Abstract
The development of culture systems that facilitate ex vivo maintenance and expansion of transplantable hematopoietic stem cells (HSCs) is vital to stem cell research. Establishment of such culture systems will have significant impact on ex vivo manipulation and expansion of transplantable stem cells in clinical applications such as gene therapy, tumor cell purging, and stem cell transplantation. We have recently developed a stromal-based culture system that facilitates ex vivo expansion of transplantable human HSCs. In this stromal-based culture system, 2 major contributors to the ex vivo stem cell expansion are the addition of leukemia inhibitory factor (LIF) and the AC6.21 stromal cells. Because the action of LIF is indirect and mediated by stromal cells, we hypothesized that LIF binds to the LIF receptor on AC6.21 stromal cells, leading to up-regulated production of stem cell expansion promoting factor (SCEPF) and/or down-regulated production of stem cell expansion inhibitory factor (SCEIF). Here we demonstrate a secreted SCEPF activity in the conditioned media of LIF-treated AC6.21 stromal cell cultures (SCM-LIF). The magnitude of ex vivo stem cell expansion depends on the concentration of the secreted SCEPF activity in the SCM-LIF. Furthermore, we have ruled out the contribution of 6 known early-acting cytokines, including interleukin-3, interleukin-6, granulocyte macrophage colony-stimulating factor, stem cell factor, flt3 ligand, and thrombopoietin, to this SCEPF activity. Although further studies are required to characterize this secreted SCEPF activity and to determine whether this secreted SCEPF activity is mediated by a single factor or by multiple growth factors, our results demonstrate that stromal cells are not required for this secreted SCEPF activity to facilitate ex vivo stem cell expansion.
During the last 2 decades, hematopoietic stem cell transplantation (HSCT) has been shown to provide definitive benefit for a variety of malignant and nonmalignant hematologic diseases and myelopoietic support for patients undergoing high-dose chemotherapy.1,2 However, several inherent limitations associated with HSCT have restricted its general use.3,4These limitations include: (1) lack of sufficient donors for all recipients; (2) requirement for either operative bone marrow (BM) harvests or pheresis procedures to obtain sufficient stem cells to achieve benefit after transplant; (3) a period of BM aplasia leading to severe, prolonged neutropenia and thrombocytopenia; and (4) the potential for tumor contamination in autologous HSCT. An increasing interest exists in strategies to manipulate HSCs and hematopoietic progenitor cells in vitro for clinical purposes. The ability to generate and expand transplantable HSCs ex vivo from a small number of HSCs could have enormous potential in a variety of clinical settings.5 Ex vivo generated and expanded HSCs could support multiple cycles of chemotherapy, provide transplantation options for patients without matched donors, facilitate transduction of vectors into HSCs for gene therapy, and provide a tumor-free product for transplantation. Finally, transplantation with ex vivo expanded stem cells might abrogate the extended neutropenia and thrombocytopenia.6-8
We have previously developed a stromal-based culture system that facilitates ex vivo expansion of CD34+ thy-1+cells using long-term hematopoietic reconstitution in severe combined immunodeficient (SCID)-hu mice as an in vivo assay for transplantable human HSCs.9 The addition of leukemia inhibitory factor (LIF)10 to purified CD34+thy-1+ cells isolated from human fetal BM on AC6.21 stroma,11 a murine BM-derived stromal cell line, caused expansion of cells with the CD34+ thy-1+phenotype.9 Addition of other cytokines, including interleukin-3 (IL-3), interleukin-6 (IL-6), granulocyte macrophage colony-stimulating factor (GM-CSF), and stem cell factor (SCF), to LIF in the cultures caused a 150-fold expansion of cells retaining the CD34+ thy-1+ phenotype.9 The ex vivo expanded fetal BM CD34+ thy-1+ cells gave rise to multilineage differentiation, including myeloid, T, and B cells, when transplanted into SCID-hu mice.9 Another human HSC candidate, CD34+ CD38−cells,12,13 displayed a similar magnitude of phenotypic and functional expansion,9 suggesting that ex vivo expansion of transplantable HSCs under this culture system is a general phenomenon. We have previously demonstrated that both murine LIF, which cannot bind to human LIF receptor, and human LIF, which can bind to murine LIF receptor, are equally capable of facilitating the expansion of transplantable human HSCs in this culture system, which suggests that LIF mediates its action through the murine stroma.9 On the basis of these previous results, we have hypothesized that binding of LIF to the receptor on AC6.21 stromal cells leads to up-regulated production of stem cell expansion promoting factor (SCEPF) and/or down-regulated production of stem cell expansion inhibitory factor (SCEIF). The present studies were undertaken to test this hypothesis. Here we report the detection of a secreted, LIF-mediated, stromal derived SCEPF activity in the conditioned media (SCM-LIF) of AC6.21 stromal cell cultures. This secreted SCEPF activity is able to expand transplantable human CD34+thy-1+ cells without the stromal cells. The magnitude of CD34+ thy-1+ cell expansion depends on the concentration of the SCEPF in the SCM-LIF. The absolute number of transplantable CD34+ thy-1+ cells increases more than 200-fold after 3 weeks of culture. Furthermore, our data demonstrate that 6 known prominent stem cell cytokines, including IL-3, IL-6, GM-CSF, SCF, flt3 ligand (FL),14,15 and thrombopoietin (TPO),16-18 either alone or in combinations, cannot account solely for this secreted SCEPF activity in the SCM-LIF.
Materials and methods
Preparation of stroma-conditioned media from untreated (SCM) and LIF-treated stromal cell cultures (SCM-LIF)
Stroma-conditioned media were harvested from a confluent layer of mouse stromal cell line AC6.21. Briefly, 5 × 105 to 1 × 106 stromal cells were distributed into a T75 flask in long-term culture medium (LTCM) consisting of RPMI 1640 (GIBCO/BRL, Gaithersberg, MD), 5 × 10−5 mol/L 2-mercaptoethanol, 10 mmol/L HEPES, penicillin (50 U/mL) and streptomycin (50 mg/mL), 2 mmol/L sodium pyruvate, 2 mmol/L glutamine, and 10% fetal calf serum (FCS) at 37°C in a humidified atmosphere with 5% CO2. Confluent stromal layers formed after 1 week. A complete medium change was made with fresh LTCM containing 10 ng/mL of LIF when the layers were confluent, after which half the medium conditioned by the stromal layers was harvested every 3 days and replaced with fresh LTCM containing 10 ng/mL of LIF for a total of 4 weeks. The SCM-LIF was centrifuged at 1300 rpm for 10 minutes to remove nonadherent cells and filtered through a 0.45-μm pore filter with low protein binding (Sterivex-HV; Millipore, Bedford, MA). SCM-LIF was stored at −20°C until use. In some cases, concentrated SCM-LIF was prepared. Pooled crude supernatants were first concentrated with a DC10 concentrator using a 100 000-d nominal molecular weight cutoff (NMWC)19 hollow-fiber cartridge (Amicon Inc, Danvers, MA), and then the concentrate was clarified by refiltering with a 5000 NMWC cartridge.19 20 These concentration steps reduced the volume 40-fold. Stroma-conditioned media (SCM) from AC6.21 cultures without LIF were also harvested and processed with the same protocols and used in experiments as described in the text.
Preparation of human HSCs
Human fetal bones were dissected from 21- to 24-week-old fetuses obtained by elective abortion with approved consent (Anatomic Gift Foundation, White Oak, GA). To purify human HSCs, BM cell suspensions were prepared by flushing split long bones with RPMI 1640 containing 2% heat-inactivated FCS (Gemini Bio-Products, Inc, Calabasas, CA). Low-density (< 1.077 g/mL) mononuclear cells were isolated (Lymphoprep; Nycomed Pharma, Oslo, Norway) and washed twice in staining buffer (SB) consisting of Hanks' balanced salt solution (HBSS) with 2% heat-inactivated FCS and 10 mmol/L HEPES. Samples were then incubated for 10 minutes with 1 mg/mL heat-inactivated human gammaglobulin (Gamimune; Miles Inc, Elkhart, IN) to block Fc receptor binding of mouse antibodies. Fluorescein isothiocyanate (FITC)-labeled CD34 monoclonal antibodies (MoAbs) and phycoerythrin (PE)-labeled thy-1 MoAbs were then added at 0.5 to 1 μg/106 cells in 0.1 to 0.3 mL SB for 20 minutes on ice. Control samples were incubated in a cocktail of FITC-labeled and PE-labeled isotype-matched MoAbs. Cells were washed twice in SB, resuspended in SB containing 1 μg/mL propidium iodide (Molecular Probes Inc, Eugene, OR), and sorted using the tri-laser fluorescence-activated cell sorter (FACS) MoFlo (Cytomation, Inc, Fort Collins, CO). Live cells (ie, those excluding propidium iodide) were always greater than 95%. Sort gates were set based on the mean fluorescence intensity of the isotype control sample. Cells were collected in 12- or 24-well plates in RPMI 1640 containing 10% FCS and 10 mmol/L HEPES, counted, and reanalyzed for purity in every experiment. Typically, 450 000 to 500 000 CD34+thy-1+ cells were obtained from a single donor. MoAbs for CD34 were purchased from Becton Dickinson (Mountain View, CA). MoAbs for thy-1 and isotype controls were purchased from Pharmingen (San Diego, CA).
Stromal-based HSC expansion culture system
Sorted cells were cultured on a preestablished monolayer of a mouse stromal cell line, AC6.21, as described previously.9Briefly, 3 × 104 to 4 × 104stromal cells were plated in 24-well plates 1 week before the experiment in 1 mL of LTCM. Twenty or 300 CD34+ thy-1+ cells were distributed in 1 mL of LTCM into each well in 24-well plates with a preestablished AC6.21 monolayer. A cytokine cocktail including IL-3, IL-6, GM-CSF, and SCF was added immediately after seeding the sorted cells into the 24-well plates at a final concentration of 10 ng/mL of each growth factor. LIF was then added to the positive control wells at a final concentration of 10 ng/mL. The LTCM in the negative control wells contained only the cytokine cocktail without LIF. Human recombinant IL-3, IL-6, GM-CSF, SCF, and LIF were purchased from R&D Systems (Minneapolis, MN). Half of the culture medium was replaced weekly with fresh LTCM containing the same cytokine cocktail with or without LIF for positive and negative control wells, respectively.
SCM-based HSC expansion culture system
Twenty or 300 freshly purified CD34+ thy-1+cells were distributed into each well in a 24-well plate with 2 mL of LTCM containing 10 ng/mL of IL-3, IL-6, GM-CSF, SCF, and different concentrations of SCM-LIF. Culture media containing 5%, 10%, and 25% of SCM-LIF were prepared by mixing fresh LTCM with appropriate amounts of unconcentrated SCM-LIF. Culture media containing 50%, 100%, 200%, and 400% of SCM-LIF were prepared by mixing fresh LTCM with respective amounts of concentrated SCM-LIF. A complete medium change was made every 3 days and replaced with fresh LTCM containing the cytokine cocktail and respective amounts of SCM-LIF. The proliferative and phenotypic characteristics of these cultures were analyzed 3 weeks later.
For antibody-blocking experiments, neutralizing antibody against mouse IL-3, IL-6, GM-CSF, SCF, FL, and TPO (R&D Systems) or control goat immunoglobulin (R&D Systems) was used at 0.1 to 10 μg/mL in 200% SCM-LIF. Cultures were fed daily by replacement of half of the medium. The proliferative and phenotypic characteristics of the cultures were analyzed 3 weeks later. The neutralizing activity of the antibody for each cytokine has been determined by R&D Systems under a specific set of conditions. The neutralizing activities for each antibody as determined by R&D Systems and standardized to a 1-μg/mL dose are the following: 7.5, 5, 25, 25, 25, and 3 ng/mL for GM-CSF, IL-3, IL-6, SCF, FL, and TPO, respectively.
To determine the effect of exogenous cytokines on ex vivo stem cell expansion, we added each of the 6 cytokines, either alone or in various combinations, to the culture medium (either 200% SCM plus 10 ng/mL of LIF or 200% SCM-LIF) at concentrations of 10, 50, and 100 ng/mL. A complete medium change was made every 3 days, and cultures were analyzed 3 weeks later for their proliferative and phenotypic characteristics. Recombinant human FL and TPO were purchased from R&D Systems.
Proliferative analysis, phenotypic analysis, and sorting of ex vivo expanded human fetal HSCs
The extent to which different concentrations of SCM-LIF supported in vitro expansion of purified human fetal BM stem cells was determined by counting the total number of hematopoietic cells present in 10 individual wells in each culture. At the end of the 3-week culture period, hematopoietic cells were harvested individually from these wells, cell number was counted, and then cells were analyzed for lineage content by flow cytometry. Half of the cells from each well were stained with FITC- or PE-labeled MoAbs against CD19 and CD33, and the other half were stained with antibodies against CD34 and thy-1. Analysis was gated on the hematopoietic cells, excluding the stromal cells for the positive and negative control samples, and the quadrants were set based on the mean fluorescence intensity of the isotype control samples. FITC- and PE-labeled MoAbs against CD19 and CD33 were purchased from Pharmingen. Cells were analyzed on a FACScan fluorescent cell analyzer. To purify the ex vivo expanded HSCs from those cultures, cells from each culture condition including positive control, negative control, and SCM-based cultures were pooled, cell number was counted, and then cells were sorted for CD34+ thy-1+phenotype as described earlier.
In vivo reconstitution assay in SCID-hu mice
Human fetal bone, thymus, and liver tissues were dissected from 18- to 24-week-old fetuses obtained by elective abortion with approved consent (Anatomic Gift Foundation). A sample of each received fetal tissue was stained with a panel of MoAbs to HLA to establish the donor allotype. These fetal tissues were used for construction of the SCID-hu mice. C.B-17 scid/scid mice were bled in our facility under sterile conditions. Mice used for human tissue transplantation were 6 to 8 weeks of age, and SCID-hu thymus/liver (thy/liv) and bone-model mice were constructed as previously described9,21 22 and in accordance with the guidelines set forth by the City of Hope Research Animal Care Committee. At the time of surgery, the animals were weighed and anesthetized with a mixture of ketamine (50 mg/kg) and xylazine HCl (25 mg/kg) administered intraperitoneally. For thy/liv mice, individual pieces (1 to 2 mm) of human fetal thymus and autologous liver were placed under the kidney capsule of C.B-17 scid/scid mice and allowed to engraft for 3 months before stem cell reconstitution. For bone-model mice, pieces of fetal bone were placed subcutaneously and allowed to vascularize for 2 to 3 months. Animals were preconditioned by total-body irradiation with 350 rads 4 to 6 hours before they were subjected to stem cell reconstitution. The ability of the purified human fetal BM HSCs, CD34+ thy-1+ population (stem cell donor is always selected to be HLA-MA2.1–positive), either freshly purified or ex vivo expanded, to reconstitute thymus and BM was tested by direct inoculation into irradiated grafts (thy/liv and bone; the graft is always selected to be HLA-MA2.1–negative). For reconstitution experiments, 10 000 cells were used because we have previously established that 10 000 CD34+thy-1+ cells, either purified from fresh fetal BM or after ex vivo expansion in the stromal-based culture system, can reproducibly establish long-term hematopoietic reconstitution in more than 90% of SCID-hu mice and give rise to about 50% donor-derived hematopoietic cells in reconstituted animals. Control animals were injected with HBSS only. Engraftment was analyzed at 3 to 4 months after injection. Human bones were removed and split open to flush the marrow cavity with SB. Collected cells were spun down, and the pellet was resuspended for 5 minutes in a red blood cell lysing solution. Cells were washed twice in SB and counted before being stained for 2-color immunofluorescence with directly labeled MoAbs against HLA allotypes in combination with CD19 and CD33. Human thymus grafts were recovered, reduced to a cellular suspension, and subjected to 2-color immunofluorescence analysis using directly labeled MoAbs against HLA allotypes in combination with CD3, CD4, and CD8. FITC- and PE-conjugated irrelevant mouse immunoglobulins were used as negative controls. Cells were analyzed on a FACScan fluorescent cell analyzer. FITC- or PE-labeled CD19, CD33, CD3, CD4, and CD8 were purchased from Pharmingen.
Quantitative measurement of cytokines by enzyme-linked immunosorbent assay (ELISA)
The amount of various cytokines in the SCM and SCM-LIF was determined by the sandwich ELISA technique, using combinations of unlabeled and biotinylated MoAbs to different epitopes of each cytokine. Colorimetric ELISA kits for murine IL-3, IL-6, GM-CSF, SCF, and TPO were purchased from R&D Systems, and assays were performed according to the manufacturer's instructions. For murine FL, an affinity-purified goat polyclonal antibody raised against a peptide mapping at the amino terminus of murine FL (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) was used as capture antibody. Immulon 4 plates (Dynatech Laboratories, Inc, Chantilly, VA) were coated overnight at room temperature with 0.4 to 2 μg/mL of the above capture antibody. A biotinylated anti-mouse FL polyclonal antibody from R&D Systems was used as the detection antibody at 50 ng/mL, and assays were performed according to the manufacturer's instructions. Recombinant murine FL was purchased from R&D Systems and used to establish the standard titration.
Results
Effect of SCM-LIF on ex vivo proliferation and differentiation of human fetal BM CD34+ thy-1+ cells
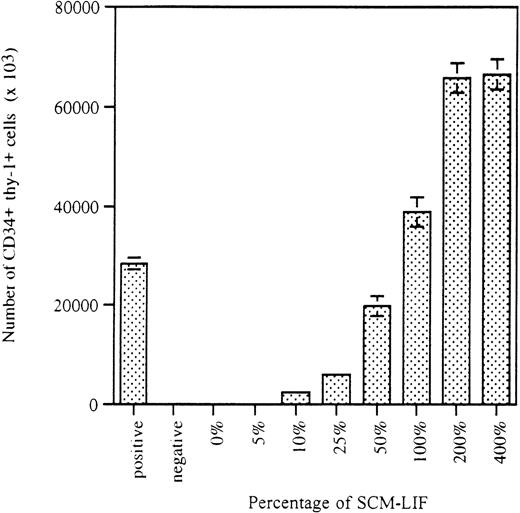
The initial studies were focused on the detection of any potential SCEPF activity in SCM-LIF. As reported previously, only 10% of the wells initiated with 20 CD34+ thy-1+ cells per well in co-culture with AC6.21 stromal cells were CD34+thy-1+–positive after 5 weeks of culture.9 To minimize the number of wells in the assay, we used 300 CD34+ thy-1+ cells per well. On the basis of the binomial distribution, we expected that every well in the culture should be CD34+ thy-1+–positive. The addition of IL-3, IL-6, GM-CSF, and SCF to the LTCM was used to shorten the time required for the assay from 7 weeks to 3 weeks.9 Three thousand freshly purified CD34+ thy-1+ cells were distributed into 10 wells (300 cells per well in a 24-well plate with 1 mL of LTCM) on a preestablished AC6.21 stromal layer in the presence of LIF (10 ng/mL) and a cytokine cocktail including 10 ng/mL of IL-3, IL-6, GM-CSF, and SCF. These 10 wells served as the positive control for the quality of the sorted CD34+thy-1+ cells and the activity of the stromal-based culture system to establish ex vivo expansion of CD34+thy-1+ cells.9 Another 3000 freshly purified CD34+ thy-1+ cells were similarly distributed but without LIF. These 10 wells served as the negative control.9 To investigate the responsiveness of CD34+ thy-1+ cells to SCM-LIF, we used 8 different concentrations, ranging from 0% to 400%, of SCM-LIF. For each concentration of SCM-LIF, 3000 CD34+thy-1+ cells were distributed into 10 wells (300 cells per well) in a 24-well plate without stroma. The proliferative and phenotypic characteristics of these cultures were analyzed 3 weeks later. As shown in Figure 1, the proliferative potential of purified CD34+thy-1+ cells in the SCM-based culture system was proportional to the concentration of SCM-LIF. The total number of hematopoietic cells apparently increased from 0% to 200% SCM-LIF and reached a plateau at 200% SCM-LIF, which was very similar to the total number of cells in the positive control cultures.
Effects of SCM-LIF on the proliferative potential of human fetal BM CD34+ thy-1+ cells in vitro.
Stromal-based cultures in the presence of cytokine cocktail (10 ng/mL of each cytokine), including IL-3, IL-6, GM-CSF, and SCF, were used as controls. Positive control and negative control are cultures with or without exogenous LIF (10 ng/mL), respectively. Data are presented as the total number of hematopoietic cells per well (total of 10 wells) in each culture condition for 3-week cultures. Results are expressed as the mean ± SD.
Effects of SCM-LIF on the proliferative potential of human fetal BM CD34+ thy-1+ cells in vitro.
Stromal-based cultures in the presence of cytokine cocktail (10 ng/mL of each cytokine), including IL-3, IL-6, GM-CSF, and SCF, were used as controls. Positive control and negative control are cultures with or without exogenous LIF (10 ng/mL), respectively. Data are presented as the total number of hematopoietic cells per well (total of 10 wells) in each culture condition for 3-week cultures. Results are expressed as the mean ± SD.
To assess the effects of SCM-LIF on the differentiation potential of purified CD34+ thy-1+ cells in this SCM-based culture system, we analyzed cells from individual wells by flow cytometry for the presence of CD33-positive myeloid cells and CD19-positive B lymphocytes. As expected from our previous report,9 all wells (with 300 cells per well), regardless of treatments, were lymphoid/myeloid wells (data not shown). The percentage of CD33-positive myeloid cells within these mixed lymphoid/myeloid wells ranged from 46% of those treated with 200% SCM-LIF to 54% of those treated with 0% SCM-LIF; the percentage of CD19-positive cells ranged from 15% of those treated with 200% SCM-LIF to 10% of those treated with 0% SCM-LIF (data not shown). To better evaluate the effect on differentiation, experiments were repeated at a limiting-dilution density (20 cells per well), and the results showed that CD33-positive and CD19-positive cells were generated at similar levels (about 50% of the wells) in these cultures regardless of different treatments (data not shown). These results suggest that SCM-LIF is capable of providing a suitable environment for multipotential CD34+ thy-1+ cells to differentiate into both B cells and myeloid cells similar to the stromal-based culture system.
Effect of SCM-LIF on ex vivo expansion of cells with CD34+ thy-1+ phenotype
To determine whether SCM-LIF is capable of facilitating the maintenance and expansion of cells with CD34+thy-1+ phenotype, we performed flow cytometric analyses to detect and quantify the number of CD34+ thy-1+cells in each individual well in these cultures. As expected from our previous report with the stromal-based culture system,9 cells with CD34+ thy-1+phenotype were detected only in all of the positive control wells and not in the negative control cultures (Table1). The frequency of CD34+thy-1+–positive wells in the SCM-based cultures was proportional to the concentration of SCM-LIF in the cultures. The frequency increased from 0% of cultures treated with 0% SCM-LIF to 100% of cultures treated with more than 100% of SCM-LIF (Table 1). The percentage of CD34+ thy-1+ cells within the CD34+ thy-1+–positive wells among those SCM-based cultures also depended upon the concentration of SCM-LIF. The percentage of CD34+ thy-1+ cells increased significantly from 3.6% in cultures treated with 10% SCM-LIF to 18% in cultures treated with more than 200% of SCM-LIF (Table 1;P < .00001). These results demonstrate that SCM-LIF alone is sufficient to facilitate ex vivo expansion of cells with CD34+ thy-1+ phenotype and that the magnitude of expansion of CD34+ thy-1+ cells is proportional to the concentration of SCM-LIF in these SCM-based cultures. These results suggest that it is a secreted, LIF-mediated, stromal cell–derived SCEPF in the SCM-LIF that facilitates ex vivo expansion of CD34+ thy-1+ cells in both the stromal-based and SCM-based culture systems.
Effects of SCM-LIF on the maintenance and expansion of freshly purified human fetal BM CD34+thy-1+ cells in vitro
| Culture Conditions . | Frequency of CD34+ thy-1+–Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| Positive control | 100% (10/10) | 8 ± 2 |
| Negative control | 0% (0/10) | N/A |
| 0% SCM-LIF | 0% (0/10) | N/A |
| 5% SCM-LIF | 0% (0/10) | N/A |
| 10% SCM-LIF | 10% (1/10) | 3.6 |
| 25% SCM-LIF | 40% (4/10) | 5 ± 3 |
| 50% SCM-LIF | 70% (7/10) | 10 ± 4 |
| 100% SCM-LIF | 100% (10/10) | 14 ± 4 |
| 200% SCM-LIF | 100% (10/10) | 18 ± 4 |
| 400% SCM-LIF | 100% (10/10) | 18 ± 3 |
| Culture Conditions . | Frequency of CD34+ thy-1+–Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| Positive control | 100% (10/10) | 8 ± 2 |
| Negative control | 0% (0/10) | N/A |
| 0% SCM-LIF | 0% (0/10) | N/A |
| 5% SCM-LIF | 0% (0/10) | N/A |
| 10% SCM-LIF | 10% (1/10) | 3.6 |
| 25% SCM-LIF | 40% (4/10) | 5 ± 3 |
| 50% SCM-LIF | 70% (7/10) | 10 ± 4 |
| 100% SCM-LIF | 100% (10/10) | 14 ± 4 |
| 200% SCM-LIF | 100% (10/10) | 18 ± 4 |
| 400% SCM-LIF | 100% (10/10) | 18 ± 3 |
A well is scored as CD34+ thy-1+–positive only if it has detectable (>1%) CD34+ thy-1+cells in the well. Numbers in parentheses indicate the total number of CD34+ thy-1+–positive wells divided by the total number of wells analyzed in the experiments. Data for the percentage of CD34+ thy-1+ cells in the CD34+ thy-1+–positive wells are presented as the mean ± SD of the total number of CD34+thy-1+–positive wells in each culture condition. N/A means not applicable.
To further confirm that the mechanism is the up-regulated production of SCEPF and not the down-regulated production of SCEIF, we mixed 200% of SCM-LIF with either 200% or 400% SCM and determined the activity of these conditions to facilitate ex vivo stem cell expansion. Table2 clearly demonstrates that it is the presence of SCEPF and not the absence of SCEIF in SCM-LIF that is responsible for facilitating ex vivo stem cell expansion. Cultures treated with 200% SCM-LIF displayed the highest magnitude of CD34+ thy-1+ cell expansion, and 200% SCM-LIF was derived from a concentration step by collecting proteins with molecular weight between 5000 and 100 000 d (see “Materials and methods”).19,20 These results show that the size of the protein for this secreted SCEPF is between 5000 and 100 000 d. Because there are approximately 350 000 cells per well within these CD34+ thy-1+–positive wells in those cultures treated with more than 200% of SCM-LIF (Figure 1), the absolute number of CD34+ thy-1+ cells in each well of these cultures averaged about 65 000 (Table 1 and Figure2), representing a greater than 200-fold expansion in this population during the 3 weeks in culture (each well was initiated with 300 purified CD34+ thy-1+cells). Because cultures treated with 200% SCM-LIF gave rise to the highest magnitude of expansion, this was selected as the optimal condition for subsequent experiments. To better evaluate the degree of stem cell expansion in SCM-LIF, experiments were repeated at a limiting-dilution density (20 initial cells per well) with 200% SCM-LIF. As expected from our previous report,9 only about 10% (13/125) of the wells were CD34+thy-1+–positive, with an average of 18% (18% ± 4%) of CD34+ thy-1+ cells within these wells (data not shown). Because there are approximately 250 000 cells per well in each CD34+thy-1+–positive well (data not shown), the absolute number of CD34+ thy-1+ cells in each well averaged about 45 000, representing a 2250-fold expansion. The overall bulk equivalent is in excess of a 225-fold expansion of CD34+thy-1+ cells in the cultures with 200% SCM-LIF. These results demonstrate that SCM-LIF is capable of providing a suitable environment for ex vivo expansion of CD34+thy-1+ cells similar to the stromal-based culture system.
SCM-LIF maintains its activity to facilitate ex vivo expansion of freshly purified human fetal BM CD34+thy-1+ cells in the presence of SCM
| Culture Conditions . | Frequency of CD34+ thy-1+–Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| Positive control | 100% (10/10) | 9 ± 3 |
| Negative control | 0% (0/10) | N/A |
| 200% SCM | 0% (0/10) | N/A |
| 400% SCM | 0% (0/10) | N/A |
| 200% SCM-LIF | 100% (10/10) | 17 ± 3 |
| 200% SCM-LIF + 200% SCM | 100% (10/10) | 17 ± 4 |
| 200% SCM-LIF + 400% SCM | 100% (10/10) | 18 ± 6 |
| Culture Conditions . | Frequency of CD34+ thy-1+–Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| Positive control | 100% (10/10) | 9 ± 3 |
| Negative control | 0% (0/10) | N/A |
| 200% SCM | 0% (0/10) | N/A |
| 400% SCM | 0% (0/10) | N/A |
| 200% SCM-LIF | 100% (10/10) | 17 ± 3 |
| 200% SCM-LIF + 200% SCM | 100% (10/10) | 17 ± 4 |
| 200% SCM-LIF + 400% SCM | 100% (10/10) | 18 ± 6 |
See Table 1 for details.
The number of CD34+ thy-1+cells in the cultures is proportional to the concentration of SCM-LIF.
Data for the number of CD34+ thy-1+ cells in the CD34+ thy-1+–positive wells are presented as the mean ± SD of the total number of CD34+thy-1+–positive wells in each culture condition. See Figure 1 legend for additional information.
The number of CD34+ thy-1+cells in the cultures is proportional to the concentration of SCM-LIF.
Data for the number of CD34+ thy-1+ cells in the CD34+ thy-1+–positive wells are presented as the mean ± SD of the total number of CD34+thy-1+–positive wells in each culture condition. See Figure 1 legend for additional information.
In vivo transplantation potential of CD34+thy-1+ cells before and after cultures
To determine whether ex vivo expanded CD34+thy-1+ cells in the SCM-based cultures maintained their in vivo transplantation potential and to further quantify the number of transplantable CD34+ thy-1+ cells from SCM-based cultures, we compared the in vivo engrafting activity among freshly purified CD34+ thy-1+ cells and ex vivo expanded CD34+ thy-1+ cells from both stromal-based and SCM-based cultures from the same donors. Data from 3 independent experiments using cells from different donors were compiled. As expected from our previous report,9 there was no donor effect on the ex vivo expansion of CD34+thy-1+ cells in the stromal-based cultures (positive control; Table 3). Similarly, cells from different donors did not display a donor effect on the ex vivo expansion of CD34+ thy-1+ cells in the SCM-based culture system (Table 3). As expected from our previous report,9 when 10 000 freshly purified human fetal BM CD34+ thy-1+ cells were injected into SCID-hu mice, it gave rise to about a 90% reconstitution rate in both thy/liv and bone-model mice with 40% to 50% donor-derived cells in the reconstituted animals for all 3 donors (Table4). Ex vivo expanded CD34+thy-1+ cells from both stromal-based cultures (positive control) and SCM-based cultures (200% SCM-LIF) gave almost identical results as the freshly purified CD34+ thy-1+cells from the same donors in terms of both the frequency of reconstitution and the percentage of donor-derived cells in the reconstituted animals (Table 4). These results indicate that ex vivo expanded CD34+ thy-1+ cells in either the stromal-based or SCM-based culture system are very similar to the freshly purified CD34+ thy-1+ cells from the same donors, both qualitatively and quantitatively. These results further demonstrate that the secreted SCEPF activity in the conditioned medium of LIF-treated AC6.21 stromal cell cultures is clearly capable of facilitating, qualitatively and quantitatively, ex vivo expansion of transplantable HSCs as the stromal co-culture system in the presence of LIF, and show that this secreted, LIF-mediated, stromal cell–derived SCEPF is responsible for ex vivo expansion of transplantable HSCs in both the stromal-based culture system and SCM-based culture system.
HSCs from different donors demonstrate no donor effect in this SCM-based culture system
| BM Samples . | Culture Conditions . | Cell Number . | Frequency of CD34+thy-1+– Positive Wells . | Percentage of CD34+ thy-1+ Cells . |
|---|---|---|---|---|
| Donor #11 | Positive control | 335 000 ± 8000 | 43/48 | 9 ± 3 |
| Negative control | 88 000 ± 6000 | 1/48 | 1.6 | |
| 200% SCM-LIF | 358 000 ± 8000 | 46/48 | 18 ± 4 | |
| Donor #12 | Positive control | 346 000 ± 6000 | 42/48 | 8 ± 2 |
| Negative control | 80 000 ± 8000 | 2/48 | 1.8 ± 0.6 | |
| 200% SCM-LIF | 382 000 ± 9000 | 44/48 | 19 ± 4 | |
| Donor #13 | Positive control | 352 000 ± 10 000 | 43/48 | 10 ± 3 |
| Negative control | 90 000 ± 9000 | 1/48 | 1.2 | |
| 200% SCM-LIF | 378 000 ± 9000 | 43/48 | 19 ± 3 |
| BM Samples . | Culture Conditions . | Cell Number . | Frequency of CD34+thy-1+– Positive Wells . | Percentage of CD34+ thy-1+ Cells . |
|---|---|---|---|---|
| Donor #11 | Positive control | 335 000 ± 8000 | 43/48 | 9 ± 3 |
| Negative control | 88 000 ± 6000 | 1/48 | 1.6 | |
| 200% SCM-LIF | 358 000 ± 8000 | 46/48 | 18 ± 4 | |
| Donor #12 | Positive control | 346 000 ± 6000 | 42/48 | 8 ± 2 |
| Negative control | 80 000 ± 8000 | 2/48 | 1.8 ± 0.6 | |
| 200% SCM-LIF | 382 000 ± 9000 | 44/48 | 19 ± 4 | |
| Donor #13 | Positive control | 352 000 ± 10 000 | 43/48 | 10 ± 3 |
| Negative control | 90 000 ± 9000 | 1/48 | 1.2 | |
| 200% SCM-LIF | 378 000 ± 9000 | 43/48 | 19 ± 3 |
See Table 1 for details. These data were compiled from 3 independent experiments using different donor BM samples. For HSCs from each donor, two 24-well plates were initiated for each culture condition including positive control, negative control, and 200% SCM-LIF.
Comparative quantitation of the number of transplantable CD34+ thy-1+ cells among freshly purified CD34+ thy-1+ cells and ex vivo expanded CD34+ thy-1+ cells derived from stromal-based (positive control) and SCM-based culture systems from the same donors
| BM Samples . | SCID-hu Models . | Freshly Purified Human Fetal CD34+ thy-1+Cells . | Ex vivo Expanded CD34+thy-1+ Cells from Positive Control . | Ex vivo Expanded CD34+ thy-1+ Cells from 200% SCM-LIF Cultures . | |||
|---|---|---|---|---|---|---|---|
| Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . | ||
| Donor #11 | Thy/liv | 19/20 | 52 ± 8 | 19/20 | 50 ± 6 | 19/20 | 50 ± 10 |
| bone | 18/20 | 43 ± 8 | 18/20 | 41 ± 6 | 19/20 | 43 ± 8 | |
| Donor #12 | Thy/liv | 18/20 | 52 ± 6 | 18/20 | 53 ± 8 | 18/20 | 51 ± 6 |
| bone | 17/20 | 43 ± 9 | 19/20 | 43 ± 10 | 17/20 | 41 ± 9 | |
| Donor #13 | Thy/liv | 19/20 | 54 ± 11 | 19/20 | 53 ± 11 | 19/20 | 50 ± 11 |
| bone | 20/20 | 44 ± 12 | 19/20 | 42 ± 10 | 19/20 | 40 ± 10 | |
| BM Samples . | SCID-hu Models . | Freshly Purified Human Fetal CD34+ thy-1+Cells . | Ex vivo Expanded CD34+thy-1+ Cells from Positive Control . | Ex vivo Expanded CD34+ thy-1+ Cells from 200% SCM-LIF Cultures . | |||
|---|---|---|---|---|---|---|---|
| Frequency . | Percentage . | Frequency . | Percentage . | Frequency . | Percentage . | ||
| Donor #11 | Thy/liv | 19/20 | 52 ± 8 | 19/20 | 50 ± 6 | 19/20 | 50 ± 10 |
| bone | 18/20 | 43 ± 8 | 18/20 | 41 ± 6 | 19/20 | 43 ± 8 | |
| Donor #12 | Thy/liv | 18/20 | 52 ± 6 | 18/20 | 53 ± 8 | 18/20 | 51 ± 6 |
| bone | 17/20 | 43 ± 9 | 19/20 | 43 ± 10 | 17/20 | 41 ± 9 | |
| Donor #13 | Thy/liv | 19/20 | 54 ± 11 | 19/20 | 53 ± 11 | 19/20 | 50 ± 11 |
| bone | 20/20 | 44 ± 12 | 19/20 | 42 ± 10 | 19/20 | 40 ± 10 | |
Purified human fetal BM CD34+ thy-1+ cells from each sample (average 450 000 to 500 000 cells) were divided into 3 fractions. One fraction (approximately 400 000 cells) was used to inject 40 SCID-hu mice (20 thy/liv mice and 20 bone-model mice; 10 000 cells per graft). The other 2 fractions (about 10 000 cells for each fraction) were used to initiate stromal-based cultures (positive control) and SCM-based cultures (300 cells per well in two 24-well plates for each system). Three weeks later, ex vivo expanded CD34+ thy-1+ cells were sorted separately from both stromal-based cultures (approximately 1 million CD34+thy-1+ cells) and SCM-based cultures (approximately 2.5 million CD34+ thy-1+ cells), and cells from each culture system were injected into 40 SCID-hu mice (again, 20 thy/liv mice and 20 bone-model mice; 10 000 cells per graft). The frequency of donor-reconstituted animals and percentage of donor-derived hematopoietic cells in the reconstituted animals were determined at 3 to 4 months after stem cell injection. These data were compiled from 3 independent experiments using different donor BM samples. The frequency of reconstituted animals is presented as the total number of reconstituted animals divided by the total number of animals used in the experiments. The percentage of donor-derived cells represents the mean ± SEM of all reconstituted animals from the same experiments.
Effect of LIF on the production of 6 known prominent stem cell cytokines by AC6.21 stromal cells
It has been reported previously that LIF treatment of stromal cells up-regulates a large number of known cytokines and down-regulates many others.23 Our first step to characterize this secreted SCEPF activity was to rule out or rule in the contribution from various known early-acting factors. It was reported previously that the addition of LIF to SyS-1 stromal cells enabled the in vitro maintenance of competitive repopulating murine stem cells.23 Evidence was presented to suggest that synergy between IL-6 and SCF, both of which are up-regulated by LIF on SyS-1 stroma, most likely accounted for the LIF-mediated activity in maintaining competitive repopulating murine stem cells in vitro.23 Flt3 is a recently discovered member of the class III tyrosine kinase receptor family.24,25 The flt3 receptor appears to be selectively expressed on primitive murine HSC and progenitor cells26and is largely restricted in human hematopoietic cells to the CD34+ progenitor population.27 FL has been shown to enhance the proliferation of HSC28,29 and progenitor cells30,31 in vitro and to mobilize HSC and progenitor cells in vivo.32-34 Recently, Shah et al35 have demonstrated that FL is able to induce proliferation of quiescent human CD34+CD38−cells that are not responsive to other early-acting cytokines and to improve the maintenance of progenitor cells in vitro. TPO has been shown to function not only as a proliferative and differentiative factor for megakaryocytes, but also as a survival factor for highly purified HSCs from both mouse36-38 and human39-41 in vitro. Several lines of evidence demonstrate the effect of TPO in stem cell proliferation in vivo. Administration of TPO has been shown to shorten the time for hematopoietic recovery in myelosuppressed animals.42 Targeted disruptions of TPO or of its receptor, c-mpl, result not only in thrombocytopenia and megakaryocytopenia, but also in a decreased number of hematopoietic stem or progenitor cells.43,44 In this study, experiments were performed to determine whether IL-6, SCF, FL, TPO, and 2 other cytokines including GM-CSF and IL-3 (which were used in our previous study),9either alone or in combinations, might account solely for the secreted SCEPF activity in the SCM-LIF. Because this secreted SCEPF activity is mediated by LIF on stromal cells, the logical criterion for a cytokine to be essential for this SCEPF activity is that the expression of the candidate cytokine must be up-regulated by the stromal cells upon LIF stimulation. ELISA was used to determine whether the expression of these 6 cytokines was up-regulated in the LIF-stimulated AC6.21 stromal cells and to measure the amount of these cytokines in the SCM and SCM-LIF. Among these 6 cytokines, IL-6 and SCF were the only 2 that could be detected by ELISA, and both were up-regulated by the stromal cells upon LIF stimulation. Because the amount of IL-6 in SCM was below the sensitivity of ELISA for IL-6 (3.1 pg/mL), the production of IL-6 by the stromal cells was up-regulated more than 40-fold upon LIF stimulation (Table 5). There was a 3-fold increase in SCF production by the LIF-stimulated stromal cells (Table5). This suggests that both IL-6 and SCF may represent components in this secreted SCEPF activity. Although the other 4 cytokines were not detectable by ELISA in either SCM or SCM-LIF (Table 5), we cannot completely rule out the possibility that these 4 cytokines were being generated by the stromal cells and were up-regulated by LIF treatment, albeit at extremely low concentrations that were below the sensitivity of ELISA. Therefore, each of these 4 cytokines, even though at low concentrations, might remain possible components in this secreted SCEPF activity.
Quantitative measurement of the 6 known prominent stem cell cytokines in SCM and SCM-LIF
| Cytokine . | Amount (pg/mL) in SCM . | Amount (pg/mL) in SCM-LIF . |
|---|---|---|
| GM-CSF | <1 | <1 |
| IL-3 | <2.5 | <2.5 |
| IL-6 | <3.1 | 130 ± 18 |
| SCF | 10 ± 2 | 30 ± 4 |
| FL | <5.5 | <5.5 |
| TPO | <20 | <20 |
| Cytokine . | Amount (pg/mL) in SCM . | Amount (pg/mL) in SCM-LIF . |
|---|---|---|
| GM-CSF | <1 | <1 |
| IL-3 | <2.5 | <2.5 |
| IL-6 | <3.1 | 130 ± 18 |
| SCF | 10 ± 2 | 30 ± 4 |
| FL | <5.5 | <5.5 |
| TPO | <20 | <20 |
The results show the mean ± SD of 4 independent experiments. “<” indicates less than the sensitivity of ELISA for each cytokine.
Neutralizing antibody to each of the 6 known prominent stem cell cytokines cannot block ex vivo stem cell expansion
To directly examine the role of these 6 cytokines in facilitating ex vivo stem cell expansion, CD34+ thy-1+ cells purified from human fetal BM were cultured on 200% SCM-LIF for 3 weeks in the absence or presence of 0.1 to 10 μg/mL of neutralizing antibody against each cytokine. All cultures, regardless of treatment, gave similar results. That is, 100% of wells (20/20) are CD34+ thy-1+-positive, with an average of 9% (9 ± 2%) of CD34+ thy-1+ cells in each well (data not shown). This result shows that ex vivo stem cell expansion was not affected by the addition of various concentrations (0.1, 1, and 10 μg/mL) of each neutralizing antibody against GM-CSF, IL-3, IL-6, SCF, FL, and TPO to the cultures in both the frequency of CD34+ thy-1+–positive wells and the percentage of CD34+ thy-1+ cells in the wells. This result demonstrates that neutralizing antibody to each of the 6 known prominent stem cell cytokines cannot block the ex vivo stem cell expansion facilitated by the SCEPF activity in the SCM-LIF and suggests that none of these 6 cytokines is essential for the SCEPF activity in the SCM-LIF.
GM-CSF, IL-3, IL-6, SCF, FL, and TPO, either alone or in various combinations, cannot facilitate ex vivo stem cell expansion
To further demonstrate that these 6 known prominent stem cell cytokines are not essential for the SCEPF activity in the SCM-LIF, we performed experiments with the addition of these 6 cytokines to the cultures. CD34+ thy-1+ cells purified from human fetal BM were cultured in 200% SCM containing 10 ng/mL of LIF for 3 weeks in the presence of 10 ng/mL of these 6 cytokines, either alone or in various combinations. Cells were also cultured with 200% SCM-LIF as a positive control. Results from this set of experiments showed that cells with CD34+ thy-1+ phenotype could be detected only in the positive control culture and not in any other culture conditions treated with cytokines, including 6 with a single cytokine, 15 with any combination of 2 cytokines, 20 with any combination of 3 cytokines, 11 with any combination of 4 cytokines, 3 with any combination of 5 cytokines, and 1 with all 6 cytokines together (data not shown). Consistent with the antibody-blocking experiments above, these results demonstrate that these 6 cytokines, either alone or in various combinations, are not sufficient to facilitate ex vivo stem cell expansion (data not shown). Similar data were obtained when higher concentrations of cytokines, at 50 and 100 ng/mL, were used. Taken together, these results further demonstrate that these 6 known prominent stem cell cytokines, including IL-3, IL-6, GM-CSF, SCF, FL, and TPO, are not the essential components for the SCEPF activity in the SCM-LIF.
Several combinations of the 6 cytokines can enhance the proportion of CD34+ thy-1+ cells in cultures with SCM-LIF
In our previous study with the stromal-based culture system, we found that the addition of GM-CSF, IL-3, IL-6, and SCF to the stromal cells in the presence of LIF was capable of increasing the proportion of CD34+ thy-1+ cells as compared with the culture with LIF alone.9 Although the addition of the 6 known stem cell cytokines, either alone or in any possible combinations, to SCM was not capable of maintaining cells with CD34+ thy-1+ phenotype in the cultures, experiments were performed to determine whether the addition of these 6 cytokines, either alone or in any possible combinations, to SCM-LIF had any effect on the proportion of CD34+thy-1+ cells in this SCM-based culture system. As expected, 100% of the wells (20/20) in all cultures were CD34+ thy-1+–positive (data not shown). The percentage of CD34+ thy-1+ cells in each well averaged about 9% (9% ± 2%) in most of the cultures, including the control (cultures without any exogenous cytokine). However, the addition of several combinations of cytokines to SCM-LIF in this SCM-based culture system was clearly capable of enhancing the proportion of CD34+ thy-1+cells, similar to the phenomenon observed in the stromal-based culture system. Table 6 shows the combinations of cytokines that significantly increased the proportion of CD34+ thy-1+ cells in these cultures from 9% to about 18%. Addition of IL-3 and IL-6 or TPO to SCF significantly increased the proportion of CD34+ thy-1+ cells from 9% to 14% (P = .0001). Although the addition of more cytokines to IL-3 + IL-6 + SCF or to TPO + SCF seemed to give a greater proportion of CD34+ thy-1+ cells, overall the differences were not significant (P = .32).
Conditions that significantly enhance the proportion of cells with CD34+ thy-1+ phenotype in the cultures
| Treatments . | Frequency of CD34+ thy-1+– Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| None | 100% (20/20) | 9 ± 2 |
| IL-3+IL-6+SCF | 100% (20/20) | 14 ± 2 |
| IL-3+IL-6+SCF+FL | 100% (20/20) | 17 ± 3 |
| GM-CSF+IL-3+IL-6+SCF | 100% (20/20) | 18 ± 4 |
| GM-CSF+IL-3+IL-6+SCF+FL | 100% (20/20) | 18 ± 4 |
| TPO+SCF | 100% (20/20) | 14 ± 2 |
| TPO+FL+SCF | 100% (20/20) | 16 ± 2 |
| TPO+FL+SCF+IL-3 | 100% (20/20) | 18 ± 4 |
| TPO+FL+SCF+IL-6 | 100% (20/20) | 18 ± 3 |
| TPO+FL+SCF+IL-3+IL-6 | 100% (20/20) | 20 ± 4 |
| Treatments . | Frequency of CD34+ thy-1+– Positive Wells . | Percentage of CD34+ thy-1+Cells . |
|---|---|---|
| None | 100% (20/20) | 9 ± 2 |
| IL-3+IL-6+SCF | 100% (20/20) | 14 ± 2 |
| IL-3+IL-6+SCF+FL | 100% (20/20) | 17 ± 3 |
| GM-CSF+IL-3+IL-6+SCF | 100% (20/20) | 18 ± 4 |
| GM-CSF+IL-3+IL-6+SCF+FL | 100% (20/20) | 18 ± 4 |
| TPO+SCF | 100% (20/20) | 14 ± 2 |
| TPO+FL+SCF | 100% (20/20) | 16 ± 2 |
| TPO+FL+SCF+IL-3 | 100% (20/20) | 18 ± 4 |
| TPO+FL+SCF+IL-6 | 100% (20/20) | 18 ± 3 |
| TPO+FL+SCF+IL-3+IL-6 | 100% (20/20) | 20 ± 4 |
All treatments were performed in 200% SCM-LIF. Cells from individual wells were analyzed by flow cytometry after 3 weeks in culture. See Table 1 for additional information.
Discussion
The hematopoietic stem cell is characterized by its ability to self-renew and to generate cells of all hematopoietic lineages. The mechanisms that regulate stem cell self-renewal versus differentiation are poorly understood. In vivo, hematopoiesis occurs close to the BM microenvironment, which presumably provides all the signals necessary for proliferation and differentiation of stem cells. Long-term bone marrow cultures (LTBMC) closely mimic many aspects of the BM microenvironment45 and have been shown to be capable of supporting HSC self-renewal, proliferation, and differentiation in vitro.46,47 However, stromal layers derived from LTBMC consist of a heterogeneous mixture of cells and present difficulties for the identification of cytokines that may promote HSC self-renewal or differentiation in this setting.48 Studies using cloned murine stromal cell lines have further confirmed that stromal cells are functionally heterogeneous in terms of their ability to support lymphoid and/or myeloid differentiation11,49,50 and proliferation of HSCs.23,51,52 It has been hypothesized that distinct stromal cells form niches within the microenvironment that selectively regulate stem cell functions.48,53-55 We have previously shown that the AC6.21 stromal cell line provides an environment for a single multipotential CD34+thy-1+ cell to differentiate into both B lymphocyte and myeloid cells, a phenomenon similar to the in vivo BM environment.9 This AC6.21 stromal cell line might represent a specific and relatively rare subpopulation of stromal cells that constitute the stem cell–supporting niches in the BM microenvironment.48,53 Using AC6.21 stromal cells, we have recently developed an in vitro culture system in which purified human fetal BM CD34+ thy-1+ cells are expanded 150-fold in the presence of LIF.9 Furthermore, we have demonstrated that LIF facilitates ex vivo CD34+thy-1+ cell expansion indirectly via AC6.21 stromal cells.9 LIF is a polyfunctional regulator of cell growth and has been shown to have a broad spectrum of effects on a variety of cell types.56,57 Prior studies have shown that LIF has little or no effect on murine hematopoietic progenitor cell growth yet enhances hematopoiesis in vivo, suggesting that LIF might have an indirect role in hematopoiesis.58-61
On the basis of our own results and those of others, we have hypothesized that LIF stimulates the AC6.21 stromal cells either to produce SCEPF or to inhibit the production of SCEIF. In this study, we focused on the detection and characterization of the putative SCEPF or SCEIF. We demonstrated that SCM-LIF is sufficient to support ex vivo proliferation (Figure 1) and multilineage differentiation of purified CD34+ thy-1+ cells and to cause ex vivo expansion of CD34+ thy-1+ cells independent of AC6.21 stromal cells (Table 1). The magnitude of CD34+thy-1+ cell proliferation (Figure 1) and expansion (Table1) was proportional to the concentration of SCM-LIF. These results suggest that there is an SCEPF activity in the SCM-LIF. Results from mixing SCM-LIF and SCM (Table 2) clearly confirmed that it is the presence of SCEPF and not the absence of SCEIF in SCM-LIF that is responsible for facilitating ex vivo stem cell expansion. Furthermore, we demonstrated that this secreted SCEPF activity can support ex vivo proliferation and differentiation of purified CD34+thy-1+ cells and promote the expansion of cells with CD34+ thy-1+ phenotype (Figure 2). In addition, these ex vivo expanded CD34+ thy-1+ cells not only maintained the input CD34+ thy-1+phenotype, but also preserved their in vivo transplantation potential as freshly purified CD34+ thy-1+ cells (Tables3 and 4). Our data from hematopoietic reconstitution in SCID-hu mice showed that ex vivo expanded cells from both stromal-based and SCM-based culture systems and freshly purified CD34+thy-1+ cells were qualitatively and quantitatively similar (Table 4). In our previous studies in the stromal-based culture system, there was a greater than 200-fold expansion of CD34+thy-1+ cells in 5-week cultures. Similarly, there was a 217-fold expansion of CD34+ thy-1+ cells in the 3-week SCM-based cultures initiated with 300 CD34+thy-1+ cells per well (Table 1). These results demonstrate that the degree of stem cell expansion supported by the SCM-based culture system is as effective as that in the stromal-based culture system. The degree of stem cell expansion in the SCM-based culture system was further examined at a limiting-dilution density (20 cells per well), and our results showed a 225-fold expansion under this culture condition (data not shown). Although the cultures initiated at 20 cells per well had a higher degree (225-fold) of stem cell expansion than the cultures initiated with 300 cells per well (217-fold), the difference in the degree of stem cell expansion between these conditions was not statistically significant (P = .825). These results indicate that there is no inhibitory or enhancing effect on ex vivo stem cell expansion by the interactions among hematopoietic cells in this SCM-based culture system and suggest that this SCM-based culture system might be ready for scaled-up expansion in a clinical application.
To characterize the nature of this secreted SCEPF activity, we investigated the possible contribution from 6 known prominent stem cell cytokines, including GM-CSF, IL-3, IL-6, SCF, FL, and TPO, either alone or in combinations, to this secreted SCEPF activity. It has been reported that addition of LIF to SyS-1 stromal cells enabled the in vitro maintenance of competitive repopulating murine stem cells.23 Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to demonstrate that macrophage colony-stimulating factor (M-CSF), interleukin-7 (IL-7), SCF, flt3/flk2 receptor, interleukin-2 (IL-2), IL-6, granulocyte colony-stimulating factor (G-CSF), and LIF were up-regulated on SyS-1 stromal cells upon LIF stimulation.23 Evidence was presented to suggest that synergy between IL-6 and SCF, both of which are up-regulated by LIF on SyS-1 stroma, most likely accounts for the LIF-mediated activity in maintaining competitive repopulating murine stem cells in vitro.23 Several studies have shown that FL is capable of facilitating proliferation of hematopoietic stem and progenitor cells in vitro28-31 and mobilization of hematopoietic stem and progenitor cells in vivo.32-35 TPO, although a potent factor for megakaryocytopoiesis,18 also stimulates division of primitive human hematopoietic stem and progenitor cells.39-41 Because this secreted SCEPF activity is mediated by LIF on stromal cells, the logical criterion for a cytokine to be essential for this secreted SCEPF activity is that its expression must be up-regulated by the stromal cells upon LIF stimulation. ELISA was used to determine not only whether the expression of the cytokine was up-regulated upon LIF treatment, but also the actual amount of the cytokine in the conditioned media from LIF-treated stromal cell cultures, which is a prerequisite for knowing the amount of antibody needed in antibody-blocking experiments. As shown in Table 5, only IL-6 and SCF gave measurable amounts of cytokines by ELISA, and both were up-regulated by the stromal cells upon LIF stimulation. Although the amount of proteins for the other 4 cytokines could not be determined by ELISA, it remains possible that these 4 cytokines might be up-regulated by the stromal cells upon LIF stimulation, but at extremely low concentrations that are below the sensitivity of ELISA. To directly examine the possible contribution from these 6 cytokines to this secreted SCEPF activity, we added neutralizing antibodies against each cytokine to the 200% SCM-LIF and determined their activity to facilitate ex vivo expansion of CD34+ thy-1+cells. Results from this set of experiments showed that neutralizing antibody to each of the 6 known prominent stem cell cytokines cannot inhibit the ex vivo stem cell expansion facilitated by the SCEPF activity in the SCM-LIF. Based on the amount of the cytokines present in the SCM-LIF determined by ELISA and the neutralizing activity of the antibody against each cytokine, 1 μg/mL of each neutralizing antibody was already above saturation level for each cytokine. These results demonstrate that none of these 6 cytokines is essential for the SCEPF activity in the SCM-LIF. To further rule out the contribution from these 6 known cytokines to this SCEPF activity, all possible combinations of these 6 cytokines at 10, 50, and 100 ng/mL were added to 200% SCM plus 10 ng/mL of LIF, and their activity to maintain and expand cells with CD34+ thy-1+ phenotype was determined. Our results demonstrate that these 6 cytokines, either alone or in various combinations, are not capable of maintaining CD34+ thy-1+ cells in the cultures (data not shown). We have recently used the more sensitive RT-PCR method to further investigate the expression of IL-3, FL, TPO, and GM-CSF in the stromal cells before and after LIF treatment (data not shown). SCF was used as the positive control to define the conditions for RT-PCR. We have established an optimal condition in which there is a 3-fold increase in SCF expression upon LIF stimulation, consistent with the result from ELISA. Under this RT-PCR condition, there is no detectable signal for IL-3, GM-CSF, and TPO in the stromal cells with or without LIF treatment. The expression of FL is detectable by RT-PCR and is not altered upon LIF stimulation. We conclude that these 6 known prominent stem cell cytokines, including IL-3, IL-6, GM-CSF, SCF, FL, and TPO, are not required for the SCEPF activity in the SCM-LIF.
Recently, a number of cytokines and different cytokine combinations have been shown to inhibit apoptosis of hematopoietic stem and progenitor cells. TPO promotes clonal growth of murine marrow Sca+Lin− cells in vitro by suppression of apoptosis.62 SCF can directly promote survival of hematopoietic progenitor cells in the absence of cell division.63,64 Combinations of cytokines including TPO, SCF, and other early-acting cytokines have been demonstrated to facilitate the survival of human CD34+ cells following cell division ex vivo.39-41 In this study, several combinations of cytokines including TPO, SCF, and other early-acting cytokines have been identified that significantly increased the proportion of CD34+ thy-1+ cells in these cultures from 9% to about 18% (Table 6). It is very likely that the addition of these cytokines to the cultures can promote the survival of cells with CD34+ thy-1+ phenotype and subsequently increase the proportion of CD34+ thy-1+ cells in these cultures as compared with the cultures without these cytokines.
In this study, we have demonstrated that 6 known early-acting cytokines, including GM-CSF, IL-3, IL-6, SCF, FL, and TPO, are not required for the secreted SCEPF activity in the SCM-LIF, and suggest the presence of other secreted, LIF-mediated, stromal cell–derived factor(s) in the SCM-LIF that promote ex vivo expansion of transplantable human HSCs. At the present time, we have no data to demonstrate that a single novel protein is the candidate for this putative SCEPF activity in facilitating ex vivo expansion of transplantable human fetal BM HSCs, and it remains possible that this putative SCEPF activity might involve a synergism among multiple factors, including known and novel cytokines. Based on the method we have used to concentrate the SCM-LIF, our results suggest that the molecular weight for the factor(s) of this secreted SCEPF activity is between 5000 and 100 000 d. Although the nature of the putative SCEPF activity is not yet defined, the results presented in this study support the hypothesis that binding of LIF to the receptor on AC6.21 stromal cells leads to the production of a secreted SCEPF activity that facilitates ex vivo expansion of transplantable HSCs, and that this secreted SCEPF activity is not mediated by cell–cell or cell–extracellular matrix interactions. The identification of the component(s) for this secreted SCEPF activity will undoubtedly lead to a better understanding of the intricate regulatory process governing the development of all hematopoietic lineages from HSCs. Furthermore, the ability to reconstitute this SCEPF activity in vitro with defined components will facilitate ex vivo manipulation and expansion of transplantable HSCs in several critical clinical applications, including gene therapy, tumor cell purging, and stem cell transplantation.
Acknowledgments
We thank Drs David DiGiusto, John Rossi, John Zaia, and Ravi Bhatia for review of the manuscript; Drs Jeffrey Longmate and Joycelynne Palmer for their assistance in statistical analysis; Lucy Brown and Jim Bolen for assistance in FACS analysis; Drs Steve Novak and Tom LeBon for their assistance in the preparation of the manuscript; supportive team members in the Department of Molecular Biology at Beckman Research Institute at City of Hope for their administrative assistance; and members in the Animal Research Center at City of Hope for their assistance in animal care.
Supported by grants from the National Cancer Institute: NCI PPG CA 30206, NCI CA 33572, and NCI CA 71.866.
Reprints:Chu-Chih Shih, Department of Hematology/Bone Marrow Transplantation, City of Hope National Medical Center, and Department of Molecular Biology, Beckman Research Institute at City of Hope, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: cshih@coh.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal