Abstract
Chronic granulomatous disease (CGD) is an inherited disease caused by defects in the superoxide-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase of phagocytes. Genetic lesions in any of 4 components of this antimicrobial enzyme have been detected. Family-specific mutations are found in 3 of 4 forms of CGD due to deficiencies of the gp91-phox, p22-phox, andp67-phox genes. In p47-phox–deficient CGD (autosomal recessive form A47°) patients, a GT deletion (▵GT) at the beginning of exon 2 of the p47-phox gene has been reported in 19 of 20 alleles. This GT deletion is also characteristic for the recently identified p47-phox pseudogenes. To explore a possible link between these findings, a sequence analysis of 28 unrelated, racially diverse A47° CGD patients and 37 healthy individuals was performed. The GT deletion in exon 2 was present on all alleles in 25 patients. Only 3 patients but all healthy individuals contained the GTGT and ▵GT sequences. A total of 22 patients carried additional pseudogene-specific intronic sequences on all alleles, either only in intron 1 or in intron 1 and intron 2, which lead to different types of chimeric DNA strands. It is concluded that recombination events between the p47-phox gene and its highly homologous pseudogenes result in the incorporation of ▵GT into the p47-phox gene, thereby leading to the high frequency of GT deletion in A47° CGD patients.
Phagocytic cells play a central role in the cellular host defense system due to their ability to release large amounts of superoxide in the respiratory burst upon stimulation with bacterial toxins and other reagents. The subsequent conversion of superoxide to toxic oxygen radical derivatives, such as hydrogen peroxide, hydroxyl radicals, and hypochlorous acid, is critical for microbicidal activity.1 The multicomponent enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is largely responsible for this antimicrobial defense system.2 Upon activation, the cytosolic factors p47-phox and p67-phox and the low molecular weight GTP-binding protein Rac translocate to the membrane, where they associate with the 2 membrane-bound subunits of cytochrome b558, p22-phox and gp91-phox.3-7 This allows electron flux from NADPH via the flavin-containing heme protein to molecular oxygen leading to the generation of superoxide.8,9 Recently an additional protein, p40-phox, was identified, and it associates with p47-phox and p67-phox in a 240-kd preformed complex within the cytoplasm of neutrophils under resting conditions.10 This newly identified protein may have a negative regulatory role on NADPH oxidase function.11
The essential role of NADPH oxidase in cellular host defense is clearly demonstrated by patients suffering from a rare inherited disorder known as chronic granulomatous disease (CGD).12 Due to genetic lesions affecting any of the 4 components, gp91-phox,p22-phox, p47-phox, or p67-phox, phagocytes from CGD patients are unable to generate superoxide upon stimulation.13,14 Typically, these patients suffer from recurrent and life-threatening bacterial and fungal infections.15 The study of patients with CGD has highlighted the functional importance of each of the 4 NADPH oxidase components and has led to the identification of their respective gene structure. In turn, this has permitted analysis of individual patients and provided insight into the molecular genetics of each subtype of CGD.
Mutations in the X-linked gp91-phox gene, leading to functional impairment or complete loss of gp91-phox, account for approximately 60% of all CGD cases. A spectrum of mutations distributed over the gp91-phox gene has been reported including insertions, substitutions, or deletions within exons, at splice junctions, or within the 5′ upstream regulatory region.13,16-18 In contrast to this heterogeneous group of patients, in the second most common form of CGD (autosomal recessive form A47° CGD), which accounts for 25%-30% of all cases, the lack of functional p47-phox is caused by an unusually uniform mutation pattern. A single defect in the p47-phox gene, such as a GT deletion (ΔGT) at the beginning of exon 2, which predicts for a premature stop codon at amino acid 51, has been identified in 19 of 20 alleles reported to date.19-21Remarkably, these A47° CGD patients were unrelated and of different racial backgrounds.
Several hypotheses have been raised to explain this peculiarity. The hypotheses mainly focus on the tandem repeat at the beginning of exon 2 and the surrounding sequence, which could represent a mutational hot spot for either DNA strand slippage or the formation of hair pin loops.18-21 However, these speculations do not sufficiently explain the unusually high frequency of ΔGT in A47° CGD patients.
We reported the presence of at least 1 duplicated copy of thep47-phox pseudogene, which localizes to the identical region of chromosome 7q11.23 as the p47-phox gene.22Hockenhull et al23 have established that there are 2 copies of the highly homologous pseudogene in this region. The possibility exists that there might be 3 or more copies in selected individuals. By definition, all pseudogenes contain ΔGT at the beginning of exon 2, exactly at the same position as is found in A47° CGD patients. The aim of this study was to elucidate a probable relationship between the high frequency of detecting only the ΔGT in nearly all A47° CGD patients and the closely linked p47-phox pseudogenes. We performed a genomic sequence analysis of exon 2 in a series of 28 A47° CGD patients and a group of 37 healthy individuals. In addition we concentrated on characterizing previously identified pseudogene markers in intron 1 and intron 2 in our cohort of 28 A47° CGD patients and looking for possible sites or patterns of recombination. Our findings provide strong evidence that the high frequency of ΔGT in A47° CGD patients is due to recombination events between the gene and pseudogenes.
Materials and methods
Patient samples
A total of 28 A47° CGD patients from 28 racially divergent families was studied. Blood samples were obtained from CGD patients and family members by one of the investigators (J.T.C.). Procedures and consent forms were approved by the Committees on the Protection of Human Subjects Research of the Scripps Research Institute (La Jolla, CA) and the Stanford University School of Medicine (Stanford, CA). Neutrophils from these patients did not contain p47-phox by Western blot analysis. The control group comprised 37 healthy individuals representing a comparable racial mix.
Polymerase chain reaction
Genomic DNA from A47° CGD patients and control donors was isolated from whole blood stored in ethylenediaminetetraacetic acid (EDTA) using a DNA extractor (Applied Biosystems, Foster City, CA). Similarly, genomic DNA from 6 chimpanzees and 1 rhesus monkey was obtained by standard methods. Oligonucleotide primers were synthesized (DNA synthesizer Model 394, Applied Biosystems). Polymerase chain reaction (PCR) using 100 ng genomic DNA as a template was performed in 100-μL reactions containing the following: 5 μL 20 × PCR buffer with 670 mmol/L Tris-HCl (tris[hydroxymethyl] aminomethane hydrochloride; pH 8.8), 67 mmol/L MgCl2(magnesium dichloride), 166 mmol/L (NH4)2SO4, and 1.7 μL/μL BSA (bovine serum albumin); 0.5 mmol/L dNTP (nucleoside 5′-triphosphate) mix (Pharmacia, Piscataway, NJ); 5% DMSO (dimethyl sulfoxide) (Sigma Chemical, St. Louis, MO); and 5 ng of each primer (Table 1). DNA was denatured at 98°C for 7 minutes prior to the addition of 1 unit Thermus aquaticus (Taq) polymerase (Boehringer Mannheim, Indianapolis, IN). PCR was performed (GeneAmp 9600 thermal cycler; Perkin Elmer, Norwalk, CT) for 30 cycles using the following amplification conditions: 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds followed by an extension at 72°C for 7 minutes.
Oligonucleotide primers for exon 2 and the flanking intronic regions of the p47-phox gene and its pseudogene(s)
| 1LA: 5′-CTGGGGCGTGGCAGAACTTGGGT |
| 1LB: 5′-CACGTTTGTGCCCTTTCTGCAA |
| 2LA: 5′-TTCCTCCAGTGGGTAGTGGGAT |
| 2LB: 5′-AGCAAAGCCTCTTTGGAGGCTGAATGGGG |
| 2RA: 5′-GCTATGATTGCGCCCCTGCACTGCAA |
| 2RB: 5′-ACCGTGGGGGCTCATGCCTGTAATC |
| 2RC: 5′-AGAGAACATGAGGTGTTCAGAGT |
| 2RD: 5′-AGCTGGAACGGTGGGTCCCTGT |
| 1LA: 5′-CTGGGGCGTGGCAGAACTTGGGT |
| 1LB: 5′-CACGTTTGTGCCCTTTCTGCAA |
| 2LA: 5′-TTCCTCCAGTGGGTAGTGGGAT |
| 2LB: 5′-AGCAAAGCCTCTTTGGAGGCTGAATGGGG |
| 2RA: 5′-GCTATGATTGCGCCCCTGCACTGCAA |
| 2RB: 5′-ACCGTGGGGGCTCATGCCTGTAATC |
| 2RC: 5′-AGAGAACATGAGGTGTTCAGAGT |
| 2RD: 5′-AGCTGGAACGGTGGGTCCCTGT |
INTRONIC PRIMERS ARE USED TO AMPLIFY AND SEQUENCE EXON 2 AND THE FLANKING INTRONIC REGIONS OF THE P47-PHOX GENE AND ITS PSEUDOGENE(S). THE PCR PRODUCTS GENERATED CONTAIN SEQUENCES THAT DIFFER BETWEEN WILD-TYPE GENES AND PSEUDOGENES.
Sequencing
Cycle sequencing was performed using an fmol DNA cycle sequencing system (Promega, Madison, WI). Oligonucleotide primers (82.5 ng) were labeled in an 11-μL reaction containing 1 μL32P γATP (adenosine 5′-triphosphate; 22.2×1013 Bq/mmol [6000 Ci/mmol]) (DuPont), 1 μL 10 × T4 polynucleotide kinase buffer containing 500 mmol/L Tris-HCl (pH 8.3), 100 mmol/L MgCl2, 50 mmol/L dithiothreitol, and 1 mmol/L spermidine; and 1 μL T4 polynucleotide kinase (equal to 1 unit). The reaction was run for 30 minutes at 37°C and stopped at 90°C for 2 minutes. Genomic DNA was amplified by PCR as described above, and an aliquot of 2.5 μL from the first 100-μL PCR reaction was amplified with a set of nested primers under identical conditions. PCR fragments were purified over microcon 100 filters (Amicon, Beverly, MA) by centrifuging at 500g for 10 minutes. The eluate (1 μL) was diluted in a 100 μL solution of 10 mmol/L Tris-HCl (pH 8.3) and 1 mmol/L EDTA. The eluate was then precipitated with 260 μL ethanol and 7.3 μL 3 M ammonium acetate at −80°C for 10 minutes. The precipitate was centrifuged for 12 minutes at 16 000g at 4°C. The pellet was vacuum dried, dissolved in 14.5 μL 5 × sequencing buffer (250 mmol/L Tris-HCl [pH 9] and 10 mmol/L MgCl2), and then mixed with 5 units sequencing grade Taq polymerase and 2.5 μL labeled sequencing primer. Aliquots of this mixture (4 μL) were added to 2 μL of each d/ddNTP. After an initial denaturing step at 95°C for 1 minute, 30 cycles were performed of the following: 95°C for 20 seconds, 42°C for 20 seconds, and 70°C for 30 seconds, and a 7-minute extension at 72°C completed the process. Stop solution (3 μL) provided with the kit was used to terminate each reaction. The samples were heated at 72°C for 2 minutes, immediately chilled on ice, and then loaded on an 8% denaturing polyacrylamide gel. Electrophoresis was performed with a constant current of 50 W in 1 × Tris-borate/EDTA (TBE). Sequencing gels were vacuum dried, and autoradiography was performed overnight at −70°C.
Single-strand configuration polymorphism analysis
Single-strand configuration polymorphism (SSCP) analysis of each of the 11 exons of the p47-phox gene was performed using amplicons generated by nested intronic primers according to the standard protocol, as described previously, but with only 15 cycles. Oligonucleotide primers (1 μg) were labeled in a 5-μL reaction containing 6.105 MBq (165 μCi) 32P γATP (DuPont), 0.5 μL 10 × T4 polynucleotide kinase buffer, and 0.6 units T4 polynucleotide kinase by incubation for 30 minutes at 37°C. The reaction was stopped at 90°C for 2 minutes, and 10 μL H2O were added. Using the conditions described previously, 1 μL primary PCR product was reamplified for 30 cycles with the following: 0.5 μL 20 × PCR buffer, 0.2 μL 10 mmol/L dNTP, 1 μL of each labeled sense and antisense oligonucleotide, 0.25 units Taq polymerase, and 6.3 μL H2O. The labeled PCR products were diluted 1:50 in SSCP gel loading buffer containing 20 mmol/L EDTA, 0.1% SDS, 0.04% xylene cyanol, and 0.4% Bromophenol blue dissolved in formamide. After denaturation at 80°C for 5 minutes, samples were loaded on a 4.9% acrylamide/1% bis-acrylamide gel in 0.5 × TBE containing 5% glycerol and 0.05% ammonium persulfate and then electrophoresed in 0.5 × TBE at 35 W for 3 hours while cooling the gel. After the gels were vacuum dried, autoradiography was performed overnight at −70°C.
Reverse transcriptase–polymerase chain reaction
Poly(A)-RNA was isolated from 107-108peripheral lymphocytes and/or monocytes or Epstein-Barr virus–transformed (EBV–transformed) B cell lines derived from healthy individuals and patients (FastTrack kit; Invitrogen, San Diego, CA) according to the manufacturer's instructions. Briefly, the cells were lysed with the provided buffer, and poly(A)-RNA was isolated by binding to oligo(dT) cellulose. After 2 washing steps with high and low salt buffers (to remove DNA, proteins, cell debris, and nonpolyadenylated RNA), RNA was eluted under nonsalt conditions, precipitated, dissolved in ribonuclease-free (RNase-free) H2O, and stored at −70°C until used. Reverse transcriptase–PCR (RT-PCR) was performed (Strata Script RT-PCR kit; Stratagene, La Jolla, CA) according to the manufacturer's instructions. In a 59-μL reaction, 20-200 μg poly(A)-RNA was reverse transcribed with 50 units of RNase H reverse transcriptase using random primers to generate first-strand complimentary DNA (cDNA). PCR amplification of first-strand cDNA (1μL) was followed by sequence analysis using the same conditions described above for genomic DNA. For this analysis, the following nested primers were used: (1) 2 from exon 1: 5′-ATGGGGGACACCTTCATCCGTCACA and 5′-TCGCCCTGCTGGGCTTTGAGAAGCGC, (2) 1 from exon 9: 5′-CGTTGGGCCTGGGACACGTCTTG, and (3) 1 from the 3′UTR: 5′-CGTCCAGACGCCAGGCTCTATAC.
Restriction analysis
Restriction digest analysis was performed in a 10-μL reaction using 1-μL labeled PCR products of either genomic or cDNA material amplified with nested primers (as described above). The following enzymes were used in each reaction: 5 units Bsp 1407l (MBI Ferments, Buffalo, NY) and 2.5 units Aci1 or 1.5 units DraIII (New England Bolas, Beverly, MA). Commercially available buffers supplied by the manufacturers were used for restriction digestion at 37°C for 3 hours. The reaction products were analyzed by electrophoresis on a 4.9% acrylamide/1% bis-acrylamide gel in 0.5 × TBE containing 5% glycerol and 0.05% ammonium persulfate.
Results
Genomic DNA derived from a total of 28 A47° CGD patients and 37 healthy donors was analyzed following nested amplification (Table 1primers) of the region flanking and including exon 2 (Figures1 and 2). Because thep47-phox wild type gene and pseudogenes are highly homologous in exonic and intronic regions, DNA strands from wild-type genes and pseudogenes were necessarily coamplified. Indeed, consistent with previous findings,22 sequence analysis of these PCR products revealed that all 37 healthy donors contained both wild-type gene and pseudogene sequences. In 25 of the 28 CGD patients, on the other hand, only the ΔGT characteristically found in the pseudogenes was detected on all alleles (Figures 2 and3). The remaining 3 patients had both the ΔGT and wild-type GTGT sequences. SSCP analysis of exon 2 showed that all A47° CGD patients with ΔGT/ΔGT sequences were lacking a band seen in all normal controls who had both the GTGT and ΔGT sequence (Figure 4). There were no consistent differences observed in the other 10 exons of thep47-phox gene, which suggests that SSCP is a reliable method to screen for ΔGT/ΔGT sequences in A47° CGD patients. In addition, these data were confirmed by restriction analysis with Bsp 1407I, an enzyme known to digest only the wild type GTGT sequences (not shown). However, this latter method is complicated by heteroduplex formation between the p47-phox gene and pseudogene DNA strands, which cannot be cut.
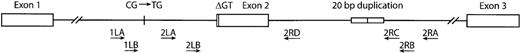
Localization of oligonucleotide primers.
The arrows indicate the position and direction of the primers used to amplify exon 2 and the flanking intronic regions.
Localization of oligonucleotide primers.
The arrows indicate the position and direction of the primers used to amplify exon 2 and the flanking intronic regions.
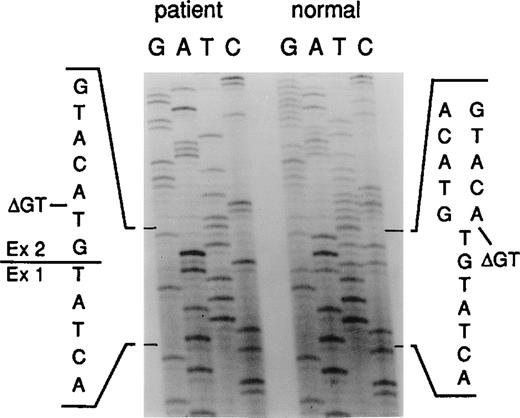
Sequence analysis of the intron 1/exon 2 border of thep47-phox gene.
Genomic DNA derived from a normal donor and an A47° CGD patient was amplified using primers 2LA, 2LB, 2RC, and 2RD. The PCR product contained the 3′ end of intron 1 (small letters) and the 5′ end of exon 2 (capital letters). The position of the GT deletion is marked as ΔGT.
Sequence analysis of the intron 1/exon 2 border of thep47-phox gene.
Genomic DNA derived from a normal donor and an A47° CGD patient was amplified using primers 2LA, 2LB, 2RC, and 2RD. The PCR product contained the 3′ end of intron 1 (small letters) and the 5′ end of exon 2 (capital letters). The position of the GT deletion is marked as ΔGT.
Distribution of p47-phox wild-type and pseudogene markers.
The distribution of wild-type–specific (w) and pseudogene-specific (p) sequence markers is shown at each sequence location in intron 1, exon 2, and intron 2 in a total of 28 A47° CGD patients, 37 healthy donors, 6 chimpanzees, and 1 rhesus monkey. Intron 2 could not be amplified in the primates with human primers, and the CG sequence was not conserved in the rhesus monkey.
Distribution of p47-phox wild-type and pseudogene markers.
The distribution of wild-type–specific (w) and pseudogene-specific (p) sequence markers is shown at each sequence location in intron 1, exon 2, and intron 2 in a total of 28 A47° CGD patients, 37 healthy donors, 6 chimpanzees, and 1 rhesus monkey. Intron 2 could not be amplified in the primates with human primers, and the CG sequence was not conserved in the rhesus monkey.
SSCP of PCR amplified genomic DNA strands of exon 2.
SSCP was performed on genomic DNA samples from normal donors (n1, n2, and n3) and A47° CGD patients (p1, p2, and p3) using the nested primer pairs 2LA/2LB and 2RC/2RD. The amplification products containing the 3′ end of intron 1 and the 5′ end of exon 2 were electrophoresed on a nondenaturing polyacrylamide gel. The figure shows a typical SSCP analysis, where the diagnostic band (−>) was missing in all patients carrying ΔGT.
SSCP of PCR amplified genomic DNA strands of exon 2.
SSCP was performed on genomic DNA samples from normal donors (n1, n2, and n3) and A47° CGD patients (p1, p2, and p3) using the nested primer pairs 2LA/2LB and 2RC/2RD. The amplification products containing the 3′ end of intron 1 and the 5′ end of exon 2 were electrophoresed on a nondenaturing polyacrylamide gel. The figure shows a typical SSCP analysis, where the diagnostic band (−>) was missing in all patients carrying ΔGT.
Because the transcript for the p47-phox gene is detected in all normal controls, we analyzed the p47-phox transcripts in A47° CGD patients.22 Sequence analysis of cDNA derived from samples of 12/25 ΔGT/ΔGT patients confirmed the importance of the ΔGT sequence; it was seen exclusively in each of the 12 patients analyzed, which indicates that the ΔGT transcript is highly expressed (Figure 5). In cDNA samples derived from mononuclear white blood cells (n = 5) or EBV-transformed B cells (n = 2) from healthy donors, the GTGT and the ΔGT sequences were always detected. This is consistent with our previous findings that at least 1 p47-phox pseudogene is transcribed.22 Restriction digestion with DraIII, which only recognizes the wild type cDNA sequence (CACNNN/GTG),22 confirmed the sequence analysis (data not shown). Taken together, these findings demonstrate that the majority of A47° CGD patients investigated in this study had only thep47-phox pseudogene sequence in exon 2 at the genomic and messenger RNA (mRNA) levels.
Sequence analysis of the exon 1/exon 2 border of p47-phox.
cDNA from an A47° CGD patient and a normal donor was PCR amplified and sequenced. The position of the GT deletion is marked as ΔGT.
Sequence analysis of the exon 1/exon 2 border of p47-phox.
cDNA from an A47° CGD patient and a normal donor was PCR amplified and sequenced. The position of the GT deletion is marked as ΔGT.
In control and A47° CGD patients with only the ΔGT/ΔGT, we analyzed the flanking regions of exon 2, in intron 1 and intron 2, looking for specific sequence differences between the gene and the pseudogenes previously characterized.22 We concentrated on 2 sites: (1) intron 1, where there is a CG in the wild type gene and TG in the pseudogenes, 122–base pair (bp) upstream from the 5′ end of exon 2, and (2) a 20-bp duplication in the pseudogenes but not the wild-type gene, 176-bp downstream from the 5′ end of intron 2. As predicted in all control samples, both the wild-type gene and the pseudogene sequences in intron 1 (Figure6) and intron 2 (Figure 7) were detected easily. In 22 of the 28 CGD patients, only the pseudogene marker TG in intron 1 was detected in all alleles, whereas in 6 patients both CG and TG were seen (Figure 6). Restriction digestion with Aci1, which specifically cuts the wild type C/CGC sequence, was consistent with the sequence analysis (data not shown).
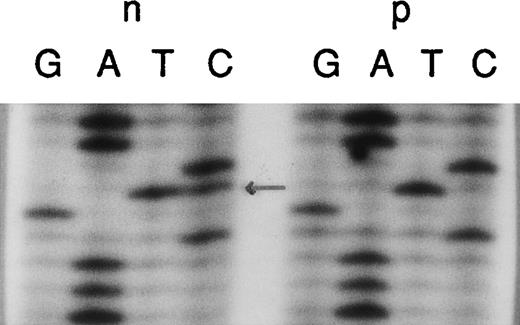
Identification of the “pseudogene marker” TG in intron 1.
Genomic DNA from a normal individual (n) and an A47° CGD patient (p) was amplified using the primers 1LA, 1LB, 2RC, and 2RD. The PCR products contained the 3′ end of intron 1 and the entire exon 2. In the patient sample, only the “pseudogene marker” TG located in intron 1 122-bp upstream from the 5′ end of exon 2 is present. The normal individual typically showed both sequences, the wild type CG and the pseudogene TG (arrow). Sequence analysis of the antisense DNA strand or restriction analysis with Aci1 (c/cgc) confirmed these data (not shown).
Identification of the “pseudogene marker” TG in intron 1.
Genomic DNA from a normal individual (n) and an A47° CGD patient (p) was amplified using the primers 1LA, 1LB, 2RC, and 2RD. The PCR products contained the 3′ end of intron 1 and the entire exon 2. In the patient sample, only the “pseudogene marker” TG located in intron 1 122-bp upstream from the 5′ end of exon 2 is present. The normal individual typically showed both sequences, the wild type CG and the pseudogene TG (arrow). Sequence analysis of the antisense DNA strand or restriction analysis with Aci1 (c/cgc) confirmed these data (not shown).
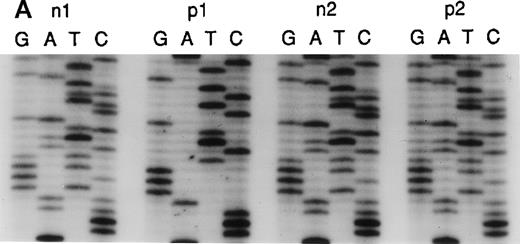
Sequence analysis of the 5′ region of intron 2.
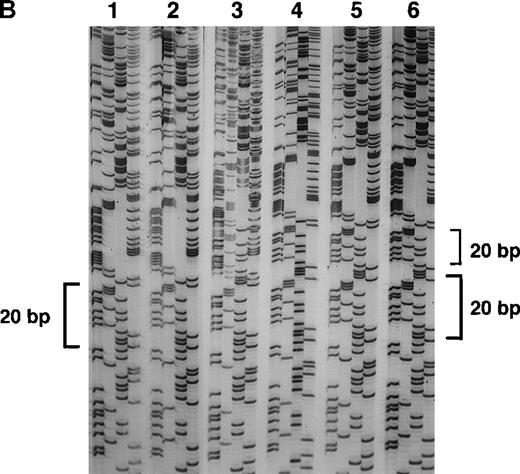
(A) Genomic DNA from healthy individuals (n1 and n2) and A47° CGD patients (p1 and p2) was amplified using the primers 2LA, 2LB, 2RA, and 2RB. The PCR product of the pseudogene contained the 20-bp duplication located 176-bp downstream of the 5′ end of intron 2. Patient p1 had only the 20-bp duplication characteristic for the pseudogenes, whereas patient p2 and healthy individuals showed both the single 20-bp sequence of the wild-type gene and the 20-bp duplication, as indicated by 2 overlying sequences in intron 2. (B) Direct sequence analysis of fragments amplified across the 5′ region of intron 2 using the above primers and directly subcloned into pTA (Invitrogen). Sequence analysis used the reverse primer, and the orientation of the nucleotides is T, G, C, and A across the 4 lanes for each reaction. The DNA template for PCR was normal genomic DNA, and individual clones were analyzed in lanes 1, 2, 4, 5, and 6. Lane 3 represents a 1:1 mixing of the clones sequenced in lanes 1 and 4. Lanes 1 and 2 demonstrate the wild-type sequence, which includes only 1 copy of the 20-bp repeat unit CAGGGTCTTGCTCTGTCACC, whereas lanes 4, 5, and 6 show the repeat (indicated by brackets).
Sequence analysis of the 5′ region of intron 2.
(A) Genomic DNA from healthy individuals (n1 and n2) and A47° CGD patients (p1 and p2) was amplified using the primers 2LA, 2LB, 2RA, and 2RB. The PCR product of the pseudogene contained the 20-bp duplication located 176-bp downstream of the 5′ end of intron 2. Patient p1 had only the 20-bp duplication characteristic for the pseudogenes, whereas patient p2 and healthy individuals showed both the single 20-bp sequence of the wild-type gene and the 20-bp duplication, as indicated by 2 overlying sequences in intron 2. (B) Direct sequence analysis of fragments amplified across the 5′ region of intron 2 using the above primers and directly subcloned into pTA (Invitrogen). Sequence analysis used the reverse primer, and the orientation of the nucleotides is T, G, C, and A across the 4 lanes for each reaction. The DNA template for PCR was normal genomic DNA, and individual clones were analyzed in lanes 1, 2, 4, 5, and 6. Lane 3 represents a 1:1 mixing of the clones sequenced in lanes 1 and 4. Lanes 1 and 2 demonstrate the wild-type sequence, which includes only 1 copy of the 20-bp repeat unit CAGGGTCTTGCTCTGTCACC, whereas lanes 4, 5, and 6 show the repeat (indicated by brackets).
Agarose gel electrophoresis of PCR products containing exon 2 and the flanking region of intron 2 revealed 2 fragments in all control samples that differed by 20 bp, again indicating the presence of wild-type gene and pseudogene sequences (Figure 8). Sequence analysis confirmed that the larger fragment contained the 20-bp duplication specific for the pseudogenes. In 10 of the 25 ΔGT/ΔGT patients, only 1 band the size of the pseudogene fragment was detected (Figure 8). This suggests that only the pseudogene sequence with the 20-bp duplication was present in these patients. Analysis of the remaining 18 patients indicates that each one has both the wild-type and pseudogene sequences in intron 2, as shown by the presence or absence of the 20-bp duplication beginning 176-bp downstream of the 5′ end of intron 2.
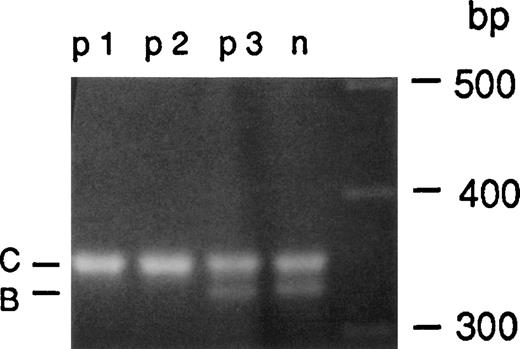
PCR amplification of intron 2.
Genomic DNA from a healthy individual (n1) and 3 A47° CGD patients (p1, p2, and p3) was amplified using primers 2LB and 2RC. A 330-bp amplification product containing the single bp sequence 176-bp downstream from the 5′ end of intron 2 (B) as well as a 350-bp fragment containing the 20-bp duplicated sequence from the pseudogene (C) were detected in normal individuals. Only the 350-bp fragment indicative for the pseudogene sequence was amplified from patients p1 and p2, whereas patient p3 showed both the 330- and 350-bp PCR product indicating the presence of wild-type and pseudogene alleles.
PCR amplification of intron 2.
Genomic DNA from a healthy individual (n1) and 3 A47° CGD patients (p1, p2, and p3) was amplified using primers 2LB and 2RC. A 330-bp amplification product containing the single bp sequence 176-bp downstream from the 5′ end of intron 2 (B) as well as a 350-bp fragment containing the 20-bp duplicated sequence from the pseudogene (C) were detected in normal individuals. Only the 350-bp fragment indicative for the pseudogene sequence was amplified from patients p1 and p2, whereas patient p3 showed both the 330- and 350-bp PCR product indicating the presence of wild-type and pseudogene alleles.
In summary, in 10 of 25 ΔGT/ΔGT patients, only the pseudogene marker sequences, the TG in intron 1, the ΔGT in exon 2, and the 20-bp duplication in intron 2 were detected in all alleles (Figure 3). There were 12 patients who had only pseudogene sequences in intron 1 and exon 2 (but not in intron 2), whereas 3 patients showed both wild-type and pseudogene sequences in intron 1 and 2 flanking the ΔGT mutation. Our data indicate that there is a heterogeneity in the flanking markers around the ΔGT in patients who appear to have only the ΔGT sequence in A47° CGD (Figure 3). Some strands were pure pseudogene alleles identical in this region to previously characterized genomic pseudogene clones.22 On other strands, ΔGT was variably linked to either wild-type or pseudogene sequences in the flanking introns leading to wild-type and/or pseudogene chimers. In 3 patients, both wild-type and pseudogene sequences were present at all 3 positions, which suggests that these patients have other mutations than ΔGT. The precise mutation analysis of these patients is currently under investigation.
Whereas at least 2 p47-phox pseudogenes are present in the human genome, nothing is known about the presence of p47-phoxpseudogenes in other primates. We therefore performed sequence analysis of genomic DNA from 6 chimpanzees and 1 rhesus monkey using human primers. The wild-type p47-phox sequence of exon 2 and the flanking region of intron 1 were completely conserved in the chimpanzees. In the rhesus monkey, several nucleotide exchanges were found in exon 2, and the sequence surrounding the CG→TG pseudogene marker in intron 1 was not conserved (Figure9). Both primates, however, showed the GTGT sequence in exon 2, and the chimpanzees also contained the wild- type CG sequence in intron 1. No evidence for the presence of pseudogene sequences could be found, indicating that these primates only have thep47-phox wild-type gene. These results suggest that thep47-phox gene duplications (or at least the formation of pseudogenes) occurred late in evolution after the branching of the hominids from the common line of ancestors. This evolutionarily recent event is a possible explanation for the high homology between wild-type genes and pseudogenes and the presence of pseudogene mRNA in all normal controls.
Sequence comparison of exon 2 and adjacent intronic regions of p47-phox genes.
Sequence of exon 2 (capital letters) and the flanking introns is shown from the human p47-phox wild-type gene (N), the humanp47-phox pseudogenes (P), the chimpanzee p47-phox gene (C), and the rhesus monkey p47-phox gene (R). The start of the sequence available is indicated by 5′, and 3′ indicates the end of the sequence. Note that the CG sequence in intron 1 was not conserved in the rhesus monkey and that intron 2 could not be amplified with human primers in the chimpanzee nor the rhesus monkey.
Sequence comparison of exon 2 and adjacent intronic regions of p47-phox genes.
Sequence of exon 2 (capital letters) and the flanking introns is shown from the human p47-phox wild-type gene (N), the humanp47-phox pseudogenes (P), the chimpanzee p47-phox gene (C), and the rhesus monkey p47-phox gene (R). The start of the sequence available is indicated by 5′, and 3′ indicates the end of the sequence. Note that the CG sequence in intron 1 was not conserved in the rhesus monkey and that intron 2 could not be amplified with human primers in the chimpanzee nor the rhesus monkey.
Discussion
We recently demonstrated the presence of more than 1 highly homologous p47-phox pseudogene, which characteristically contains ΔGT at the beginning of exon 2, leading to a premature termination codon at amino acid 51.22 In previous studies, 9 of 10 A47° CGD patients were found to be “homozygous” for ΔGT.19-21 In this study, we confirm that in the majority of A47° CGD patients, only the ΔGT allele in exon 2 can be detected. We provide an explanation at the molecular level for the unusually high frequency of a single mutation in an unrelated racially diverse population. Previously, the high number of A47° CGD patients with the ΔGT mutation had been ascribed to the sequence surrounding the beginning of exon 2. It has been hypothesized that the dinucleotide repeat might be especially susceptible to mutations by DNA strand slippage at this site by DNA polymerase, thereby generating deletions of the dinucleotide unit.19,21 Furthermore, it has been speculated that the palindromic sequence surrounding the tandem repeat might allow the formation of a hairpin loop, with a mismatch resulting in the dinucleotide deletion by slipped mispairing during DNA replication.21 However, the GT deletion appears too small to be caused by the deletion of an entire hairpin loop. Moreover, small hairpin loops require a large amount of energy to form and are consequently not favored.24
Although these mechanisms cannot be completely ruled out, they do not explain the finding that the majority of our patients carried at least 1 additional pseudogene marker in the flanking intronic regions on all alleles present (Figures 3 and 9). Since the highly homologousp47-phox pseudogenes carrying ΔGT colocalize to the same chromosomal band 7q11.23, it appears very likely that recombination events between the p47-phox gene and pseudogenes, such as crossing-over and/or gene conversion, take place. Such mechanisms would allow the incorporation of the ΔGT mutation into the p47-phoxwild-type gene, thus rendering p47-phox nonfunctional. Crossing-over leads to reciprocal exchange of homologous DNA stretches, and gene conversion leads to nonreciprocal exchange of these stretches. Such exchanges involve an alteration of an allele at a specific locus in such a way that an internal portion of its sequence has been replaced by a homologous segment copied from another allele or locus.25 Possible recombination events include crossing-over and/or gene conversion with or without deletions at various breakpoints, leading to chimeric genes with pseudogene fragments of different sizes. Such mechanisms would be consistent with our observation that different combinations of intronic gene and pseudogene markers occur in A47° CGD patients with only the ΔGT allele. Gene conversion events between genes and their homologous pseudogenes have also been described in the pathogenesis of several other genetic disorders such as 21-hydroxylase deficiency,26 von Willebrand disease,27 Gaucher disease,28 and Hunter syndrome.29
The size of the putative converted sequence element can be estimated for those patients who contain ΔGT but do demonstrate wild-type and pseudogene sequences in the flanking intronic sites. The gene conversion product in these patients could be as small as 377 bp, based upon the subsets of patients who are heterozygous for the CG to GT exchange in intron 1 and the presence of both the duplicated and nonduplicated region in intron 2. The size of 377 bp is approximately the same as that reported for a microconversion confined to a tract of no more than 390 nucleotides in a subset of patients with 21-hydroxylase deficiency.30 On the other hand, in those patients who have pseudogene sequences exclusively in all 3 marker positions on all alleles, the converted element must be larger than 377 bp. Alternatively, the p47-phox wild-type gene might be partially or completely deleted in these cases. However, the complete deletion of the wild-type gene can be ruled out in those patients who carry only ΔGT or ΔGT and TG in intron 1 but who also carry the wild-type sequence in intron 2. These findings would be again consistent with the presence of chimeric DNA strands resulting from recombination events of a sequence stretch of a yet unknown size. They can also be explained by the presence of fusion DNA strands with or without a partial deletion of the 5′ portion of thep47-phox wild-type gene. However, to further elucidate the mechanisms leading to chimeric DNA strands in A47° CGD patients, it will be necessary to study the presence of additional pseudogene markers upstream of the TG sequence in intron 1 and downstream of the 20-bp duplication in intron 2.
Several observations further support our hypothesis that recombination events occur between the p47-phox wild-type gene and pseudogenes. First, the degree of sequence similarity appears to be closely related to the frequency of recombination events.31The p47-phox wild-type gene and pseudogenes are approximately 99% homologous in exonic and intronic regions.22 The 21-hydroxylase gene and its pseudogene, for example, are 98% homologous in their exonic and 96% homologous in their intronic regions.26 Second, hot spots for recombination, such as the Chi sequence and the human minisatellite, repeat sequences, and repetitive elements (such as Alu repeats) appear to stimulate and facilitate recombination events.32-34 Indeed, recombination hot spots andAlu sequences are frequently found in the p47-phox gene and pseudogenes.22 Third, the p47-phox wild-type gene and pseudogenes are located within a cluster of duplicated sequences at chromosomal band 7q11.23.22 Most importantly, there is no explanation other than recombination events for the presence of chimeric DNA strands composed of wild-type and pseudogene sequences in most of our A47° CGD patients.
In contrast to other diseases caused by recombination events between the wild-type gene and pseudogene, where multiple deleterious mutations are found within the pseudogene,26 27 only 1 deleterious mutation, namely ΔGT, is present in the p47-phox pseudogenes. All other sequence differences between wild type and pseudogenes in the exonic regions are single bp substitutions. Most of them are silent and would therefore, when transformed to the wild-type gene, not be expected to cause a notable loss of function of p47-phox. However, in those cases where the converted element contains ΔGT in exon 2, a truncated p47-phox will be encoded, assuming that the transcription is initiated at the same site as in the wild-type gene. The presence of only 1 putative deleterious mutation in the p47-phoxpseudogenes would explain the unusually high incidence of a single mutation affecting unrelated, racially diverse patients with a rare autosomal recessive disease.
Frequently occurring recombination events between the p47-phoxgene and its pseudogenes within different populations provides not only an explanation for the increased incidence of the ΔGT mutation but might also explain the high frequency of carriers for A47° CGD. Because A47° CGD accounts for approximately 25% of CGD patients, and the incidence for CGD is estimated at 1:500 000,14approximately 1 in 2 million newborns suffer from p47-phox–deficient CGD. The carrier frequency can then be calculated to be approximately 1 in 700. In the other autosomal inherited CGD forms, A22° CGD (p22-phox deficiency) and A67° CGD (p67-phox deficiency), carrier frequencies are estimated to be 1:5000 and below, respectively. In contrast to the reports of these latter forms, the parents of A47° CGD patients are rarely consanguineous (none of our families were). At present, there appears to be no evolutionary advantage for A47° CGD carriers. Furthermore, no founder effect has been observed.
Whereas there is evidence that recombination events are involved in 25 of 28 patients, 3 of these patients had a detectable wild-type sequence in exon 2; no other mutations in the coding region have been found that could explain the absence of the p47-phox protein in these patients. Although we currently do not know the genetic defects in these patients, we speculate that they might have de novo mutations. In previous reports of A47° CGD patients, only 1 of 10 patients had a second mutation, namely deletion of a G at bp 502.19-21 These data are consistent with our observations that approximately 10% of A47° CGD patients might have de novo mutations. Considering that approximately 25% of all CGD patients do not have functional p47-phox, approximately 2.5% of all CGD patients might carry new mutations in the p47-phox gene other than the homozygous state for the ΔGT mutation. Thus, the incidence of de novo mutations in A47° CGD would be in a similar range as that observed in the other autosomal recessive CGD forms deficient of p22-phox or p67-phox, where the incidence of de novo mutations is below 5%.12-14
Lastly, it is possible to diagnose A47° CGD patients who have only the ΔGT mutation by using a combination of sequence, restriction digest, and SSCP analysis. However, the diagnosis of a carrier state is problematic because the pseudogene alleles are present in all normal individuals. Further elucidation of the molecular mechanism of A47° CGD will perhaps identify other significant changes or differences within or linked to the p47-phox gene and pseudogenes that will be useful for developing an acceptable test to detect p47-phox carriers.
Acknowledgments
The authors wish to thank Drs Pauline Lee, Andrew Cross, and Paul Heyworth for helpful suggestions. Special thanks to Inga Roesler for helping type the manuscript.
Supported by a grant from Deutscher Akademischer Austauschdienst, Bonn, Germany (A. Goerlach), and a grant from Deutsche Forschungsmeinschaft, Bonn, Germany (J. Roesler).
Reprints:John T. Curnutte, Department of Immunology, Genentech Inc, 1 DNA Way, Building 12, Mailstop 34, South San Francisco, CA 94080; e-mail: curnutte.john@gene.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal