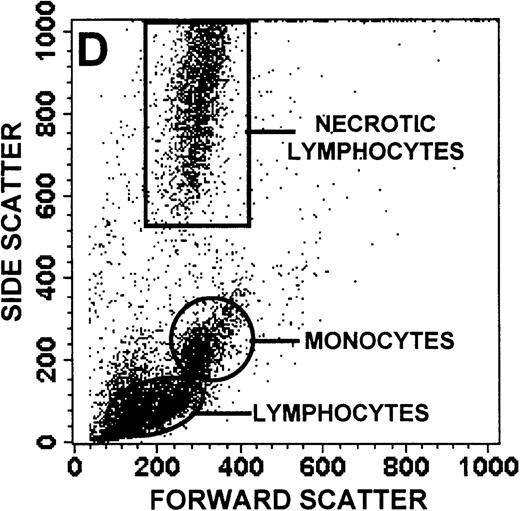
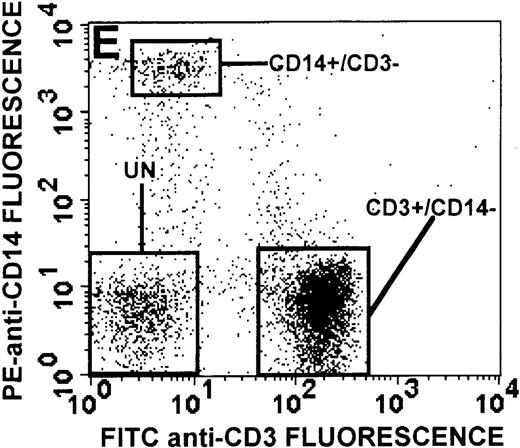
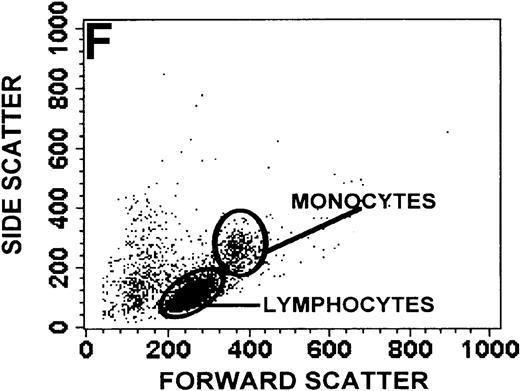
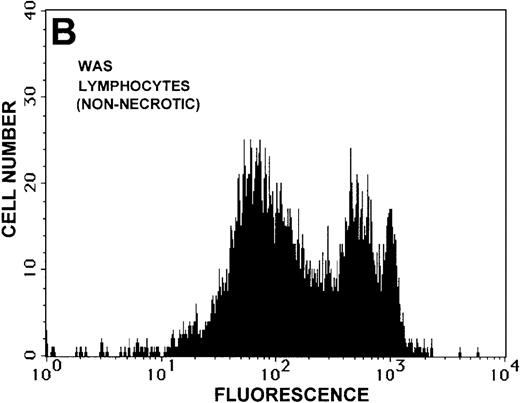
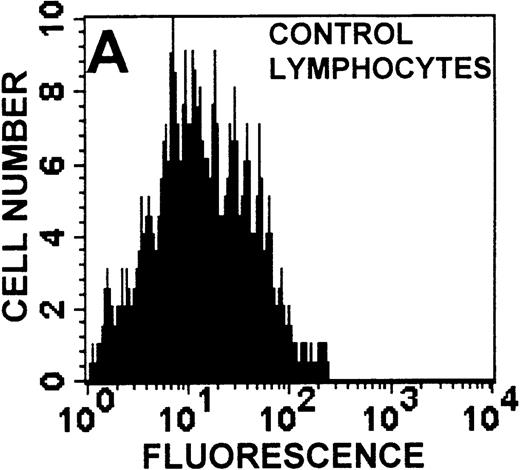
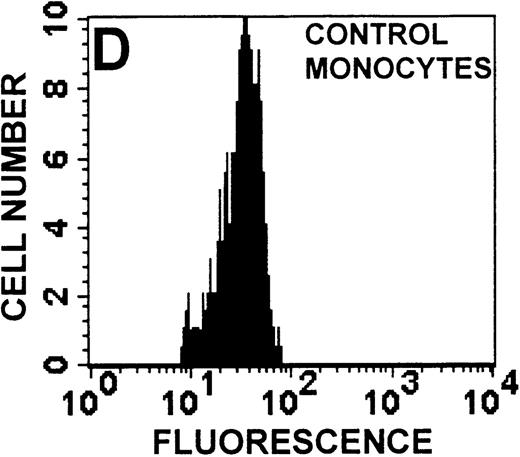
The monocyte/lymphocyte band was removed and used for these studies. In addition to using their light-scattering properties to distinguish the monocyte population from the lymphocytes, we labeled cells with PE–anti-CD14 antibody and fluorescein-isothiocyanate (FITC)–anti-CD3 antibody and analyzed them on a Becton Dickinson FACScan (Figure1). This analysis was carried out for cells isolated immediately after venipuncture (Figures 1A, 1B) and for cells isolated from 24-hour-old blood (Figures 1C to 1F), allowing for identification of the monocyte and lymphocyte populations. An additional population of necrotic lymphocytes that had unique scattering properties was identified in cells isolated from 24-hour-old blood. We could not detect a distinct necrotic monocyte population, and the necrotic lymphocyte population did not contain monocytes. This analysis allowed for unambiguous identification of the monocyte and T-lymphocyte populations. Because T and B lymphocytes have identical scattering properties, the scattering parameters of the FITC-anti-CD3–labeled cell population were used to gate electronically for all lymphocytes. The scattering parameters of the PE-anti-CD14–labeled cell population were used to gate electronically for all monocytes. For the purposes of the actin and caspase-3 assays, the necrotic lymphocyte population was excluded.
Cell stimulation and quantification of F-actin content
Platelets were stimulated as previously described.21 The sole modification was the addition of 1% Triton X-100 to the staining cocktail to enhance uptake of the fluorescent probe N-(7-nitrobenz-2-oxa-1, 3-diazole 4-yl) (NBD)-phallicidin. Stimulus was added directly to stirred platelet-rich plasma at 37°C. An aqueous stock of stimulus was prepared on the day of the experiment as a 1:100 or 1:10 dilution in MGB of a frozen, concentrated stock solution originally made in dimethyl sulfoxide (DMSO). This aqueous stock was added as a 1:100 dilution to platelet-rich plasma. Thus, the final concentration of DMSO added to the cells was 0.1% or less.
Lymphocytes, monocytes, and neutrophils were stimulated essentially as previously described.24 Briefly, cells were resuspended at a concentration of 2 × 106 cells/mL in MGB in the presence of calcium (10 mmol/L HEPES, 5 mmol/L KCl, 0.22 mmol/L KH2PO4, 1.1 mmol/L Na2HPO4, 5.5 mmol/L glucose, 0.3 mmol/L MgSO4, 1 mmol/L MgCl2, 1.5 mmol/L CaCl2, 147 mmol/L NaCl, pH 7.4) and stirred at 37°C for 10 minutes before the addition of stimulus prepared as described for platelets. Stimulus was added as a 1:100 dilution to stirred cells in suspension. Thus, the final concentration of DMSO added to cells was 0.1% or less. After stimulation, aliquots of cells were removed and fixed in 3.7% formalin at various time points during the course of the response. These samples were subsequently stained for F-actin with NBD-phallicidin and quantified by flow cytometry.
For analysis of the CD3 receptor-mediated actin polymerization response in lymphocytes, cells were incubated at a concentration of 4 × 106 cells/mL in MGB containing 8 μg/mL OKT3 anti-CD3 antibody for 30 minutes at 4°C. After this incubation, cells were stirred at 37°C for 10 minutes before stimulation. Cells were then stimulated with 80 μg/mL goat antimouse antibody to cross-link the primary antibody bound to the receptor, which resulted in the initiation of actin polymerization.
For analysis of the FCRIII receptor– and CD-18 receptor–mediated actin polymerization responses in neutrophils, cells were incubated at a concentration of 5 × 107 cells/mL in MGB containing the appropriate antibody (3G8 and IB4, respectively) for 30 minutes at 4°C. Neutrophils were incubated with 3G8 at a concentration of 5 μg/mL and with IB4 at a concentration of 10 μg/mL. Subsequent to this incubation, cells were suspended at a concentration of 2 × 106 cells/mL in MGB and stirred at 37°C for 10 minutes before stimulation. Stimulation of these cells was achieved using 50 μg/mL goat antimouse antibody to cross-link the primary antibody bound to the receptor, which resulted in the initiation of actin polymerization.
Identification of apoptotic cells
Viable apoptotic and necrotic lymphocytes were identified as directed using the apoptosis detection kit (R&D Systems, Minneapolis, MN). Briefly, cells were labeled with FITC-annexin-V and propidium iodide and analyzed on a Becton Dickinson FACScan. An excitation wavelength of 488 nm was used; emissions were read at 530 nm for the FITC-annexin-V and at more than 650 nm for the propidium iodide. Necrotic cells were defined as those that bound annexin-V and propidium iodide. Apoptotic cells were defined as those that bound annexin-V but excluded propidium iodide. Viable cells were defined as those that did not bind either stain.
Determination and inhibition of caspase-3 activity
Caspase-3 activity was measured as previously described using the caspase-3 assay kit.25 Briefly, cells were incubated in 10 μmol/L PhiPhiLux-G2D2 substrate solution. This substrate is cleaved to a fluorescent product by caspase-3. After incubation for 1 hour in this solution, cells were analyzed using a Becton Dickinson FACScan. An excitation wavelength of 488 nm was used, and emission was read at 585 nm. Non-necrotic lymphocytes were selected using light-scattering parameters, and the fluorescence of this population was measured. This fluorescence was reflective of caspase-3 activity.
To inhibit caspase-3, whole blood drawn from patients with WAS was mixed with 50 μmol/L zVAD-fmk immediately after venipuncture. This concentration of inhibitor was used because it has been shown to inhibit significantly the signature events seen in apoptosis.26 The blood was then incubated at room temperature for 24 hours before lymphocytes were isolated.
Determination of CD95 receptor expression
Lymphocytes isolated from peripheral blood as described above were labeled with FITC–anti-CD95 and Cy5–anti-CD3 (to identify T lymphocytes) or FITC–anti-CD95 and PE–anti-CD14 (to identify monocytes) and were analyzed by flow cytometry to quantify CD95 expression. Briefly, 1 × 106 cells were suspended in 50 μL MGB. To this suspension, 20 μL FITC–anti-CD95 antibody and 5 μL Cy5–anti-CD3 (to identify T lymphocytes) or 20 μL FITC–anti-CD95 antibody and 10 μL PE–anti-CD14 (to identify monocytes) antibody were added and maintained on ice for 20 minutes. Cells were washed and resuspended in MGB and analyzed on a Becton Dickinson FACScan.
Kinetics of WAS and XLT lymphocyte apoptosis
To determine the kinetics of apoptosis in WAS, XLT, and patient lymphocytes, cells were resuspended at a concentration of 1 × 106 cells/mL in T-cell media (RPMI 1640, 8% fetal bovine serum, 2% human serum) and maintained at 37°C. Cells were then removed at indicated time intervals and analyzed for viability as determined by annexin-V and propidium iodide exclusion as described above.
Cytochalasin-B incubation of lymphocytes
After purification, lymphocytes and monocytes were incubated at a concentration of 1 × 106 cells/mL with the indicated concentration of cytochalasin B, cytochalasin B and zVAD-fmk, or vehicle control at room temperature for the indicated time intervals. Lymphocytes were then analyzed for apoptosis and caspase-3 activity as described above.
Results
Quantitative and qualitative assessments of stimulated actin polymerization response in platelets isolated from patients with WAS and XLT
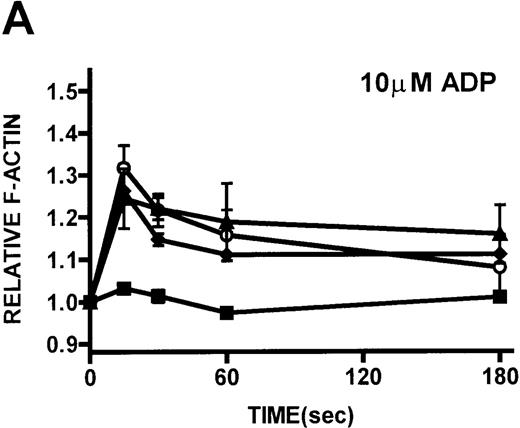
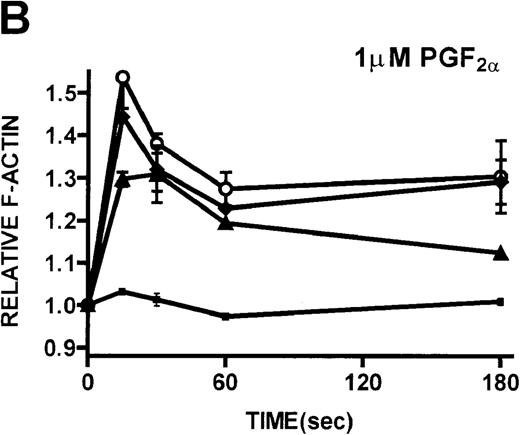
Platelets isolated from patients with WAS and XLT and from controls were stimulated with either PGF2α or ADP. These compounds, which activate G-protein–dependent signaling pathways,27 have been shown to induce rapid actin polymerization and depolymerization in platelets isolated from healthy volunteers.21 The kinetics of actin polymerization in platelets isolated from patients with WAS and XLT, in response to stimulation with these compounds, was determined by fixing and staining the F-actin with NBD-phallacidin during the time course of the response (Figures 2A, 2B). No notable difference in the kinetics of actin cytoskeletal remodeling of patient platelets relative to platelets from healthy volunteers was observed. After taking into account their smaller size, it was found that resting F-actin content in WAS platelets was not lower than that in control platelets (data not shown).
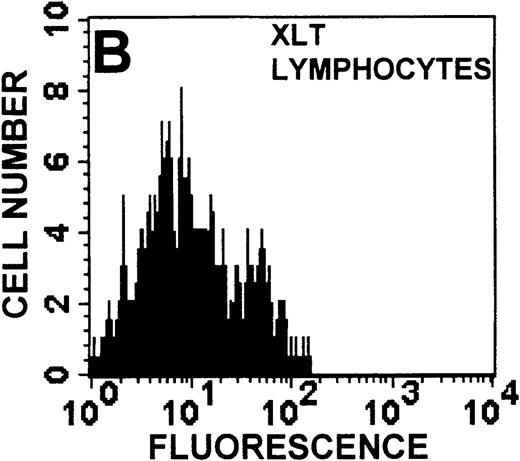
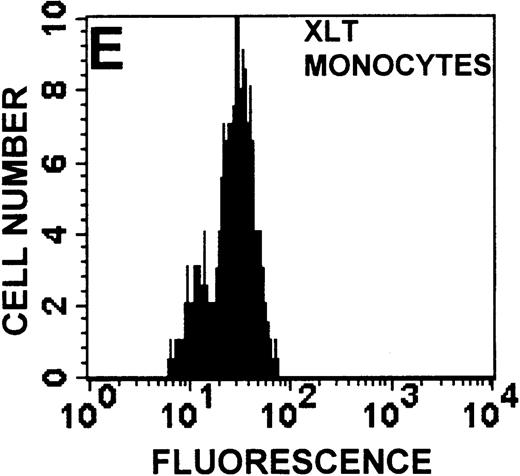
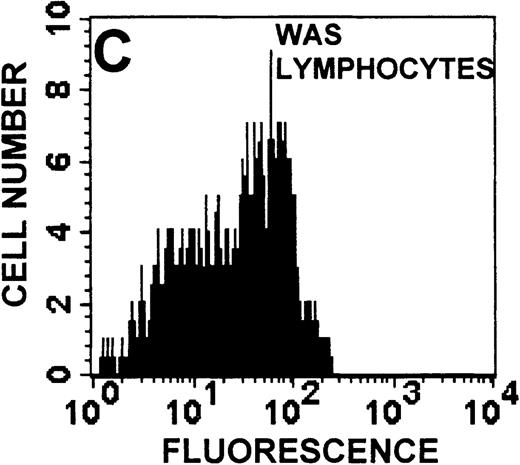
Identification of lymphocyte and monocyte subpopulations in cells isolated from WAS and control blood immediately and 24 hours after venipuncture.
(A, C, E) Identification of unlabeled, FITC-anti-CD3–labeled, and PE-anti-CD14–labeled populations in cells isolated from WAS patient blood immediately (A) and 24 hours (C) after venipuncture and in cells isolated from control blood 24 hours (E) after venipuncture. Four different subpopulations were identified in these cells: T lymphocytes (CD3+/CD14-), monocytes (CD14+/CD3-), unlabeled (UN), and necrotic lymphocytes (CD3+/CD14-(N)-seen only in panel C). These graphs are representative of data collected from 2 patients with WAS and at least 5 control donors. (B, D, F) Dot plots showing forward and side-scatter data for cells isolated from WAS patient blood immediately (B) and 24 hours (D) after venipuncture and in cells isolated from control blood 24 hours (F) after venipuncture. Three different subpopulations were identified in these cells: lymphocytes, necrotic lymphocytes (only in C), and monocytes. These graphs are representative of data collected from 2 patients with WAS and at least 5 control donors.
Identification of lymphocyte and monocyte subpopulations in cells isolated from WAS and control blood immediately and 24 hours after venipuncture.
(A, C, E) Identification of unlabeled, FITC-anti-CD3–labeled, and PE-anti-CD14–labeled populations in cells isolated from WAS patient blood immediately (A) and 24 hours (C) after venipuncture and in cells isolated from control blood 24 hours (E) after venipuncture. Four different subpopulations were identified in these cells: T lymphocytes (CD3+/CD14-), monocytes (CD14+/CD3-), unlabeled (UN), and necrotic lymphocytes (CD3+/CD14-(N)-seen only in panel C). These graphs are representative of data collected from 2 patients with WAS and at least 5 control donors. (B, D, F) Dot plots showing forward and side-scatter data for cells isolated from WAS patient blood immediately (B) and 24 hours (D) after venipuncture and in cells isolated from control blood 24 hours (F) after venipuncture. Three different subpopulations were identified in these cells: lymphocytes, necrotic lymphocytes (only in C), and monocytes. These graphs are representative of data collected from 2 patients with WAS and at least 5 control donors.
To assess qualitatively the actin cytoskeletal function in platelets isolated from patients with WAS and XLT, scanning electron micrograph images of these cells were obtained during the time course of stimulation with PGF2α (Figures 2C to 2F).28Although the patient platelets (Figures 2E, 2F) were clearly smaller than platelets isolated from healthy volunteers (Figures 2C, 2D), they produced cytoskeletal extensions strikingly similar to those produced by control platelets once stimulated. It is important to note that in the patients with classic WAS included in these studies, we found no detectable levels of WASP.
Quantification of stimulated actin polymerization in neutrophils and monocytes isolated from patients with WAS and XLT
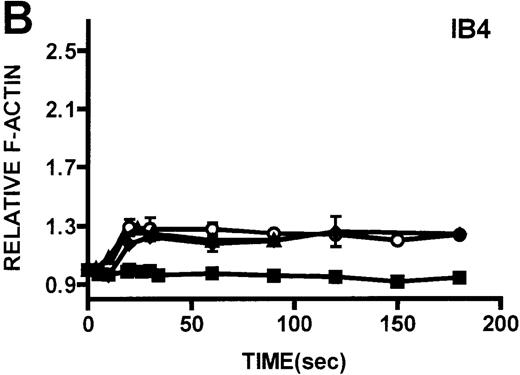
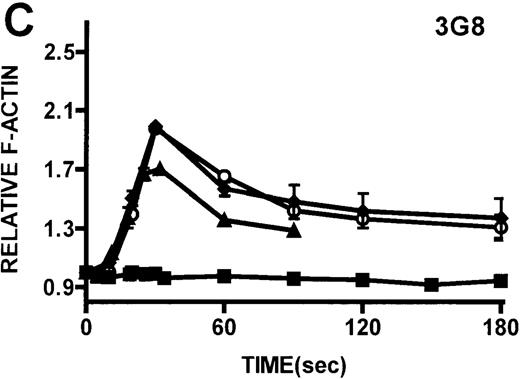
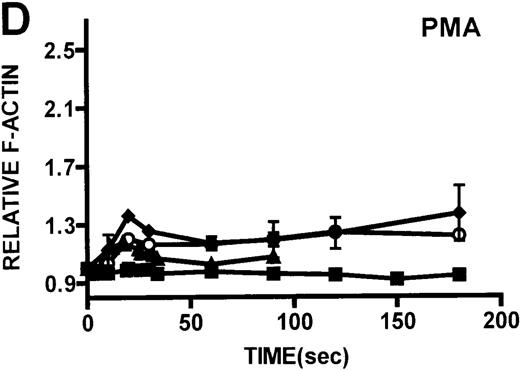
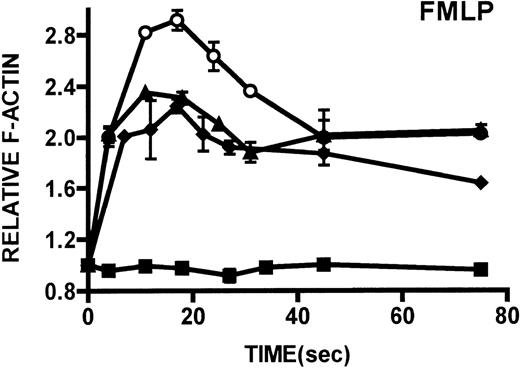
Neutrophils and monocytes isolated from patients with WAS and XLT were stimulated by different pathways known to result in actin polymerization in these cells (Figures3, 4). FMLP is a potent chemoattractant that activates actin polymerization in neutrophils and monocytes.16,17 It has been shown to activate actin polymerization in neutrophils through a pertussis toxin-sensitive G-protein–dependent pathway.16 PMA activates actin polymerization in neutrophils through a membrane receptor-independent pathway.29 Ligation of the FCRIII receptor and of the β2-integrin receptor has been shown to result in actin polymerization in neutrophils, likely by way of pathways important for phagocytosis and adhesion.30 31 Activation of neutrophils isolated from patients with WAS and XLT with these compounds resulted in an actin polymerization response that was indistinguishable from the response in control neutrophils (Figure 3). There were no notable differences in either the kinetics or the magnitude of the actin polymerization response between patient and control neutrophils stimulated with these compounds. Additionally, the stimulation of monocytes isolated from WAS and XLT cells in patients with FMLP resulted in an actin polymerization response that was the same as the response seen in control cells (Figure 4). It is also important to note that resting levels of F-actin were identical in WAS, XLT, and control neutrophils and monocytes (data not shown).
Actin polymerization in platelets from patients with WAS and XLT and from controls.
(A, B) Platelets isolated from whole blood and maintained at 37°C in autologous platelet-rich plasma were stimulated with either PGF2α, ADP, or buffer (▪)at the indicated concentrations.21 Data reflect the typical responses from each patient group and the control group. Error bars represent the SEM for duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS (○), 2 patients with XLT (⧫), and 5 control donors (▴). (C-F) Representative scanning electron micrographs of platelets fixed before (C, E) and 180 seconds after stimulation (D, F) with 1 μmol/L PGF2α from a patient with classic WAS (E, F) and control (C, D). Images are 8-μm fields, and samples were prepared as previously described.28 Platelets isolated from patients with WAS were consistently smaller, as previously reported.2 Images are representative of data collected from 2 patients with WAS and 3 control donors.
Actin polymerization in platelets from patients with WAS and XLT and from controls.
(A, B) Platelets isolated from whole blood and maintained at 37°C in autologous platelet-rich plasma were stimulated with either PGF2α, ADP, or buffer (▪)at the indicated concentrations.21 Data reflect the typical responses from each patient group and the control group. Error bars represent the SEM for duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS (○), 2 patients with XLT (⧫), and 5 control donors (▴). (C-F) Representative scanning electron micrographs of platelets fixed before (C, E) and 180 seconds after stimulation (D, F) with 1 μmol/L PGF2α from a patient with classic WAS (E, F) and control (C, D). Images are 8-μm fields, and samples were prepared as previously described.28 Platelets isolated from patients with WAS were consistently smaller, as previously reported.2 Images are representative of data collected from 2 patients with WAS and 3 control donors.
Kinetics of actin polymerization in WAS and XLT neutrophils.
Actin polymerization in WAS (○), XLT(⧫), and control (▴)neutrophils in response to stimulation with FMLP (A), IB4 (B), 3G8 (C), PMA (D), or buffer control (A-D, ▪). Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS, 3 patients with XLT. and at least 5 control donors.
Kinetics of actin polymerization in WAS and XLT neutrophils.
Actin polymerization in WAS (○), XLT(⧫), and control (▴)neutrophils in response to stimulation with FMLP (A), IB4 (B), 3G8 (C), PMA (D), or buffer control (A-D, ▪). Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS, 3 patients with XLT. and at least 5 control donors.
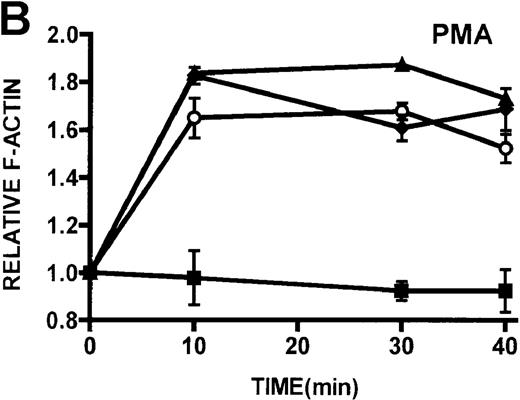
Quantification of stimulated actin polymerization in lymphocytes isolated from patients with WAS and XLT
The ability of patient lymphocytes to dynamically remodel their actin cytoskeleton in response to stimuli was examined. Activation of protein kinase C has been shown to induce shape changes in lymphocytes.16 Two potent stimuli of lymphocytes by protein kinase C–mediated pathways, PMA and bryostatin, were used, and F-actin levels were detected by binding of NBD-phallacidin.16,19,24 Additionally, lymphocytes were stimulated through ligation of the CD3 receptor, which has previously been shown to induce actin polymerization in lymphocytes.32PMA and bryostatin induced actin polymerization responses in lymphocytes from patients with WAS and XLT that were indistinguishable from those of control lymphocytes (Figures5A, 5B). Activation of the CD3 receptor resulted in actin polymerization in lymphocytes isolated from healthy volunteers (Figure 5C). Ligation of the CD3 receptor also induced an actin polymerization response in lymphocytes isolated from patients with WAS and XLT that was similar to that of control. The kinetics of the actin polymerization response in the WAS and XLT lymphocytes was essentially indistinguishable from that of the control lymphocytes. Furthermore, though the magnitude of the actin polymerization response in XLT lymphocytes was indistinguishable from that of control cells, the magnitude of the response in WAS lymphocytes appeared diminished. This diminishment, however, was not statistically significant as determined by paired Student t test analysis. It is also important to note that resting levels of F-actin were identical in control and patient lymphocytes (data not shown).
Kinetics of actin polymerization in WAS, XLT, and control monocytes.
Actin polymerization in WAS (○), XLT (⧫), and control (▴) neutrophils in response to stimulation with 100 nmol/L FMLP. (Buffer, ▪.) Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS, 3 patients with XLT, and at least 5 control donors.
Kinetics of actin polymerization in WAS, XLT, and control monocytes.
Actin polymerization in WAS (○), XLT (⧫), and control (▴) neutrophils in response to stimulation with 100 nmol/L FMLP. (Buffer, ▪.) Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 2 patients with WAS, 3 patients with XLT, and at least 5 control donors.
Identification of lymphocyte and monocyte subpopulations in cells isolated from WAS patient blood immediately and 24 hours after venipuncture
To determine the feasibility of using blood from patients with WAS that had been shipped overnight for our studies, whole blood was stored at room temperature for 24 hours before the isolation of lymphocytes and monocytes (Figure 1). After purification, the lymphocyte/monocyte mixture was labeled with FITC–anti-CD3 and PE–anti-CD14 antibodies and was analyzed by flow cytometry to identify each subpopulation. In cells isolated from WAS whole blood immediately after venipuncture (Figures 1A, 1B), 3 distinct subpopulations of cells were identified. The CD3+/CD14-population was identified as T lymphocytes because these cells express the CD3 antigen.33 Similarly, the CD14+ population was identified as monocytes because these cells express the CD14 antigen.34 There was also an unlabeled subpopulation of cells that consisted primarily of B lymphocytes. These 3 subpopulations were also present in cells isolated from WAS blood 24 hours after venipuncture (Figures 1C, 1D). However, an additional subpopulation was identified (CD3+/CD14-(N)). This subpopulation was not present in cells isolated from control blood 24 hours after venipuncture (Figures 1E, 1F). Because nonactivated monocytes do not significantly shed CD14 within 24 hours,35 this new subpopulation was identified as lymphocytes. Therefore, this subpopulation of T lymphocytes was present in cells isolated from WAS blood 24 hours after venipuncture (Figures 1C, 1D), but was not present in cells isolated from 24-hour-old control blood (Figures 1E, 1F).
Kinetics of actin polymerization in WAS, XLT, and control lymphocytes.
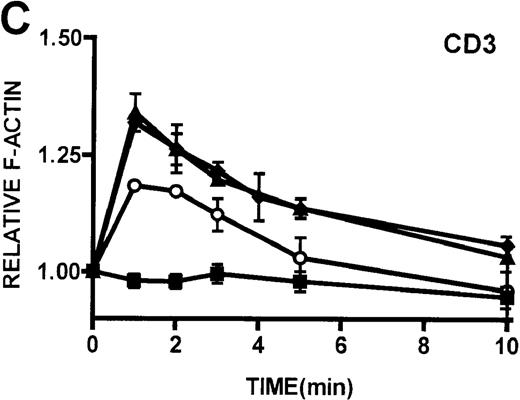
(A, B) Actin polymerization in lymphocytes in response to stimulation with 100 nmol/L PMA, 100 nmol/L bryostatin (Bryo), and a buffer control (▪). Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, ▴; WAS, ○; or XLT, ⧫). These graphs are representative of data collected from 2 patients with WAS, 5 patients with XLT, and 5 control donors. (C) Actin polymerization in lymphocytes in response to stimulation with OKT3 anti-CD3-receptor antibody and a buffer control. Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 1 patient with WAS, 1 patient with XLT, and 2 healthy volunteers.
Kinetics of actin polymerization in WAS, XLT, and control lymphocytes.
(A, B) Actin polymerization in lymphocytes in response to stimulation with 100 nmol/L PMA, 100 nmol/L bryostatin (Bryo), and a buffer control (▪). Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, ▴; WAS, ○; or XLT, ⧫). These graphs are representative of data collected from 2 patients with WAS, 5 patients with XLT, and 5 control donors. (C) Actin polymerization in lymphocytes in response to stimulation with OKT3 anti-CD3-receptor antibody and a buffer control. Error bars represent SEM of duplicate simultaneous determinations for a single donor (control, WAS, or XLT). These graphs are representative of data collected from 1 patient with WAS, 1 patient with XLT, and 2 healthy volunteers.
Viability of lymphocytes isolated from 24-hour-old blood from patients with WAS and XLT
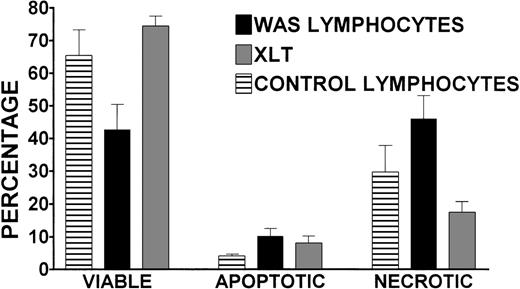
To better understand the effect of storing blood at room temperature for 24 hours before lymphocyte isolation, the viability of cells isolated from 24-hour-old WAS, XLT, and control blood was determined. We used an assay that identified viable, apoptotic, and necrotic cells (see “Patients and Methods” for greater detail). There were no statistically significant differences in the percentages of viable, apoptotic, or necrotic lymphocytes isolated from 24-hour-old XLT and control blood (Figure 6). However, the 24-hour incubation had a significant effect on the viability of WAS lymphocytes, which on average was 42%, whereas control and XLT lymphocyte viability rates were significantly (P < .05) higher at 65% and 74%, respectively (Figure 6). After 24 hours, there was a significant increase in apoptotic lymphocytes in cells isolated from the blood of patients with WAS compared with control blood (10% compared with 4%). This trend was also apparent when comparing the percentage of apoptotic lymphocytes isolated from WAS and XLT blood (10% compared with 8%); however, the difference was not statistically significant (Figure 6). There was a significantly higher percentage of necrotic WAS lymphocytes (46%) compared with control (29%) and XLT lymphocytes (17%) isolated from 24-hour-old blood (Figure 6).
Viability, apoptosis, and necrosis in WAS, XLT, and control lymphocytes isolated 24 hours after venipuncture.
Percentages of viable, apoptotic, and necrotic lymphocytes isolated from 24-hour-old WAS, XLT, and control blood were determined by annexin-V binding/propidium iodide exclusion. Lymphocytes were purified 24 hours after venipuncture by gradient centrifugation of partially purified whole blood. WAS data represent the average of 5 separate determinations performed in 2 patients with WAS. Control data represent the average of 5 experiments in 4 volunteers run concurrently with experiments in the patients with WAS. XLT data represent the average of 3 experiments in 3 patients. Error bars represent SEM.
Viability, apoptosis, and necrosis in WAS, XLT, and control lymphocytes isolated 24 hours after venipuncture.
Percentages of viable, apoptotic, and necrotic lymphocytes isolated from 24-hour-old WAS, XLT, and control blood were determined by annexin-V binding/propidium iodide exclusion. Lymphocytes were purified 24 hours after venipuncture by gradient centrifugation of partially purified whole blood. WAS data represent the average of 5 separate determinations performed in 2 patients with WAS. Control data represent the average of 5 experiments in 4 volunteers run concurrently with experiments in the patients with WAS. XLT data represent the average of 3 experiments in 3 patients. Error bars represent SEM.
Quantification of stimulated actin polymerization in non-necrotic lymphocytes isolated from 24-hour-old blood from patients with WAS and XLT
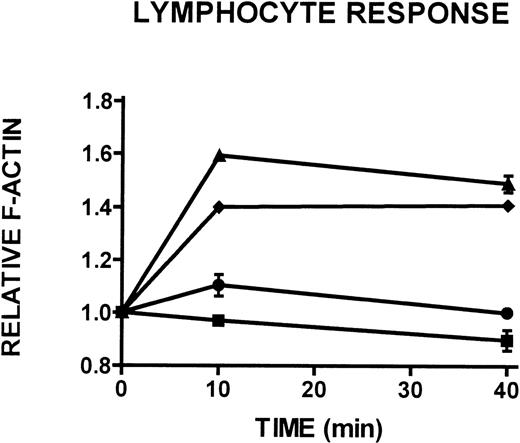
To better characterize the CD3+/CD14- lymphocyte population isolated from 24-hour-old WAS, XLT, and control blood (see Figures 1C, 1D), the actin polymerization response to stimulation with PMA in these cells was examined. This population of cells was identified as containing primarily viable and some apoptotic lymphocytes and will heretofore be referred to as the non-necrotic lymphocyte population. Whereas control and XLT non-necrotic lymphocytes were able to produce a robust actin polymerization response on stimulation with 100 nmol/L PMA (Figure 7), WAS non-necrotic lymphocytes were essentially incapable of responding to stimulation (Figure 7).
Actin polymerization in WAS, XLT, and control lymphocytes isolated 24 hours after venipuncture.
Actin cytoskeletal remodeling in response to stimulation with 100 nmol/L PMA or buffer (▪) in WAS (•), XLT (⧫), and control (▴) lymphocytes isolated from whole blood incubated at room temperature for 24 hours after venipuncture. Aliquots of cells were removed and analyzed for F-actin content at the indicated times. Error bars reflect SEM of duplicate simultaneous determinations from 1 donor (control or WAS). The graph is representative of data collected from 2 patients with WAS, 3 patients with XLT, and 5 control donors.
Actin polymerization in WAS, XLT, and control lymphocytes isolated 24 hours after venipuncture.
Actin cytoskeletal remodeling in response to stimulation with 100 nmol/L PMA or buffer (▪) in WAS (•), XLT (⧫), and control (▴) lymphocytes isolated from whole blood incubated at room temperature for 24 hours after venipuncture. Aliquots of cells were removed and analyzed for F-actin content at the indicated times. Error bars reflect SEM of duplicate simultaneous determinations from 1 donor (control or WAS). The graph is representative of data collected from 2 patients with WAS, 3 patients with XLT, and 5 control donors.
Determination of caspase-3 activity and effect of caspase-3 inhibition in non-necrotic lymphocytes isolated from 24-hour-old blood from patients with WAS
To test the hypothesis that the non-necrotic lymphocytes isolated from 24-hour-old WAS patient blood were in the early stages of apoptosis, we determined the caspase-3 activity in this population of cells. Caspase-3 activation is among the earliest signature events in apoptosis in lymphocytic cell lines, and it has been shown to occur before the expression of phosphatidylserine on the outer membrane.36-38 Therefore, this assay allows for identification of early apoptotic cell populations that would escape detection by annexin-V binding. As shown in Figures8A and 8B, caspase-3 activity was higher in the 24-hour-old non-necrotic WAS lymphocytes than in control lymphocytes isolated from 24-hour-old blood.
Caspase-3 activity and actin polymerization response in zVAD-fmk-treated and untreated WAS lymphocytes isolated 24 hours after venipuncture.
(A, B) Activity of caspase-3 in lymphocytes isolated from whole blood that had been incubated at room temperature for 24 hours. Data are expressed as a histogram with fluorescence intensity on the x-axis and cell number on the y-axis. Mean fluorescence intensities of the control and patient lymphocyte populations were 116 and 290, respectively. Graphs are representative of data collected from a single WAS patient and a control. (C, D) Caspase-3 activity in lymphocytes isolated from whole blood (from a single patient with WAS) that had been treated for 24 hours with and without 50 μmol/L zVAD-fmk. (C) Caspase-3 activity in untreated lymphocytes. (D) Caspase-3 activity in treated cells. Note the marked decrease in fluorescence in the treated lymphocytes. Mean fluorescence intensities in untreated and zVAD-fmk–treated WAS lymphocytes were 403 and 223, respectively. Because the instrumental settings of the flow cytometer varied from day to day, these data cannot be compared directly with those shown in A and B. (E) Percentages of viable, apoptotic, and necrotic lymphocytes from WAS lymphocytes isolated from 24-hour-old blood incubated with and without 50 μmol/L zVAD-fmk as defined previously. Graphs are representative of data taken from a single WAS patient and a single control. (F) Actin polymerization response in WAS lymphocytes isolated from 24-hour-old blood incubated with and without 50 μmol/L zVAD-fmk. The actin polymerization response of WAS lymphocytes was stimulated with 100 nmol/L PMA for 40 minutes at 37°C. Error bars represent SEM of duplicate determinations from a single donor (WAS). This graph is representative of data collected from a single patient with WAS.
Caspase-3 activity and actin polymerization response in zVAD-fmk-treated and untreated WAS lymphocytes isolated 24 hours after venipuncture.
(A, B) Activity of caspase-3 in lymphocytes isolated from whole blood that had been incubated at room temperature for 24 hours. Data are expressed as a histogram with fluorescence intensity on the x-axis and cell number on the y-axis. Mean fluorescence intensities of the control and patient lymphocyte populations were 116 and 290, respectively. Graphs are representative of data collected from a single WAS patient and a control. (C, D) Caspase-3 activity in lymphocytes isolated from whole blood (from a single patient with WAS) that had been treated for 24 hours with and without 50 μmol/L zVAD-fmk. (C) Caspase-3 activity in untreated lymphocytes. (D) Caspase-3 activity in treated cells. Note the marked decrease in fluorescence in the treated lymphocytes. Mean fluorescence intensities in untreated and zVAD-fmk–treated WAS lymphocytes were 403 and 223, respectively. Because the instrumental settings of the flow cytometer varied from day to day, these data cannot be compared directly with those shown in A and B. (E) Percentages of viable, apoptotic, and necrotic lymphocytes from WAS lymphocytes isolated from 24-hour-old blood incubated with and without 50 μmol/L zVAD-fmk as defined previously. Graphs are representative of data taken from a single WAS patient and a single control. (F) Actin polymerization response in WAS lymphocytes isolated from 24-hour-old blood incubated with and without 50 μmol/L zVAD-fmk. The actin polymerization response of WAS lymphocytes was stimulated with 100 nmol/L PMA for 40 minutes at 37°C. Error bars represent SEM of duplicate determinations from a single donor (WAS). This graph is representative of data collected from a single patient with WAS.
To determine whether the elevated caspase-3 activity had an effect on lymphocyte viability, we added the caspase-3 inhibitor zVAD-fmk to WAS patient whole blood before the 24-hour incubation. At a concentration of 50 μmol/L, zVAD-fmk decreased caspase-3 activity in non-necrotic WAS lymphocytes (Figures 8C, 8D), and this resulted in a 15% increase in WAS lymphocyte viability (Figure 8E). As expected, this inhibitor also decreased the number of apoptotic and necrotic lymphocytes in cells isolated from patient blood (Figure 8E). Addition of this inhibitor had no notable effect on the viability of control lymphocytes (2% increase in viability). Additionally, lymphocytes isolated from zVAD-fmk–treated whole blood exhibited a more robust actin polymerization response to stimulation with PMA than that observed in lymphocytes isolated from untreated WAS patient blood (Figure 8F).
CD95 expression and kinetics of lymphocyte apoptosis
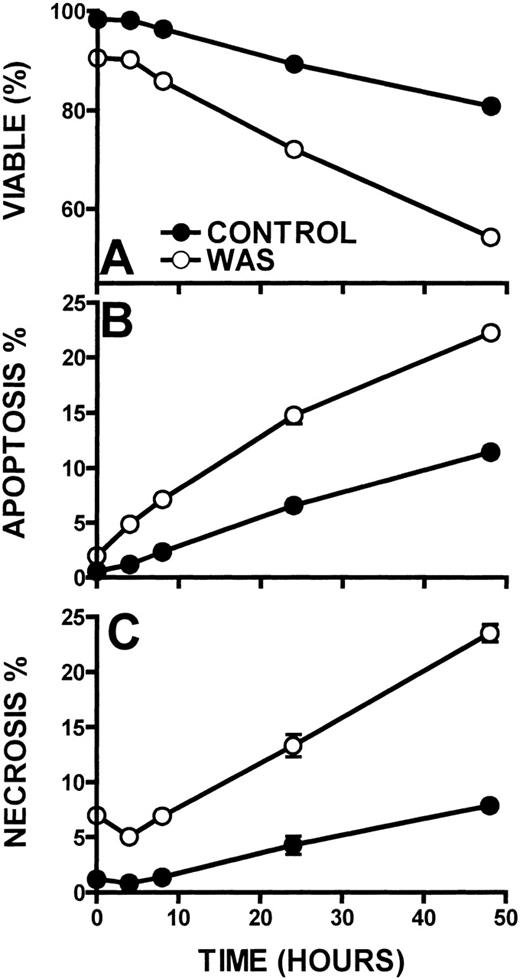
The kinetics of lymphocyte cell death in WAS lymphocytes was also determined (Figure 9). When compared with control lymphocytes, WAS lymphocytes displayed approximately a 2-fold greater cell death rate. Although control lymphocytes exhibited a 9% lymphocyte death rate per day, WAS lymphocytes succumbed at a rate of 19% per day as measured out to 48 hours (Figure 9A). WAS lymphocytes also exhibited 2-fold higher apoptosis and necrosis (10% and 10% per day, respectively) than control lymphocytes (4% and 5% per day, respectively) (Figures 9B, 9C). XLT lymphocytes did not exhibit increased apoptosis or necrosis when compared with control (5% and 5% per day, respectively). That WAS lymphocyte necrosis exhibited the same kinetics as the apoptosis of these cells indicates that the necrosis is likely secondary to lymphocyte apoptosis.39 40
Kinetics of cell death in WAS and control lymphocytes.
Viability (A), apoptosis (B), and necrosis (C) were examined in WAS and control lymphocytes. Lymphocytes were incubated at 37°C. Error bars represent SEM of triplicate determinations from a patient with WAS and a control.
Kinetics of cell death in WAS and control lymphocytes.
Viability (A), apoptosis (B), and necrosis (C) were examined in WAS and control lymphocytes. Lymphocytes were incubated at 37°C. Error bars represent SEM of triplicate determinations from a patient with WAS and a control.
Ligation of the CD95, or Fas, receptor has been shown to initiate lymphocyte-programmed cell death.41 Therefore, Fas receptor expression on lymphocytes isolated from patients with WAS was determined by flow cytometry (Figures 10A to10C). WAS T-lymphocytes were found to express CD95 at almost 2-fold higher levels than those of control cells and XLT lymphocytes. This increased Fas receptor expression was not observed in WAS monocytes (Figures 10D to 10F).
CD95 receptor expression in WAS, XLT, and control T lymphocytes and monocytes.
CD95 receptor expression as measured by flow cytometry on control (A, D), XLT (B, E), and WAS (C, F) lymphocytes (A-C) and monocytes (D-F). Data are expressed as a histogram with fluorescence intensity on the x-axis and cell number on the y-axis. Mean fluorescence intensities of the control and patient lymphocyte populations were 20 and 43, respectively. Graphs are representative of data collected from a patient with WAS and a control.
CD95 receptor expression in WAS, XLT, and control T lymphocytes and monocytes.
CD95 receptor expression as measured by flow cytometry on control (A, D), XLT (B, E), and WAS (C, F) lymphocytes (A-C) and monocytes (D-F). Data are expressed as a histogram with fluorescence intensity on the x-axis and cell number on the y-axis. Mean fluorescence intensities of the control and patient lymphocyte populations were 20 and 43, respectively. Graphs are representative of data collected from a patient with WAS and a control.
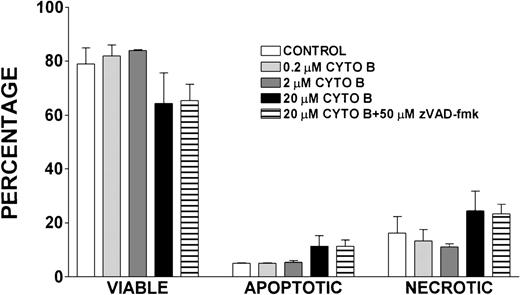
Effect of cytochalasin B treatment on lymphocyte viability, apoptosis, and necrosis
Because cytochalasin B has been shown to inhibit actin polymerization,42 we determined the effect of treatment with lymphocytes isolated from healthy volunteers with this compound on the viability, apoptosis, and necrosis of these cells (Figure11). After a 24-hour incubation with varying concentrations of cytochalasin B, there were no notable differences in the viability, apoptosis, or necrosis of control lymphocytes and those treated with 0.2 μmol/L and 2 μmol/L cytochalasin B. There was a slight decrease in viability and increase in necrosis and apoptosis observed in lymphocytes treated with 20 μmol/L cytochalasin B and in cells treated with 20 μmol/L cytochalasin B and 50 μmol/L zVAD-fmk when compared with control; however, these differences were not statistically significant.
Effect of cytochalasin B treatment on viability, apoptosis, and necrosis of lymphocytes.
Percentages of viable, apoptotic, and necrotic lymphocytes in cells isolated from healthy volunteers and incubated for 24 hours with indicated concentrations of cytochalasin B (CYTO B), zVAD-fmk, or vehicle control. Error bars reflect SEM of duplicate simultaneous determinations from 2 healthy control donors.
Effect of cytochalasin B treatment on viability, apoptosis, and necrosis of lymphocytes.
Percentages of viable, apoptotic, and necrotic lymphocytes in cells isolated from healthy volunteers and incubated for 24 hours with indicated concentrations of cytochalasin B (CYTO B), zVAD-fmk, or vehicle control. Error bars reflect SEM of duplicate simultaneous determinations from 2 healthy control donors.
Discussion
Platelets are affected in all patients who have mutations of theWAS gene, regardless of clinical phenotype. To determine whether these platelet abnormalities are related to an inability to polymerize actin on activation of signaling pathways, platelets isolated from patients with WAS and XLT were stimulated with ADP and PGF2α. The observation that platelets isolated from patients with WAS and XLT are capable of producing a normal actin polymerization response subsequent to stimulation suggested that dynamic actin cytoskeletal function was not universally impaired in these cells. Further, the observation that resting F-actin content in platelets isolated from patients with WAS and XLT was not significantly different from that of control platelets suggested that mutation of the WAS gene did not affect the resting F-actin pool. Because platelets isolated from healthy volunteers produce cytoskeletal extensions such as filopodia and microspikes on stimulation,43 we assessed the ability of WAS platelets to produce these cytoarchitectural features subsequent to stimulation. As Figures 2C to 2F show, platelets isolated from WAS patients were smaller than control platelets, but on stimulation they were capable of producing the same cytoskeletal extensions produced by platelets isolated from a healthy volunteer. These data suggested that mutation of the WAS gene did not universally impair stimulated platelet actin polymerization or the ability of these cells to exhibit morphologic changes on stimulation.
One of the primary distinctions between classic WAS and XLT is that patients with WAS have pronounced cellular immune dysfunction. For this reason, we examined the actin polymerization response in leukocytes isolated from patients with WAS and patients with XLT. Because WASP is expressed in neutrophils,3 we conducted detailed examinations of the actin polymerization response in these cells through the activation of different pathways known to result in actin polymerization. We examined the actin polymerization response in neutrophils to stimuli that activate pathways important for chemotaxis (FMLP), phagocytosis, 3G8 adhesion (IB4), and receptor-independent pathways (PMA). The observation that neutrophils from patients with WAS and XLT were capable of producing a robust actin polymerization response on stimulation with any of these compounds suggests that WASP mutation does not result in any universal defect in the pathways regulating actin polymerization in these cells. Additionally, the observation that monocytes from patients with WAS and XLT stimulated with FMLP were capable of producing an actin polymerization response that was indistinguishable from that of control cells suggests that WASP mutation does not universally affect the ability of these cells to polymerize actin.
Because cytoarchitectural abnormalities have been identified in lymphocytic cell lines expressing mutant WASP,11 we examined the ability of lymphocytes isolated from patients with WAS and XLT to produce an actin polymerization response. The observation that lymphocytes isolated from patients with WAS and XLT were able to produce a robust actin polymerization response on stimulation with either PMA or bryostatin suggests that WASP mutation does not universally impair this response in these cells. Furthermore, lymphocytic cell lines expressing mutant WASP have been shown to be incapable of polymerizing actin in response to CD3 receptor stimulation.44 Therefore, we examined CD3 receptor-stimulated actin polymerization in lymphocytes isolated from patients with WAS and XLT. In contrast to the observations in T-cell lines, lymphocytes isolated from patients with WAS and XLT were capable of producing an actin polymerization response to CD3 receptor stimulation (Figure 5D). Interestingly, in accordance with previously published observations,44 WAS lymphocytes did not proliferate in response to CD3 receptor stimulation (data not shown). This suggests that though certain aspects of T-cell receptor signaling are defective in WAS lymphocytes, the receptor is still capable of signaling actin cytoskeletal reorganization.
To summarize, we examined the actin polymerization response in the hematopoietic lineages most significantly affected in patients expressing mutant WASP, namely platelets, neutrophils, monocytes, and lymphocytes. We were unable to identify any detectable defect in the actin polymerization response in any of these cells on stimulation of a variety of different pathways with a variety of different ligands. We also examined the resting F-actin content in these cells and found that there was no difference in resting F-actin levels between cells expressing mutant WASP and control cells. This strongly suggested that mutation of the WAS gene did not universally affect the ability of these cells to polymerize actin. Further, the observation that WAS platelets were able to produce hallmark cytoarchitectural features on stimulation suggested that dynamic actin cytoskeletal function was preserved in these cells.
To include additional patients with WASP in our study, we examined the effect of overnight incubation of whole blood before lymphocyte isolation on these cells. This incubation resulted in significant lymphocyte death in cells isolated from patients with WAS (Figures 1,6). That this cell death was not observed in patients with XLT is consistent with the fact that these patients do not suffer from the profound immune dysfunction that patients with WAS exhibit. Further, the increase in necrotic lymphocytes observed in cells isolated from 24-hour-old WAS blood may be a result of secondary necrosis subsequent to apoptosis of these cells.39,40 Additionally, we examined the actin polymerization response in the non-necrotic lymphocyte population isolated from 24-hour-old WAS and XLT blood (Figure 7). These cells produced a significantly attenuated actin polymerization response when compared with XLT and control lymphocytes. A possible explanation for this attenuation is that the WAS lymphocytes were in the earliest stages of apoptosis. Such a mechanism would be compatible with the observation that apoptotic cells have been shown to have impaired functional responses when compared with non-apoptotic cells.45 46 The observation of elevated caspase-3 activity in lymphocytes isolated from 24-hour-old WAS blood suggested that these cells may indeed have been in the early stages of apoptosis. Further, the observation that inhibition of caspase-3 in WAS lymphocytes resulted in increased viability and decreased apoptosis and necrosis in these cells strongly suggested that apoptosis was accelerated in these lymphocytes (Figure 8). Finally, the observation that the inhibition of caspase-3 in lymphocytes isolated from 24-hour-old WAS blood resulted in enhancement of the actin polymerization response to stimulation with PMA indicated that apoptosis was the underlying cause of the observed attenuation of the actin polymerization response in these cells (Figure 7).
To better characterize the accelerated apoptosis observed in WAS lymphocytes, we determined the kinetics of cell death in WAS, XLT, and control lymphocytes. WAS lymphocytes exhibited an apoptosis rate 2-fold greater than control or XLT lymphocytes (Figure 9). To determine the underlying mechanism driving the accelerated apoptosis observed in WAS lymphocytes, we quantified CD95 receptor expression in these cells (Figure 10). The fact that WAS lymphocytes had roughly a 2-fold higher membrane density of CD95 receptor than control or XLT lymphocytes indicated that this might have been the underlying cause of the increased programmed cell death observed in WAS lymphocytes. This also suggested that the Fas-mediated cell death pathway might have been up-regulated in WAS.
Finally, we addressed whether an underlying defect in actin polymerization could have led to the accelerated apoptosis we observed in WAS lymphocytes. To address this, we treated lymphocytes with cytochalasin B, a potent inhibitor of actin polymerization,42 and examined the effect of this treatment on the viability of these cells. The observation that treatment of lymphocytes with cytochalasin B (0.2 μmol/L-20 μmol/L) did not result in a significant decrease in lymphocyte viability or increase in apoptosis or necrosis of these cells suggests that the accelerated apoptosis we observed was not caused by an underlying defect in actin polymerization (Figure 11).
Based on these observations, we hypothesize that WASP plays a role in delaying apoptosis and that WASP mutation interferes with the regulation of the apoptotic response. Accelerated apoptosis could explain many of the clinical features that have been observed in WAS. Immune dysfunction, a characteristic feature of classic WAS, is partly a consequence of the progressive T-cell depletion observed in these patients over time.47 This progressive lymphopenia may be a direct result of the accelerated apoptosis and secondary cell clearance of WAS lymphocytes. The fact that XLT lymphocytes did not exhibit increased CD95 receptor, but did exhibit an increased rate of apoptosis compared with control, also correlates with the clinical observation of significant immune deficiency in patients with WAS but not with XLT. Finally, the association of WASP with the actin cytoskeleton may have little significance for the structural function of cytoskeleton itself but may have consequences for the regulation of cell death. There is increasing evidence linking cytoskeletal-associated proteins such as gelsolin with key roles in regulating apoptosis.48,49Similarly, the potential role of WASP in delaying apoptosis may be mediated through its cytoskeletal attachments. Thus, the cytoskeletal abnormalities that have been observed in cells from patients with WAS and in cells expressing mutant WASP likely reflect the cytoskeletal changes that are associated with apoptosis.50
Our data indicated that a defect in the dynamic function of the actin cytoskeleton plays a minor role, if any, in the pathogenesis of WAS and XLT because mutations of WASP do not appear to affect the ability of hematopoietic cells to polymerize actin in response to stimulation. In addition, platelets from patients with WAS were able to produce the cytoarchitectural features associated with the stimulation of normal platelets. A possible explanation for the complex abnormalities observed in WAS lies in the observation that Fas receptor expression and apoptosis were increased in lymphocytes isolated from patients with WAS. This observation suggests the possibility that the primary consequence of mutations in WASP is defective regulation of apoptosis and not abnormal cytoskeletal function.
Acknowledgments
We thank Dr A. Al-Katib for generously donating bryostatin (synthesized by Dr G. Petit) and Dr Anu Srinivasan for helpful suggestions during the course of the project. We also thank Dr T. Futatani for determining the CD3-induced proliferation response in lymphocytes. Finally, we thank E. Calomeni, M. Young, and A. Tantri for excellent technical assistance.
Supported by the Office of Research and Development, Medical Research Service, Department of Veteran Affairs, and by grants from the Arthritis Foundation, the National Science Foundation (BES 9410403), and the National Institutes of Health (AI20065; training grants T32 GM07767/Pharmacological Sciences Training Program and T32 GM07863/Medical Scientist Training Program). Supported also by grants from the National Institutes of Health, the March of Dimes Birth Defects Foundation, and the DiJoria Wiskott-Aldrich Research Fund for that part of the study conducted at the Clinical Research Center, University of Washington, Seattle, WA.
Reprints:Geneva M. Omann, Research Services (11R), Room G1, Building 22, Veterans Administration Medical Center, 2215 Fuller Rd, Ann Arbor, MI 48105-2399; e-mail: gmomann@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.










This feature is available to Subscribers Only
Sign In or Create an Account Close Modal