Thirty-five patients with myelodysplastic syndrome (MDS) were registered on protocol MDS 96-02 and were receiving continuous therapy with pentoxifylline 800 mg 3 times a day and ciprofloxacin 500 mg twice a day by mouth; dexamethasone was added to the regimen for the partial responders and the nonresponders after 12 weeks at a dose of 4 mg by mouth every morning for 4 weeks. Amifostine was administered intravenously 3 times a week at 3 dose levels (200 mg/M2, 300 mg/M2, and 400 mg/M2) to cohorts of 10 patients each. Therapy has been continued for 1 year in responders. Twenty-nine have completed at least 12 weeks of therapy and are available for response evaluation. Of the 21 men and 8 women (median age, 67 years), 20 had refractory anemia (RA), 3 had RA with ringed sideroblasts (RARS), 5 had RA with excess blasts (RAEB), and 1 had chronic myelomonocytic leukemia (CMMoL). Five had secondary MDS. No differences were noted in response rates among the 3 dose levels. Seven patients did not respond at all, and 22 showed an improvement in cytopenias (76%). Three had a triple lineage response, 10 had a double lineage response, and 9 had a single lineage response (8 of 9 in absolute neutrophil count [ANC] and 1 had more than a 50% reduction in packed red blood cell transfusions). Fifteen patients responded only after the addition of dexamethasone, whereas 7 responded before. When examined by lineage, 19 of 22 showed improved ANC, 11 of 22 demonstrated more than 50% reduction in blood transfusions, improved Hb levels, or both, and 7 of 22 showed improvement in platelet counts. Interestingly, the responses were frequently slow to appear, and continued improvement in counts was seen up to 12 months of therapy and beyond. This study supports the feasibility of treating patients with MDS with the unique approach of cytoprotection and anticytokine therapies as well as the principle that prolonged commitment to treatment is desirable when noncytotoxic agents are administered.
No single therapeutic approach appears to have made a significant impact on survival of patients with myelodysplastic syndromes (MDS).1,2 Allogeneic bone marrow (BM) transplantation,3,4 a choice available to few patients given that the median age at diagnosis is approximately 70 years, is the only exception. Options range from supportive care to the use of stem cell transplantation. Based on the assumption that the cytopenias may reflect a primary bone marrow failure, colony-stimulating growth factors with overlapping activities designed to stimulate proliferation of hematopoietic progenitors have been extensively investigated.5-7 The problem is that administered as single agents, granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF rarely improves the anemia and the thrombocytopenia so commonly the pathognomonic features of MDS. Erythropoietin alone produces an improvement in the anemias of approximately 20% of patients, which increases to almost 50% when combined with G-CSF.8,9However, only a proportion of patients respond, the response is usually temporary, and there is some concern related to an incidence of accelerated transformation.10
Acute leukemia-like intensive induction therapies have been attempted in patients with high-risk MDS (those with excess blasts or chronic myelomonocytic leukemia), with as many as half the patients achieving complete remission.11 12 Short duration of remission marked by a relentless return of MDS cells in most patients, treatment-related complications or mortality, frequent encounters with drug-resistant clones, and the morbidity caused by the appearance of unexpected and unusual opportunistic infections reflecting the enormously compromised state of the immune system in these patients make the intensive chemotherapy option less desirable. In summary, save for allogeneic transplantation, MDS is a universally fatal illness, and no single approach has either altered the natural history of the disease or improved survival.
Given the biologic complexity and the unpredictable course of the disease ranging from chronic, insidious, and slowly progressive cytopenia to a rapidly evolving, lethal transformation to acute leukemia, it is not surprising that therapeutic options range widely between supportive care to intensive induction-type chemotherapy. Clearly, a better understanding of the basis for cytopenias in MDS is critical to design therapies tailored for individual needs. Recent biologic studies have demonstrated that cytokine-mediated excessive intramedullary apoptosis of hematopoietic cells may form this basis in most patients with MDS.13-16 This insight offers a novel therapeutic window of opportunity because it naturally follows that suppression of the proapoptotic cytokines should lead to an improvement in cytopenias. The proinflammatory/proapoptotic cytokines that have so far been demonstrated to be candidates for this role are tumor necrosis factor α (TNF-α), transforming growth factor β (TGF-β), and interleukin 1b (IL-1b).17-20Because the pathologic course most likely results from the activity of a cascade of cytokines, suppression of any single cytokine by specific antibodies would not be the most desirable therapy. Rather, agents that interfere with the activity of several cytokines would be preferred. We chose to use pentoxifylline (PTX), a xanthine derivative known to interfere with the lipid-signaling pathway used by TNF-α, TGF-β and IL-1b21 and thus reduces the activity of these cytokines.22-24 Ciprofloxacin (Cipro) was concomitantly administered because it reduces the hepatic degradation of PTX,25 and dexamethasone (Decadron) was added to down-regulate the translation of mRNA for TNF-α.26 This pentoxifylline–ciprofloxacin–dexamethasone (PCD) therapy resulted in encouraging hematopoietic responses in 18 of 43 patients with MDS,27 and the mechanism of action was found to be cytokine related because responders showed the most sustained reductions in TNF-α levels.28
More recently, the cytoprotective agent amifostine has been found to have substantial activity in improving cytopenias of patients with MDS.29 In the current study, therefore, the anticytokine and cytoprotective approaches were combined to determine whether the gains in improving ineffective hematopoiesis of MDS could be further enhanced. This article reports on the first trial that combined all 4 agents namely, pentoxifylline, ciprofloxacin, amifostine, and dexamethasone.
Patients and methods
All patients were entered on the protocol MDS 96-02. The protocol was reviewed and approved by the Institutional Review Board (IRB) of the Rush–Presbyterian–St. Luke's Medical Center and by the IRBs of other participating institutions. All patients considered potential candidates for treatment on MDS 96-02 had the protocol explained to them by the Principal Investigator, and if they agreed to participate in the study, they signed an informed consent form before therapy began. All patients underwent a bone marrow examination before the start and after approximately 12 weeks of therapy. Weekly complete blood counts with differentials were obtained on all the patients; only adults older than 18 years of age were eligible for the study. All pretherapy and posttherapy bone marrow examination results were reviewed at Rush University by a hematopathologist.
Clinical studies
Thirty-five patients with MDS were formally registered on the protocol MDS 96-02 after a bone marrow examination confirmed the diagnosis. Twenty-nine have completed at least 12 weeks of therapy and are available for a response evaluation. All patients began by taking pentoxifylline 400 mg by mouth 3 times a week for 1 week. This was increased to 800 mg by mouth 3 times a week from the second week until the termination of the protocol. Ciprofloxacin (Cipro) was started at a dose of 500 mg by mouth twice a week from the 3rd week. Amifostine was administered 3 times per week (Monday, Wednesday, Friday) at 3 dose levels to cohorts of 10 patients each. The first cohort received 200 mg/M2, the second cohort received 300 mg/M2, and the third cohort received 400 mg/M2 intravenously 3 times/week. After 12 weeks of therapy with pentoxifylline, Cipro, and amifostine, responses were evaluated according to the criteria described below. Partial responders, nonresponders, or both were then given dexamethasone at 4 mg by mouth every morning in addition to the other drugs for a period of 4 weeks. After this 4-week course, dexamethasone was tapered and stopped, and then a maintenance dose of 4 mg by mouth was given for 5 days every month after 6 weeks.
The protocol was written to continue all drugs for a period of 6 months and then to reduce the amifostine frequency to twice a week and continue all drug administration for a total of 1 year. These drug durations and schedules were chosen for a variety of reasons. PTX and Cipro have been safely administered to patients with MDS for up to 3 years in our previous study27 and therefore were continued for 1 year at full dose. Because the administration of dexamethasone at 4 mg by mouth every morning for 12 weeks was associated with many of the expected side effects,27 in the current protocol this was changed to a 5-day per month intermittent schedule after continuous daily administration for 4 weeks. After 6 months of thrice weekly amifostine, the dose was reduced to twice weekly mainly for the convenience of the patients.
Response criteria
Responses were defined according to criteria previously reported.29 30 Restoration of normal hematopoiesis with normal peripheral blood counts was defined as complete remission. Partial remission was defined as improvement in 1 of the following parameters: (1) a decrease in monthly packed red blood cell (PRBC) transfusions by at least 50% was defined as a partial response; (2) an increase in hemoglobin by 2 g/dL over pretreatment value was considered a good response, whereas an increase by 1 g/dL was considered a partial response and anything less as no response; (3) an increase in platelet count by more than 30 000/μL above pretreatment value if the pretreatment count was less than 150 000/μL was considered a good response, and an increase by 10 000/μL was a partial response; (4) an increase in granulocyte count by 500/pL over pretreatment value or a 50% increase over pretreatment value; (5) disappearance of 1 or more cytogenetic abnormalities.
Cytogenetic studies
Standard karyotypic analysis using GTG banding was performed on every case before therapy was started and each time a marrow was performed thereafter.
Statistical analysis
Mann-Whitney U tests were used for 2 sample comparisons of continuous variables. Contingency tables with χ2statistics or the Fisher exact test were used for analysis.
Results
Thirty-five patients with a confirmed diagnosis of MDS were registered on protocol 96-02, and 29 patients could be evaluated because they completed the minimum specified period of 12 weeks on the study. Of the 29 patients who are the subject of this report, there were 21 men and 8 women, 27 were white, 1 was Hispanic, and 1 was African American. The median age was 67 years (range, 46-81 years), and 5 patients had a history of toxic exposure (secondary MDS). Of the 5 patients with secondary MDS, patient 2 had a history of myelofibrosis but did not receive any cytotoxic therapy (Table1), patient 17 underwent autologous stem cell transplantation for non-Hodgkin's lymphoma, patient 19 underwent autologous bone marrow transplantation for AML 10 years before the diagnosis of MDS, patient 23 had breast cancer and underwent 6 cycles of chemotherapy 1 year before the diagnosis, and patient 29 underwent multiple cytotoxic therapies for chronic lymphocytic leukemia. Twenty patients had refractory anemia (RA) according to the French-American-British (FAB) classification, 3 had RA with ringed sideroblasts (RARS), 5 had RA with excess blasts (RAEB), and 1 had chronic myelomonocytic leukemia (CMMoL). These data are shown in Table1.
Clinical and laboratory characteristics of MDS patients on protocol
| S. No . | Age (y) . | Sex . | FAB . | Baseline . | Week 12/Before Dexamethasone . | Week 24/After Dexamethasone . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | |||||
| 1 | 72 | F | RA | 0.43 | 9.8 | — | 54 | NA | 8.90 | — | 27 | 1.33 | 7.20 | — | 21 | ANC + D |
| 2 | 63 | M | RA | 0.26 | 10 | — | 99 | 0.36 | 9.00 | — | 106 | OFF STUDY | NR | |||
| 3 | 49 | M | RA | 1.50 | 7.5 | 2q1wk | 44 | NA | 7.70 | 2q1wk | 35 | 3.116 | 7.60 | 2q1wk | 21 | ANC + D |
| 4 | 67 | M | RA | 1.86 | 8.8 | 3q3wk | 115 | 2.32 | 6.50 | 2q2wk | 112 | OFF STUDY | ANC | |||
| 5 | 58 | M | RA | 0.18 | 9.6 | — | 54 | 1.12 | 9.00 | 3q8wk | 148 | 1.99 | 10.60 | 1q6wk | 123 | Trilineage |
| 6 | 82 | M | RARS | 0.26 | 7.3 | 2q1wk | 44 | 0.19 | 6.70 | 2q1wk | 49 | 0.30 | 7.30 | 1q6wk | 43 | pRBC >50% + D |
| 7 | 52 | M | RA | 1.50 | 7.7 | 2q1wk | 14 | 0.88 | 8.00 | 2q1wk | 12 | 3.11 | 10.50 | 1q1wk | 28 | Trilineage + D |
| 8 | 73 | M | RAEB | 9.20 | 9.2 | 2q2wk | 89 | 6.96 | 8.00 | 1q2wk | 136 | 6.28 | 8.00 | 2q4wk | 160 | Bilineage (plts + trans.) |
| 9 | 78 | F | RARS | 1.12 | 9.1 | 1q2wk | 434 | 2.9 | 10.40 | 1q2wk | 426 | 3.75 | 9.30 | 1q3wk (8wk gap) | 447 | Bilineage (ANC + trans.) |
| 10 | 61 | F | RA | 0.36 | 6.8 | 1q3wk | 151 | 0.42 | 8.50 | 1q8wk | 150 | OFF STUDY | Bilineage (ANC + trans.) | |||
| 11 | 59 | M | RARS | 2.19 | 6.8 | 2q1wk | 242 | 1.24 | 9.90 | 2q1wk | 93 | 5.02 | 9.60 | 2q6wk (after 24 wks) | 179 | Bilineage (trans. (Hb) + ANC + D) |
| 12 | 71 | M | RAEB | 1.45 | 7.2 | 2q2wk | 48 | 0.65 | 8.00 | 2q4wk | 27 | 3.28 | 10.30 | nil | 65 → 84 | Trilineage (transfusion without D; ANC + plt were with D) |
| 13 | 74 | M | CMMoL | 2.39 | 11.4 | — | 33 | NA | 8.50 | — | 34 | 4.08 | 9.10 | — | 45 | ANC + D; blasts 30% to <5% |
| 14 | 81 | M | RA | 2.41 | 7.3 | 2q1wk | 110 | 2.01 | 10.00 | 2q1wk | 138 | 2.54 | 13.40 | — | 133 | Bilineage (trans. Hb + ANC) |
| 15 | 52 | F | RA | 1.60 | 6.9 | 2q2wk | 229 | 1.94 | 7.90 | 2q2wk | 161 | 5.58 | 8.70 | 2q2wk | 242 | Bilineage ANC + plt + D |
| 16 | 73 | M | RAEB | 0.29 | 6.7 | 3q1wk | 21 | 0.16 | 8.50 | 3q1wk | 21 | NA | 10.10 | 3q3wk | 115 → 242 | Bilineage pRBC >50% + plt + D |
| 17 | 56 | M | RA | 0.55 | 7.9 | 1q4wk | 159 | 0.32 | 7.60 | 2q2wk | 81 | 0.64 | 9.50 | 2q2wk | 49 | NR |
| 18 | 69 | M | RAEB | 0.91 | 7.6 | 2q1wk | 6 | 0.93 | 7.70 | 2q1wk | 20 | 1.56 | 8.00 | 2q2wk | 18 | Bilineage + D (ANC + trans.) |
| 19 | 47 | M | RA | 3.48 | 9.4 | nil | 27 | 1.98 | 8.10 | nil | 19 | 5.77 | 9.30 | nil | 26 | ANC + D |
| 20 | 69 | M | RA | 1.04 | 9.3 | 3q4wk | 41 | 0.68 | 7.80 | 3q4wk | 28 | 4.2 | 7.00 | 3q4wk | 36 | ANC + D |
| 21 | 68 | M | RA | 0.31 | 9.2 | NA | 63 | 0.176 | 6.40 | 2q4wk | 66 | 1.18 | 8.80 | 2q4wk | 105 | Bilineage ANC + plt + D |
| 22 | 75 | M | RA | 1.43 | 9.8 | nil | 84 | 1.36 | 8.50 | nil | 65 | 1.3 | 10.20 | nil | 57 | NR |
| 23 | 52 | F | RA | 0.67 | 9 | 3q2wk | 16 | 2.32 | 7.60 | 3q2wk | 17 | 3.02 | 8.10 | 3q2wk | 18 | NR |
| 24 | 66 | M | RAEB | 1.97 | 13.2 | NA | 42 | NA | 11.00 | NA | 38 | NR | ||||
| 25 | 66 | F | RA | 1.31 | 9 | 1q1wk | 201 | 0.75 | 8.20 | 1q2wk | 89 | 4.27 | 9.10 | 1q2wk | 45 | NR |
| 26 | 67 | M | RA | 2.17 | 7.4 | 2q2wk | 66 | 2.05 | 7.50 | 2q2wk | 47 | 10.1 | 8.20 | 2q2wk | 80 | ANC + D |
| 27 | 78 | F | RA | 2.32 | 7.1 | 2q3wk | 358 | 2.16 | 6.40 | 2q3wk | 280 | 4.04 | 6.60 | 2q3wk | 297 | ANC + D |
| 28 | 66 | M | RA | 1.91 | 8.5 | 2q1wk | 172 | 2.55 | 8.60 | 3q3wk | 51 | 0.86 | 8.20 | 3q3wk | 29 | NR |
| 29 | 66 | F | RA | 0.42 | 9.3 | 2q2wk | 16 | 0.48 | 8.70 | 1q3wk → none | 47 | OFF STUDY | Bilineage trans. + plt | |||
| S. No . | Age (y) . | Sex . | FAB . | Baseline . | Week 12/Before Dexamethasone . | Week 24/After Dexamethasone . | Responses . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | ANC . | Hb (g/dL) . | RBC Trans. (units) . | Plt . | |||||
| 1 | 72 | F | RA | 0.43 | 9.8 | — | 54 | NA | 8.90 | — | 27 | 1.33 | 7.20 | — | 21 | ANC + D |
| 2 | 63 | M | RA | 0.26 | 10 | — | 99 | 0.36 | 9.00 | — | 106 | OFF STUDY | NR | |||
| 3 | 49 | M | RA | 1.50 | 7.5 | 2q1wk | 44 | NA | 7.70 | 2q1wk | 35 | 3.116 | 7.60 | 2q1wk | 21 | ANC + D |
| 4 | 67 | M | RA | 1.86 | 8.8 | 3q3wk | 115 | 2.32 | 6.50 | 2q2wk | 112 | OFF STUDY | ANC | |||
| 5 | 58 | M | RA | 0.18 | 9.6 | — | 54 | 1.12 | 9.00 | 3q8wk | 148 | 1.99 | 10.60 | 1q6wk | 123 | Trilineage |
| 6 | 82 | M | RARS | 0.26 | 7.3 | 2q1wk | 44 | 0.19 | 6.70 | 2q1wk | 49 | 0.30 | 7.30 | 1q6wk | 43 | pRBC >50% + D |
| 7 | 52 | M | RA | 1.50 | 7.7 | 2q1wk | 14 | 0.88 | 8.00 | 2q1wk | 12 | 3.11 | 10.50 | 1q1wk | 28 | Trilineage + D |
| 8 | 73 | M | RAEB | 9.20 | 9.2 | 2q2wk | 89 | 6.96 | 8.00 | 1q2wk | 136 | 6.28 | 8.00 | 2q4wk | 160 | Bilineage (plts + trans.) |
| 9 | 78 | F | RARS | 1.12 | 9.1 | 1q2wk | 434 | 2.9 | 10.40 | 1q2wk | 426 | 3.75 | 9.30 | 1q3wk (8wk gap) | 447 | Bilineage (ANC + trans.) |
| 10 | 61 | F | RA | 0.36 | 6.8 | 1q3wk | 151 | 0.42 | 8.50 | 1q8wk | 150 | OFF STUDY | Bilineage (ANC + trans.) | |||
| 11 | 59 | M | RARS | 2.19 | 6.8 | 2q1wk | 242 | 1.24 | 9.90 | 2q1wk | 93 | 5.02 | 9.60 | 2q6wk (after 24 wks) | 179 | Bilineage (trans. (Hb) + ANC + D) |
| 12 | 71 | M | RAEB | 1.45 | 7.2 | 2q2wk | 48 | 0.65 | 8.00 | 2q4wk | 27 | 3.28 | 10.30 | nil | 65 → 84 | Trilineage (transfusion without D; ANC + plt were with D) |
| 13 | 74 | M | CMMoL | 2.39 | 11.4 | — | 33 | NA | 8.50 | — | 34 | 4.08 | 9.10 | — | 45 | ANC + D; blasts 30% to <5% |
| 14 | 81 | M | RA | 2.41 | 7.3 | 2q1wk | 110 | 2.01 | 10.00 | 2q1wk | 138 | 2.54 | 13.40 | — | 133 | Bilineage (trans. Hb + ANC) |
| 15 | 52 | F | RA | 1.60 | 6.9 | 2q2wk | 229 | 1.94 | 7.90 | 2q2wk | 161 | 5.58 | 8.70 | 2q2wk | 242 | Bilineage ANC + plt + D |
| 16 | 73 | M | RAEB | 0.29 | 6.7 | 3q1wk | 21 | 0.16 | 8.50 | 3q1wk | 21 | NA | 10.10 | 3q3wk | 115 → 242 | Bilineage pRBC >50% + plt + D |
| 17 | 56 | M | RA | 0.55 | 7.9 | 1q4wk | 159 | 0.32 | 7.60 | 2q2wk | 81 | 0.64 | 9.50 | 2q2wk | 49 | NR |
| 18 | 69 | M | RAEB | 0.91 | 7.6 | 2q1wk | 6 | 0.93 | 7.70 | 2q1wk | 20 | 1.56 | 8.00 | 2q2wk | 18 | Bilineage + D (ANC + trans.) |
| 19 | 47 | M | RA | 3.48 | 9.4 | nil | 27 | 1.98 | 8.10 | nil | 19 | 5.77 | 9.30 | nil | 26 | ANC + D |
| 20 | 69 | M | RA | 1.04 | 9.3 | 3q4wk | 41 | 0.68 | 7.80 | 3q4wk | 28 | 4.2 | 7.00 | 3q4wk | 36 | ANC + D |
| 21 | 68 | M | RA | 0.31 | 9.2 | NA | 63 | 0.176 | 6.40 | 2q4wk | 66 | 1.18 | 8.80 | 2q4wk | 105 | Bilineage ANC + plt + D |
| 22 | 75 | M | RA | 1.43 | 9.8 | nil | 84 | 1.36 | 8.50 | nil | 65 | 1.3 | 10.20 | nil | 57 | NR |
| 23 | 52 | F | RA | 0.67 | 9 | 3q2wk | 16 | 2.32 | 7.60 | 3q2wk | 17 | 3.02 | 8.10 | 3q2wk | 18 | NR |
| 24 | 66 | M | RAEB | 1.97 | 13.2 | NA | 42 | NA | 11.00 | NA | 38 | NR | ||||
| 25 | 66 | F | RA | 1.31 | 9 | 1q1wk | 201 | 0.75 | 8.20 | 1q2wk | 89 | 4.27 | 9.10 | 1q2wk | 45 | NR |
| 26 | 67 | M | RA | 2.17 | 7.4 | 2q2wk | 66 | 2.05 | 7.50 | 2q2wk | 47 | 10.1 | 8.20 | 2q2wk | 80 | ANC + D |
| 27 | 78 | F | RA | 2.32 | 7.1 | 2q3wk | 358 | 2.16 | 6.40 | 2q3wk | 280 | 4.04 | 6.60 | 2q3wk | 297 | ANC + D |
| 28 | 66 | M | RA | 1.91 | 8.5 | 2q1wk | 172 | 2.55 | 8.60 | 3q3wk | 51 | 0.86 | 8.20 | 3q3wk | 29 | NR |
| 29 | 66 | F | RA | 0.42 | 9.3 | 2q2wk | 16 | 0.48 | 8.70 | 1q3wk → none | 47 | OFF STUDY | Bilineage trans. + plt | |||
M, male; F, female; FAB, French-American-British classification; RA, refractory anemia; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts; ANC, absolute neutrophil count/mL; Hb, hemoglobin in g/dL; RBC Trans, number of units of packed red blood cells transfused; q, every; wk, weeks; Plt, platelets in thousands per microliter; NA, not available for that date; +D, with dexamethasone; Responses: ANC, response in neutrophils; Plt, response in platelet counts; Hb, response in hemoglobin levels; pRBC > 50%, decrease in packed red blood cell transfusion requirements by 50%; NR, no response.
Protocol compliance and toxicity
Of the 35 patients registered on MDS 96-02, 3 died before 12 weeks of therapy could be completed, 1 discontinued therapy because of intolerable nausea, 1 had a myocardial infarction and discontinued therapy within 4 weeks, and 1 was registered but never started treatment. Of the 29 patients who could be evaluated for response because they completed at least 12 weeks of therapy, 9 were treated on the 200 mg/M2 dose of amifostine, 8 on the 300 mg/M2 dose, and 12 on the 400 mg/M2. Twelve patients received the highest dose of amifostine because 3 patients in the lower dose groups could not be evaluated. No differences were noted in response rates among these groups. Responses were seen in 22 of 29 (76%) patients, 7 of 9 (78%) received the lowest dose of amifostine, 6 of 8 (75%) received the intermediate dose, and 9 of 12 (76%) received the highest dose of amifostine (P = .98). Although 29 patients completed 12 weeks of therapy, only 8 patients completed 6 months, 5 completed 9 months, and 3 completed the full year of treatment specified in the protocol. Sixteen patients stopped treatment because there was no further improvement in their cytopenias, 5 stopped because of intolerable side effects, 5 showed progression of disease, and 3 completed the full year of therapy. Approximately half the treated patients experienced some side effects from the drugs (Table2). Briefly, 57% patients experienced nausea and 10% vomiting. Among the patients who experienced nausea, vomiting, or both there was a difference in those who received the higher doses of amifostine compared with those who received the lowest dose. For example, in the 200 mg/M2 amifostine dose group, the incidence for nausea was 11% compared with 25% and 26% at the higher doses. Similarly, though 7% of patients at the lowest dose of amifostine experienced vomiting, 14% had vomiting at both the higher doses. From 17% to 20% of patients experienced decreased appetite, hypotension, rash, and fever, whereas depression (13%) and anxiety (3%) were rarer. Once again, all these side effects were experienced primarily in the 2 higher dose groups rather than the lowest dose amifostine group (Table 2).
Adverse effects of amifostine by dose groups
| Symptom . | Group . | Grade (% Patients) . | |
|---|---|---|---|
| Grade 1 . | Grade 2 . | ||
| Nausea | 1 | 3.6 | 0 |
| 2 | 14 | 3.6 | |
| 3 | 11 | 3.6 | |
| Vomiting | 1 | 3.6 | 0 |
| 2 | 7 | 7 | |
| 3 | 3.6 | 7 | |
| Decreased appetite | 1 | 0 | 0 |
| 2 | 0 | 3.6 | |
| 3 | 3.6 | 0 | |
| Hypotension | 1 | 0 | 0 |
| 2 | 0 | 7 | |
| 3 | 0 | 7 | |
| Rash | 1 | 0 | 0 |
| 2 | 3.6 | 3.6 | |
| 3 | 0 | 3.6 | |
| Fever | 1 | 0 | 0 |
| 2 | 0 | 7.1 | |
| 3 | 0 | 0 | |
| Depression | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| Anxiety | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 3.6 | |
| Symptom . | Group . | Grade (% Patients) . | |
|---|---|---|---|
| Grade 1 . | Grade 2 . | ||
| Nausea | 1 | 3.6 | 0 |
| 2 | 14 | 3.6 | |
| 3 | 11 | 3.6 | |
| Vomiting | 1 | 3.6 | 0 |
| 2 | 7 | 7 | |
| 3 | 3.6 | 7 | |
| Decreased appetite | 1 | 0 | 0 |
| 2 | 0 | 3.6 | |
| 3 | 3.6 | 0 | |
| Hypotension | 1 | 0 | 0 |
| 2 | 0 | 7 | |
| 3 | 0 | 7 | |
| Rash | 1 | 0 | 0 |
| 2 | 3.6 | 3.6 | |
| 3 | 0 | 3.6 | |
| Fever | 1 | 0 | 0 |
| 2 | 0 | 7.1 | |
| 3 | 0 | 0 | |
| Depression | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 0 | |
| Anxiety | 1 | 0 | 0 |
| 2 | 0 | 0 | |
| 3 | 0 | 3.6 | |
Hematologic responses
Of the 29 evaluable patients, 7 had no response after at least 12 weeks of therapy whereas 22 of 29 (76%) showed partial response in that there was improvement in their cytopenias. There were no complete responders. Seven patients showed some improvement before the addition of dexamethasone, and 15 only responded after PCD + amifostine. The median time to response varied depending on the lineage and on whether the patient received dexamethasone. Nineteen patients showed an improvement in ANC, 11 in hemoglobin or transfusion requirements, and 7 in platelet count. Overall, there were 3 triple lineage responders, 10 double lineage responders, and 9 single lineage responders (8 of 9 in ANC only; 1 showed more than 50% reduction in PRBC transfusions). The details of these responses and the precise blood counts are shown in Table 1. In summary, two-thirds of the responding patients had improved ANC, half showed improvement in the erythroid lineage, and one-third showed improvement in their platelet counts. Improvements in these cytopenias were noted more rapidly after the addition of dexamethasone, whereas a more gradual improvement occurred in the patients who did not receive the additional steroid therapy.
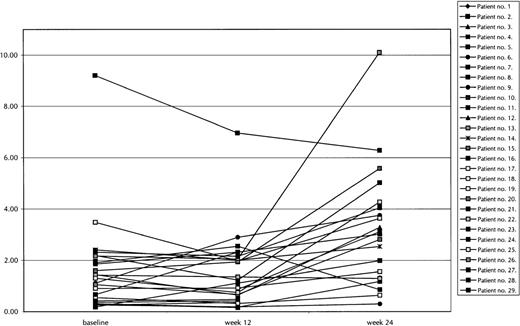
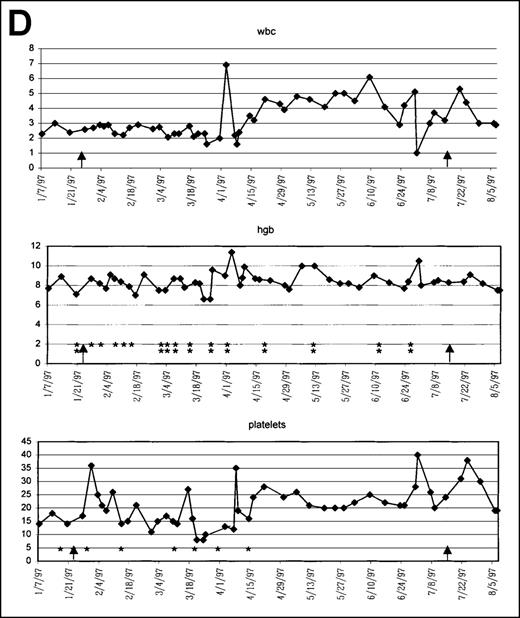
Significant statistical improvement was seen in ANC after 16 weeks (P = .01) and 24 weeks (P = .02) of therapy. The erythroid and platelet count responses were not statistically significant (P = .52 and P = .72, respectively, at 24 weeks). Figure 1 graphically depicts the serial ANC counts in all 29 patients. Figure2 graphically demonstrates the hematologic responses in 4 responding patients. These 4 patients were chosen for more detailed description because they represent a variety of responses after therapy with PCD + amifostine:
Graphic presentation of absolute neutrophil counts in patients with MDS during therapy.
Graphic presentation of absolute neutrophil counts in patients with MDS during therapy.
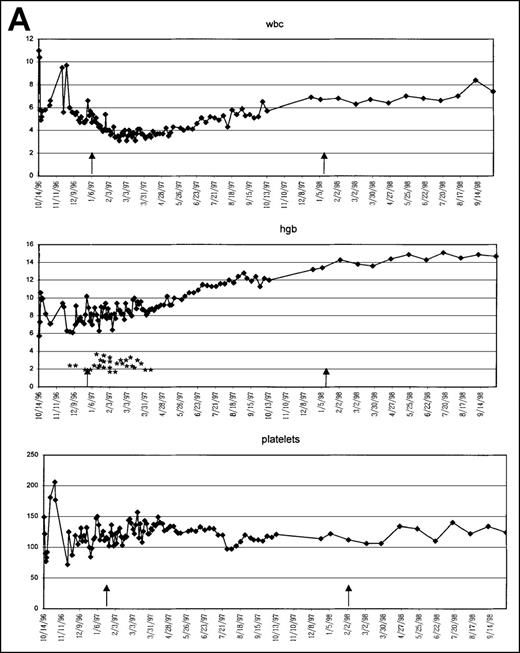
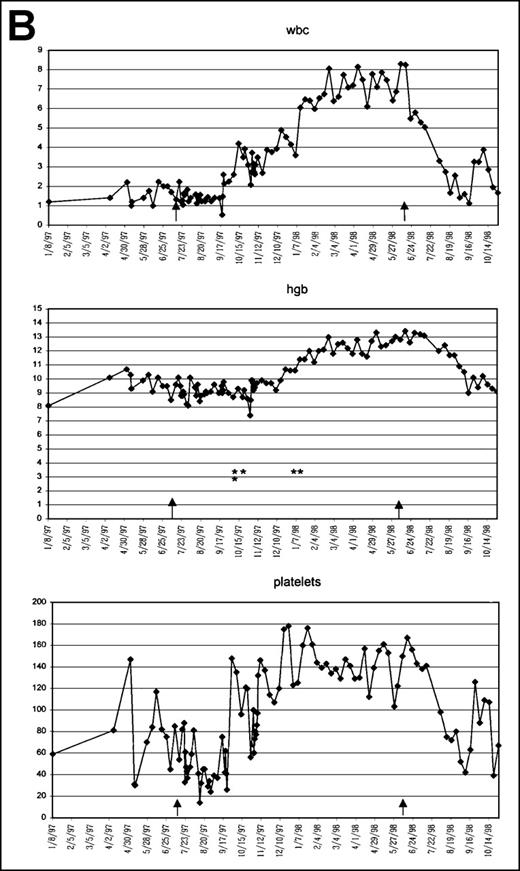
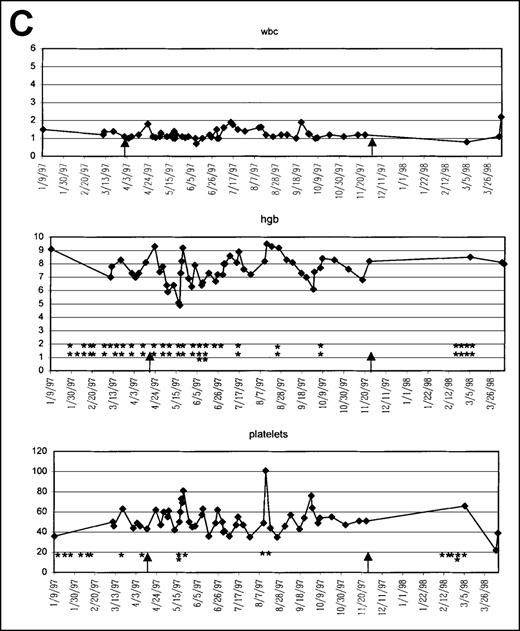
Graphic presentation of peripheral blood indices in 4 (A-D) patients treated with PCD and amifostine.
*2 U PRBC transfusion.
Graphic presentation of peripheral blood indices in 4 (A-D) patients treated with PCD and amifostine.
*2 U PRBC transfusion.
Patient 14
This 8l-year-old white man was diagnosed with RA on 11/1/96 (Table 1, Figure 2a). He had a hypercellular BM and normal cytogenetics. At the time of diagnosis, his white blood cell count was 4200/μL, Hb level was 5.7 g/dL, and platelet count was 149 000/μL. He received 5 U PRBC, which brought his Hb level to 10 g/dL. Patient started treatment approximately 6 weeks after diagnosis (12/16/96) and continued to require 2 to 3 U PRBC each week until March 1997. Of note, however, was the gradual increase in his hemoglobin values between transfusions. Once the transfusion requirements stopped, the hemoglobin continued to increase until the end of therapy at 1 year, as shown in the graph. The patient has been off therapy since 01/06/98, and his latest values on 9/10/98 were Hb, 14.9 g/dL; WBC, 8 400/μL; and platelet count, 134 000/μL. He feels well.
Patient 12
This 58-year-old white man was diagnosed with RA in June 1997 when he sought treatment for profound pancytopenia and severe fatigue (Table1, Figure 2b). He had a hypercellular BM and cytogenetic abnormality 46, XY, del20(qll .2ql3.3)/46 × Y). After several PRBC transfusions, his Hb level increased to 9.6 g/dL, WBC was 1500/μL, and platelet count 54 000/μL when he started on the protocol. As seen in Figure 2B, he did require PRBCs twice in the next 3 months, but then his Hb level continued to improve, reaching a maximum of 13.9 g/dL. His WBC and platelet counts also improved (8200 μL and 180 000 μL, respectively). After approximately 11 months of treatment, the patient experienced a severe hypotensive episode after a routine amifostine injection. All study drugs were stopped at this point (6/12/98), and the patient began to experience a slow decline in all his counts within 6 weeks of halting therapy. By October, he was placed on PCD therapy because his Hb fell to 8.5 g/dL and his platelet count decreased to the 70 000/μL range. He has been showing response to this therapy.
Patient 6
This 82-year-old white man was diagnosed with RARS on 9/9/96 (Table1, Figure 2C). He was started on MDS 96-02 on 3/31/97, at which time his WBC count was 1100/μL, Hb level was 7.3 g/dL, and platelet count was 44 000/dL. He had normal cytogenetics and hypercellular BM with 3% blasts. He required 2 U PRBC almost every 7 to 10 days and platelets every 2 to 3 weeks. After treatment with amifostine + PC, the patient continued to require the same level of transfusions until dexamethasone was added. At that point, he showed a dramatic response by becoming transfusion independent for 5 months. After approximately 8 months of therapy, the patient was taken off all medications because no further improvement was noted in the cytopenias. He began to require transfusions within 8 weeks of halting therapy and was started on another protocol. His condition eventually transformed to AML 6 months later, and he died on 8/13/98.
Patient 7
This 52-year-old white man was diagnosed with RA in 1992 and underwent multiple therapies for MDS before he started on this protocol (Table l, Figure 2D). He began treatment on 1/13/97 when his Hb was 8.9 g/dL (after PRBC transfusion), WBC count was 3000/μL, and platelet count was 18 000/μL. He was receiving 2 U PRBC every 7 to 10 days and platelet transfusions every 1 to 3 weeks. His BM was hypocellular (10% cellularity), and cytogenetics showed an abnormal karyotype with 46XY, de(7) t(1;7) (q10; p10)/46, XYde(14) t(1;14) (q10; p10)(2)/46XY(14). He continued to require both blood and platelet transfusions until the dexamethasone was added on 5/21/97. After approximately 2 months of therapy with APC + D, this patient became completely transfusion independent. Eventually, amifostine + PC was stopped (7/9/97), and the patient has been maintained on a 5 day per month cycle of dexamethasone at 4 mg by mouth 4 times a day. He has only required blood and platelet transfusions twice in the last year, both times because he was undergoing elective hip replacement surgery. At present, he continues to be transfusion independent.
Cytogenetic studies
Detailed karyotypes were performed in every patient. Fourteen patients had normal karyotypes when therapy was begun, and 15 patients showed abnormal chromosomes. The most frequent abnormalities affected chromosome 5 or 7 (8 patients), 2 had del20 abnormality, 1 had an isochromosome 17 (ql0), and 4 had other cytogenetic anomalies. Serial studies were performed when possible and showed clonal evolution with the appearance of new abnormalities in 4 patients. No cytogenetic responses were observed in this group of 29 patients.
Discussion
Myelodysplastic syndromes are universally fatal disorders. Because erythroid, myeloid, and megakaryocytic cells, and occasionally B lymphocytes, have been found to be clonal in nature, it is likely that the transforming event(s) has occurred at a pluripotential stem cell stage.31 One approach to treating this illness with a curative intent would be to target the abnormal clone directly by using intensive chemotherapy, stem cell transplantation, or both. The associated prohibitive morbidity and mortality, however, especially in an elderly group of patients, render these procedures applicable only to a select subgroup of MDS patients. An alternative approach, which may not be curative but could provide substantial palliation, would be to suppress the cause of cytopenias in these patients. We have observed the presence of extensive apoptosis in the bone marrows of as many as 75% of patients with MDS.14 The parallel high levels of TNF-α18 and IL-1b17 in these marrows suggested that cytokine-mediated apoptosis played a significant role in the genesis of cytopenias.32 Accordingly, we treated patients with pentoxifylline and ciprofloxacin with or without dexamethasone, demonstrating that the administration of these therapies resulted in a reduction in the level of TNF-α in the bone marrows of patients with MDS28 and improvement in the cytopenias of approximately 40% patients.29
Amifostine, or ethyol, is an organic thiophosphate that exists as a pro-drug.33 Alkaline phosphatase in the cell dephosphorylates it into an active form. Because normal cells rather than tumor cells have higher alkaline phosphatase levels, more active drug is available to them.33 In the presence of chemotherapeutic agents, the free thiol in amifostine provides an alternative target for reactive molecules of alkylating or platinum agents and can act as a potent scavenger of oxygen-free radicals. These protective effects are more pronounced in normal cells because more active drug is available to them.33,34 In addition, amifostine has been found to stimulate hematopoiesis in humans.35 The precise mechanism of this hematopoietic promoting activity is unclear; however, preincubation exposure to amifostine was associated with profound stimulation and enhanced survival of MDS progenitors in vitro.35 In vivo, amifostine has been useful in stimulating hematopoiesis in patients with MDS as well.29 In a group of acute myeloid leukemia patients who have poor prognoses, amifostine was found to suppress apoptosis, TNF-α, IL-6 production, and telomerase expression.36Because these are desirable therapeutic effects to be achieved in patients with MDS, the current study was designed to test whether a combination of anti-cytokine (PCD) and cytoprotective (amifostine) strategies would be more useful than either strategy alone. The results indicate that although this novel approach may not prove to be curative in the long run, it provides substantial palliative support for at least some of the patients.
The salient findings of this trial can be summarized as follows. Of the 29 evaluable patients, none achieved complete remission; however, 22 showed partial response. Two thirds of the patients had improved ANC, half showed an erythroid response, and one third had improved platelet counts. The median time to response was long, 11 to 12 weeks for erythroid and platelet responses even in the group administered dexamethasone. Two thirds of the responses occurred after the addition of dexamethasone, and one third occurred only in response to amifostine, pentoxifylline, and ciprofloxacin. Although the responders often required blood and platelet transfusions for 2 to 3 months while undergoing therapy, a gradual improvement in their cytopenias was noted that often continued for several months. No complete responses or cytogenetic responses were seen. Although the protocol was designed to provide continued therapy for 1 year, only 3 patients completed that duration, and only 16 patients completed 6 months of therapy. The main reason to discontinue therapy was a lack of continued effective response (16 patients). Others included intolerable side effects (5 patients) and disease progression (5 patients). For 12 to 16 weeks therapy was fairly well tolerated, and no differences were noted in the response rates among the 3 dose schedules. Toxicity, especially nausea, was higher in the highest dose group. There was a response seen in every FAB category, including 1 patient with CMMoL.
Amifostine + PCD therapy appears to be a positive addition to treatment options for patients with MDS. The results of this combination are better than those seen with PCD alone27 or amifostine alone,29 though the long-term outcome of the patients is unknown. It is difficult to compare the current study with the other published reports of patients with MDS treated with amifostine29,37 because of the differences in the duration of treatment and the combination of amifostine with PCD in our study. We based our study duration on previous experience with using PTX + Cipro by another group,25 which we thought was short, our own encouraging experience using PCD for a longer duration in a pilot study,27 and the use of interferon for chronic myeloid leukemia if prolonged, continuous therapy appears to be better than short-term trials. Whenever biologic response modifiers are used to treat malignancies, consideration must be given to the feasibility of long-term therapy. The approach proved to be reasonable because many patients did not show a response for 12 to 16 weeks, yet the responses could prove to be substantial once they did appear. The current study indicates that a combination of PCD + amifostine may be better than either PCD alone or amifostine alone and that prolonged, uninterrupted therapy may be better than short courses of treatment. Whether the outcome would have been this good with continuous amifostine alone is doubtful because two thirds of the responses occurred after the addition of dexamethasone at the 3-month mark. Obviously, this does not answer the question of whether pentoxifylline and Cipro add anything to the regimen or whether a combination of amifostine and dexamethasone would have produced similar results.
The mechanism of response can only be speculated on at this time. Possibilities include the suppression of proinflammatory cytokines such as TNF-α with the consequent suppression of apoptosis in hematopoietic cells, suppression of telomerase in the transformed cells with resultant regression of the MDS clone and stimulation of hematopoiesis in the normal cells, and, finally, protection of progenitor cells from pro-apoptotic signals. Regardless of the precise mechanism, the responses observed in this study provide a clinical endorsement for the strategy of using noncytotoxic therapies in MDS. The absence of serious toxicity with this combination is a decided improvement on the potentially lethal side effects of cytotoxic agents. Although patients with high-risk MDS, such as those with excess blasts, unquestionably respond to chemotherapies, it is unclear whether their survival is extended by such aggressive approaches.11 A trial of noncytotoxic drugs, such as the current trial aimed at modulating cell behavior rather than killing off the expanded clone, may be warranted as a first step for patients with high-risk MDS. The impressive erythroid response, even though seen in only a third of the treated patients, certainly makes this strategy an attractive choice for patients who have refractory anemias without excess blasts.
There are several limitations to the study reported. First of all, even in absence of serious toxic side effects, only 16 of the 29 patients were able to complete barely 6 months of the proposed 12-month trial. The single most important factor contributing to this attrition was that most patients were tired of coming 3 times a week for the intravenous administration of amifostine, especially in the absence of a response that allowed them to become transfusion independent. Another was that the use of multiple agents in this clinical trial essentially precluded a precise dissection of the mechanism of response. Yet another was that the use of dexamethasone can be a particularly confounding variable because some neutrophilia could result from either its de-margination effect or from direct stimulation of myeloid precursors. We do not believe this to be the case for several reasons. First, in our pilot study aimed at suppressing apoptosis, we found that some patients responded to PC alone without the addition of dexamethasone.27 Second, steroids as single agents have produced severe toxicity and a poor response rate in the range of 10% in patients with MDS.38 Rather, the anti-TNF synergy between pentoxifylline and dexamethasone, observed in vitro26 because the former down-regulates mRNA for TNF-α and the latter prevents its translation, appears to be the case in vivo as well.
In summary, various clinical responses were observed after therapy with PCD + amifostine. Obviously, the treatment produces different biologic effects in different patients. It may mean that in some patients the genesis of cytopenias is cytokine dependent, whereas in others cytokines not affected by this drug combination are involved, and in still others the dominant abnormality may reside within the autonomously proliferating stem cells with little contribution from cytokines. It is also possible that the anticytokine therapies used affect the production of cytokines by the BM microenvironment differently from that produced by the clonal cells themselves. Because no complete remissions were seen, it is clear that such approaches are inadequate by themselves. Perhaps a step-wise reversal of MDS can be envisioned for the future, with optimum use of anticytokine and cytoprotective agents to maximize improvement in cytopenias followed by the use of cytotoxic agents to address the stem cell defect. Our investigation into the cause of MDS may yield newer therapeutic possibilities.39 40 In the meantime, the palliative benefits of these nontoxic therapies should not be underestimated.
Acknowledgments
We thank Dr Rohit Shah and Dr Israel Wiznitzer (Lake Forest Hospital, Lake Forest, IL) for study participation. We also thank Ms Lakshmi Venugopal and Ms Sandra Howery for excellent administrative and secretarial assistance. The drug amifostine was provided free of charge to the patients by a grant from the Alza Corporation.
Supported by the National Cancer Institute (grant PO1CA 75606), The Markey Charitable Trust, and the Dr Roy Ringo Grant for basic research in myelodysplastic syndrome.
Reprints:Azra Raza, Pre-Leukemia and Leukemia Program, Rush Cancer Institute, Rush–Presbyterian–St. Luke's Medical Center, 2242 West Harrison Street, Suite 108, Chicago, IL 60612-3515; e-mail:araza@rush.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal