Abstract
The course of mycosis fungoides (MF) is indolent except when transformation to a large T-cell lymphoma occurs. The diagnosis of transformed MF (T-MF) relies on the presence of more than 25% of large cells on biopsy of an MF lesion. We analyzed 45 patients with T-MF recorded by the French Study Group on Cutaneous Lymphomas to better determine clinicopathological features of MF transformation and to analyze their impact on prognosis. Median time from diagnosis of MF to transformation was 6.5 years. Extracutaneous progression was present in 20 patients. Mean survival from transformation to death was 22 months. In univariate analysis, only an extracutaneous progression was associated with a worse prognosis (5-year actuarial survival: 7.8% versus 32%). Neither sex, age, clinical and skin disease stage at transformation, transformation speed, nor percentage of large cells or CD30 expression (14 of 45) had a prognostic value. When performing multivariate analysis, age (at least 60 years), and extracutaneous spreading were found to be associated with a poor prognosis. There was no difference between survival curves of patients with T-MF and with pleomorphic large T-cell CD30− lymphomas. The main diagnostic pitfall was “histiocytic-rich” MF, requiring CD68 staining for the diagnosis of T-MF. Out of 45 patients, 6 presented an histologic transformation before clinical progression, suggesting that an early histopathological diagnosis may be performed by histological follow-up. The prognostic value of such early histopathological diagnosis must be confirmed by prospective studies.
Large cell transformation in mycosis fungoides (MF) is rare and is associated with an aggressive clinical course and shortened survival.1 MF transformation (T-MF) is defined on the following histopathological basis: presence of large cells exceeding 25% of the infiltrate throughout or forming microscopic nodules.2 This transformation has been shown to represent an evolution of the original malignant clone.3,4Clinicopathologic or biologic criteria predictive of transformation are unknown except for the expression of CD25 antigen (interleukin 2 receptor), which may identify a subset of MF patients at risk.5 The diagnosis of transformation may be difficult because histopathologic criteria have a low reproducibility, and several differential clinical and/or histopathologic diagnoses exist. The diagnosis of T-MF is almost always made on cutaneous biopsies because a clinical progression (tumor or infiltrated patches) occurs. Owing to the lack of prospective studies with histological follow-up, it is impossible to determine the impact on survival of an earlier histopathologic diagnosis of transformation in the absence of clinical progression. Recently, a statistical study performed on 26 T-MF patients showed that clinical features associated with a poor survival were (1) early transformation (less than 2 years from the diagnosis versus 2 years or longer) and (2) advanced clinical stage at the time of T-MF diagnosis (IIB-IV vs I-IIA).1
The aim of our study was to better determine clinical and histopathological parameters associated with MF transformation and to analyze their impact on prognosis. Therefore, we statistically analyzed several recently analyzed clinical criteria,1 as well as morphological (percentage of large T-cells) and phenotypical (CD30, CD20, CD68, p53, or MiB1 expression) features.
Patients and methods
Inclusion criteria
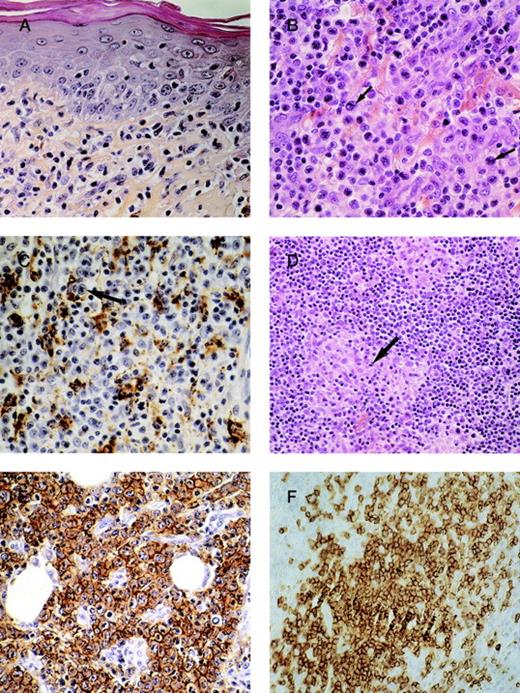
We retrieved all patients with diagnosis of MF transformation from the registry of the French Study Group on Cutaneous Lymphomas from January 1, 1992, to September 1, 1998. These patients were selected among the 419 cases of MF registered by our group. For each case, clinical features and pathologic slides were reviewed by 2 dermatologists (M.B.B., L.V.) and 3 pathologists (B.V., A.M., J.W.). We included patients with (1) clinical diagnosis of MF and (2) histopathologic transformation in at least 1 skin biopsy according to the criteria described previously2: large cells (≥ 4 times the size of a small lymphocyte) exceeding 25% of the infiltrate throughout or forming microscopic nodules (Figure1A, B). This cytological transformation was analyzed before and after CD3 (pan–T-lymphocyte antigen) and CD68 (histiocytic antigen) immunostaining in order to differentiate histiocytes from large T-cells (Figure 1C) and to more precisely evaluate the percentage of large lymphomatous cells.
Histopathologic and immunohistochemical features.
(A) Mycosis fungoides before transformation: proliferation of small-sized lymphocytes in the upper dermis with epidermotropism. (B) The same case after transformation: the lymphoid infiltrate has become tumoral and polymorphous with more than 25% of large-sized lymphocytes (arrows). (C) Analysis of percentage of large lymphomatous cells (cytologic transformation) after CD68 immunostaining: between histiocytic components (CD68 positive cells), large lymphomatous cells (arrows) are CD68 negative. (D) The main differential diagnosis of transformed-MF: “histiocytic-rich” or granulomatous MF. Note that the lymphocytes between histiocytic granulomas (arrows) remain small. (E) Immunostaining with the Ber-H2 antibody in a case of transformed-MF: CD30 expression by more than 75% of large cells. (F) Immunostaining with the CD20 antibody (anti-B lymphocytes): nodular infiltrate of CD20-positive lymphocytes.
Histopathologic and immunohistochemical features.
(A) Mycosis fungoides before transformation: proliferation of small-sized lymphocytes in the upper dermis with epidermotropism. (B) The same case after transformation: the lymphoid infiltrate has become tumoral and polymorphous with more than 25% of large-sized lymphocytes (arrows). (C) Analysis of percentage of large lymphomatous cells (cytologic transformation) after CD68 immunostaining: between histiocytic components (CD68 positive cells), large lymphomatous cells (arrows) are CD68 negative. (D) The main differential diagnosis of transformed-MF: “histiocytic-rich” or granulomatous MF. Note that the lymphocytes between histiocytic granulomas (arrows) remain small. (E) Immunostaining with the Ber-H2 antibody in a case of transformed-MF: CD30 expression by more than 75% of large cells. (F) Immunostaining with the CD20 antibody (anti-B lymphocytes): nodular infiltrate of CD20-positive lymphocytes.
Out of 64 patients selected, 19 were excluded because of (1) clinical criteria (initial presentation as large T-cell lymphoma without proven previous clinical and histologic MF, n = 6; and lymphomatoid papulosis with MF instead of T-MF, n = 2) and (2) histopathologic criteria (3 cases were reclassified as granulomatous MF [Figure 1D]; 8 were “histiocytic rich MF” and had fewer than 25% of large lymphomatous T-cells after CD68 immunostaining).
Clinical evaluation
In all patients, staging included physical evaluation, blood cell count, chest radiographs or thoracic CT scan, abdominal ultrasound tomography or abdominal CT scan, and bone marrow biopsy. Patients with clinically significant adenopathy had lymph node biopsy. Staging criteria used were those of the Mycosis Fungoides Cooperative Group.6 The types of cutaneous lesions, the transformation on the patches or elsewhere, and the treatments were recorded. The occurrence of extracutaneous spreading and actual status (complete remission, disease limited to the skin, extracutaneous progression, died of lymphoma or other cause) were recorded.
Histopathological and immunohistochemical analysis
After inclusion, some morphological parameters were analyzed: percentage of large T-lymphocytes (classified in 3 groups: 25%-49%, 50%-79%, and 80%-100%), epidermal changes (hyperplasia, ulceration, etc), epidermotropism of large cells, pattern (patchy or diffuse) and thickness of the lymphomatous infiltrate, and presence of an inflammatory component (plasmocytes, histiocytes, eosinophils, etc). When patients had a previous skin biopsy before T-MF, we compared the 2 biopsies (before and after the diagnosis of transformation). In all cases we performed immunohistochemical studies on paraffin sections with a 3-stage streptavidin peroxidase procedure (labeled streptavidin-biotin, Dako, Trappes, France) using the following antibodies (Dako, Trappes, France): anti-CD3, CD4, CD8, CD20 (L26), CD68 (KP1 and PG-M1), CD30, p53 (D07), and MiB1 (nuclear proliferation antigen). The expression of the CD30 antigen was analyzed as negative, positive with fewer than 75% of positive tumoral cells, or positive with more than 75% of tumoral cells. CD25 (interleukin 2 receptor α) expression was not studied because fresh frozen tissue was not available for all cases.
Data analysis
Survival duration was calculated from diagnosis of MF transformation to either September 1, 1998, or death of the patient (whatever the cause). Survival curves were plotted using the Kaplan-Meier product-limit method. Differences between survival curves were tested by the log-rank test. The following variables (recorded at the date of diagnosis of MF transformation) were analyzed as potential prognostic characteristics: age (less than or at least 60 years), sex, clinical staging (I-IIA versus IIB-IV; IIB versus IV), stage of skin disease: generalized (T2) plaque disease vs tumoral (T3) stage, interval between diagnosis of MF and transformation (less than or at least 2 years), interval between appearance of first skin lesions and diagnosis of transformation, percentage of large cells (25%-49% versus 50%-79% or versus 80%-100%), and expression of CD30, CD68, and CD20 antigens. Moreover, we analyzed the prognostic value of extracutaneous spreading measured during the follow-up. A proportional hazards regression model was used to estimate the independent effect of the clinical and histologic variables measured at baseline on survival. All variables associated with survival in the univariate analysis withP < .25 or known from the literature as highly prognostic were introduced into the first model. A reduced model was produced by backward elimination. Results are expressed in terms of the hazard ratio (HR), which estimates how each independent variable affects the baseline instantaneous risk of death. The proportional hazards assumption was checked using graphic methods by examining log(-log[survival probability]) versus log(time) plots for each of the covariates in the final model.
Moreover, the survival curve of patients with T-MF was compared with 2 series of patients with non-MF cutaneous lymphoma who were registered in the French Group database during the same study period7: patients with CD30 negative pleomorphic large T-cell cutaneous lymphoma (n = 15) and those with CD30 positive large T-cell cutaneous lymphoma (n = 30). STATA software, version 5.1 (STATA Corp, College Station, TX), was used for statistical analysis.
Results
Clinical data
About 10 percent of registered patients presented a transformation (45 of 419; Table 1). These included 26 men and 19 women. Their median age at transformation was 65 years (range, 31-90 years). Transformation occurred before (n = 10) or after (n = 35) the first 2 years following the initial diagnosis of MF (median time: 6.5 years). Eight patients were diagnosed with transformation at their initial presentation but had a long history of a dermatitis (diagnosed as eczema or psoriasis) but clinically suggestive of MF without initial biopsy. The mean duration between the first cutaneous lesions of MF (reported by patients) and transformation was 14 years (range, 1-49 years). For all patients the first site of transformation was the skin. At the time of MF diagnosis, most patients had stage I or IIA (32 of 45) disease. At transformation, most had a tumor stage (T3: 40 of 45). Only 5 patients presented a cytologic transformation on patches without any clinical transformation. For 2 other patients, biopsies performed 2 years before diagnosis of transformation were reviewed and already showed a cytologic transformation, whereas the patients had only patches and no tumor. The outcome of these 6 patients was not different from that of the other T-MF (3 patients died). In 15 patients, biopsies were performed at the same time both on tumors and plaques and showed transformation either only on tumors (n = 5) or both on tumors and plaques (n = 10). An extracutaneous progression (nodal for 19, meningeal for 1) was present in 20 patients either at the time of histologic transformation or in the following 6 months. Treatments were heterogeneous with 10 patients treated by methotrexate, alone (n = 2) or associated (n = 8) with topical treatments such as nitrogen mustard or electrontherapy for tumoral lesions. Sixteen patients were treated by polychemotherapy (cyclophosphamide, vincristine, prednisone), and in 3 cases this was followed by autologous bone marrow transplantation. Five patients were treated by interferon. Fourteen were treated only by topical treatment (nitrogen mustard, electrontherapy, UVA).
Principal clinical, morphological and immunohistochemical data of 45 patients with transformed mycosis fungoides (T-MF)
| Patient, Sex (M/F), Age (y) . | Interval from Diagnosis of MF to T-MF (y) . | Stage at T-MF . | % of Large Cells at T-MF* . | Expression of CD30 at T-MF† . | Survival After T-MF (mo) . | Extracutaneous Involvement . | Patient Status‡ . |
|---|---|---|---|---|---|---|---|
| 1. M, 74 | 0 | IVA (T2) | 3 | 0 | 36 | yes | D |
| 2. M, 83 | 0 | IIB (T3) | 2 | 0 | 30 | no | D |
| 3. M, 49 | 3 | IIB (T3) | 3 | 0 | 57 | no | A |
| 4. F, 73 | 2 | IIB (T3) | 3 | 0 | 48 | no | D |
| 5. F, 78 | 8 | IVA (T3) | 2 | 0 | 48 | yes | D |
| 6. F, 70 | 2 | IIB (T3) | 2 | 1 | 48 | no | D |
| 7. F, 87 | 7 | IIB (T3) | 3 | 2 | 12 | no | D |
| 8. F, 69 | 9 | IIB (T3) | 3 | 2 | 33 | no | A |
| 9. F, 36 | 32 | IIB (T3) | 2 | 2 | 105 | no | A |
| 10. F, 77 | 18 | IB (T2) | 1 | 0 | L | L | L |
| 11. M, 50 | 10 | IVA (T3) | 2 | 0 | 31 | yes | A |
| 12. M, 64 | 15 | IIB (T3) | 3 | 0 | 12 | yes | D |
| 13. F, 75 | 6 | IIB (T3) | 1 | 1 | 24 | no | A |
| 14. F, 47 | 11 | IIB (T3) | 3 | 0 | 31 | no | A |
| 15. M, 40 | 0 | IVA (T2) | 2 | 1 | 7 | yes | D |
| 16. F, 72 | 7 | IVA (T3) | 2 | 0 | 12 | yes | D |
| 17. M, 40 | 5 | IVA (T3) | 3 | 0 | 33 | yes | D |
| 18. M, 76 | 7 | IVA (T3) | 1 | 0 | 10 | yes | A |
| 19. M, 61 | 2 | IVA (T3) | 3 | 0 | 30 | yes | D |
| 20. M, 57 | 3 | IVA (T3) | 1 | 0 | 15 | yes | D |
| 21. M, 79 | 2 | IIB (T3) | 3 | 0 | 15 | no | D |
| 22. M, 67 | 15 | IVA (T3) | 2 | 0 | 25 | yes | A |
| 23. M, 90 | 5 | IIB (T3) | 3 | 2 | 15 | no | D |
| 24. M, 85 | 4 | IVA (T3) | 3 | 0 | 4 | yes | D |
| 25. M, 31 | 8 | IVA (T3) | 3 | 1 | 71 | yes | A |
| 26. F, 81 | 2 | IIB (T3) | 3 | 1 | 18 | no | A |
| 27. F, 68 | 4 | IB (T2) | 2 | 0 | 35 | no | A |
| 28. M, 75 | 1 | IIB (T3) | 2 | 0 | 12 | yes | D |
| 29. M, 67 | 1 | IVA (T3) | 3 | 0 | 10 | yes | A |
| 30. M, 56 | 1 | IIB (T3) | 1 | 0 | 18 | no | A |
| 31. F, 72 | 7 | IVA (T3) | 2 | 0 | 12 | yes | D |
| 32. F, 81 | 2 | IVA (T2) | 2 | 0 | 3 | yes | D |
| 33. M, 69 | 11 | IVA (T3) | 3 | 0 | 8 | yes | D |
| 34. M, 80 | 16 | IIB (T3) | 3 | 0 | 5 | no | D |
| 35. M, 45 | 4 | IIB (T3) | 2 | 0 | 36 | yes | D |
| 36. F, 43 | 3 | IIB (T3) | 3 | 0 | 96 | no | A |
| 37. F, 75 | 32 | IIB (T3) | 3 | 2 | 144 | no | A |
| 38. F, 82 | 11 | IIB (T3) | 2 | 1 | 60 | no | D |
| 39. F, 72 | 10 | IIB (T3) | 2 | 2 | 38 | no | D |
| 40. M, 43 | 0 | III (T3) | 2 | 0 | 22 | yes | D |
| 41. M, 71 | 0 | IIB (T3) | 2 | 0 | 36 | no | A |
| 42. M, 50 | 0 | IVA (T3) | 1 | 0 | 4 | no | D |
| 43. M, 37 | 0 | IVA (T3) | 2 | 1 | 16 | no | A |
| 44. F, 75 | 15 | IIB (T3) | 1 | 0 | 1 | no | D |
| 45. M, 62 | 10 | IIB (T3) | 2 | 2 | 3 | no | A |
| Patient, Sex (M/F), Age (y) . | Interval from Diagnosis of MF to T-MF (y) . | Stage at T-MF . | % of Large Cells at T-MF* . | Expression of CD30 at T-MF† . | Survival After T-MF (mo) . | Extracutaneous Involvement . | Patient Status‡ . |
|---|---|---|---|---|---|---|---|
| 1. M, 74 | 0 | IVA (T2) | 3 | 0 | 36 | yes | D |
| 2. M, 83 | 0 | IIB (T3) | 2 | 0 | 30 | no | D |
| 3. M, 49 | 3 | IIB (T3) | 3 | 0 | 57 | no | A |
| 4. F, 73 | 2 | IIB (T3) | 3 | 0 | 48 | no | D |
| 5. F, 78 | 8 | IVA (T3) | 2 | 0 | 48 | yes | D |
| 6. F, 70 | 2 | IIB (T3) | 2 | 1 | 48 | no | D |
| 7. F, 87 | 7 | IIB (T3) | 3 | 2 | 12 | no | D |
| 8. F, 69 | 9 | IIB (T3) | 3 | 2 | 33 | no | A |
| 9. F, 36 | 32 | IIB (T3) | 2 | 2 | 105 | no | A |
| 10. F, 77 | 18 | IB (T2) | 1 | 0 | L | L | L |
| 11. M, 50 | 10 | IVA (T3) | 2 | 0 | 31 | yes | A |
| 12. M, 64 | 15 | IIB (T3) | 3 | 0 | 12 | yes | D |
| 13. F, 75 | 6 | IIB (T3) | 1 | 1 | 24 | no | A |
| 14. F, 47 | 11 | IIB (T3) | 3 | 0 | 31 | no | A |
| 15. M, 40 | 0 | IVA (T2) | 2 | 1 | 7 | yes | D |
| 16. F, 72 | 7 | IVA (T3) | 2 | 0 | 12 | yes | D |
| 17. M, 40 | 5 | IVA (T3) | 3 | 0 | 33 | yes | D |
| 18. M, 76 | 7 | IVA (T3) | 1 | 0 | 10 | yes | A |
| 19. M, 61 | 2 | IVA (T3) | 3 | 0 | 30 | yes | D |
| 20. M, 57 | 3 | IVA (T3) | 1 | 0 | 15 | yes | D |
| 21. M, 79 | 2 | IIB (T3) | 3 | 0 | 15 | no | D |
| 22. M, 67 | 15 | IVA (T3) | 2 | 0 | 25 | yes | A |
| 23. M, 90 | 5 | IIB (T3) | 3 | 2 | 15 | no | D |
| 24. M, 85 | 4 | IVA (T3) | 3 | 0 | 4 | yes | D |
| 25. M, 31 | 8 | IVA (T3) | 3 | 1 | 71 | yes | A |
| 26. F, 81 | 2 | IIB (T3) | 3 | 1 | 18 | no | A |
| 27. F, 68 | 4 | IB (T2) | 2 | 0 | 35 | no | A |
| 28. M, 75 | 1 | IIB (T3) | 2 | 0 | 12 | yes | D |
| 29. M, 67 | 1 | IVA (T3) | 3 | 0 | 10 | yes | A |
| 30. M, 56 | 1 | IIB (T3) | 1 | 0 | 18 | no | A |
| 31. F, 72 | 7 | IVA (T3) | 2 | 0 | 12 | yes | D |
| 32. F, 81 | 2 | IVA (T2) | 2 | 0 | 3 | yes | D |
| 33. M, 69 | 11 | IVA (T3) | 3 | 0 | 8 | yes | D |
| 34. M, 80 | 16 | IIB (T3) | 3 | 0 | 5 | no | D |
| 35. M, 45 | 4 | IIB (T3) | 2 | 0 | 36 | yes | D |
| 36. F, 43 | 3 | IIB (T3) | 3 | 0 | 96 | no | A |
| 37. F, 75 | 32 | IIB (T3) | 3 | 2 | 144 | no | A |
| 38. F, 82 | 11 | IIB (T3) | 2 | 1 | 60 | no | D |
| 39. F, 72 | 10 | IIB (T3) | 2 | 2 | 38 | no | D |
| 40. M, 43 | 0 | III (T3) | 2 | 0 | 22 | yes | D |
| 41. M, 71 | 0 | IIB (T3) | 2 | 0 | 36 | no | A |
| 42. M, 50 | 0 | IVA (T3) | 1 | 0 | 4 | no | D |
| 43. M, 37 | 0 | IVA (T3) | 2 | 1 | 16 | no | A |
| 44. F, 75 | 15 | IIB (T3) | 1 | 0 | 1 | no | D |
| 45. M, 62 | 10 | IIB (T3) | 2 | 2 | 3 | no | A |
M, male; F, female.
1 indicates 25%-49%; 2, 50%-79%; 3, ≥80%.
0 indicates negative expression; 1, less than 75% of positive cells; 2, at least 75% of positive cells.
A, alive; D, dead; L, lost to follow-up.
Histopathologic and immunologic data
The percentage of large T-lymphocytes (evaluated after CD68 immunostaining) from all lymphomatous cells was between 25% and 49% for 7 patients, between 50% and 79% for 19 patients, and more than 80% for 19 patients (Table 1). Transformation on biopsy-proven tumor lesions (compared with plaques) was always associated with an increase in the number of large cells (more than 50%) and a decrease in epidermotropism. Only 1 patient presented a cytologic transformation localized to the pilotropic lymphocytes. In all cases, transformed large cells had a T-cell phenotype. Only 1 case was CD8 (T-suppressor cell marker) positive, the others being CD4 (T-helper cell marker) positive. CD30 expression by large cells was found in 14 cases (31%) with a positivity of more than 75% of cells in 7 of them (Figure 1E). A large histiocyte (CD68+) or B-cell lymphocyte (CD20+) component was observed in 27 of 40 (67%) and 18 of 40 cases (45%), respectively. Both components appeared at the stage of transformation and were not found in the corresponding previous biopsies of MF. Analysis of CD68 expression was particularly interesting for 1 patient. He simultaneously presented 2 clinically similar tumors; however, 1 (on the face) corresponded histologically to a granulomatous MF, and the other (on the arm), to MF transformation. Initial exclusion of 11 patients from the analysis was based on CD68 immunostaining (3 cases reclassified as granulomatous MF and 8 with fewer than 25% of large cells). All of these 11 patients were alive with complete remission or stabilization (n = 10) or lymph node involvement (without cytologic transformation; n = 1). In general, CD20 antigen was expressed not only by small reactive cells but also by large lymphocytes. The infiltrate of CD20-positive lymphocytes had either a band or a nodular (Figure 1F) pattern or was scattered. The p53 oncoprotein was expressed in only 1 T-MF. Nuclear proliferation antigen (MiB1) was expressed by fewer than 10% of tumoral cells in 11 cases and more than 50% of tumoral cells in 3 cases. The other cases were MiB1 negative.
Statistical analysis: outcome and prognostic variables
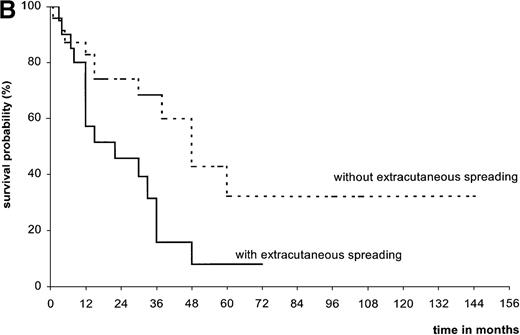
Overall, the median follow-up of T-MF was 26.5 months (Table2). At the end of the study, only 1 patient was lost to follow-up, 18 were alive (5 with extracutaneous spreading), and 26 were deceased (22 of lymphoma, 4 of other causes). Median survival time from transformation was 36 months (range, 1-60 months; mean, 22 months). Survival from diagnosis of transformation was 61.3% at 2 years, 43.4% at 3 years, and 20.8% at 5 years (Figure2A). In univariate analysis, extracutaneous spreading was the only clinical feature associated with a worse survival at 5 years (7.8%, versus 32% in the absence of progression; Figure 2B). Neither age, sex, clinical stage at transformation nor stage of skin disease were statistical prognostic indicators for survival. Although this was not significant, there was a trend for a worse prognosis in patients presenting an early transformation compared to others (3-year survival 11.4%, versus 49.9%). Considering histologic features, a low percentage of large cells or the presence of CD30, CD20, or CD68 positive cells were not associated with a better prognosis. Moreover, even with a low percentage of large cells (25% to 50%) the prognosis was poor (3 patients out of 6 died).
Clinical and morphologic data of the 45 patients with T-MF and statistical survival analysis
| Grouping . | No. of Patients . | No. of Death . | Median Survival (mo) . | % Patients Alive at 2 Yr (95% CI) . | % Patients Alive at 5 Yr (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Men | 26 | 16 | 30 | 52.5 | 18.9 | .09 |
| Women | 19 | 10 | 48 | 72.2 | 27.1 | |
| Age | ||||||
| Younger than 60 y | 14 | 6 | 36 | 69.8 | 46.6 | .09 |
| At least 60 y | 31 | 20 | 30 | 56.5 | 6.8 | |
| Interval from MF diagnosis to transformation | ||||||
| Less than 2 y | 10 | 6 | 30 | 51.4 | — | .31 |
| At least 2 y | 35 | 20 | 38 | 62.9 | 23.9 | |
| Stage at transformation | ||||||
| I-IIA | 2 | 0 | — | — | — | * |
| IIB | 24 | 13 | 48 | 69.3 | 26.9 | |
| III | 1 | 1 | — | — | — | |
| IV | 18 | 12 | 30 | 52.5 | 10.6 | |
| Transformation staging | ||||||
| T2 | 5 | 3 | 7 | — | — | .24 |
| T3 | 40 | 23 | 36 | 62.3 | 22.5 | |
| Extracutaneous spreading | ||||||
| No | 24 | 11 | 48 | 74.1 | 32.0 | .02 |
| Yes | 20 | 15 | 22 | 45.7 | 7.8 | |
| Percentage large T-cells | ||||||
| 25%-49% | 7 | 3 | 15 | 44.4 | — | .47 |
| 50%-79% | 19 | 12 | 38 | 66.3 | 10.1 | |
| 80%-100% | 19 | 11 | 33 | 61.8 | 32.1 | |
| CD30 staining | ||||||
| None | 31 | 20 | 30 | 54.3 | 14.1 | .14 |
| Positivity at fewer than 75% | 7 | 3 | 60 | 85.7 | 28.6 | |
| Positivity at 75% or more | 7 | 3 | 38 | 66.6 | 44.4 | |
| CD20 staining† | ||||||
| Negative | 22 | 16 | 30 | 47.4 | 13.9 | .27 |
| Positive | 18 | 8 | 38 | 75.3 | 27.8 |
| Grouping . | No. of Patients . | No. of Death . | Median Survival (mo) . | % Patients Alive at 2 Yr (95% CI) . | % Patients Alive at 5 Yr (95% CI) . | P . |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Men | 26 | 16 | 30 | 52.5 | 18.9 | .09 |
| Women | 19 | 10 | 48 | 72.2 | 27.1 | |
| Age | ||||||
| Younger than 60 y | 14 | 6 | 36 | 69.8 | 46.6 | .09 |
| At least 60 y | 31 | 20 | 30 | 56.5 | 6.8 | |
| Interval from MF diagnosis to transformation | ||||||
| Less than 2 y | 10 | 6 | 30 | 51.4 | — | .31 |
| At least 2 y | 35 | 20 | 38 | 62.9 | 23.9 | |
| Stage at transformation | ||||||
| I-IIA | 2 | 0 | — | — | — | * |
| IIB | 24 | 13 | 48 | 69.3 | 26.9 | |
| III | 1 | 1 | — | — | — | |
| IV | 18 | 12 | 30 | 52.5 | 10.6 | |
| Transformation staging | ||||||
| T2 | 5 | 3 | 7 | — | — | .24 |
| T3 | 40 | 23 | 36 | 62.3 | 22.5 | |
| Extracutaneous spreading | ||||||
| No | 24 | 11 | 48 | 74.1 | 32.0 | .02 |
| Yes | 20 | 15 | 22 | 45.7 | 7.8 | |
| Percentage large T-cells | ||||||
| 25%-49% | 7 | 3 | 15 | 44.4 | — | .47 |
| 50%-79% | 19 | 12 | 38 | 66.3 | 10.1 | |
| 80%-100% | 19 | 11 | 33 | 61.8 | 32.1 | |
| CD30 staining | ||||||
| None | 31 | 20 | 30 | 54.3 | 14.1 | .14 |
| Positivity at fewer than 75% | 7 | 3 | 60 | 85.7 | 28.6 | |
| Positivity at 75% or more | 7 | 3 | 38 | 66.6 | 44.4 | |
| CD20 staining† | ||||||
| Negative | 22 | 16 | 30 | 47.4 | 13.9 | .27 |
| Positive | 18 | 8 | 38 | 75.3 | 27.8 |
CI = confidence interval.
P = .40 for I-IIA versus IIB-IV. P = .07 for IIB versus IV.
Only 40 of 45 cases tested.
Statistical analysis.
(A) Overall survival of patients since MF transformation. (B) Survival of patients with and without extracutaneous spreading. (C) Survival of patients with transformed MF compared with that of 15 patients with pleomorphic large T-cell lymphoma since MF transformation.
Statistical analysis.
(A) Overall survival of patients since MF transformation. (B) Survival of patients with and without extracutaneous spreading. (C) Survival of patients with transformed MF compared with that of 15 patients with pleomorphic large T-cell lymphoma since MF transformation.
When variables were considered together in multivariate analysis (Table3), both age of at least 60 years and extracutaneous spreading (clinical stage IV) at the time of transformation were found to be associated with a poor prognosis.
Multivariate analysis of prognostic factors for survival in 44 patients with T-MF
| Characteristics . | Univariate Analysis . | Multivariate Analysis (final model) . | ||
|---|---|---|---|---|
| HR . | P . | HR (95% CI) . | P . | |
| Age | ||||
| Younger than 60 y | 1.00 | 1.00 | ||
| At least 60 y | 2.10 | .11 | 2.81 (1.03-7.61) | .04 |
| Sex | ||||
| Male | 1.00 | |||
| Female | 0.51 | .11 | ||
| Clinical stage | ||||
| IIb | 1.00 | 1.00 | ||
| IV | 1.96 | .10 | 2.22 (0.99-4.98) | .05 |
| Stage of skin disease | ||||
| T2 | 1.00 | |||
| T3 | 0.49 | .26 | ||
| Delay MF/T-MF | ||||
| Less than 2 y | 1.00 | |||
| At least 2 y | 0.62 | .33 | ||
| Extracutaneous spreading (follow-up) | ||||
| Yes | 1.00 | |||
| No | 2.49 | .02 | ||
| % of large cells | ||||
| 25%-49% | 1.00 | |||
| 50%-79% | 0.51 | .32 | ||
| 80%-100% | 0.45 | .25 | ||
| CD30 expression | ||||
| No staining | 1.00 | |||
| Positivity at less than 75% | 0.39 | .14 | ||
| Positivity at 75% or more | 0.43 | .18 | ||
| Characteristics . | Univariate Analysis . | Multivariate Analysis (final model) . | ||
|---|---|---|---|---|
| HR . | P . | HR (95% CI) . | P . | |
| Age | ||||
| Younger than 60 y | 1.00 | 1.00 | ||
| At least 60 y | 2.10 | .11 | 2.81 (1.03-7.61) | .04 |
| Sex | ||||
| Male | 1.00 | |||
| Female | 0.51 | .11 | ||
| Clinical stage | ||||
| IIb | 1.00 | 1.00 | ||
| IV | 1.96 | .10 | 2.22 (0.99-4.98) | .05 |
| Stage of skin disease | ||||
| T2 | 1.00 | |||
| T3 | 0.49 | .26 | ||
| Delay MF/T-MF | ||||
| Less than 2 y | 1.00 | |||
| At least 2 y | 0.62 | .33 | ||
| Extracutaneous spreading (follow-up) | ||||
| Yes | 1.00 | |||
| No | 2.49 | .02 | ||
| % of large cells | ||||
| 25%-49% | 1.00 | |||
| 50%-79% | 0.51 | .32 | ||
| 80%-100% | 0.45 | .25 | ||
| CD30 expression | ||||
| No staining | 1.00 | |||
| Positivity at less than 75% | 0.39 | .14 | ||
| Positivity at 75% or more | 0.43 | .18 | ||
HR = hazard ratio; CI = confidence interval.
Compared with the series of 15 patients with CD30 negative pleomorphic large T-cell cutaneous lymphoma (without diagnosis of MF) registered in the group database,7 no difference in survival was found with our cohort (5-year survival 20.8%, versus 21%;P = .97; Figure 2C). Moreover, there was a worse prognosis for CD30 positive (more than 75% of positive tumoral cells) transformed MF (5-year survival, 44.4%) than for CD30 positive large T-cell cutaneous lymphoma without MF (registered in the group database7; 5-year survival, 87%). But this difference did not reach a statistically significant level (P = .61).
Discussion
The usual good prognosis of mycosis fungoides (MF) may be altered by transformation into large T-cell lymphoma (T-MF).1 But the incidence of this transformation is highly variable between series, ranging from 8%8 to 55%9 of patients with MF, pointing the need of a strict clinicopathological definition. Dmitrovsky et al classified MF as transformed if there were more than 50% of large cells.8 On the other hand, Cerroni et al evaluated transformation only at a tumor stage; so the probability to find cytologic transformation was higher.9 The criteria for T-MF are now better defined with at least 1 skin biopsy showing large cells (≥ 4 times the size of a small lymphocyte) exceeding 25% of the infiltrate throughout or forming microscopic nodules.2Our study confirms the bad prognosis of patients with 25% to 50% of large cells and confirms that no difference was found according to the percentage of large cells above 25%. However, the histologic diagnosis must avoid 3 main pitfalls.
First, it is difficult to differentiate histiocyte macrophages from large T-lymphocytes if immunohistochemistry with (CD3 and CD68 staining) is not performed. The prognosis of “histiocyte-rich MF” or “granulomatous MF” is better than T-MF and similar to that of MF without transformation.10-12 Clinically, the tumor may be similar in “granulomatous MF” and in “T-MF” (as in 1 of our patients who simultaneously presented both lesions). Eleven of the patients initially diagnosed with T-MF were excluded after immunohistochemical study. Moreover, the good prognosis of patients with “histiocytic-rich MF” confirmed the clinical relevance of using CD68 staining. Among histiocytic markers, the most routinely used is the lysosome-associated protein CD68.13 Many monoclonal antibodies allow the detection of CD68 antigen in paraffin-embedded tissue. But the KP1 staining of certain human peripheral blood T-cells led us to use PG-M1 as an alternative anti-CD68 antibody.14 15
Second, T-MF (with CD30 positive cells) may be difficult to differentiate from MF associated with a CD30+ lymphoproliferative disorder (such as lymphomatoid papulosis or CD30+ large-cell cutaneous lymphoma). The prognosis of CD30 lymphoproliferative disorders is quite good (87% to 100% 5-year survival for patients with primary cutaneous CD30+ large cells lymphoma) contrary to T-MF, even if large cells are CD30 positive (44.4% 5-year survival for our patients with more than 75% of CD30 positive large cells).7 16 This differential diagnosis is obvious if the 2 types of cutaneous lesions are clinically distinct but may raise several problems in clinical practice. In our study 3 patients with MF and CD30+ lymphoma were excluded because the 2 types of lesions were quite different. But another patient who presented MF associated with a tumor histologically suggestive of CD30+ large cell cutaneous lymphoma was included after review because both lesions (MF and CD30+ lymphoma) were intermingled at the same localization. This patient, however, had the best prognosis in our study (alive after 12 years). The diagnosis of CD30+ T-MF is “certain” if (1) clinical transformation occurs on a MF lesion (such as patches) and (2) if cellular pleomorphism is observed with cerebriform T lymphocytes mixed with fewer than 75% of CD30 positive large T-cells. When more than 75% of large cells are CD30+, it is very difficult to distinguish these 2 entities. Only the patients' evolution may reveal the difference between CD30 positive T-MF and MF associated with primary CD30+ large cell lymphoma.
The third histological pitfall in the diagnosis of T-MF is the presence of B-lymphocytes. Such clusters of B-lymphocytes were previously reported,9 raising the question of whether these CD20 positive cells are reactive or tumoral. In our cases, CD20 antigen was expressed not only by small lymphocytes but also by large tumoral cells. Moreover, the combined analysis of CD3 and CD20 staining showed that some lymphomatous cells may co-express both antigens (data not shown). Therefore, we hypothesize that some T-lymphomatous cells in transformed-MF may have a CD20 phenotype, as reported in other rare T-cell lymphomas.17-19
The molecular mechanisms by which some MF undergo transformation are to date undefined. Contrary to our finding, Li et al found overexpression of p53 protein in T-MF, but this expression was not correlated with p53 gene mutation.20 The t(2;5)(p23;q35) chromosomal translocation reported in CD30 + anaplastic large-cell lymphoma is not involved in the molecular pathogenesis of MF transformation.21 Another deletion (codeletion of CDKN2 and MTAP genes) reported in extracutaneous transformed large cell lymphoma could be implicated in MF transformation.22
The bad prognosis of T-MF was emphasized by Diadamandidou et al: median survival from initial diagnosis of MF is 37 months for patients with T-MF instead of 163 months for patients with untransformed MF.1 Indeed, in our study, the prognosis of T-MF was closely akin to that of pleomorphic large-T cell CD30 negative cutaneous lymphoma. Median survival time from transformation was from 2 months8 to 36 months in our study (19.4 months for the recent study of Diamandinou1, 27 months for Greer et al23). Statistical studies have shown that the main poor prognostic factor is an extracutaneous progression (as observed in MF).1,24,25 Moreover, an initial extracutaneous transformation is associated with a worse prognosis than an initial cutaneous transformation.26 Contrary to our study, Diamandinou found that advanced stage (IIB-IV versus I-IIA) at the time of transformation had a bad prognostic value. This discrepancy is probably due to the fact that almost all of our patients (43 of 45) had an advanced stage IIB-IV at the time of transformation. We only found a predictive value of stage IV compared with stage IIB at the time of transformation, thus reflecting the role of extracutaneous involvement. Age was not previously found as a prognostic factor.1Indeed, age was not statistically significant in our univariate analysis but became a prognostic factor when variables were considered together in multivariate analysis. There was a trend for a worse prognosis in patients presenting an early transformation (< 2 years from diagnosis of MF), but this did not reach a statistically significant level, unlike in the report of Diamandinou et al.1
An important question to determine is the respective prognostic value of tumoral stage (T3) and cytologic transformation. Six of our patients presented an histologic transformation before clinical progression; for the others, tumoral progression was associated with transformation. In the absence of clinical progression, biopsies are not systematically performed. Therefore, it is not possible to retrospectively analyze the role of cytologic transformation. Moreover, survival of MF at tumoral (T3) stage has been analyzed without evaluation of cytologic transformation in most series.24,27 However, 5-year survival of all MF patients at T3 stage was found by others to be better (45%) than that of our T-MF with T3 stage (22.5%).27 Only 2 reports have analyzed the impact of cytologic transformation on tumoral stage. Diamandinou et al have compared the median survival of transformed tumor stage (T3) patients (n = 13) to that of nontransformed ones (n = 15) and found a trend for a worse prognosis (24.5 versus 35 months).1,28 Cerroni et al demonstrated that survival rates after tumor onset did not significantly differ between nontransformed and transformed tumoral MF (5-year survival of 23%, versus 11.2%), but overall survival from initial diagnosis of MF was worse for the latter (10-year survival rate 11.2%, versus 46.6%; P < .05).9 Another argument for the prognostic significance of cytologic transformation is that median survival was not statistically different between our patients with generalized (T2) plaque disease or with tumor (T3) stage. Therefore, our study suggests that an earlier histological diagnosis of T-MF could be performed before clinical tumor transformation. The impact on survival of such an earlier histological diagnosis should be confirmed by prospective studies of MF patients, with regular systematic biopsies (whenever there are no signs of clinical progression) in order to determine if histological diagnosis of T-MF before clinical progression may be relevant for therapeutic strategies.
Supported by a Programme Hospitalier de Recherche Clinique, CHU Bordeaux, and by the Region Aquitaine.
Reprints:B. Vergier, EA 2406 Equipe Histologie et Pathologie du Système Immunitaire, BP 8, Université de Bordeaux 2, 33076 Bordeaux, France; e-mail:beatrice.vergier@chu-aquitaine.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal