The BCL-6 proto-oncogene is involved in the genesis of non-Hodgkin lymphoma (NHL). Rearrangements due to chromosomal translocations and somatic mutations of the 5′ noncoding regulatory region of the BCL-6 gene are potential mechanisms for altering its expression in NHL. To further elucidate the nature of the somatic mutations in the regulatory region of this gene, we have studied 10 healthy donors and 11 NHL biopsy samples by extensive molecular cloning and sequencing. In addition, we analyzed the BCL-6 genes of tumor and nontumor cells from 2 of the cases. The germ line sequence of this region was defined, which differs in 7 positions from that previously reported. In addition, 1 polymorphic variation at position 397(G or C) was identified. Deletions, insertions, and repeated substitution mutations were detected among the molecular isolates in 8 tumor specimens, with a mutational incidence ranging from 1.3 × 10−3 to 1.3 × 10−2/bp (base pair). A total of 20 distinct substitution mutations, 1 insertion and 3 deletions were observed. One of these deletion mutations and 2 of the substitutions were observed in more than 1 tumor specimen from different individuals. In 3 tumor samples, identical mutations affecting both alleles were observed. These findings suggest the presence of mutational hot spots and hot specific events, a finding supported by our compilation of previously published data. In 6 samples, the nucleotide sequences showed evidence of intraclonal heterogeneity, consistent with a stepwise ongoing mutational process affecting the BCL-6 gene in the tumor cells. These mutations accumulating in the regulatory region of the BCL-6 gene could play a role in lymphoma progression and in the transformation of follicular lymphomas to more aggressive large cell lymphomas.
The BCL-6 proto-oncogene, identified by virtue of its involvement in chromosomal translocations affecting band 3q27, encodes a POZ/Zinc finger sequence-specific transcriptional repressor.1-3 It is normally expressed in B cells and CD4+ T cells within germinal center4 and controls germinal center formation as well as represses T helper cell type 2 mediated inflammatory responses.5,6 Clonal BCL-6 rearrangements cluster within a highly conserved 4.0 kilobase (kb) regulatory region, spanning the first noncoding exon containing the promoter and the 5′ region of the first intron—the major breakpoint region (MBR).2,7,8 These rearrangements that are observed in 30% to 40% of diffuse large B-cell lymphomas (DLBCL) and 6% to 10% of follicular lymphomas (FL)7,9,10 result in BCL-6 expression by formation of chimeric transcripts driven by a heterologous promoter from the partner chromosomes.11 In addition, recent studies disclosed small deletions12,13 and somatic point mutations in 70% of DLBCL and 45% of FL.14-16 Although these mutations occur in the subdomain of the BCL-6 regulatory region that overlap with the MBR, their occurrence is independent of translocation generated rearrangements.15
The BCL-6 gene mutations show predominance of transitions over transversions and may affect both alleles.14,15 Some similarities are observed in the hypermutational processes affecting the BCL-6 and the immunoglobulin (Ig) genes.14 Initial studies on tumor-derived BCL-6 alleles indicate that some mutations can significantly deregulate BCL-6 expression, whereas others are apparently functionally irrelevant.14 However, the precise nature of all the deregulating mutations is still unknown.
To further elucidate the nature of BCL-6 gene mutations, we have analyzed by extensive molecular cloning multiple BCL-6 gene sequences derived from non-Hodgkin lymphoma (NHL) specimens.
Materials and methods
Tumor specimens and blood mononuclear cells from healthy persons
Biopsy specimens from 11 NHL patients with follicular center lymphoma (FCL)8 and DLBCL3 that were stored as frozen viable single-cell suspensions were used in this study. Tumor samples were routinely immunophenotyped by flow cytometry for expression of Ig heavy and light chains and B- and T-cell markers. For B-cell enrichment, tumor cells were incubated with anti-CD 19 microbeads (Miltenyi Biotec, Auburn, CA) for 15 minutes at 4°C according to the manufacturer's protocol. After incubation, the cells were passed through a 70-μm nylon cell strainer (Becton Dickinson, San Jose, CA) and were enriched by magnetic cell separation using MidiMacs system (Miltenyi Biotec). Cells from every specimen were stained with anti-CD 20 and anti-CD 5 or anti-CD 3 antibodies (Becton Dickinson) before the enrichment, after enrichment, and in the flow-through part of the specimen. Tumor B cells were enriched to more than 95% in all the specimens. Normal T lymphocytes from 2 tumor specimens were enriched by CD 3 microbeads (Miltenyi Biotec). Blood mononuclear cells from 10 healthy persons were isolated by Ficoll-Isopaque density centrifugation Amersham Pharmacia Biotech, Piscataway, NJ.
DNA synthesis and polymerase chain reaction
High molecular-weight DNA was extracted from 5.0 × 106 cells with a commercially available kit as described by the manufacturer (QIAamp Tissue Kit; Qiagen, Valencia, CA). The first intron region of the BCL-6 gene that has previously been shown to undergo extensive mutation in B cells was amplified by polymerase chain reaction (PCR) using 0.5 to 1 μg of genomic DNA and the following primers: 5′-CCGCTGCTCATGATCATTATTT and 5′-TAGACACGATACTTCATCTCAT.
The reaction was primed from bp 1702, approximately 650 base pairs (bp) 3′ of the TATA box, and extended 790 bp downstream. The PCR was performed in a final volume of 50 μL containing a final concentration of 0.5 μmol of each primer, 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 200 μmol/L of each dNTP, and 2.5 units of Taq polymerase (GibcoBRL). The PCR conditions were as follows: 94°C for 5 minutes, 57°C for 30 seconds, 75°C for 1 minute, 1 cycle; 94°C for 30 seconds, 57°C for 30 seconds, 75°C for 1 minute, 30 cycles; and 75°C for 6 minutes, 1 cycle. For each PCR, a control with no added template was used to check for contamination. PCR products were analyzed by 2% agarose gel electrophoresis and staining with ethidium bromide. Bands of appropriate size were excised from the gels and purified by adsorption to a silica matrix (QIAquick columns, Qiagen).
Cloning and sequencing of PCR products
The purified PCR amplicons were cloned into a TA-PCR cloning vector (Invitrogen, Carlsbad, CA). After the transformation of competentEscherichia coli (1 Shot INV αF′, Invitrogen, Carlsbad, CA) and plating on selective agar (50 μg/mL kanamycin, 40 μg/mL × gal), 10 to 12 white colonies were picked per sample and used in a second round of PCR. At least 5 amplicons were selected for sequencing. DNA sequencing of PCR amplicons was performed on a 373 automatic DNA sequencer (Applied Biosystems, Foster City, CA) using ABI Prism Big Dye Terminator Kit (Perkin Elmer, Foster City, CA) as recommended by the manufacturer. The same primers used for the PCR were used for forward and backward sequencing.
Sequence analysis was performed using the programs MacVector and Assembly Lign (Oxford Molecular Group, Campbell, CA). Sequences were aligned with the BCL-6 hypermutation region germ line sequence sequenced from the blood mononuclear cells of the healthy people by the same methods. The Taq polymerase error frequency in our laboratory is 0.09%, which amounts to 0.71 mutations per BCL-6 clone.
Definitions
The following definitions were used in this manuscript:
Unconfirmed mutation: a substitution mutation observed in only 1 of the BCL-6 gene clones from the same tumor specimen.
Confirmed mutation: a mutation observed more than once in the BCL-6 gene clones from the same tumor specimen.
Recurrent mutation: a mutation observed in the BCL-6 gene clones from 2 or more tumor specimens from different patients.
Homozygous mutation: mutation status in tumor specimens in which only 1 identically mutated BCL-6 gene sequence was observed in all the tested BCL-6 gene clones.
Heterozygous mutation: mutation status in which 2 or more different BCL-6 gene sequences were observed in the genes cloned from a given tumor.
The number of single unconfirmed substitution mutations in each tumor specimen is included in Table 1, but these mutations were disregarded in our determination of the tumor mutation status, because at least some of them could have resulted from Taq polymerase error, as described later.
Characteristics of changes in the 5′ noncoding regulatory region of the BCL-6 gene in non-Hodgkin lymphoma
| Sample . | Diagnosis . | Number of Evaluated BCL-6 Gene Clones . | Nucleotide Changes in Deletions, Insertions and Confirmed Substitution Mutations . | Number of Additional Single Unconfirmed Substitution Mutations . | Allelic Status . | Intraclonal Heterogeneity . |
|---|---|---|---|---|---|---|
| 1 | FCL | 8 | 75 (T → C), 105 (T → C), 123 (C → T), 190 (T → C),397 (G → C), 479 (G → C), 484 (T → C), 494 (T → G), 520 ▵T, 627 (C → G) | 5 | Heterozygous | + |
| 2 | FCL | 11 | 331 (G → A), 371 (C → T), 403 (G → A), 520 ▵T | 15 | Heterozygous | + |
| 3 | FCL | 8 | 397 (G → C),520 ▵T, 577 + C, 729 ΔC | 12 | Heterozygous | + |
| 4 | FCL | 7 | 397 (G → C), 437 (G → T) | 7 | Heterozygous | — |
| 5 | FCL | 9 | 123 (C → T), 173 (C → A), 292 (G → A), 611 (G → T), 690 (A → C) | 7 | Heterozygous | + |
| 6 | FCL | 5 | 397 (G → C) | 1 | Heterozygous | — |
| 7 | FCL | 8 | 186 (T → C) | 9 | Heterozygous | — |
| 8 | FCL | 8 | 186 (T → C), 586 (C → A), 671 (T → A) | 6 | Heterozygous | + |
| 9 | DLBCL | 9 | 520 ▵T, 639 (C → T), 411 ΔCTCGCGCT | 7 | Heterozygous | + |
| 10 | DLBCL | 8 | 4 | Homozygous | — | |
| 11 | DLBCL | 5 | 1 | Homozygous | — |
| Sample . | Diagnosis . | Number of Evaluated BCL-6 Gene Clones . | Nucleotide Changes in Deletions, Insertions and Confirmed Substitution Mutations . | Number of Additional Single Unconfirmed Substitution Mutations . | Allelic Status . | Intraclonal Heterogeneity . |
|---|---|---|---|---|---|---|
| 1 | FCL | 8 | 75 (T → C), 105 (T → C), 123 (C → T), 190 (T → C),397 (G → C), 479 (G → C), 484 (T → C), 494 (T → G), 520 ▵T, 627 (C → G) | 5 | Heterozygous | + |
| 2 | FCL | 11 | 331 (G → A), 371 (C → T), 403 (G → A), 520 ▵T | 15 | Heterozygous | + |
| 3 | FCL | 8 | 397 (G → C),520 ▵T, 577 + C, 729 ΔC | 12 | Heterozygous | + |
| 4 | FCL | 7 | 397 (G → C), 437 (G → T) | 7 | Heterozygous | — |
| 5 | FCL | 9 | 123 (C → T), 173 (C → A), 292 (G → A), 611 (G → T), 690 (A → C) | 7 | Heterozygous | + |
| 6 | FCL | 5 | 397 (G → C) | 1 | Heterozygous | — |
| 7 | FCL | 8 | 186 (T → C) | 9 | Heterozygous | — |
| 8 | FCL | 8 | 186 (T → C), 586 (C → A), 671 (T → A) | 6 | Heterozygous | + |
| 9 | DLBCL | 9 | 520 ▵T, 639 (C → T), 411 ΔCTCGCGCT | 7 | Heterozygous | + |
| 10 | DLBCL | 8 | 4 | Homozygous | — | |
| 11 | DLBCL | 5 | 1 | Homozygous | — |
Recurrent mutations are in bold; polymorphic changes, in italics.
FCL = follicular center lymphoma; DLBCL = diffuse large B-cell lymphoma.
Δ represent deletion.
The first nucleotide of the amplified BCL-6 gene region, corresponding to the first nucleotide of the sense primer was arbitrarily defined as position +1.
Results
Sequence of the BCL-6 first intron
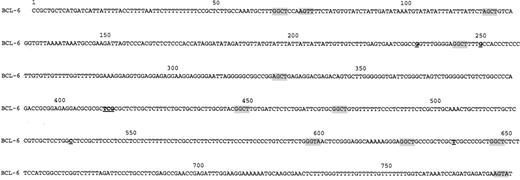
An identical sequence of the BCL-6 first intron region was obtained from multiple molecular clones derived from the blood mononuclear cells of 10 healthy donors (Figure 1). This sequence differs at 7 positions from the previously published BCL-6 first intron sequence.12
Nucleotide sequence of BCL-6 genomic DNA derived from MNC of healthy individuals.
Shaded tetranucleotide sequences indicate RGYW motifs. Differences from the previously published sequence12 are underlined.
Nucleotide sequence of BCL-6 genomic DNA derived from MNC of healthy individuals.
Shaded tetranucleotide sequences indicate RGYW motifs. Differences from the previously published sequence12 are underlined.
Unconfirmed single BCL-6 gene substitution mutations in tumor samples
Single but unconfirmed substitution changes from the established germ line BCL-6 gene sequence were observed in all the tested tumor specimens. Their number ranged from 1 to 15 per specimen, with an incidence of 0.2 to 1.5 bp/BCL-6 gene clone. In 7 samples (samples 1,5,6,8,9,10, and 11 in Table 1), the incidence of the single base-pair substitutions was similar to or lower than the 0.71 bp/ BCL-6 gene clone Taq error rate. Thus, the unique base-pair substitutions observed in these samples most probably originated from Taq polymerase error. In 4 samples (samples 2,3,4, and 7 in Table 1), the incidence of the single substitutions was higher than the Taq polymerase error rate, suggesting that at least some of these substitutions were real mutations. Because it is impossible to discriminate between real single substitution mutations and Taq polymerase errors, we decided to be conservative in our analytical approach and to exclude all the single unconfirmed substitution mutations from our subsequent analyses. In contrast, deletions and insertions that were observed only once were included in the subsequent analyses, because they cannot be attributed to Taq polymerase errors.
Confirmed unique and recurrent BCL-6 gene mutations in tumor samples
Deletions, insertions, and repeated confirmed nucleotide substitution changes from the established germ line BCL-6 gene sequence were detected in all FCL specimens and in 1 of 3 DLBCL specimens (Table1). The number of these changes varied from 1 to 10 per specimen, with an incidence ranging from 1.3 × 10−3 to 1.3 × 10−2/bp. A total of 21 distinct confirmed substitution changes from the established germ line BCL-6 gene sequence, 3 deletions, and 1 insertion were disclosed in the tested tumor samples. Eleven of these substitution changes were transitions, whereas 10 were transversions. One deletion mutation (Δ T 520) and 3 substitutions (123 [C→T], 186 [T→C], 397 [G→C]) were recurrently observed in more than 1 tumor specimen from different individuals, but in none of the BCL-6 gene sequences from healthy normal individuals studied in our laboratory. One report has noted that deletion Δ T 520 and substitution 397 [G→C] may be polymorphisms.15 None of these recurrent BCL-6 gene changes were observed in the G nucleotide of the RGYW motif (where R = purine, Y = pirimidine, and W = A or T) that is preferentially mutated in Ig genes and represents a mutational hot spot.17 Only 1 of these recurrent substitutions occurred in another well-established mutational hot spot in Ig genes — GC or TA dinucleotide motifs (position 123).18
Polymorphism versus somatic mutations
To examine the possibility that inherited polymorphism may have accounted for these recurrent changes, the BCL-6 gene sequence was determined in normal T cells from samples 1 and 8 (Table 1). These 2 tumors together demonstrated all the 4 recurrent changes that we had observed. The BCL-6 gene sequences derived from T cells of sample 8 were identical to the established germ line sequence, thus demonstrating that all the observed changes in this sample, including the recurrent 186 (T→C) change, represent real mutations. In the T cells from sample 1, 2 distinct BCL-6 gene sequences that varied at position 397 (G or C) were found. Therefore, the G→C change at position 397 represents polymorphism, as was previously reported.15 All the remaining changes, including recurrent 123 (C→T) and (Δ T 520) were absent from the normal cells and, thus, represent somatic mutations in the tumor.
Significance of recurrent somatic mutations
The recurrent occurrence of identical mutations at the same nucleotide may suggest the presence of mutational hot spots. To further evaluate the recurrent nature and the potentially skewed distribution of BCL-6 gene mutations, we combined our data with all the published mutations in NHL and Hodgkin lymphoma for which nucleotide change and exact mutation positions have been previously reported (Figure2).15,19-22 A total of 249 nucleotide substitutions, deletions, and insertions were found, including the mutations presented here. From this compilation, it is evident that certain nucleotide positions are frequently effected by mutations. The summary of the nucleotide changes at these positions is presented in Table 2. Some of the recurrent mutations observed in our study (positions 123 and 186) were also observed in previous studies,15 21 but previously they had not been noted to be recurrent.
Distribution of reported mutations in 5′ regulatory region of BCL-6 gene in NHL and Hodgkin lymphoma.
The first nucleotide of the amplified BCL-6 gene region, corresponding to the first nucleotide of the sense primer was arbitrarily defined as position 1. The characteristics of mutations repeatedly effecting the same position are presented in Table 2.
Distribution of reported mutations in 5′ regulatory region of BCL-6 gene in NHL and Hodgkin lymphoma.
The first nucleotide of the amplified BCL-6 gene region, corresponding to the first nucleotide of the sense primer was arbitrarily defined as position 1. The characteristics of mutations repeatedly effecting the same position are presented in Table 2.
Summary of mutations recurrently affecting the same nucleotide in the 5′ regulatory region of the BCL-6 gene
| Position . | Mutation . | Number of Reported Mutations . | Reference . |
|---|---|---|---|
| 84 | G → A | 2 | 15, 26 |
| G → C | 1 | 26 | |
| G → T | 1 | 27 | |
| 122 | G → C | 1 | 29 |
| G → A* | 3 | 26, 27 | |
| 123 | C → T | 4 | Present study, 15, 26 |
| C → G | 2 | 15, 26 | |
| 186 | T → C | 3 | Present study, 26 |
| 190 | T → C | 1 | Present study |
| T → A | 2 | 15, 29 | |
| 365 | G → A | 2 | 29 |
| ΔA | 1 | 15 | |
| 423 | C → G | 2 | 15, 27 |
| ΔC | 1 | 29 | |
| 437 | G → T* | 2 | Present study, 15 |
| G → A | 2 | 15, 26 | |
| 520 | ΔT | 4 | Present study |
| 748 | ΔT | 2 | 15, 28 |
| +T | 2 | 15, 28 | |
| T → G | 1 | 27 |
| Position . | Mutation . | Number of Reported Mutations . | Reference . |
|---|---|---|---|
| 84 | G → A | 2 | 15, 26 |
| G → C | 1 | 26 | |
| G → T | 1 | 27 | |
| 122 | G → C | 1 | 29 |
| G → A* | 3 | 26, 27 | |
| 123 | C → T | 4 | Present study, 15, 26 |
| C → G | 2 | 15, 26 | |
| 186 | T → C | 3 | Present study, 26 |
| 190 | T → C | 1 | Present study |
| T → A | 2 | 15, 29 | |
| 365 | G → A | 2 | 29 |
| ΔA | 1 | 15 | |
| 423 | C → G | 2 | 15, 27 |
| ΔC | 1 | 29 | |
| 437 | G → T* | 2 | Present study, 15 |
| G → A | 2 | 15, 26 | |
| 520 | ΔT | 4 | Present study |
| 748 | ΔT | 2 | 15, 28 |
| +T | 2 | 15, 28 | |
| T → G | 1 | 27 |
Only nucleotide positions affected by mutations 3 or more times are included in the table.
Denotes mutations that were also reported in normal germinal center lymphocytes (14).
Allelic heterozygous-homozygous status
All the 8 mutated lymphoma samples were heterozygous (Table 1) with retention of germ line sequence in 6. In 5 of these samples, an additional 2 or more distinct BCL-6 gene sequences were observed. In these samples, the retained germ line BCL-6 gene sequence may have originated from the unmutated BCL-6 gene within some of the tumor cell subclones or from normal cells infiltrating the tumor. The unmutated DLBCL cases were homozygous, whereas the unmutated FCL sample was heterozygous for 397(G or C) polymorphism.
Intraclonal heterogeneity
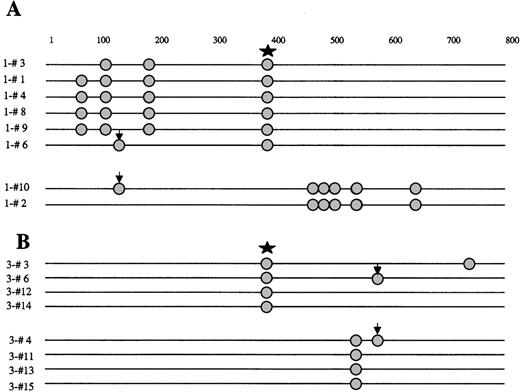
In 6 NHL samples (5 FCL and 1 DLBCL), the nucleotide sequences showed evidence of intraclonal heterogeneity (Table 1) consistent with a stepwise ongoing mutational process affecting the BCL-6 gene in the tumor cells. Representative examples of these mutations and the intraclonal heterogeneity of the tumor clones are demonstrated in Figures 3 and 4.
Schematic representation of the BCL-6 5′ noncoding region mutations in molecular clones derived from NHL samples, demonstrating intraclonal diversification and biallelic mutations.
Molecular clones are derived from NHL samples 1 (A) and 3 (B). Each sequence is represented as a horizontal line. The first nucleotide of the amplified BCL-6 gene region, corresponding to the first nucleotide of the sense primer is arbitrarily defined as position +1. In each sequence, mutations are indicated as circles. The nature of each mutation is specified in Table 1. Arrows indicate identical biallelic mutations. Star indicates 397 C polymorphism.
Schematic representation of the BCL-6 5′ noncoding region mutations in molecular clones derived from NHL samples, demonstrating intraclonal diversification and biallelic mutations.
Molecular clones are derived from NHL samples 1 (A) and 3 (B). Each sequence is represented as a horizontal line. The first nucleotide of the amplified BCL-6 gene region, corresponding to the first nucleotide of the sense primer is arbitrarily defined as position +1. In each sequence, mutations are indicated as circles. The nature of each mutation is specified in Table 1. Arrows indicate identical biallelic mutations. Star indicates 397 C polymorphism.
Genealogic tree of the mutation events in the 5′ nocoding regulatory region of the BCL-6 gene derived from DLBCL.
DLBCL is sample 4 in Table 1. Clonal relationship of the molecular clone sequences (ovals) is shown. Number of mutations is indicated along the lines in bold. The mutations are listed by location as specified in “Materials and Methods” and the legend to Figure1.
Genealogic tree of the mutation events in the 5′ nocoding regulatory region of the BCL-6 gene derived from DLBCL.
DLBCL is sample 4 in Table 1. Clonal relationship of the molecular clone sequences (ovals) is shown. Number of mutations is indicated along the lines in bold. The mutations are listed by location as specified in “Materials and Methods” and the legend to Figure1.
Biallelic mutations
By examining the sequences from a given case for the presence of the polymorphism or the pattern of mutations, we could assign the 2 alleles of the tumor. Mutations occurred in both alleles in 6 of the 8 mutated lymphomas. Moreover, in 3 tumor samples, identical mutations affecting both alleles were observed (Figure 3).
Discussion
After recent reports on somatic mutation in the 5′ noncoding regulatory region of the BCL-6 gene, we examined this region for mutation in lymphomas by evaluation of multiple molecular clones of the BCL-6 gene from each lymphoma sample. In previous reports, BCL-6 mutation analysis was performed by the technique of single strand conformational polymorphism (SSCP). Although the SSCP method is rapid, it is less sensitive for the detection of mutations than extensive actual sequencing of molecular clones, and it may bias the analysis or underestimate the extent of the mutational process.23 By applying a sequencing approach, we demonstrate (a) a germ line sequence different from that previously published12; (b) a polymorphism of G-C at position 397; (c) ongoing BCL-6 gene mutation in 6 of the 11 tested NHL samples; and (d) the presence of recurrent mutations observed repeatedly in lymphoma samples from different patients.
An ongoing mutation process at the Ig locus is a hallmark of germinal center derived lymphomas, such as FL,24,25 Burkitt lymphoma,26 and a subset of DLBCL.27,28 In the current study, we have shown that the untranslated regulatory region of the BCL-6 gene – the single non-Ig gene currently known to undergo somatic hypermutation,29 also shows intraclonal heterogeneity in some FCL and DLBCL cases. The extent of the intraclonal heterogeneity is conservatively estimated here because we excluded single unconfirmed substitution mutations from the analysis. The functional significance of this intraclonal divergence of the BCL-6 regulatory region is currently unknown. Previously, it had been shown that some mutations can significantly deregulate BCL-6 expression.15 Therefore, it is possible that, like in the Ig genes, in which mutations are selected by the antigenic stimulation permitting preferential lymphocyte survival,24 BCL-6 gene mutations could be selected by growth advantage afforded by the lymphomatous state. Ongoing BCL-gene mutations may eventually lead to a subclone with the BCL-6 gene as deregulated as if it were translocated to another chromosome and provide a critical event in lymphoma progression or in the conversion of FL to a higher grade malignancy.
The observed incidence of the BCL-gene mutations and the excess of transitions over transversions are in agreement with previous reports.14-16 However, we have detected the presence of recurrent mutations and identical biallelic mutations in some of the lymphoma samples. Identical biallelic mutations in the BCL-6 gene were recently reported in 2 cases of primary effusion lymphoma.21 One potential problem with comparing gene sequences from different individuals is that of inherited polymorphism. In the case of the human BCL-6 hypermutational region, there is only 1 published DNA sequence.12 The normal sequence that we determined has a total of 7 differences from the published sequence, none of which occurred at the positions of recurrent substitution mutations observed in our tumor samples. To examine the possibility of polymorphism, we sequenced the BCL-6 gene from T cells derived from 2 tumor samples that exhibited the 4 recurrent changes. Only the 397(G→C) change was observed in these cells, thus representing a polymorphic change, whereas 186(T→C), 123(C→T), and (Δ T 520) are nonpolymorphic recurrent mutations observed in our NHL cohort. Furthermore, review of the literature15 19-22confirmed the recurrent occurrence of the mutations at positions 123 and 186 and demonstrated presence of recurrent mutations at additional positions (Figure 2 and Table 2).
The significance of these recurrent mutations is unknown. It is possible that they occur at mutational hot spots similar to the hot spots present in Ig genes.17 However, identical types of mutation at the same position in more than 1 lymphoma sample argues not just for hot spots but for hot specific events. That is, there must be a propensity to change not just at certain position but to make certain specific mutations. These recurrent mutations could result from mutational events that for mechanistic reasons are more probable, or they could be selected for by their effect on the regulation of BCL-6 gene, leading to expansion and survival of tumor clones containing these mutations. The nucleotide motifs predisposing to these recurrent mutations in the BCL-6 gene are still unknown, but most probably they are different from the mutational hot spot motifs of the Ig gene, such as RGWY and dinucleotides GC and TA.17 18 To evaluate the potential influence of these mutations on BCL-6 regulation, we searched for possible transcription factors binding sites in and around the sites of recurrent mutations observed by us (TFSEARCH on the Internet). Mutations 186(T→C) and 123(C→T) occurred in the vicinity of potential binding sites of GATA 2 and PBX-1 transcription factors, respectively. Whether these transcription factors have any role in BCL-6 gene regulation is unknown.
In conclusion, the current study shows intraclonal heterogeneity of the BCL-6 gene regulatory region in NHL samples due to the process of ongoing somatic mutation. Furthermore, our data suggest the presence of mutational hot spots in the BCL-6 gene. Further studies are needed to determine the functional significance of recurrent mutations and the potential role of the BCL-6 gene regulatory region mutations in tumor progression and transformation.
Supported by grants CA33399 and CA34233 from the USPHS-NIH. R.L. is an American Cancer Society Clinical Research Professor.
Reprints:Ronald Levy, Stanford University School of Medicine, Division of Oncology M207, Stanford, CA 94305-5306.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal