The human erythrocyte membrane skeleton consists of hexagonal lattices with junctional complexes containing F-actin protofilaments of approximately 33-37 nm in length. We hypothesize that complexes formed by tropomodulin, a globular capping protein at the pointed end of actin filaments, and tropomyosin (TM), a rod-like molecule of approximately 33-35 nm, may contribute to the formation of protofilaments. We have previously cloned the human tropomodulin complementary DNA and identified human TM isoform 5 (hTM5), a product of theγ-TM gene, as one of the major TM isoforms in erythrocytes. We now identify TM5b, a product of the -TM gene, to be the second major TM isoform. TM5a, the alternatively spliced isoform of the-TM gene, which differs by 1 exon and has a weaker actin-binding affinity, however, is not present. TM4, encoded by the δ-TM gene, is not present either. In sodium dodecyl sulfate–polyacrylamide gel electrophoresis, hTM5 comigrated with the slower TM major species in erythrocyte membranes, and hTM5b comigrated with the faster TM major species. TM5b, like TM5, binds strongly to tropomodulin, more so than other TM isoforms. The 2 major TM isoforms, therefore, share several common features: They have 248 residues, are approximately 33-35 nm long, and have high affinities toward F-actin and tropomodulin. These common features may be the key to the mechanism by which protofilaments are formed. Tropomodulin-TM5 or tropomodulin-TM5b complexes may stabilize F-actin in segments of approximately 33-37 nm during erythroid terminal differentiation and may, therefore, function as a molecular ruler. TM5 and TM5b further define the hexagonal geometry of the skeletal network and allow actin-regulatory functions of TMs to be modulated by tropomodulin.
The erythrocyte membrane skeletal network is a spectrin-actin–based 2-dimensional protein network anchored to the endoface of the membrane. It provides the mechanical stability and elastic deformability for the lipid bilayer. The protein network is organized into hexagonal lattices (Figure1A), with 6 long spring-like spectrin tetramers (approximately 200 nm) binding to 1 short actin filament (approximately 33-37 nm) at the junctional complex (indicated by arrows).1-3 Several other actin-binding proteins, such as myosin, tropomyosin4 (TM), and tropomodulin,5 6 exist in erythrocytes. The properties, kinetics, and expression of these actin-associated proteins may play important roles in regulating the length of actin filaments and thus the spectrin-actin organization and the mechanical properties of the erythrocyte membrane.
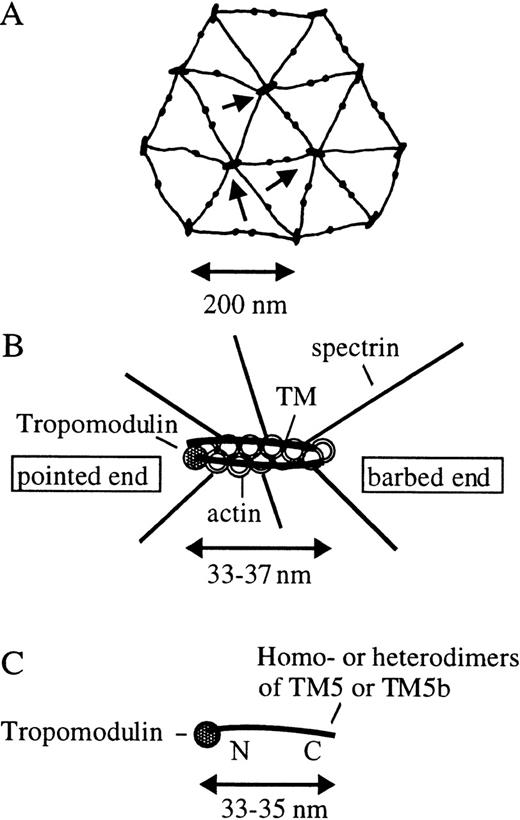
Hexagonal lattices, protofilament, and tropomodulin-TM complex of the erythrocyte membrane skeletal network.
(A) The top view of the hexagonal network based on the electron micrographs.1-3 As indicated by an arrow, 6 spectrin tetramers are associated with 1 junctional complex. The junctional complexes are approximately 33-37 nm in length. Each spectrin tetramer is approximately 200 nm long and comprises 2 αβ spectrin dimers associated with a head-to-head fashion. The 2 tail ends of the spectrin tetramer join the junctional complexes, and the 2 head ends meet with each other in the middle. The pair of smaller complexes in the mid regions of the spectrin tetramers are protein 4.2/band 3/ankyrin complexes, which hang the membrane skeletal network to the lipid bilayer. (B) The molecular model of an actin protofilament in the erythrocyte membrane skeleton. The filament's length is approximately 6-7 G-actin and is associated with only 2 TM molecules, 1 in each groove of the actin filament, and 1 tropomodulin molecule at the pointed end. The barbed end may either be uncapped or capped by adducin,7 gelsolin,8 or another barbed-end capping protein. The number of G-actin limits the number of spectrin binding to the protofilament and defines the hexagonal geometry of the membrane network. (C) The model of the tropomodulin-TM complex that stabilizes the actin protofilament. Tropomodulin binds near the N-terminal of TM5 or TM5b (homodimer or heterodimer approximately 33-35 nm in length, 6 actin-binding sites), at the pointed end of the actin filament. The complex functions as a measuring device, determining the number of G-actin to be protected. N and C stand for the N-terminal and C-terminal of the TM molecule, respectively.
Hexagonal lattices, protofilament, and tropomodulin-TM complex of the erythrocyte membrane skeletal network.
(A) The top view of the hexagonal network based on the electron micrographs.1-3 As indicated by an arrow, 6 spectrin tetramers are associated with 1 junctional complex. The junctional complexes are approximately 33-37 nm in length. Each spectrin tetramer is approximately 200 nm long and comprises 2 αβ spectrin dimers associated with a head-to-head fashion. The 2 tail ends of the spectrin tetramer join the junctional complexes, and the 2 head ends meet with each other in the middle. The pair of smaller complexes in the mid regions of the spectrin tetramers are protein 4.2/band 3/ankyrin complexes, which hang the membrane skeletal network to the lipid bilayer. (B) The molecular model of an actin protofilament in the erythrocyte membrane skeleton. The filament's length is approximately 6-7 G-actin and is associated with only 2 TM molecules, 1 in each groove of the actin filament, and 1 tropomodulin molecule at the pointed end. The barbed end may either be uncapped or capped by adducin,7 gelsolin,8 or another barbed-end capping protein. The number of G-actin limits the number of spectrin binding to the protofilament and defines the hexagonal geometry of the membrane network. (C) The model of the tropomodulin-TM complex that stabilizes the actin protofilament. Tropomodulin binds near the N-terminal of TM5 or TM5b (homodimer or heterodimer approximately 33-35 nm in length, 6 actin-binding sites), at the pointed end of the actin filament. The complex functions as a measuring device, determining the number of G-actin to be protected. N and C stand for the N-terminal and C-terminal of the TM molecule, respectively.
While the actin filaments in nonmuscle cells are generally long and vary in length, the actin filaments in erythrocyte membranes, as revealed by electron microscopy, were relatively short and uniform, approximately 33-37 nm in length. The mechanism by which such short actin protofilaments is generated is not clear. Since 33-37 nm approximates the length of a TM molecule, it was thought that the protofilament is the result of actin-TM complexing.2 It was not clear, however, why the actin-TM complex should stop with only 1 TM molecule.
TM is a family of actin-binding proteins present in the thin filaments of muscle cells and in the microfilaments of nonmuscle cells. TM molecules comprise 2 monomers and polymerize in a head-to-tail fashion along the grooves of the actin double helix. TM molecules function to stiffen the filament, stabilize its polymerization, and regulate its interaction with other actin-binding proteins. There are several TM isoforms encoded by at least 4 distinct genes in humans: α, β, γ, and δ.9-11 The diversity of TM is further generated by alternative promoters and alternative splicing of primary RNA. Tissue-restriction or specific expression of TM suggests that different TM isoforms may perform distinct functions in different tissues, especially in regulating cell motility and in organizing actin filaments.9-14
In human erythrocytes, TMs of Mr 29 000 andMr 27 000 (molar ratio of 3:1) have been reported.4 There are approximately 70 000 copies of TM molecules per ghost. We have previously identified 1 major TM isoform to be human TM isoform 5 (hTM5), which comigrated with the slower migrating TM species.15 It is a gene product of the humanγ-TM gene, and it is approximately 33-35 nm in length. It was not clear what is the other major faster migrating TM isoform(s) expressed in human erythrocyte membranes.
Tropomodulin is a 359-residue16 TM-binding protein first discovered in human erythrocyte membranes.5 There are approximately 30 000 copies of tropomodulin per ghost. Tropomodulin binds to the N-terminal end of the TM molecule6,15 and has the ability to inhibit the cooperativity of TM and the TM binding to actin.6 Together with TM, tropomodulin inhibits the elongation and depolymerization of the actin filaments at the pointed end. It is, therefore, a capping protein17 that binds to the pointed end of the actin filaments18 and is involved in the regulation of actin filament length.19 20
We have cloned the first tropomodulin complementary DNA (cDNA) from human fetal liver and reticulocytes16 and mapped the human tropomodulin gene to chromosome 9, long arm, band 22 (9q22).21Northern blot analysis of human tissues revealed that in addition to erythrocytes, tropomodulin is highly expressed in the heart and skeletal muscle, with moderate or very little expression in some other issues.21 The function of tropomodulin, therefore, is important not only in erythrocytes but also in several other cell types. For example, myofibril degeneration caused by tropomodulin overexpression has been shown to lead to dilated cardiomyopathy in juvenile mice.22
We have previously demonstrated that tropomodulin binds to hTM5 more strongly than other TM isoforms (eg, hTM2, hTM3, chicken skeletal muscle isoforms, and chicken smooth muscle isoforms).15 It is possible that tropomodulin may play an important role in regulating the organization of actin filaments by preferentially binding to specific TM isoforms at the pointed end of the actin filaments. Based on our findings that tropomodulin binds to the N-terminal of hTM5 and that hTM5 is one of the major TM isoforms in human erythrocytes, we have previously proposed that tropomodulin complexed with hTM5 may contribute to the protofilament formation in erythrocyte membranes.15
Here we report that TM5b, one of the alternatively spliced gene products of the α-TM gene, is the second major TM isoform in human erythrocytes. The fact that TM5 and TM5b are the 2 TM isoforms that have not only the highest tropomodulin affinities but also the highest F-actin affinities (among known low-molecular weight [LMW] TM isoforms) suggests that the tight association of tropomodulin/TM/F-actin may be the key to the mechanism of protofilament formation. Since both TM5 and TM5b are LMW TM isoforms with 6 actin-binding sites, they also favor the association of 6 spectrin molecules to 1 protofilament and, thus, the formation of hexagonal lattices in the erythrocyte membrane skeletal network. TM5, TM5b, and tropomodulin, therefore, are important molecules in the development and organization of the membrane skeletal network.
Materials and methods
Antibodies
Mouse monoclonal antibodies (mAbs) CG3 (immunoglobulin M [IgM]),23 LC1 (IgG),11,24 Pep3-43 (IgG),25 and Tmod-20415 have been described. The rabbit antiserum R-41 was raised by using a combination of recombinant hTM5 and chimeric hTM5/4 isoforms as the immunogen. The peptide-specific antibody against tropomodulin was raised in rabbits using a peptide corresponding to residues 70-84 of human erythrocyte tropomodulin,16 conjugated to a protein carrier, keyhole limpet hemocyanin.
Recombinant TM isoforms
Western blot analysis
Human and rat erythrocyte ghost membranes were prepared27 and dissolved in equal volumes of sodium dodecyl sulfate–solubilization (SDS-solubilization) solution containing 0.2% bromophenol blue, 20% glycerol, 4% SDS, 100 mmol/L Tris-HCl (tris(hydroxymethyl) aminomethane hydrochloride) (pH 6.8), and 200 mmol/L dithiothreitol. Using 10% SDS-PAGE28(polyacrylamide gel electrophoresis), 30 μg host membranes and 2 μg recombinant TMs were electrophoresed and transblotted onto a nitrocellulose membrane.29 The transblot was incubated with either mouse mAbs CG3, LC1, Pep3-43, or LC24 or rabbit antiserum R-41 followed by a horse radish peroxidase (HRP)-conjugated secondary antibody to mouse IgG or IgM or rabbit IgG. The transblot was then developed enzymatically.
Sequential Western blot analysis
Human erythrocyte ghost membranes (30 μg) were electrophoresed using 12% SDS-PAGE and transblotted onto a nitrocellulose membrane. The same strip was subjected to 2 distinct rounds of Western blotting with 2 different primary antibodies. Specifically, mAb Pep3-431:100 was used, followed by an HRP-conjugated secondary antibody specific for mouse IgG.1:1000 The strip was developed enzymatically. After development, the same strip was incubated with mAb LC1,1:1000 followed by an HRP-conjugated secondary antibody1:1000 specific for mouse IgG and enzymatic development.
Recombinant human tropomodulin
Human tropomodulin cDNA clone 1016 was subcloned into an expression vector, pMal-c230 31 (New England BioLabs, Beverly, MA). The constructed plasmid pMBP/Tmod-10 was used to transform Escherichia coli (E coli) DH5α. The induction of expression by isopropyl β-D-thiogalactopyranoside (IPTG) and purification of the fusion protein, MBP/Tmod-10, were performed according to the manufacturer's protocol, with a modification so that glucose was not added in the culture media.
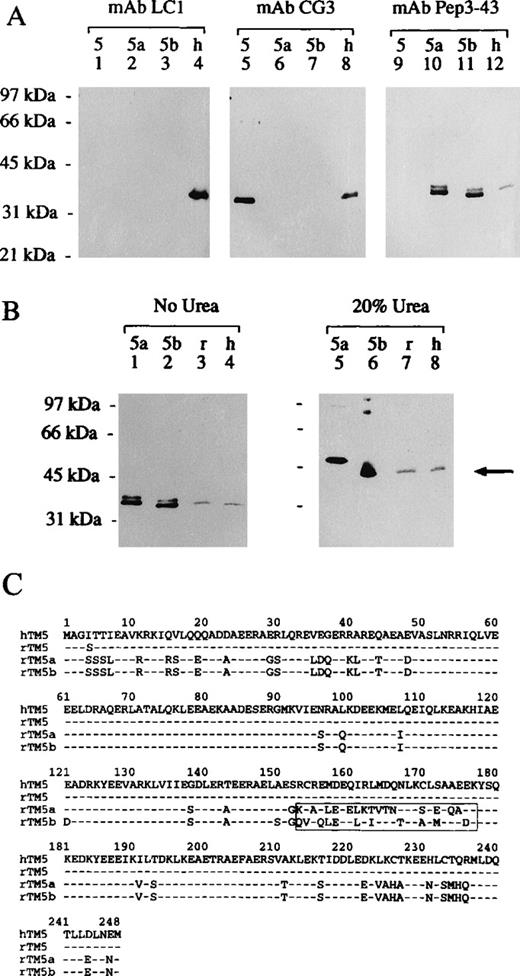
Specificity of mAbs CG3, LC1, and Pep3-43 and the detection of TM5b in human erythrocyte ghost membranes.
(A) Using SDS-PAGE, 2 μg each of rTM5, rTM5a, and rTM5b and 30 μg human erythrocyte ghost membranes were separated on a 10% gel. Three identical sets of proteins were transblotted onto nitrocellulose membranes and probed either with mAbs CG31:1000 (panel labeled “mAb CG3”), LC11:500 (panel labeled “mAb LC1”), or Pep3-431:100 (panel labeled mAb “Pep3-43”). Secondary antibodies conjugated with HRP were used, and the signals were developed enzymatically. Molecular weight standards (lane 1) are phosphorylase b (97 kd), bovine serum albumin (66 kd), ovalbumin (45 kd), carbonic anhydrase (31 kd), and soybean trypsin inhibitor (21 kd). (B) This panel demonstrates the presence of TM5b, but not TM5a, in human erythrocyte ghost membranes. Using SDS-PAGE, we separated 2 μg each of purified rTM5a (lanes 1 and 5) and rTM5b (lanes 2 and 6) and 30 μg ghost membranes prepared from rat (lanes 3 and 7) and human (lanes 4 and 8) erythrocytes on an 10% gel in the absence (panel labeled “No Urea”) and presence (panel labeled “20% Urea”) of 20% urea. Both transblots were probed with mAb Pep3-431:100 and an HRP-conjugated secondary antibody against mouse IgG; the color was developed enzymatically. The arrow points to the band that comigrated with rTM5b in human and rat ghost membranes. (C) Amino acid (1 letter code) sequences of hTM5,35 rTM5,25 rTM5a, and rTM5b36are shown; numbers on the top refer to the amino acid residues. The − indicates residue identical with those in hTM5, with human (h) and rat (r) identified. rTM5a is identical to rTM5b except for the boxed region (residues 152-177) encoded by an alternative exon.
Specificity of mAbs CG3, LC1, and Pep3-43 and the detection of TM5b in human erythrocyte ghost membranes.
(A) Using SDS-PAGE, 2 μg each of rTM5, rTM5a, and rTM5b and 30 μg human erythrocyte ghost membranes were separated on a 10% gel. Three identical sets of proteins were transblotted onto nitrocellulose membranes and probed either with mAbs CG31:1000 (panel labeled “mAb CG3”), LC11:500 (panel labeled “mAb LC1”), or Pep3-431:100 (panel labeled mAb “Pep3-43”). Secondary antibodies conjugated with HRP were used, and the signals were developed enzymatically. Molecular weight standards (lane 1) are phosphorylase b (97 kd), bovine serum albumin (66 kd), ovalbumin (45 kd), carbonic anhydrase (31 kd), and soybean trypsin inhibitor (21 kd). (B) This panel demonstrates the presence of TM5b, but not TM5a, in human erythrocyte ghost membranes. Using SDS-PAGE, we separated 2 μg each of purified rTM5a (lanes 1 and 5) and rTM5b (lanes 2 and 6) and 30 μg ghost membranes prepared from rat (lanes 3 and 7) and human (lanes 4 and 8) erythrocytes on an 10% gel in the absence (panel labeled “No Urea”) and presence (panel labeled “20% Urea”) of 20% urea. Both transblots were probed with mAb Pep3-431:100 and an HRP-conjugated secondary antibody against mouse IgG; the color was developed enzymatically. The arrow points to the band that comigrated with rTM5b in human and rat ghost membranes. (C) Amino acid (1 letter code) sequences of hTM5,35 rTM5,25 rTM5a, and rTM5b36are shown; numbers on the top refer to the amino acid residues. The − indicates residue identical with those in hTM5, with human (h) and rat (r) identified. rTM5a is identical to rTM5b except for the boxed region (residues 152-177) encoded by an alternative exon.
Solid-phase binding assay
Recombinant TMs (2 μg) were electrophoresed in 10% SDS-PAGE28 and transblotted onto a nitrocellulose membrane.29 The binding assay was identical to an established procedure for Western blot analysis (Bio-Rad, Hercules, CA) except that the transblot was incubated with 26 μg/mL recombinant tropomodulin in Tris-buffered saline (pH 7.4) for 1.5 hours before immunological analyses. For detection, a rabbit peptide-specific antibody to tropomodulin was used, followed by an HRP-conjugated secondary antibody to rabbit IgG and enzymatic development.
Results
Detection of TM5a and/or TM5b isoforms in human erythrocyte membranes
To search for additional unknown TM isoforms in human erythrocytes, we first investigated the specificity of a group of mAbs (ie, CG3, LC1, and Pep3-43) and then used them to detect specific TM isoforms in human erythrocyte membranes (Figure 2). The mAbs CG3 and LC1 were previously used to identify hTM5, a gene product of theγ-TM gene, in human erythrocytes.15 In search for a TM isoform, which may migrate faster than hTM5 in SDS-PAGE, mAb Pep3-43 was chosen to identify hTM5a and/or TM5b, the 2 alternatively spliced gene products of the α-TM gene, in human erythrocyte membranes.
TM5 and TM5b are the 2 major TM isoforms in human erythrocyte membranes.
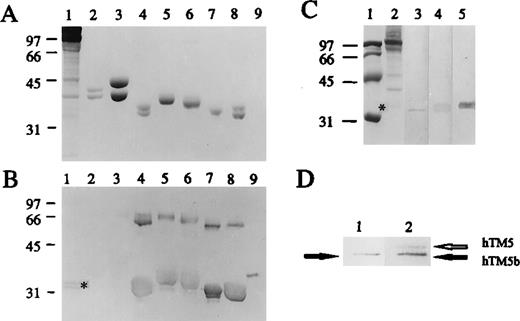
(A) A Coomassie blue stain of several purified TM isoforms and their chimeric molecules separated on a 12% gel using SDS-PAGE. (B) The Western blot analysis of the above, with antibody R-41. Samples are 30 μg human ghost membrane (lane 1), chicken leg TM (lane 2), chicken gizzard TM (lane 3), hTM5/2 (lane 4), hTM2 (lane 5), hTM3 (lane 6), hTM5 (lane 7), hTM5/3 (lane 8), and hTM3/5 (lane 9). Approximately 10 μg TM isoforms (except hTM3/5, which has less) are applied. (C) The following lanes depict the Western blot analysis of 30 μg human ghost membrane by mAb Pep3-431:100 (lane 3), antibody R-411:100 (lane 4), and mAb LC11:500(lane 5). The panel also depicts the Coomassie blue stains of human ghost membrane (lane 2) and the molecular weight standards (lane 1). (D) A sequential Western blot analysis demonstrates the distinction between hTM5 and hTM5b. Lane 1 presents the first round of Western blot with mAb Pep3-43,1:100 while lane 2 shows the same strip after the second round of Western blot with mAb LC1.1:1000
TM5 and TM5b are the 2 major TM isoforms in human erythrocyte membranes.
(A) A Coomassie blue stain of several purified TM isoforms and their chimeric molecules separated on a 12% gel using SDS-PAGE. (B) The Western blot analysis of the above, with antibody R-41. Samples are 30 μg human ghost membrane (lane 1), chicken leg TM (lane 2), chicken gizzard TM (lane 3), hTM5/2 (lane 4), hTM2 (lane 5), hTM3 (lane 6), hTM5 (lane 7), hTM5/3 (lane 8), and hTM3/5 (lane 9). Approximately 10 μg TM isoforms (except hTM3/5, which has less) are applied. (C) The following lanes depict the Western blot analysis of 30 μg human ghost membrane by mAb Pep3-431:100 (lane 3), antibody R-411:100 (lane 4), and mAb LC11:500(lane 5). The panel also depicts the Coomassie blue stains of human ghost membrane (lane 2) and the molecular weight standards (lane 1). (D) A sequential Western blot analysis demonstrates the distinction between hTM5 and hTM5b. Lane 1 presents the first round of Western blot with mAb Pep3-43,1:100 while lane 2 shows the same strip after the second round of Western blot with mAb LC1.1:1000
Throughout the Western blot analyses (Figure 2A and 2B), samples labeled 5, 5a, and 5b are purified bacterial recombinant proteins of TM isoforms derived from the corresponding rat cDNA clones, and samples labeled h and r are total proteins of ghost membranes purified from human and rat erythrocytes, respectively. To understand the specificity of these mAbs and the results, 4 published amino acid sequences, hTM535, rat TM525(rTM525), rTM5a, and rTM5b36 are shown and aligned in Figure 2C.
The middle panel of Figure 2A, labeled mAb CG3, demonstrates that mAb CG3, which recognizes an epitope within residues 29-44 of the hTM5 amino acid sequence, was able to cross-react with the purified rTM5 but not with rTM5a or rTM5b. Thus, the mAb CG3 is specific for hTM5 and rTM5 and can distinguish between TM5 and TM5a/5b. In human erythrocyte ghost membranes (lane 8), the mAb CG3 was capable of detecting and confirming the presence of hTM5.15 Such specificity can be explained by the amino acid sequences of these TM isoforms (Figure 2C): In residues 29-44, rTM5 is identical to hTM5, whereas rTM5a and rTM5b share only a 50% sequence identity with hTM5. The mAb CG3 is, therefore, TM5 specific.
The left panel of Figure 2A, labeled mAb LC1, demonstrates the specificity of mAb LC1; mAb LC1 was able to detect hTM5 in human erythrocyte ghosts (lane 4), as previously reported.15However, mAb LC1 did not cross-react with purified rTM5 (lane 1) as did mAb CG3. The entire sequences of rTM5 and hTM5 are absolutely identical except for 1 residue: at the fourth position, rTM5 has a serine4 (Ser) and hTM5 has an isoleucine4 (Ile) (Figure 2C). We (L. A. Sung and J. J.-C. Lin, unpublished result) have previously discovered that mAb LC1 did not recognize mouse TM5 (mTM5). The entire sequences of mTM537 and hTM5 are also absolutely identical except for 1 residue, also at the fourth position, where mTM5 has a threonine4 (Thr) and hTM5 has an Ile.4The findings that the mAb LC1 did not recognize rTM5 or mTM5 indicates the absolute requirement of Ile4 for the recognition of the mAb LC1. In fact, by using TM5 molecules naturally existing in different species, we mapped the epitope of mAb LC1 to include Ile4 of hTM5. The mAb LC1 panel also showed that mAb LC1 did not cross-react with purified recombinant rTM5a (lane 2) or rTM5b (lane 3). This finding is reasonable since rTM5a and rTM5b not only have a Ser4 but also have 3 additional different residues immediately downstream from Ser4 (Figure 2C). The mAb LC1 is, therefore, not only TM5-specific but also human-specific.
Finally, the right panel of Figure 2A, labeled mAb Pep3-43, demonstrates the specificity of mAb Pep3-43 and shows that it detected a specific band in human erythrocyte membranes. ThemAb Pep3-43 recognized both rTM5a (lane 10) and rTM5b (lane 11) purified from recombinant bacteria. This finding is expected because the 2 alternatively spliced TM isoforms have an identical N-terminal sequence (Figure 2C), which contains the peptide sequence n-AGSSSLEAVERRKIRSLC-c (residues 2-19) used in generating mAb Pep3-43.25 It is important to note that mAb Pep3-43 did not cross-react with rTM5 (lane 9). In a separate experiment, mAb Pep3-43 was also shown not to cross-react with purified recombinant hTM5 (data not shown). The mAb Pep3-43 is, therefore, TM5a/5b specific. In the human erythrocyte ghost membrane (lane 12), mAb Pep3-43 was found to recognize a specific band of ∼Mr 34 000 that comigrated with the purified recombinant rTM5a and rTM5b.
This series of experiments concludes that: (1) these 3 mAbs (LC1, Pep3-43, and GC3) are capable of distinguishing between TM5 and TM5a/5b, and mAb LC1 can even distinguish between hTM5 and TM5 of other species (eg, mouse and rat); (2) mAb Pep3-43, which was made against an N-terminal peptide of rTM5a/5b, is capable of cross-reacting with hTM5a/5b; and (3) human erythrocyte membranes contain hTM5a, hTM5b, or both.
It is not surprising that mAb Pep3-43 can recognize hTM5a/5b. hTM5a/5b differs from rTM5a/5b in only 2 conserved amino acid changes at position 30 (tyrosine [Thr or T] versus Ser or S) and position 37 (histidine [His or H] versus glutamine [Gln or Q]).12In addition, the rTM5a/5b peptide used as the immunogen is located at the conserved N-terminal region, which is shared by rTM5a/5b and hTM5a/5b.
TM5a and TM5b are 2 alternatively spliced gene products from the α-TM gene. TM5a has a weaker actin-binding affinity than TM5b and differs from TM5b only in the alternatively spliced exon 6, which encodes residues 152-177 (boxed in Figure 2C).9 Their relative molecular weights, therefore, are indistinguishable under the above experimental condition. The α-TM gene also encodes skeletal muscle α-TM, which is a high molecular weight (HMW) TM and has a different N-terminal.
TM5b, not TM5a, present in human erythrocyte membranes
To determine whether TM5a, TM5b, or both are present in human erythrocyte membranes, we carried out a second set of Western blot analyses (Figure 2B). To be complete, we included rat erythrocyte membranes here as a control, since mAb Pep3-43 was made against TM5a/5b from rats. The experiments were carried out in both the presence and absence of urea. The panel labeled “No Urea” showed a single polypeptide recognized by mAb Pep3-43 in rat (lane 3) and human (lane 4) ghost membranes. Both rat and human polypeptides comigrated with the purified recombinant rTM5a (lane 1) and rTM5b (lane 2). Since rTM5a and rTM5b comigrated with each other under this experimental condition (as in Figure 2A), 20% urea was included in the other gel (panel labeled “20% Urea”). Under this condition, the migration of the denatured rTM5a and rTM5b can be distinguished from each other: rTM5a (lane 5) migrated slightly slower than rTM5b (lane 6). Under this condition, a single mAb Pep3-43–reactive polypeptide with an apparentMr of about 38 000 was found in the human ghost membrane (lane 8). This polypeptide comigrated exactly with rTM5b (lane 6) and not with rTM5a (lane 5). The results clearly demonstrated that TM5b, not TM5a, is present in the human membrane skeletal network.
Differential migration of hTM5 and hTM5b in SDS-PAGE
In human erythrocyte membranes, there are 2 closely migrating TM bands revealed by SDS-PAGE in the absence of urea.4 Because we previously reported that hTM5 is the isoform that comigrates with the major slower migrating species of the TM bands,15 it is of interest to know whether hTM5b is the isoform that comigrates with the faster migrating species.
R-41 is a polyclonal antibody that cross-reacts with several nonmuscle TM isoforms. TM2, TM3, TM5, and several chimeric TM molecules were used in a Western blot analysis to demonstrate its wide range of recognition among TM isoforms (Figure 3). R-41 was then used to demonstrate the existence of 2 major TM bands in human erythrocyte ghost membranes.
Construction, expression, and purification of human recombinant tropomodulin and its binding to rTM5 and rTM5b.
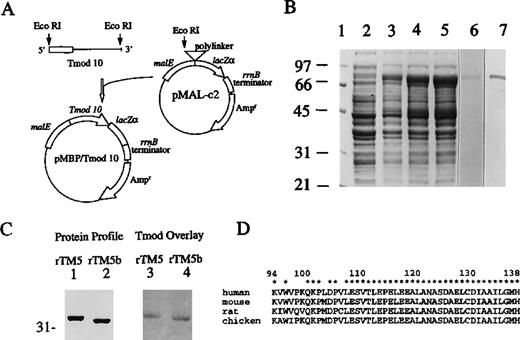
(A) Human tropomodulin cDNA clone 10 was subcloned at the EcoR1 site downstream from the MalE gene, which encodes MBP. (B) The induction of the MBP/tropomodulin fusion protein. Total proteins ofE coli were separated on a 7% gel using SDS-PAGE and stained by Coomassie blue, before the addition of IPTG (lane 2) and 1 hour (lane 3), 2 hours (lane 4), and 3 hours (lane 5) after the addition of IPTG. Affinity-purified tropomodulin fusion protein (Mr 79 000) was either stained by Coomassie blue (lane 6, 2 μg) or transferred onto a nitrocellulose membrane and detected with tropomodulin-specific antibody using Western blot analysis (lane 7, 0.1 μg). Molecular weight standards (lane 1) are the same as in Figure 2. (C) A solid-phase binding assay demonstrates the binding of human tropomodulin to rTM5 and rTM5b. Using SDS-PAGE, 2 μg of rTM5 (lanes 1 and 3) and rTM5b (lanes 2 and 4) were separated on a 10% gel, transblotted onto a nitrocellulose membrane (panel labeled “Protein Profile”), and overlaid with 26 μg/mL recombinant human tropomodulin (panel labeled “Tmod Overlay”). The presence of bound tropomodulin was then analyzed by a rabbit antibody against tropomodulin followed by HRP-antirabbit IgG, enzymatic color development, and densitometry. (D) The panel shows the partial sequence alignments of human,16 mouse,32rat,33 and chicken38 tropomodulin in the region between residues 94 and 138. One letter code for amino acids is used. *Indicates identical residues among all 4 species. Numbers on the top indicate residue numbers in human tropomodulin.
Construction, expression, and purification of human recombinant tropomodulin and its binding to rTM5 and rTM5b.
(A) Human tropomodulin cDNA clone 10 was subcloned at the EcoR1 site downstream from the MalE gene, which encodes MBP. (B) The induction of the MBP/tropomodulin fusion protein. Total proteins ofE coli were separated on a 7% gel using SDS-PAGE and stained by Coomassie blue, before the addition of IPTG (lane 2) and 1 hour (lane 3), 2 hours (lane 4), and 3 hours (lane 5) after the addition of IPTG. Affinity-purified tropomodulin fusion protein (Mr 79 000) was either stained by Coomassie blue (lane 6, 2 μg) or transferred onto a nitrocellulose membrane and detected with tropomodulin-specific antibody using Western blot analysis (lane 7, 0.1 μg). Molecular weight standards (lane 1) are the same as in Figure 2. (C) A solid-phase binding assay demonstrates the binding of human tropomodulin to rTM5 and rTM5b. Using SDS-PAGE, 2 μg of rTM5 (lanes 1 and 3) and rTM5b (lanes 2 and 4) were separated on a 10% gel, transblotted onto a nitrocellulose membrane (panel labeled “Protein Profile”), and overlaid with 26 μg/mL recombinant human tropomodulin (panel labeled “Tmod Overlay”). The presence of bound tropomodulin was then analyzed by a rabbit antibody against tropomodulin followed by HRP-antirabbit IgG, enzymatic color development, and densitometry. (D) The panel shows the partial sequence alignments of human,16 mouse,32rat,33 and chicken38 tropomodulin in the region between residues 94 and 138. One letter code for amino acids is used. *Indicates identical residues among all 4 species. Numbers on the top indicate residue numbers in human tropomodulin.
In Figure 3, the 2 major TM bands of the erythrocyte membrane in the band 7 area (indicated by *) can be clearly detected by R-41 in both Figure 3B (lane 1) and Figure 3C (lane 4) in the absence of urea. To demonstrate the positions of hTM5 and hTM5b, relative to the 2 R-41 reactive TM bands, 2 additional strips from the same transblot were processed (Figure 3C): one was incubated with mAb Pep3-43 and the other with mAb LC1. The results show that hTM5b (lane 3), as detected by mAb Pep3-43, comigrated with the faster migrating species, while hTM5 (lane 5), as detected by mAb LC1, comigrated with the slower migrating species.
To confirm the differential migration pattern of TM5 and TM5b, we did a sequential Western blot analysis (Figure 3D). In this experiment, a transblot of erythrocyte membrane proteins was first incubated with mAb Pep3-43 to reveal a single band of hTM5b. The same strip was then further incubated with mAb LC1 to reveal a specific band of hTM5, which appeared slightly above the band of hTM5b. The 2 bands were clearly distinguishable. All of the above experiments, plus the finding that hTM5 and hTM5b each comigrated with 1 of the 2 major TM bands, suggest that TM5 and TM5b are indeed the 2 major TM isoforms present in human erythrocyte membranes.
Human tropomodulin is capable of binding to rTM5 and rTM5b
Human recombinant tropomodulin was synthesized in E coli in quantity and used to study the binding of tropomodulin to various TM isoforms. Figure 4 shows the construction (panel A), expression (panel B), and purification (panel B, lane 6) of human recombinant tropomodulin clone 10. The fusion protein contains residues 39-359 of human tropomodulin (Mr 36 000), which includes the TM-binding domain (residues 39-138)16 and the maltose-binding protein (MBP, Mr 43 000). Total bacterial proteins before and after the addition of isopropylthiogalactoside (IPTG) were separated on a 7% gel by SDS-PAGE. The correct reading frame of the affinity-purified tropomodulin fusion protein (Mr 79 000) was verified with a tropomodulin-specific antibody by Western blot analysis (panel B, lane 7).
A solid-phase binding assay was used to assess whether human recombinant tropomodulin binds to purified recombinant rTM5 and rTM5b and their relative binding affinities. The left panel of Figure 4C shows the SDS-PAGE protein profiles of rTM5 (lane 1) and rTM5b (lane 2) stained with Coomassie blue. The right panel, labeled “Tmod Overlay,” shows the presence of human tropomodulin that remained bound to rTM5 and rTM5b on the transblot, as detected by a rabbit peptide-specific antibody against human tropomodulin. This result indicates that human tropomodulin is capable of binding to both rTM5 and rTM5b. The intensity of tropomodulin bound to rTM5b, however, was about 80% of that bound to rTM5, which suggests that human tropomodulin has a slightly higher affinity toward rTM5 than rTM5b. Under the identical experimental condition, human tropomodulin had no detectable binding or very little binding to other TM isoforms including hTM2, hTM3, chicken skeletal muscle isoforms, and chicken smooth muscle isoforms.15 The above finding indicates that TM5 and TM5b are the 2 TM isoforms that bind to tropomodulin with a higher affinity than other TM isoforms.
It is not surprising that tropomodulin derived from humans is capable of binding to TM5 and TM5b derived from rats because both TM isoforms and tropomodulin are highly conserved between humans and rats. On the one hand, rTM5 differs from hTM5 only at the fourth position, and rTM5a/5b differs from hTM5a/5b in only 2 conserved amino acid changes at positions 30 and 3712 outside of the tropomodulin-binding domain.34 On the other hand, the TM-binding domain in tropomodulin (residues 94-13816,38) is located in a region where a high degree of sequence identity is found among several species including human,16 mouse,32 and rat33 (Figure 4D). The tropomodulin-binding domain has been mapped to within the N-terminal 18 residues of hTM5,34 and it is now known that human tropomodulin is capable of binding to both rTM5 and rTM5b. These facts suggest that (1) residue 4 (ie, Ile4 in hTM5, Ser4 in rTM5, and Ser4 in rTM5b) is either not involved in the binding and/or is located outside of the tropomodulin-binding domain and (2) the N-terminal sequence variations between TM5 and TM5b (Figure 2C) contribute to the minor difference of their affinities toward human tropomodulin.
Protofilaments in erythrocyte membrane skeleton
Using electron microscopy, the actin filaments revealed in a negatively stained membrane skeletal network were approximately 33-37 nm in length.1,2 This length is equivalent to 6-7 G-actin in each strand of the actin filament (approximately 13 G-actin in total) or 1 TM molecule (2 strands) bound in each groove of the actin double helix. It was suggested that this is the result of actin-TM complexing.2 It is not clear, however, why the TM molecule should stop with 1 molecule.
TM, which binds to the side of F-actin, does not prevent actin polymerization from the pointed end. TM stabilizes the pointed end of actin filaments, in fact, by slowing depolymerization from the pointed end.39 In vitro, in the presence of TM, both elongation and depolymerization of the actin filaments can be inhibited by low concentrations of tropomodulin. Higher concentrations of tropomodulin alone can also inhibit the elongation, even more effectively than depolymerization.17
In the model we previously proposed for nonmuscle actin filaments, tropomodulin was shown to bind to the N-terminus of a terminal TM5 molecule positioned at the pointed end of a long actin filament.15 Based on our new findings, we now propose a molecular model of a short actin protofilament in erythrocytes (Figure1B). In this model, tropomodulin is associated near the N-terminal end of 1 TM molecule, which comprises either TM5 or TM5b (both are approximately 33-35 nm in length) in the form of either a homodimer or heterodimer. The molecule is at the pointed end of the short actin filament, which is only approximately 33-37 nm in length. Since both TM5 and TM5b are LMW TM isoforms with 6 actin-binding sites, they would stabilize 6 G-actin in 1 strand and favor the binding of 6 spectrin tetramers to 1 protofilament. TM5 and TM5b are the 2 TM isoforms that have the strongest binding affinities (among all TM isoforms we tested, including muscle and nonmuscle TM isoforms) toward tropomodulin. TM5 and TM5b are also the 2 isoforms (among LMW TM isoforms) that have the highest affinities toward F-actin. These facts suggest that a very tight association between the tropomodulin-TM complex and the actin filament may be the key to the mechanism by which protofilaments are formed and maintained in erythrocyte membranes. The protofilament may, therefore, be viewed as a tightly associated basic unit of the actin filaments, consisting of a tropomodulin, 2 TM dimeric molecules (1 in each groove of the actin filament), and a double helical actin filament with a uniform length of approximately 33-37 nm. The complex formed by a globular tropomodulin and a rod-like TM molecule of approximately 33-35 nm in length may, therefore, function as a measuring device or a molecular ruler (Figure 1C) to reduce long actin filaments to short protofilaments with a uniform length.
TM4 is not present in human erythrocyte membranes
There are several LMW TM isoforms expressed in nonmuscle cells including TM5, TM5a, TM5b, TM4, and a few neuron-specific TM isoforms.9-11 TM4, a gene product of the δ-TMgene, also consists of 248 residues. To see whether human erythrocyte membranes also contain TM4, a Western blot analysis using a TM4-specific mAb LC24 was conducted. A recombinant chimeric human TM molecule (hTM5-g/4), which contains the epitope for mAb LC24, was included as a positive control. The results showed that mAb LC24 (1:1000 dilution) detected a strong signal for the positive control, but no detectable signal for TM4 was found in human ghost membranes (data not shown). The studies on the specificity of mAbs Pep3-34, LC1, and CG3, which demonstrated that they do not cross-react with TM4, have been reported previously.11 23-25 There remains a small possibility that there may be some minor or new TM isoforms in human erythrocyte membranes that have not yet been identified.
Discussion
In this report, we identified TM5 and TM5b to be the 2 major TM isoforms in human erythrocytes. Although they are products of 2 different genes (γ-TM and α-TM genes, respectively), they share several common features. Both are LMW isoforms, have 248 residues, and are approximately 33-35 nm in length.40,41 In addition, both have a high actin-binding affinity10,12 and a high tropomodulin-binding affinity.15 These properties are in agreement with previous studies of the Mr in the TM mixtures purified from erythrocytes in SDS-PAGE,4 the length of actin protofilaments (approximately 33-37 nm) in erythrocyte membrane skeletal network,1-3 and the high F-actin affinity found in TM mixtures purified from erythrocytes.4
The common features shared by TM5 and TM5b have significant functional implications, especially in the stability of protofilaments, the hexagonal organization of the membrane skeletal network, and the remodeling of the actin cytoskeleton during terminal differentiation. The following details the F-actin affinity of TM and why it is important for the stability of protofilaments; the length of TM and how it defines the hexagonal geometry of the membrane skeleton; and the tropomodulin affinity of TM and how that modulates the function of TM isoforms and the length of the actin filaments.
High F-actin affinity allows TM5 and TM5b to form more stable protofilaments. Erythrocytes are constantly subjected to the flow dynamics of the cardiovascular system and are frequently deformed by negotiating their way through narrow capillaries. Protofilaments located in the center of the junctional complexes must be strong enough to resist the pulling of spectrin in response to stresses in order to maintain the integrity of the membrane skeletal network (Figure 1A and1B). The main function of TM is to coat and stiffen the actin filaments,42 thereby making them more resistant to depolymerization and fragmentation.43 44 TM5 and TM5b, the 2 TM isoforms that have a high affinity toward F-actin, should function better than other LMW TM isoforms in stabilizing protofilaments.
The length of TM molecules or the number of actin-binding sites along the TM molecules defines the geometry of the membrane skeletal network. In general, there are 7 actin-binding sites along HMW TM isoforms (284 residues, approximately 40-43 nm in length) and 6 sites along LMW TM isoforms (248 residues, approximately 33-35 nm in length). As LMW isoforms, TM5 and TM5b stabilize 6 G-actin (in 1 strand) and allow 6 αβ spectrin dimers to bind to 1 protofilament (presumably 1 αβ spectrin dimer binds to 1 pair of G-actin in the double helix). As a result, LMW isoforms, rather than HMW isoforms, favor the organization of hexagonal lattices observed in the membrane skeletal network (Figure1A and 1B). The hexagonal arrangement allows a seamless continuation of the spectrin-actin–based skeletal network throughout the entire cell membrane. This two-dimensional (2-D) membrane skeletal network is essential for the mechanical stability of a circulating erythrocyte, as the enucleated, biconcave mature erythrocyte no longer has a supporting 3-D actin cytoskeleton in the cytoplasm.
The high tropomodulin affinity of TM5 and TM5b makes the TM/tropomodulin complex an effective measuring device or a molecular ruler capable of metering off long actin filaments to short protofilaments. This is because TM complexed with tropomodulin is able to bind and block an actin filament at its pointed end, but it is not able to overlap with other TM isoforms in a head-to-tail fashion along the actin filaments or at the barbed end (Figure5). As a result, only the first 6 G-actin in 1 strand (or 12 G-actin in the double helix) located at the pointed end of the actin filaments are protected by TM. Given the stress and strain undergone in the erythrocyte membrane during circulation, segments of actin filaments not coated by TM are likely to be fragmented or depolymerized. Thus, only short segments of the actin filaments, which are protected by 1 TM, can survive. The complex, therefore, contributes to the formation and maintenance of protofilaments in erythrocytes. The fact that TM5 has high initial binding affinity and low cooperativity to actin filaments12should also favor the formation of protofilaments the length of only 1 TM.
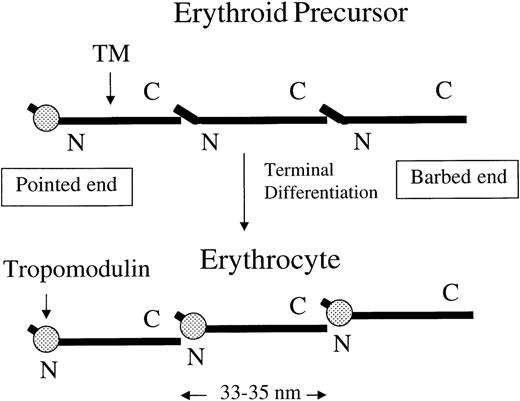
A switch of other TM isoforms to TM5 or TM5b and/or an increase of tropomodulin would favor the formation of actin protofilaments.
The tropomodulin-TM complex is able to bind to the pointed end of a long actin filament but not along it. The more tropomodulin bind to the N-terminal of homodimer or heterodimer of TM5 and TM5b (approximately 33-35 nm in length), the less the head-to-tail association of TM molecules can occur along the actin filaments. Increasing TM5, TM5b, and/or tropomodulin, therefore, would favor the stabilization of shorter actin filaments that are of the same size as an LMW TM molecule.
A switch of other TM isoforms to TM5 or TM5b and/or an increase of tropomodulin would favor the formation of actin protofilaments.
The tropomodulin-TM complex is able to bind to the pointed end of a long actin filament but not along it. The more tropomodulin bind to the N-terminal of homodimer or heterodimer of TM5 and TM5b (approximately 33-35 nm in length), the less the head-to-tail association of TM molecules can occur along the actin filaments. Increasing TM5, TM5b, and/or tropomodulin, therefore, would favor the stabilization of shorter actin filaments that are of the same size as an LMW TM molecule.
Although tropomodulin is a capping protein at the pointed end, it has more effects on the actin filaments than simply capping the pointed end. Unlike the barbed-end capping proteins that bind directly and block the G-actin at the barbed end, tropomodulin functions mainly through the mechanism of complexing with the TM molecule. TM is a regulatory rod-like molecule that overlaps with adjacent TM and stabilizes the actin filament from one end to the other. Therefore, by binding to one end (the N-terminal end) of TM, tropomodulin affects the stability of the entire actin filament and, under certain conditions, the growth from both ends. The degree to which tropomodulin destabilizes the actin filaments (except for the 1 TM-protected segment at the pointed end) depends on the proportion of TM molecules that are complexed with tropomodulin. If all TMs are complexed with tropomodulin (Figure 5) and if the actin filaments are under stress, the actin filaments can only be 1 TM in length, without growth from either end.
The erythrocyte membrane skeleton contains approximately 130 000 copies of TM (corresponding to approximately 75 000 dimeric TM molecules), approximately 400 000 copies of G-actin (corresponding to approximately 30 770 protofilaments), and approximately 30 000 copies of tropomodulin.5 The number of TM molecules is enough to stabilize all protofilaments, 1 in each groove of the actin filament. The number of tropomodulin is enough to bind to half of the TM dimeric molecules or every protofilament in the membrane skeleton. The stoichiometry of these 3 molecules, therefore, favors the formation of protofilaments that are only 1 TM molecule in length. The functional TM molecules in protofilaments are expected to be homodimers and heterodimers of TM5 and TM5b because dimers are the functional forms of the molecules and TM5 is uniquely capable of dimerizing with TM5b.25 45 TM5 and TM5b share several common features that are important for the development and organization of the membrane skeleton, and the 2 are expected to have similar functions in this respect. It is likely, however, that each may have unique functions in other aspects.
The actin cytoskeleton is remodeled during erythroid terminal differentiation. The long actin filaments are shortened into short protofilaments, and the 3-D cytoskeleton is transformed into a 2-D membrane skeleton. It is not clear how these changes are achieved, although they may be caused by an increase of complexes formed by TM5 and/or TM5b with tropomodulin (Figure 5). An increase in these complexes may be achieved by 2 mechanisms1: TM isoform switching, ie, switching from other isoforms to TM5 and/or TM5b, or by up-regulation of tropomodulin.2 Finding either or both of these mechanisms would support the functional role of tropomodulin -TM5 or -TM5b complexes in the formation of protofilaments. Examination of the levels of TM5, TM5b, and tropomodulin during erythroid terminal differentiation using a 2-phase liquid culture system would shed new light on the regulatory mechanism(s) involved in the development of erythrocyte membrane skeletons. Experiments are also in progress to determine the definitive roles of tropomodulin in the differentiation and biomechanics of erythrocytes by targeted disruption of the tropomodulin gene in the mouse embryonic stem cells.46
Acknowledgments
We thank Carlos Vera and Edgar Gutierrez for repeating some of the experiments. We also thank Matt Levitt, Ellvin Mar, and Stacy Hwang for assistance in manuscript preparation.
Supported in part by grant HL-43026 (L.A.S.) and grants DK47673 and HD18577 (J.J.-C.L.) from the National Institutes of Health, Bethesda, MD, grant MCB-9874492 (D.H.) from the National Science Foundation, and fellowship 950216 (C.J.T.-G.) from the American Heart Association, Dallas, TX. D.M.H. is an Established Investigator of the American Heart Association.
Reprints:Lanping Amy Sung, Department of Bioengineering and Center for Molecular Genetics, EBU1 6406, Mail Code 0412, University of California, San Diego, La Jolla, CA 92093-0412; e-mail:amysung@bioeng.ucsd.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal