Abstract
Although bispecific antibodies directed against malignant lymphoma have been shown to be effective in vitro and in vivo, extended clinical trials so far have been hampered by the fact that conventional approaches to produce these antibodies suffer from low yields, ill-defined byproducts, or laborious purification procedures. To overcome this problem, we have generated a small, recombinant, lymphoma-directed, bispecific single-chain (bsc) antibody according to a novel technique recently described. The antibody consists of 2 different single-chain Fv fragments joined by a glycine-serine linker. One specificity is directed against the CD3 antigen of human T cells, and the other antigen-binding site engages the pan–B-cell marker CD19, uniformly expressed on the vast majority of B-cell malignancies. The construct was expressed in Chinese hamster ovary cells and purified by its C-terminal histioline tag. Specific binding to CD19 and CD3 was demonstrated by fluorescence-activated cell sorter analysis. By redirecting unstimulated primary human T cells derived from the peripheral blood against CD19-positive lymphoma cells, the bscCD19 × CD3 antibody showed significant cytotoxic activity at very low concentrations of 10 to 100 pg/mL and at effector to target cell ratios as low as 2:1. Moreover, strong lymphoma-directed cytotoxicity at low antibody concentrations was rapidly induced during 4 hours even in experiments without any T-cell prestimulation. Thus, this particular antibody proves to be much more efficacious than the bispecific antibodies described until now. Therefore, the described bscCD19 × CD3 molecule should be a suitable candidate to prove the therapeutic benefit of bispecific antibodies in the treatment of non-Hodgkin lymphoma.
The overall survival of patients with high-grade non-Hodgkin lymphoma has been increased considerably over recent years by the introduction of high-dose chemotherapy as primary treatment. Nevertheless, about 50% of the patients ultimately succumb to their tumor.1-3 Moreover, patients with low-grade non-Hodgkin lymphoma, such as chronic lymphatic leukemia and mantle cell lymphoma, are still incurable. This bleak situation has stimulated the search for alternative therapeutic strategies, among which antibodies against B-lymphocyte differentiation antigens are playing an increasing role. Through the International Leukocyte Differentiation Workshops, these CD antigens are well defined as to their lineage specificity and overall tissue distribution. In addition, because most of the antigens have been cloned, their structures and functions are well known. Among them, CD19 holds an eminent place as a functional receptor molecule on B lymphocytes. With the important exception of stem cells, it is expressed by virtually all developmental stages of B-cell lineage, including mature B lymphocytes.4,5 Thus far, CD19 antibodies have been used in various forms for in vitro and in vivo therapeutic studies.4,6-14 Considerable effort also has been spent on the bispecific-antibody approach by joining 2 different antigen-binding sites in 1 antibody molecule to redirect effector cells to the predefined tumor target. Although bispecific antibodies are extremely efficient in recruiting cytotoxic effector cells against tumor cells in vitro and in vivo,15-19 larger randomized clinical trials as a final test of efficacy are still lacking. The main reason why these interesting antibody constructs are dramatically lagging behind intact antibodies in clinical testing is the difficulty in producing sufficient amounts of clinical-grade material. Until recently, the production and purification process has been extremely inefficient, with low yields, regardless of whether the hybrid-hybridoma approach, chemical linkage, or renaturation from bacterial inclusion bodies of recombinant Fab or Fv fragment was followed. In addition, nearly all of these procedures are plagued by contamination with ill-defined byproducts.15,16 20-23
As previously shown with a bsc17-1A × CD3 antibody, these handicaps can be overcome by the development of a molecular format linking 4 variable regions in the configuration VL1-VH1-VH2-VL2 on 1 peptide chain.24-26 The resulting recombinant 60-kd molecule produced by mammalian cells is secreted at high yield in a fully active form that requires no further renaturation. This small and compact molecule with 1 of the antigen-binding sites directed against the CD3 molecule induces remarkable and fast T-cell cytotoxicity against epithelial tumor cells without any intentional prestimulation and costimulation. Therefore, the attempt was warranted to apply the same strategy for the generation of a bscCD19 × CD3 construct for a therapeutic trial in patients with B-cell lymphomas.
Materials and methods
Cloning of variable (V) immunoglobulin domains
The V light-chain (VL) and V heavy-chain (VH) domains from the HD37 hybridoma27 were cloned according to standard polymerase chain reaction (PCR) methods.28 Synthesis of cDNA was carried out with oligo dT primers and reverse transcriptase. For amplification of the V domains via PCR, we used the primers 5′L1 and 3′K flanking the VL domain, and 5′H1 and 3′G for the heavy chain, based on primers described by Dübel et al.29
The cDNA of the anti-CD3 scFv fragment was kindly provided by A. Traunecker.30
Construction of bispecific single-chain fragments and eukaryotic expression
To obtain an anti-CD19 scFv fragment, we used the corresponding VL and VH regions cloned into separate plasmid vectors as templates for a VL- and VH-specific PCR using the oligonucleotide primer pairs 5′VLB5RRV/3′VLGS15 and 5′VHGS15/3′VHBspE1, respectively. Overlapping complementary sequences were introduced into the PCR products that combined to form the coding sequence of a 15–amino acid (G4S1)3(single-letter amino acid code) linker during the subsequent fusion PCR. This amplification step was performed with the primer pair 5′VLB5RRV/3′VHBspE1, and the resulting fusion product (or rather anti-CD19 scFv fragment) was cleaved with the restriction enzymes EcoRV and BspE1 and thus cloned into the bluescript KS vector (Stratagene, La Jolla, CA), containing the (EcoR1/Sal1-cloned) coding sequence of the bispecific single-chain antibody 17-1A × CD3.24Thereby, the anti–17-1A specificity was replaced by the anti-CD19 scFv fragment, preserving the 5–amino acid (G4S1)1 linker that connects the C-terminal anti-CD3 scFv fragment. Subsequently, the DNA fragment encoding the bispecific single-chain antibody CD19 × CD3 with the domain arrangement VLCD19-VHCD19-VHCD3-VLCD3was subcloned into the EcoR1/Sal1 sites of the described expression vector pEF-DHFR.24 The resulting plasmid DNA was transfected into DHFR-deficient Chinese hamster ovary (CHO) cells by electroporation. Selection, gene amplification, and protein production were performed as described previously.24
List of primers
Primers were as follows: 5′L1: GAAGCACGCGTAGATATCG/TTG(AC)T(GC)ACCCAA(TA)CTCCA; 3′K: GAAGATGGATCCAGCGGCCGCAGCATCAGC; 5′H1: CAGCCGGCCATGGCGCAGGT(CG)CAGCTGCAG(CG)AG; 3′G: ACCAGGGGCCAGTGGATAGACAAGCTTGGGTGTCGTTTT; 5′VLB5RRV: AGGTGTACACTCCGATATCCAGCTGACCCAGTCTCCA; 3′VLGS15: GGAGCCGCCGCCGCCAGAACCACCACCACCTTTGATCTCGAGCTTGGTCCC; 5′VHGS15: GGCGGCGGCGGCTCCGGTGGTGGTGGTTCTCAGGT(GC)(AC)A(AG)CTGCAG(GC)AGTC(AT)GG; 3′VHBspE1: AATCCGGAGGAGACGGTGACCGTGGTCCCTTGGCCCCAG.
Purification
The bsc antibody (Ab) was purified via its C-terminal H tail by affinity chromatography on a nickel-nitrilotriacetic acid (Ni-NTA) column (Qiagen, Hilden, Germany), as described previously.24
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
SDS-PAGE was carried out according to Laemmli31 with a 12% gel for analyzing the purification of the bsc-Ab. For immunoblotting, standard procedures were applied. For detection of the bsc-Ab, we used a monoclonal anti-HisTag antibody (Dianova, Hamburg, Germany). Blots were developed with the Enhanced Chemiluminescence (ECL) system (Amersham, Braunsweig, Germany).
Flow cytometric analysis
A total of 1 × 106 cells were washed with phosphate-buffered saline (PBS), resuspended in 200 μL PBS with 10% Venimmun (Centeon, Marburg, Germany) and 0.1% NaN3, and incubated for 30 minutes at 4°C. After centrifugation (100 × g, 5 minutes), the cells were incubated in 50 μL bscCD19 × CD3 (200 μg/mL in PBS with 10% Venimmun and 0.1% NaN3) for 30 minutes at 4°C. The cells were washed twice with PBS. For detection of the bsc-Ab, a fluorescein isothiocyanate (FITC)-conjugated antibody against the His-tag (Dianova, Hamburg, Germany) was used. Either the irrelevant bsc17-1A × CD3, produced by the same expression system as bscCD19 × CD3, or the His-tag antibody alone served as negative controls. Flow cytometry was performed with fluorescence-activated cell sorter analysis (FACScan, Becton Dickinson, Heidelberg, Germany).
Cell lines
The CD19-positive B-cell lines Daudi, Raji, BJAB (Burkitt lymphoma), SKW6.4 (human Epstein-Barr virus–transformed B cell), and Blin-1 (pre–B-cell line) were used in flow cytometric analysis and chromium-release assays. Jurkat is a CD3-positive T-cell line; the plasmacytoma cell lines NCI and L363 are negative for both surface molecules, CD3 and CD19. Cell lines were cultured in complete RPMI 1640 (Biochrom, Berlin, Germany) with 10% fetal calf serum (FCS) (GIBCO, Karlsruhe, Germany).
Induction of T-cell proliferation
Proliferation of unstimulated human peripheral blood mononuclear cells (PBMCs), after the addition of either phytohemagglutinin (PHA, Roche, Pensberg, Germany) + interleukin (IL)-2 (Chiton, Ratingen, Germany) (as positive control) or bispecific antibodies, was measured in a standard 3H-thymidine proliferation assay. Ninety-six-well flat-bottom plates were incubated with 250 μL PBS containing 10% FCS per well for 2 hours at 37°C. The plates were washed, and freshly isolated human PBMCs (1 × 106cells/mL) were added in a volume of 180 μL to each well, containing 20 μL of different antibodies or PHA + IL-2. As controls, the same PBMCs depleted of B cells were used (B-cell depletion by Dynabeads M-450 CD19 [Dynal, Hamburg, Germany] according to the manufacturer's protocol). After 24 hours' incubation at 37°C, 5% CO2, 20 μL of 3H-thymidine was added to each well. Cells were incubated for an additional 16 hours at 37°C, 5% CO2. The cells were then harvested and assayed for incorporated 3H-thymidine in a TopCount (Canberra Packard, Dreieich, Germany). Tests were carried out in triplicate.
Cytotoxicity assay
Human PBMCs were isolated from fresh buffy coats of random donors using Lymphoprep (Nycomed, Oslo, Norway) gradient centrifugation with subsequent 100g centrifugation steps to remove thrombocytes. CD19-positive B cells were depleted using Dynabeads M-450 CD19 (Dynal). The depleted cell populations were analyzed by flow cytometry (FACScan; Becton Dickinson), which showed 99% depletion of CD19-positive cells. The PBMCs were incubated overnight at 37°C, 5% CO2 leading to the adherence of monocytes. CD19-positive B-cell lines (Raji, Blin I, Daudi, BJAB, and SKW6.4) were used as target cells.
Cytotoxicity was measured in a standard chromium-release assay in round-bottom 96-well plates (Nunc) using RPMI 1640 complete medium (Biochrom) with 10% FCS (GIBCO).
Unstimulated peripheral blood lymphocytes (PBLs are the nonadherent fraction of PBMCs) were added as effector cells in a volume of 80 μL medium to each well, containing 20 μL of bsc-Ab in different concentrations. We then added 100 μL of 51Cr-labeled target cells (1 × 104), after which the plates were centrifuged for 3 minutes at 100 × g and incubated for 4 hours at 37°C, 5% CO2. After an additional centrifugation step, 50 μL of the supernatant was removed and assayed for released 51Cr in a gamma counter (TopCount; Canberra Packard).
Spontaneous release was measured by incubating the target cells without effector cells or antibodies, and maximal release was determined by incubating the target cells with 20 μL 10% TritonX-100. Incubation of target cells with bsc-Ab without effector cells did not result in measurable lysis. The percentage of the specific lysis was calculated as: Specific release (%) = [(cpm, experimental release) − (cpm, spontaneous release)] / [(cpm, maximal release) − (cpm, spontaneous release)] × 100. All tests were carried out in triplicate. The SD within the triplicates was below 6% in all experiments.
Results
Western blot analysis
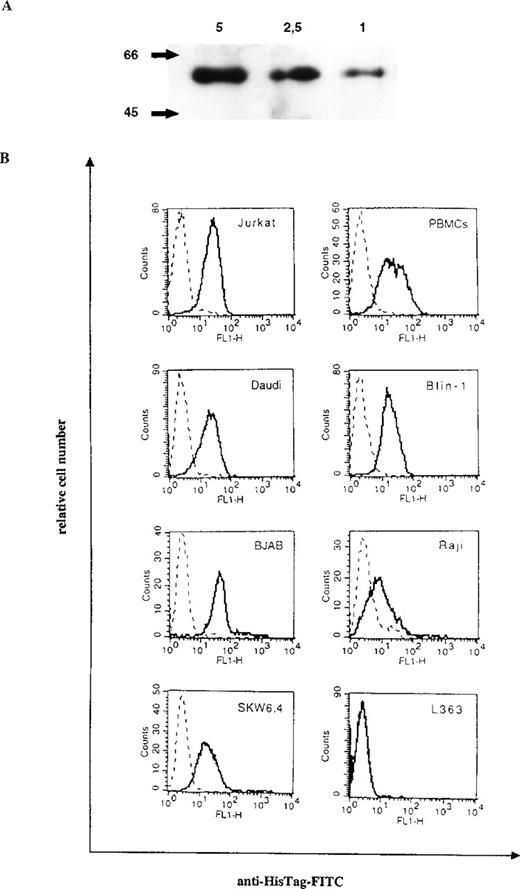
Purification of bscCD19 × CD3 from the supernatant of transfected CHO cells yielded 4 mg per liter culture supernatant. The bsc-Ab was eluted from the Ni-NTA column as a distinct peak at a concentration of 200 mmol/L imidazole. The results of Western blot analysis show the expected size of the bsc-Ab at 60 kd (Figure1A).
Purification and binding specificities of bscCD19 × CD3.
(A) Western blot analysis of the purified bscCD19 × CD3 construct detected by anti-HisTag antibodies. The molecular mass (kd) is indicated on the left; the μg protein applied on the top. (B) FACS analysis with the bscCD19 × CD3 on different CD19-positive B-cell lines (Daudi, Blin-1, BJAB, Raji, and SKW6.4), on CD3-positive Jurkat cells and primary human PBLs, and on the CD3- and CD19-negative plasmacytoma cell line L363. Broken lines are negative controls with the secondary antibody anti–HisTag-FITC alone.
Purification and binding specificities of bscCD19 × CD3.
(A) Western blot analysis of the purified bscCD19 × CD3 construct detected by anti-HisTag antibodies. The molecular mass (kd) is indicated on the left; the μg protein applied on the top. (B) FACS analysis with the bscCD19 × CD3 on different CD19-positive B-cell lines (Daudi, Blin-1, BJAB, Raji, and SKW6.4), on CD3-positive Jurkat cells and primary human PBLs, and on the CD3- and CD19-negative plasmacytoma cell line L363. Broken lines are negative controls with the secondary antibody anti–HisTag-FITC alone.
Binding properties
Binding specificities of the bsc-Ab to CD3 and CD19 were shown by flow cytometric analysis on CD3-positive Jurkat cells, human PBLs, and a number of different CD19-positive B-cell lymphoma cell lines, including Blin-1, SKW6.4, Daudi, BJAB, and Raji. No binding was detectable on the plasmacytoma cell line L363, which expresses neither CD19 nor CD3 (Figure 1B).
Induction of T-cell proliferation in the presence of autologous B cells
In a standard 3H-thymidine proliferation assay, the bscCD19 × CD3 induced proliferation of unstimulated primary human T cells only in the presence of autologous B cells. In a T-cell population depleted of B cells by immunomagnetic beads, the bscCD19 × CD3 exerted almost no proliferative stimulus. The proliferation in response to PHA and IL-2 was defined as 100%. Almost no proliferation was seen in the medium control and with the irrelevant bsc17-1A × CD3 (Figure 2A).
T-cell proliferation and cytotoxic activity of bscCD19 × CD3.
(A) Standard 3H-thymidine proliferation assay with unstimulated primary human PBMCs before and after depletion of B cells by immunomagnetic beads. (B) Cytotoxicity of bscCD19 × CD3 in a51Cr-release assay with primary human PBLs and different B-cell lines (BJAB, Raji, Blin-1, SKW6.4, and Daudi) in the presence of 60 units per mL IL-2; the E-T ratio was 10:1 and the incubation time was 4 hours. The SD in all triplicates was below 5%.
T-cell proliferation and cytotoxic activity of bscCD19 × CD3.
(A) Standard 3H-thymidine proliferation assay with unstimulated primary human PBMCs before and after depletion of B cells by immunomagnetic beads. (B) Cytotoxicity of bscCD19 × CD3 in a51Cr-release assay with primary human PBLs and different B-cell lines (BJAB, Raji, Blin-1, SKW6.4, and Daudi) in the presence of 60 units per mL IL-2; the E-T ratio was 10:1 and the incubation time was 4 hours. The SD in all triplicates was below 5%.
Cytotoxic activity against CD19-positive lymphoma cell lines
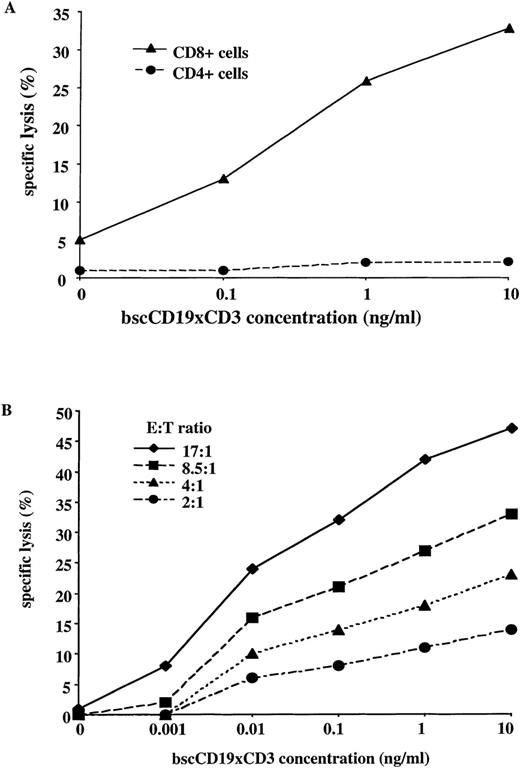
The bscCD19 × CD3 antibody proved to be highly cytotoxic for several lymphoma cell lines in a 51Cr-release assay. As a control, a bsc-Ab with different tumor specificity (bsc17-1A × CD3) showed lysis activity not significantly above medium background, although generated by the same system as the bscCD19 × CD3 antibody (Figure 2B). To approximate the in vivo conditions, we used unstimulated PBLs from healthy donors as effector cells. Rapid induction of cytotoxicity within 4 hours was observed without T-cell prestimulation. In addition, no cytotoxic activity was observed using the plasmacytoma cell lines NCI and L363 as target cells, neither of which expresses CD19 (Figure3C). In competition assays using increasing amounts of the CD19-specific parental monoclonal antibody HD37 or the CD3-specific monoclonal antibody OKT-3, cytotoxic activity of the bscCD19 × CD3 was completely blocked (Figures 3B, 3C). A CD22-specific monoclonal antibody did not affect the bscCD19 × CD3-mediated cytotoxicity (data not shown). These experiments show that bscCD19 × CD3-mediated cytotoxic effects are antigen specific. To obtain more information on the molecular mechanisms of target cell killing, we tried to block bscCD19 × CD3-mediated cytotoxicity by EGTA. Cytotoxic activity induced by bscCD19 × CD3 was completely blocked by EGTA (data not shown), indicating that lysis is mediated by T cells (probably through the perforin pathway) rather than through a direct (eg, apoptosis-inducing) effect of the antibody itself. Consistent with this observation is the finding that isolated CD8 T cells containing preformed cytolytic granules accounted for the rapid cytotoxic effect, whereas CD4 T cells remained largely inactive during the 4-hour incubation (Figure 4A).
CD-19 specificity of bscCD19 × CD3–mediated cytotoxicity.
(A) Inhibition of bscCD19 × CD3-mediated cytotoxicity of unstimulated primary human PBLs against CD19+ target cell lines by parental anti-CD19 antibody (HD37) in a standard51Cr-release assay (E-T ratio 20:1; incubation time 4 hours; target cells Blin-1). (B) Inhibition of bscCD19 × CD3-mediated cytotoxicity of unstimulated primary human PBLs against CD19+ target cell lines by anti-CD3 antibody (OKT-3) in a standard 51Cr-release assay (E-T ratio 20:1; incubation time 4 hours; target cells Blin-1). (C)51Cr-release assay with unstimulated primary human PBLs against CD19+ Daudi cell line and CD19− plasmacytoma cell lines NCI and L363 at different concentrations of bscCD19 × CD3 (E-T ratio 20:1; incubation time 8 hours).
CD-19 specificity of bscCD19 × CD3–mediated cytotoxicity.
(A) Inhibition of bscCD19 × CD3-mediated cytotoxicity of unstimulated primary human PBLs against CD19+ target cell lines by parental anti-CD19 antibody (HD37) in a standard51Cr-release assay (E-T ratio 20:1; incubation time 4 hours; target cells Blin-1). (B) Inhibition of bscCD19 × CD3-mediated cytotoxicity of unstimulated primary human PBLs against CD19+ target cell lines by anti-CD3 antibody (OKT-3) in a standard 51Cr-release assay (E-T ratio 20:1; incubation time 4 hours; target cells Blin-1). (C)51Cr-release assay with unstimulated primary human PBLs against CD19+ Daudi cell line and CD19− plasmacytoma cell lines NCI and L363 at different concentrations of bscCD19 × CD3 (E-T ratio 20:1; incubation time 8 hours).
Role of T cell subsets and E-T ratio in the cytotoxic effect.
(A) 51Cr-release assay with isolated primary human CD8+ and CD4+ T cells against cell line Blin-1 at different concentrations of bscCD19 × CD3 (E-T ratio 20:1; incubation time 4 hours). (B)51Cr-release assay with unstimulated primary human PBLs against CD19+ cell line Blin-1, with titration of bscCD19 × CD3 at different E-T ratios and incubation time of 4 hours.
Role of T cell subsets and E-T ratio in the cytotoxic effect.
(A) 51Cr-release assay with isolated primary human CD8+ and CD4+ T cells against cell line Blin-1 at different concentrations of bscCD19 × CD3 (E-T ratio 20:1; incubation time 4 hours). (B)51Cr-release assay with unstimulated primary human PBLs against CD19+ cell line Blin-1, with titration of bscCD19 × CD3 at different E-T ratios and incubation time of 4 hours.
With unstimulated T cells, a significant cytotoxic effect against Blin-1 cells was observed at antibody concentrations below 1 ng/mL (Figure 4B). The bscCD19 × CD3 antibody rapidly induced specific cytotoxic activity in freshly isolated, unstimulated T cells, even at low effector to target cell (E-T) ratios (4:1; 2:1) and at extremely low antibody concentrations of 10 to 100 pg/mL (Figure 4B). In contrast, a conventional bispecific CD19 × CD3 antibody generated by the hybrid-hybridoma technique required additional T-cell prestimulation and high antibody concentrations of approximately 100 ng/mL to induce specific T-cell cytotoxicity.6-8 32
Discussion
Other bispecific CD19 × CD3 antibodies, retargeting cytotoxic T cells at lymphoma cells, have already been shown to be effective in vitro,6,7,9-11,14 in animal models,8,33,34 and in first clinical trials.12,35,36 Those antibodies, however, were constructed either by hybrid-hybridoma techniques or by chemical linkage,37 which yield only low amounts of clinical-grade material. Therefore, it is not surprising that more extended clinical studies are still lacking. The new technique developed previously relies on the production of recombinant bispecific single-chain antibodies in mammalian cells, which circumvents the above mentioned difficulties.24 The CD19 × CD3 single-chain antibody described here was isolated to high purity from the culture supernatant of transfected CHO cells. The molecule with the 4 V-regions tightly packed (B. Steipe, unpublished results) showed several unexpected properties. (1) It induced high lymphoma-directed T-cell cytotoxicity at ultra-low concentrations (10-100 pg/mL), requiring E-T ratios of 4:1 and 2:1 only, which is in contrast to other CD19 × CD3 antibodies produced by the hybrid-hybridoma technique, which require concentrations of several nanograms per milliliter.6-8,32 Whether this surprisingly high activity is due to the peculiar anti-CD3 antibody or to the unique structure of the single-chain molecule still needs to be clarified. (2) At the indicated low concentrations, the lymphoma-directed cytotoxicity was triggered without any detectable prestimulation or costimulation of T cells. In contrast, the previously published CD19 × CD3 bispecific antibodies all require prestimulation.6-8,32 In the case of 1 other conventional CD19 × CD3 antibody, a somewhat similar effect was obtained, albeit at much higher concentrations (100 ng/mL) and with E-T ratios of 27:1.9 In addition, that antibody also depended on a 24-hour preincubation with T cells. This is in stark contrast to the rapid onset of cytotoxicity detected within 4 hours after the addition of our bispecific construct. We are not aware of a similar behavior of other bispecific antibodies. The recently described anti-p185HER2/anti-CD3 bispecific F(ab)2 antibody also required 24 hours of prestimulation with T cells and IL-2.19 Similarly, a bispecific CD19 × CD3 diabody38 needed prestimulation of the T cells with a highly mitogenic anti-CD3 antibody in the presence of IL-2 and diabody concentrations of 0.25 μg/mL or 2.5 μg/mL.
The evidence for the specificity of the construct can be summarized as follows: (1) The antibody requires the presence of B lymphocytes to stimulate T cells; (2) the target is CD19 because CD19-negative B-lineage cells such as the NCI and L363 plasmacytoma cells are resistent to lysis; and (3) cytotoxicity can be blocked by the parental anti-CD19 as well as by anti-CD3 antibody, but not by anti-CD22.
Inhibition of the perforin pathway by calcium deprivation with EGTA completely abrogated bscCD19 × CD3-mediated cytotoxicity, suggesting that T lymphocytes and not the antibody by itself execute the lytic step. The experiments with isolated CD8 and CD4 cells show that the rapid cytotoxicity seen after 4 hours may be safely attributed to the CD8 population.
Although the precise mechanism of this highly efficient and rapid induction of cytotoxicity remains enigmatic, particularly with regard to the apparent independence of a second signal (eg, via CD28), it is evident that the bscCD19 × CD3 is a promising candidate for a clinical trial in patients with non-Hodgkin lymphoma.
General support for this notion can be derived from clinical studies with conventional bispecific formats.17,39-43 An additional advantage of the presented antibody construct may be its low molecular weight, which improves penetration into tumor tissue, as has been shown for Fab or Fv antibody fragments.44 Furthermore, toxicity caused by nonspecific cytokine release can be expected to be less pronounced than with bispecific antibodies that still contain Fc fragments.36 With regard to immunogenicity of the covalently linked 4 V-regions devoid of all constant immunoglobulin domains, we anticipate a reduction in the HAMA or HACA (human anti-mouse/chimeric antibody) response that was observed with chimeric antibodies.45
A.L. and P.K. contributed equally to this work.
Reprints:Peter Kufer, Institute of Immunology, University of Munich, Goethestrasse. 31, D-80336 München, Germany; e-mail:kufer@ifi.med.uni-muenchen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal