Abstract
The drug-dependent antibody of a patient with rifampicin-induced thrombocytopenia was characterized using the antigen-capture enzyme-linked immunosorbent assay (MAIPA assay), flow cytometry, and immunoprecipitation. The antibody was found to bind glycoprotein (GP) Ib-IX but not GPIIb-IIIa because (1) it immunoprecipitated drug-dependently the former but not the latter glycoprotein complex and (2) the MAIPA assay showed strong rifampicin-dependent antibody binding when anti-GPIb-IX monoclonal antibodies (mAbs) (AK2 and FMC25) but not anti-GPIIb-IIIa mAbs (AP2, SZ21, and SZ22) were used to capture the antigen. The antibody binding site was further localized to the GPIX subunit of the GPIb-IX complex because flow cytometric analysis revealed drug-dependent antibody binding to L cells transfected with human GPIbβ and GPIX complementary DNA (L βIX cells) but not with human GPIb and GPIbβ complementary DNA (L β cells). Finally, in the MAIPA assay, the rifampicin-dependent antibody almost completely cross-blocked the binding of the anti-GPIX mAb (SZ1) to platelets. Similar cross-blocking of SZ1binding to platelets by the quinine-dependent antibodies was also observed. This finding not only confirms that the epitope of the rifampicin-dependent antibody is on GPIX but it is also identical to or located in close proximity to that of the quinine-dependent antibody and SZ1. Further characterization of the epitopes of these antibodies may have important implications for a general understanding of the mechanism of drug-induced thrombocytopenia.
Rifampicin is a widely used agent for the treatment of tuberculosis. Adverse effects from the administration of rifampicin can include interstitial nephritis, thrombocytopenia, and hemolytic anaemia. The first incidence of rifampicin-induced thrombocytopenia was reported in 1970 by Blajchman et al.1 Since then, many other cases have been reported.2,3 Several investigators have demonstrated rifampicin-dependent antiplatelet antibodies2,3but have not characterized the platelet antigen of these antibodies. Kakaiya et al2 demonstrated that rifampicin did not bind platelets directly. They suggested that the antibody first reacts with the drug to form a drug-antibody complex that then binds to platelets, resulting in their clearance through an “innocent bystander” mechanism. Martinez et al4 suggested that the interaction of the offending drug with blood cell membrane proteins leads to the formation of an antigenic complex to which the drug-dependent antibodies bind. Our observations favor the second mechanism.
In this study, we have characterized the antibody from a patient who developed rifampicin-induced thrombocytopenia. Using flow cytometry, monoclonal antibody–specific immobilization of platelet antigens (MAIPA) assay, and immunoprecipitation, we demonstrated that the antibody reacted with the glycoprotein (GP) Ib-IX complex on the platelet surface. The antibody epitope was further mapped to the GPIX subunit of the complex. More specifically, the antibody binding site was located at a site where the epitopes of 3 other drug-induced antibodies (quinine-, quinidine-, and ranitidine-induced) have been previously located. These data imply that this site on GPIX is strongly immunogenic when it combines with drugs. A careful analysis of this GPIX region may provide useful information about the pathophysiology of drug-induced thrombocytopenia in general.
Materials and methods
Materials
Bovine serum albumin (BSA), phenylmethylsulfonyl fluoride (PMSF), ethylenediaminetetraacetic acid, disodium salt (EDTA), bacitracin, benzamidine, dithiothreitol (DTT), dimethylsulfoxide, iodoacetamide, propidium iodide, and 2,2′-azinobis 3-ethylbenzthiazolinesulfonic acid (ABTS) were purchased from Sigma (St Louis, MO); 3,3′,5,5′-tetramethylbenzidine dihydrochloride from Kirkegaard & Perry (Gaithersburg, MD); sulfosuccinimidobiotin from Pierce (Rockford, IL); Western blot chemiluminescence reagents from DuPont (Boston, MA); and the sheep anti-mouse–coated Dynabeads and the sheep anti-rabbit immunoglobulin G (IgG)-coated Dynabeads from Dynal (Oslo, Norway). Rifampicin was purchased from Marion Merrell Dow (Sydney, Australia). All chemicals were of analytical reagent grade.
Antibodies
The goat anti-mouse immunoglobulin and the horseradish peroxidase (HRP)-conjugated goat anti-human immunoglobulin (Jackson, West Grove, PA), rabbit anti-mouse HRP-conjugated antibody (Dako, Carpentaria, CA), and the streptavidin-HRP conjugate (Amersham, Bucks, United Kingdom) were purchased as indicated.
All monoclonal antibodies (mAb) were of the IgG class. MOPC21 (Becton & Dickinson, San Jose, CA), a murine IgG1 myeloma protein, was used as a control immunoglobulin, and SZ1, SZ21, and SZ22 were purchased from Immunotech (Marseille, France). The sheep anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody was purchased from Silenius (Hawthorn, Australia), and the rabbit anti-human FITC-conjugated secondary antibody from Dako.
AK2 and FMC25 mAbs, which are directed against epitopes on various parts of the human GPIb-IX complex, were obtained from Dr M. Berndt (Melbourne, Australia).5 AP2, a mAb that is directed against an epitope on GPIIb-IIIa, was a kind gift from Dr T. J. Kunicki (La Jolla, CA).
Patient
Mrs N.P., a 66-year-old woman, was diagnosed with pulmonary tuberculosis and was begun on rifampicin and isoniazid. Four months later, she noted spontaneous bruising. Physical examination revealed petechiae on her legs but no other bleeding. Blood counts showed a severe thrombocytopenia: platelets 9 × 109/L, hemoglobin 165 g/L, and white blood cells 5.0 × 109/L. A diagnosis of drug-induced thrombocytopenia was made, and all drugs she was taking were stopped. She was begun on 50 mg of prednisone daily. A test for drug-dependent antibodies using an antigen-capture assay—MAIPA—demonstrated a rifampicin-dependent antiplatelet antibody with specificity against GPIb-IX complex, but no isoniazid-dependent antibody was detected. A bone marrow aspirate revealed normal numbers of megakaryocytes and erythroid and myeloid precursors, consistent with a thrombocytopenia due to increased peripheral platelet destruction. Her platelet count rose to 124 × 109/L, 189 × 109/L, and 214 × 109/L on days 4, 6, and 7, respectively, after withdrawal of antituberculosis drugs. Prednisone was then stopped.
Cell lines
Chinese hamster ovary (CHO) DUK- (dihydrofolate reductase–negative [DHFR-]) cells and mouse L (tk-) cells stably transfected with complementary DNA (cDNA) encoding the GPIb-IX subunits in various combinations were produced in one of our laboratories (J.A.L.) as previously described.6 The cloning of the cDNAs for GPIbα, GPIbβ, and GPIX has been reported previously.7-9 The 3 cDNAs (each containing the entire coding sequence and the 3′-untranslated region) were cloned separately into the eukaryotic expression vector pDX (a kind gift from Dr K. Berkner, Seattle, WA), in which transcription is driven by the adenovirus major late promoter and the SV40 enhancer.
Methods
Transfection of CHO and L cells with GPIb-IX genes.
The CHO cells were transfected with the following combinations of GPIb-IX subunit cDNAs: (a) GPIbα, GPIbβ, and GPIX, (b) GPIbα and GPIbβ, (c) GPIbα and GPIX, and (d) GPIbβ and GPIX. The L cells were transfected with (a) GPIbα and GPIbβ and (b) GPIbβ and GPIX. Expression of the GPIb-IX subunits in the cell lines was substantiated by Northern blot analysis to detect messenger RNA (mRNA) and by flow cytometry and enzyme-linked immunosorbent assay (ELISA) to ensure protein expression of the subunits on the cell surface. In all cell lines, the appropriate GPIb-IX subunits were expressed on the cell surface except in the CHO cells transfected with GPIbα and GPIX cDNA. In this cell line, GPIbα was expressed on the cell surface, but GPIX was located in the cytoplasm when detected by flow cytometry.
Enzyme-linked immunosorbent assay.
The ELISA was performed as previously described.10
MAIPA assay.
The MAIPA assay was performed as previously described with minor modifications.11 Group O platelets (2 × 107 per tube) were washed once in phosphate-buffered saline (PBS)/1% EDTA buffer, resuspended in 100 μL PBS/2% (w/v) BSA, and added to 50 μL of patient serum with or without 5 μL of rifampicin (final concentration, 70 μg/mL). The positive control for the assay was normal pooled platelets at a concentration of 2 × 107/100 μL plus 50 μL of patient serum known to contain an anti-PLA1 antibody.
Flow cytometry.
Flow cytometry was performed on a FACStar Plus flow cytometer (Becton & Dickinson) fitted with a 100-mW air-cooled argon ion laser using the 488-nm green line for fluorescence excitation. The cell emission spectra were collected on FL1 (green) using a band pass filter 530 DF 30. Dead cells were excluded using propidium iodide on FL3 using a 700 LP filter. Cells or group O platelets for flow cytometry were prepared as described previously.10 Primary antibody incubation, mAb (10 μg/mL) or patient serum (1:20 dilution), was performed for 10 minutes at room temperature in the presence or absence of rifampicin (700 μg/mL). In experiments carried out in the presence of rifampicin, the working or wash buffer contained the drug beyond this stage at a concentration of 700 or 600 μg/mL, respectively.
Biotin labeling of glycoprotein Ib-IX subunits.
The platelets (2 × 1010) were incubated with 168 μmol of sulfosuccinimidobiotin, and the free biotin was quenched with excess glycine. The platelets were then incubated with one of the following: a mouse IgG1 control (MOPC21; 10 μg/mL), the GPIX-specific mAb (GRP; 10 μg/mL), or AB or patient serum (40 μL of each with or without the drug at 700 μg/mL) for 30 minutes. In experiments performed in the presence of rifampicin, the working or wash buffer contained the drug beyond this stage at a concentration of 700 or 600 μg/mL, respectively. After 3 washes, the platelets were lysed by resuspension in 500 μL of 0.01 mol/L triethanolamine-buffered saline containing 0.5% Triton X-100, 0.05% Tween-20, and protease inhibitors (PMSF 2 mmol/L, leupeptin 10 μM/L, aprotinin 10 μM/L, EDTA 5 mM/L) and incubated for 1 hour. Following centrifugation at 12 000g for 30 minutes, the supernatant (450 μL) was collected; 1 × 107 sheep anti-mouse–coated Dynabeads, or 2.25 × 107 Dynabeads coated with goat anti-rabbit IgG linked to rabbit anti-human IgG, were added to the mAb or the human serum immunoprecipitates, respectively. After incubation for 2 hours, the Dynabeads were washed 5 times using the MPC-E-1 magnet before resuspension in 10 μL 2 × Laemmli buffer12 and storage at −80°C until analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
SDS-PAGE and detection of proteins.
The 20-μL samples for SDS-PAGE analysis were treated with 2 μL of 0.5-mol/L DTT and boiled for 5 minutes. Free DTT was quenched by 4 μL of 0.5-mol/L iodoacetamide. SDS-PAGE was performed according to the method of Laemmli12 using 12% Tris-glycine gels (Bio-Rad, Hercules, CA). After electrophoresis, the proteins were transferred to polyvinylidine difluoride (PVDF) membrane. Membranes were blocked overnight in 5% (w/v) skim milk (Diploma) and the following morning washed 5 times in PBS/0.05% Tween-20. Streptavidin HRP diluted 1:2000 in 2% BSA/PBS/0.05% Tween-20 was incubated with the membrane for 60 minutes. After 5 washes, the presence of a signal was detected using the Western blot chemiluminescence reagents according to the manufacturer's instructions.
Results
Rifampicin-dependent antibody binds to GPIb-IX and not to GPIIb-IIIa
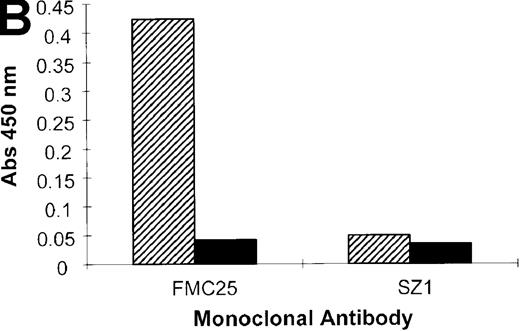
The patient serum was analyzed using the MAIPA assay. Antibody binding was only seen in the presence of rifampicin. The serum showed rifampicin-dependent antibody binding to platelets when the GPIbα-specific mAb, AK2, was used to capture the antigen (GPIb-IX complex). Similarly, strong rifampicin-dependent antibody binding occurred when the non cross-blocking GPIX-specific mAb, FMC25, captured the antigen (Figure 1). When a GPIIb-IIIa–specific mAb, AP2, SZ21, or SZ22, was used as the capture antibody, a negative result was obtained. These data indicate that the rifampicin-dependent serum contains an antibody that reacts with the GPIb-IX but not GPIIb-IIIa complex.
Binding of rifampicin-dependent antibody to platelet surface glycoproteins.
MAIPA assay, an antigen-capture ELISA, was performed using SZ21, SZ22, and AP2, anti-GPIIb-IIIa mAbs, to capture the GPIIb-IIa complex or AK2 (an anti-GPIbα mAb) or FMC25 (a non–cross-blocking anti-GPIX mAb) to capture the GPIb-IX complex. The studies were performed in the presence and absence of rifampicin with patient serum (Pt) or the control (AB serum). Samples were assayed in the presence or absence of rifampicin. Binding was not observed with the AB serum in the presence or absence of rifampicin with any of the mAbs. The patient serum did not bind in the absence of rifampicin. In the presence of rifampicin, the patient serum gave a positive result in the presence of GPIbα-specific mAb AK2 and GPIX-specific mAb FMC25. A negative result was observed with GPIIb-IIIa mAbs SZ 1, SZ22 (data not shown), and AP2. These data suggest that the antibody(ies) in the patient serum reacted with GPIb-IX complex but not with the GPIIb-IIIa complex.
Binding of rifampicin-dependent antibody to platelet surface glycoproteins.
MAIPA assay, an antigen-capture ELISA, was performed using SZ21, SZ22, and AP2, anti-GPIIb-IIIa mAbs, to capture the GPIIb-IIa complex or AK2 (an anti-GPIbα mAb) or FMC25 (a non–cross-blocking anti-GPIX mAb) to capture the GPIb-IX complex. The studies were performed in the presence and absence of rifampicin with patient serum (Pt) or the control (AB serum). Samples were assayed in the presence or absence of rifampicin. Binding was not observed with the AB serum in the presence or absence of rifampicin with any of the mAbs. The patient serum did not bind in the absence of rifampicin. In the presence of rifampicin, the patient serum gave a positive result in the presence of GPIbα-specific mAb AK2 and GPIX-specific mAb FMC25. A negative result was observed with GPIIb-IIIa mAbs SZ 1, SZ22 (data not shown), and AP2. These data suggest that the antibody(ies) in the patient serum reacted with GPIb-IX complex but not with the GPIIb-IIIa complex.
When immunoprecipitation experiments were performed using human AB or patient serum with and without rifampicin, the GPIb-IX complex components were isolated only in the presence of the patient serum and rifampicin (Figure 2). When the experiment was repeated in the presence of a nonimmune mouse IgG1 (MOPC21) and the GPIX-specific mAb, GRP, immunoprecipitation of the GPIb-IX complex components was observed with GRP in both the presence and absence of the drug, but no specific band was observed with MOPC21. The immunoprecipitation of the GPIb-IX subunits by the patient serum in the presence of rifampicin indicates that the drug-dependent antibody has specificity for the GPIb-IX complex.
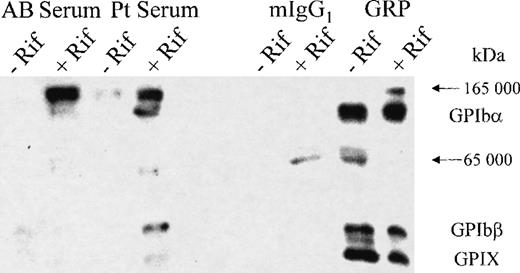
Immunoprecipitation of the GPIb-IX complex using the rifampicin-dependent antibodies.
After labeling the surface proteins of the platelets with biotin, the GPIb-IX complex was immunoprecipitated using AB serum, patient serum, a nonspecific murine IgG1 (MOPC21), and the GPIX-specific mAb (GRP), all in the presence or absence of the drug as indicated. The platelets were lysed, and the immunoprecipitated proteins were collected using Dynabeads coated with the appropriate capture antibodies. The proteins were analyzed by SDS-PAGE, transferred to PVDF membrane, and detected by streptavidin HRP and chemiluminescence reagents. The GPIb-IX complex (GPIbα, 143-kd band; GPIbβ, 24-kd band; and GPIX, 20-kd band) was only detected with the patient serum in the presence of the drug (lane 4) and with the anti-GPIX mAb (GRP) in both the absence (lane 7) and presence (lane 8) of the drug. Nonspecific bands (165 kd and 65 kd) were seen both with the test, and control antibodies in the presence or absence of the drug and were present in no particular pattern.
Immunoprecipitation of the GPIb-IX complex using the rifampicin-dependent antibodies.
After labeling the surface proteins of the platelets with biotin, the GPIb-IX complex was immunoprecipitated using AB serum, patient serum, a nonspecific murine IgG1 (MOPC21), and the GPIX-specific mAb (GRP), all in the presence or absence of the drug as indicated. The platelets were lysed, and the immunoprecipitated proteins were collected using Dynabeads coated with the appropriate capture antibodies. The proteins were analyzed by SDS-PAGE, transferred to PVDF membrane, and detected by streptavidin HRP and chemiluminescence reagents. The GPIb-IX complex (GPIbα, 143-kd band; GPIbβ, 24-kd band; and GPIX, 20-kd band) was only detected with the patient serum in the presence of the drug (lane 4) and with the anti-GPIX mAb (GRP) in both the absence (lane 7) and presence (lane 8) of the drug. Nonspecific bands (165 kd and 65 kd) were seen both with the test, and control antibodies in the presence or absence of the drug and were present in no particular pattern.
Further localization of rifampicin-dependent antibody binding to GPIX
Flow cytometry was used to test the binding of the patient antibody to platelets and CHO cells stably transfected with GPIbα, GPIbβ, and the GPIX cDNAs. A negative control of AB serum detected no specific antibody binding in the presence of the drug on platelets (Figure 3) or CHO cells (not shown). Rifampicin-dependent antibody binding was observed on both the platelets and the CHO αβIX cells. These results confirm that the rifampicin-dependent antibody binds to the GPIb-IX complex.
Binding of rifampicin-dependent antibodies to platelets and CHO βIX cells.
The platelets or CHO αβIX cells were labeled with primary antibody (patient serum or AB serum) in the presence or absence of rifampicin, followed by an FITC-conjugated secondary antibody (rabbit anti-human IgG), and examined by flow cytometry. The AB serum did not bind to the platelets in the presence or absence of the drug (A). A similar result was observed when the CHO αβIX cells were labeled with AB serum in the presence or absence of rifampicin (not shown). The patient serum bound to the platelets (B) and the CHO αβIX cells (C) in the presence of the drug. The shaded peak in each graph represents the binding of the serum in the absence of the drug. Pt indicates patient.
Binding of rifampicin-dependent antibodies to platelets and CHO βIX cells.
The platelets or CHO αβIX cells were labeled with primary antibody (patient serum or AB serum) in the presence or absence of rifampicin, followed by an FITC-conjugated secondary antibody (rabbit anti-human IgG), and examined by flow cytometry. The AB serum did not bind to the platelets in the presence or absence of the drug (A). A similar result was observed when the CHO αβIX cells were labeled with AB serum in the presence or absence of rifampicin (not shown). The patient serum bound to the platelets (B) and the CHO αβIX cells (C) in the presence of the drug. The shaded peak in each graph represents the binding of the serum in the absence of the drug. Pt indicates patient.
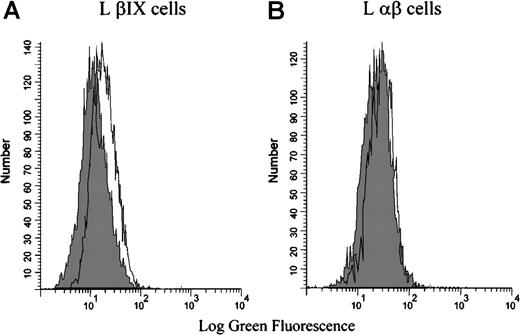
Flow cytometry was also used to test the binding of the patient antibody to mouse L cells that had been stably transfected with either the human GPIbβ and GPIX cDNA (L βIX cells) or the human GPIbα and GPIbβ cDNA (L αβ cells). Binding was not observed in the absence of rifampicin. In the presence of rifampicin, the drug-dependent antibody bound only to L βIX cells and not to the L αβ cells (Figure 4). These data indicate that the rifampicin-dependent antibody binds specifically to the GPIX subunit of the GPIb-IX complex—GPIX is the only component not present on the L αβ cells.
Binding of the rifampicin-dependent antibodies to L βIX and L β cells.
The L βIX and L αβ cells were incubated with patient serum in the presence or absence of rifampicin, followed by an FITC-conjugated secondary antibody, and examined by flow cytometry. The patient serum did not bind in the absence of the drug (shaded peak). The patient serum bound to the L βIX cells (A) but not to the L αβ cells (B) in the presence of the drug. The shaded peak in each graph represents the binding of the serum in the absence of the drug. The figure illustrates the results from 1 experiment that is representative of the results observed on the 3 occasions the experiment was performed.
Binding of the rifampicin-dependent antibodies to L βIX and L β cells.
The L βIX and L αβ cells were incubated with patient serum in the presence or absence of rifampicin, followed by an FITC-conjugated secondary antibody, and examined by flow cytometry. The patient serum did not bind in the absence of the drug (shaded peak). The patient serum bound to the L βIX cells (A) but not to the L αβ cells (B) in the presence of the drug. The shaded peak in each graph represents the binding of the serum in the absence of the drug. The figure illustrates the results from 1 experiment that is representative of the results observed on the 3 occasions the experiment was performed.
Rifampicin- and quinine-induced antibodies bind a similar or identical epitope on GPIX
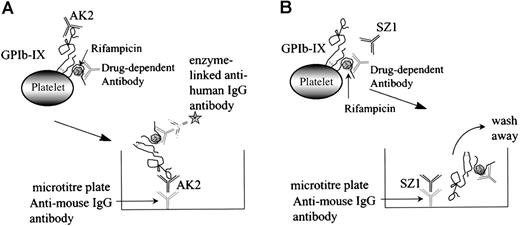
The MAIPA assay is an antigen-capture ELISA that uses an mAb to capture GPIb-IX after the human drug-dependent antibody has attached to the GP complex at a site distant from the mAb-binding site (Figure 5A). If the human antibody and the murine mAb-binding sites coincide or are in close proximity, the human antibody will cross-block the binding of the mAb, the antigen is not captured, and a negative result is obtained (Figure 5B).
Schematic representation of the MAIPA assay.
The MAIPA assay is an antigen-capture ELISA that uses a mAb to capture GPIb-IX complex to which the human drug-dependent antibody has already bound at a site distant from the mAb-binding site (A). If the human antibody and the murine mAb-binding sites coincide or are in close proximity to each other, the human antibody cross-blocks the mAb, the antigen/drug-dependent antibody complex is not captured by the anti-mouse IgG antibody, and a negative result is obtained (B).
Schematic representation of the MAIPA assay.
The MAIPA assay is an antigen-capture ELISA that uses a mAb to capture GPIb-IX complex to which the human drug-dependent antibody has already bound at a site distant from the mAb-binding site (A). If the human antibody and the murine mAb-binding sites coincide or are in close proximity to each other, the human antibody cross-blocks the mAb, the antigen/drug-dependent antibody complex is not captured by the anti-mouse IgG antibody, and a negative result is obtained (B).
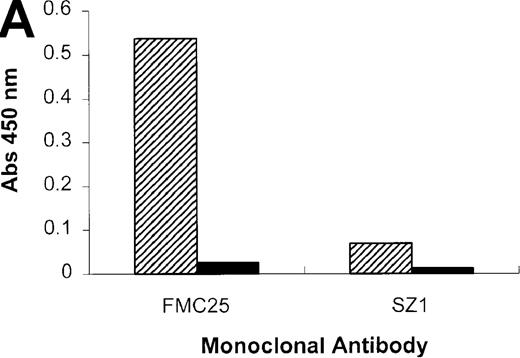
When the experiment was repeated using the anti-GPIX mAb, SZ1, a negative result was obtained because the rifampicin-dependent antibody bound to platelet GPIb-IX blocked the antigen capture by SZ21 (Figure 6A). As we have shown previously (Figure 1), antigen capture was not inhibited when other non–cross-blocking mAbs (GPIbα-specific, AK2, and GPIX-specific, FMC25) were used, and a positive result was obtained with each of these mAbs. These data confirm that the specificity of the antibody in the patient's serum is GPIX rather than GPIbα or GPIbβ. The same antibody panel was used to assay the binding to platelets of the GPIX-specific quinine-dependent antibody, and a comparable result was obtained (Figure 6B). These results indicated that the epitopes of the rifampicin- and quinine-dependent antibodies were identical or located in close proximity to each other (and also to that of mAb SZ1).
Inhibition of rifampicin- and quinine-dependent antibody binding by anti-GPIX mAbs.
MAIPA studies were performed in the presence and absence of rifampicin (A) or quinine (B) with patient serum and a mAb as indicated in the figure. (A) The results of the rifampicin-induced serum binding to platelets in the presence or absence of rifampicin and a cross-blocking anti-GPIX mAb (SZ1) or a non–cross-blocking anti-GPIX mAb (FMC25). Binding was not observed in the absence of rifampicin. The rifampicin-dependent antibody did not inhibit the binding of FMC25, giving a positive result. Conversely, the drug-dependent antibody almost completely inhibited SZ1 binding, giving a negative result. (B) The results of 1 representative quinine-induced thrombocytopenia patient's serum binding to platelets in the presence or absence of quinine and an anti-GPIX mAb (FMC25 or SZ1). No binding was observed in the absence of quinine. The quinine-dependent antibody did not inhibit the binding of FMC 25, again giving a positive result. Conversely, the quinine-dependent antibody inhibited SZ1 binding, giving a negative result. The striped box indicates binding in the presence of the drug; the filled box indicates binding in the absence of the drug; Qn, quinine; Rif, rifampicin; Pt, patient.
Inhibition of rifampicin- and quinine-dependent antibody binding by anti-GPIX mAbs.
MAIPA studies were performed in the presence and absence of rifampicin (A) or quinine (B) with patient serum and a mAb as indicated in the figure. (A) The results of the rifampicin-induced serum binding to platelets in the presence or absence of rifampicin and a cross-blocking anti-GPIX mAb (SZ1) or a non–cross-blocking anti-GPIX mAb (FMC25). Binding was not observed in the absence of rifampicin. The rifampicin-dependent antibody did not inhibit the binding of FMC25, giving a positive result. Conversely, the drug-dependent antibody almost completely inhibited SZ1 binding, giving a negative result. (B) The results of 1 representative quinine-induced thrombocytopenia patient's serum binding to platelets in the presence or absence of quinine and an anti-GPIX mAb (FMC25 or SZ1). No binding was observed in the absence of quinine. The quinine-dependent antibody did not inhibit the binding of FMC 25, again giving a positive result. Conversely, the quinine-dependent antibody inhibited SZ1 binding, giving a negative result. The striped box indicates binding in the presence of the drug; the filled box indicates binding in the absence of the drug; Qn, quinine; Rif, rifampicin; Pt, patient.
Discussion
In this study, we have shown that the antibody of a patient with rifampicin-induced thrombocytopenia interacts drug-dependently with the platelet GPIb-IX complex, and not the GPIIb-IIIa complex, using flow cytometry, the MAIPA assay, and immunoprecipitation.
GPIb is a major sialoglycoprotein on the platelet cell surface, with approximately 25 000 copies per platelet.13 GPIb is composed of 2 subunits, the α subunit of approximately 143 kd that is disulphide-bonded to a smaller β subunit of about 24 kd. GPIb is noncovalently linked to GPIX that has a molecular mass of 20 kd. The 3 glycoproteins have leucine-rich motifs, and they exist as a heterodimeric complex in the platelet membrane.5,6,14 The subunits of GPIb-IX are encoded by separate genes.7-9 15
To determine which component of the GPIb-IX complex, the rifampicin-dependent antibody, was binding to, we used CHO and mouse L cells stably transfected with various components of this complex as described in “Methods.” Drug-dependent binding of the patient antibody was observed on the CHO αβIX cells that contained all 3 components of the complex. When the mouse L cells were transfected with 2 of the 3 components of the complex, binding was only observed on the L βIX cells in the presence of the drug. Binding was not observed on the L αβ cells, indicating that neither of these components contained the epitope recognized by the drug-induced antibodies. These results indicate that the rifampicin-dependent antibodies bind an epitope on GPIX on the platelet surface. The CHO cell line transfected with GP Ibα and GPIX cDNA was not tested because it expressed only GPIbα on the cell surface, but GPIX was present in the cytoplasm (see “Methods”).
The rifampicin-dependent antibody was able to inhibit the binding of the mAb SZ1, specific for GPIX,16 in the competitive MAIPA assay. The binding of this mAb was also inhibited by binding of the quinine-dependent antibody in the same assay, shown in this study and previously reported by our group.17 It is interesting that 2 other drug-induced antibodies could also block the binding of the mAb SZ1 to platelets. Gentilini et al18 recently reported that the ranitidine-induced antibody blocked the binding of SZ1 to platelets, and we have observed the same inhibitory effect previously on the binding by quinidine-induced antibodies.17 These observations suggest that these 4 drug-induced antibodies and the mAb SZ1 bind to either the same site or sites very close to each other. Because the binding sites of 4 drug-induced antibodies have been mapped to this region of GPIX, it is possible that the epitopes of many other drug-induced antibodies may also be localized to this site. The reason for the colocalization of the binding sites of these 4 antibodies is at present unclear because the drugs involved are chemically or structurally dissimilar, except for quinine and its optic isomer, quinidine.
In summary, this is the first study to map the epitope of the rifampicin-dependent antibody to the GPIX subunit of the GPIb-IX complex—more specifically, to a domain that is also the common binding site of 3 other drug-induced antibodies. This region of GPIX obviously plays an important role in epitope formation for drug-induced antibodies. Further elucidation of the characteristics of this region on GPIX is clearly required, and it will provide useful insights into the mechanisms of drug-induced thrombocytopenias.
Acknowledgment
We wish to thank Ms Sue Evans for assistance with the MAIPA assay.
Supported by a grant from the National Health and Medical Research Council of Australia.
Reprints:Beng H. Chong, Department of Hematology, Prince of Wales Hospital High and Avoca Sts, Randwick, NSW 2031, Australia; e-mail: b.h.chong@unsw.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal