Abstract
Dendritic cells (DCs) are critical subsets of leukocytes providing antigen presentation for initiation of humoral and cellular immune responses. Their role as effector cells in tumor resistance, however, is less known. We report here that human DCs generated by culturing plastic-adherent peripheral blood monocytes in the presence of granulocyte-monocyte colony–stimulating factor (GM-CSF) and interleukin-4 have potent growth-inhibition activity in vitro on a wide spectrum of human tumor lines of different tissue origin. Proinflammatory stimuli lipopolysaccharide (LPS) and interferon-γ, but not tumor necrosis factor– and CD40 signaling, can further enhance DC-mediated inhibition of tumor growth. The growth inhibition requires contact between DCs and tumor cells while LPS treatment enhances the antitumor activity in DC culture supernatants. Our results suggest that in addition to their predominant role as regulatory cells, activated DCs are also potential effector cells in tumor immunity.
Dendritic cells (DCs) are specialized leukocytes for presenting antigens to quiescent, naive, and memory T cells, and they play pivotal roles in the induction of cell-mediated as well as humoral immune responses in vivo.1 The exceptional ability of DCs to stimulate T cells in vitro and in vivo is attributed, at least in part, to their ability to capture antigen, to migrate into lymphoid organs, and to express high levels of immunostimulatory molecules, such as major histocompatibility complex (MHC) class II, B7-1, B7-2, and interleukin (IL)–12.1,2 Immature DCs, such as Langerhans cells of the skin, capture and process antigens very efficiently.3 Upon exposure to various microbial and inflammatory products (eg, lipopolysaccharide [LPS], IL-1, tumor necrosis factor [TNF]–α), DCs mature and migrate into lymphoid tissues to interact with T and B cells.4-7 Owing to their unique capacity to activate naive T cells, DCs loaded with antigens in the form of peptides, cell lysates, or RNA have been used for active immunization to induce cell-mediated immune response against cancer.8-11 In several experimental models, it was shown that injection of DCs loaded with tumor antigens resulted in enhancement of T-cell activation and, in some cases, regression of established tumors and a significant prolongation of survival.12-14 Preliminary results from clinical trials using DCs pulsed with peptide from tumor antigen for treatment of cancer patients also showed promising results.15 16
While there is convincing evidence that DCs can execute their antitumor effect by stimulating tumor-specific T lymphocytes,9 other mechanisms may also play a role. It has been shown that DCs can produce TNF-α and express membrane FasL17,18 and a high level of nitric oxide synthase,19,20 suggesting that DCs may participate in the innate defense against infectious agents and probably malignancies. DCs can infiltrate human solid tumors,21-23 but their T-cell stimulatory ability in tumors is often suppressed.24,25 However, the density of DC infiltration may be associated with enhanced patient survival of bronchioalveollar squamous cell carcinoma.22 In addition, several studies in mouse tumor models indicate that infusion of DCs without loading specific antigens can also decrease growth of tumors to a certain degree.7,26,27 Similarly, increased responses to DC therapy were observed in a phase I clinical trial of prostate cancer where patients were treated with autologous DCs pulsed with HLA-mismatched epitope peptide from prostate-specific membrane antigens.28 Taken together, these observations suggest that DCs may inhibit tumor growth by mechanisms other than T-cell activation. One alternative mechanism was illustrated by a recent report showing that natural killer cell receptor–protein 1-positive (NKR-P1+) rat spleen DCs lysed the NK-sensitive YAC-1 tumor cell line in vitro.29
We have investigated the effector function of DCs derived from human peripheral blood monocytes (PBMCs). We demonstrated that cultured DCs from the majority of healthy donors exert potent inhibitory activity on the growth of human tumor lines in vitro. Our studies, therefore, indicate a new mechanism and provide an additional explanation for DC-mediated antitumor function.
Materials and methods
Cell lines
The following human cell lines were originally purchased from ATCC (Manassas, VA): Jurkat (acute T-cell leukemia), THP-1 (acute monocytic leukemia), Molt4 (acute lymphoblastic leukemia), U937 (histocytic lymphoma), K562 (chronic myelogenous leukemia), 293 (adenovirus-transformed kidney epithelial cells), and Raji (Burkitt lymphoma), HL60 (promyelocytic leukemia), SW620 (colon adenocarcinoma), HT29 (colon adenocarcinoma), HuTu80 (duodenum adenocarcinoma), WiDr (colon adenocarcinoma). LCL, a B-lymphoid line immortalized by Epstein-Barr virus, was established in this laboratory. The cell-culture medium used throughout was RPMI 1640 (Life Technologies, Grand Island, NE), supplemented with 10% FBS (HyClone, Logan, UT), 2 mmol/L L-glutamine (Life Technologies), 100 U/mL penicillin (Life Technologies), 100 μg/mL streptomycin (Life Technologies), and 5 × 10−3 mol/L 2-mercaptoethanol (VWR, Chicago, IL).
Generation of dendritic cells
The method for the generation of DCs from PBMCs has been previously described.30 Briefly, PBMCs were isolated from buffy coats of healthy donors by density centrifugation on Ficoll-Paque PLUS (Amersham Pharmacia Biotech, Uppsala, Sweden) for 20 minutes at 400g at room temperature. After 2 washes, cells were resuspended in the medium at a final concentration of 2 × 106 cells/mL and incubated in 100-mm tissue culture dish (Becton Dickinson, Franklin Lakes, NJ) for 2 hours. Nonadherent cells were gently washed out with warm medium. The remaining plastic-adherent cells were cultured in 5% CO2at 37°C with 800 U/mL of recombinant human granulocyte-monocyte colony–stimulating factor (GM-CSF) (Leukine, Immunex, Seattle, WA) and 5 ng/mL of recombinant human IL-4 (Bio Source International, Camarillo, CA). After 5 to 7 days of culture, nonadherent cells were harvested and used in experiments. The purity of the DC population was checked by fluorescence-activated cell sorter (FACS) analysis.
Tumor growth inhibition (tumoristasis) assay
DCs (5 × 104 cells/well) were cultured in 96-well plates (Corning, Corning, NY) with medium alone or in the presence of LPS (Sigma, St Louis, MO), recombinant human TNF-α (R&D Systems, Minneapolis, MN), interferon (IFN)–γ (R&D Systems), or anti-CD40 monoclonal antibody (mAb) (ATCC clone G28-5). At 24 hours, 100 μL of medium was withdrawn, and tumor cells (104/100 μL/well) were added to the wells. Plates were incubated (5% CO2, 37°C) for 24 hours and for an additional 24 hours in the presence of 1 μCi/well of3H-TdR, (NEN, Boston, MA). 3H-TdR incorporation was measured by means of a liquid scintillation counter (Wallac, Gaithersburg, MD). The data were presented as the percentage of inhibition calculated from the following formula: % inhibition = (1-test cpm/control cpm) × 100%, where test cpm is 3H-TdR incorporation by tumor cells cultured with DCs after various stimulations, and control CPM is 3H-TdR incorporation by tumor cells cultured without DCs. DCs with or without various stimuli did not incorporate a significant amount of3H-TdR (less than 1500 cpm) while tumor cells usually produced 20 000 to 150 000 cpm, depending on the tumor line.
51Cr-release assay
The lytic activity of DCs was measured in a standard 4-hour51Cr-release assay.31 Tumor cells (3 × 106) were labeled with Na251CrO4 (NEN) and cocultured with DCs at various effector-to-target ratios for 10 hours. The radioactivity of supernatants was measured in a γ counter (Wallac). Spontaneous release of 51Cr (incubation of target cells with media alone) was less than 10% of maximum.
Transwell cultures
The transwell chambers with 0.45 μm pore size membrane (Millipore, Bedford, MA) were used to physically separate DCs and tumor cells. DCs at 5 × 105 cells/well were incubated with or without LPS (5 μg/mL) for 24 hours while tumors at 1 × 105 cells/well were placed in the inner transwell chamber. Upon being cultured for 24 hours and pulsed with3H-TdR (10 μCi/well) for an additional 24 hours, tumor cells were brought to the suspension, and 3H-TdR incorporation was measured and calculated as described in the tumor growth–inhibition assay.
Morphological cell analysis
Adherent PBMCs cultured for 7 days with GM-CSF and IL-4 were centrifuged onto microscope slides by means of a Cytospin 2 centrifuge (Shandon Inc, Pittsburgh, PA), stained with Wright-Giemsa solution, and analyzed by light microscope (Olympus, Tokyo, Japan). Photographs were taken with an Olympus DP10 digital camera.
Flow cytometry
FACS analysis of DC surface markers was performed as described elsewhere.32 Briefly, DCs were prepared as described previously, stained with fluorescein isothiocyanate–labeled mAb specific for human CD3, CD4, CD8, CD14, CD16, CD19, CD80, CD86, and HLA-DR (Pharmingen, San Diego, CA), and analyzed by means of FACSCaliber flow cytometry (Becton Dickinson, Mountain View, CA) and CELLQuest software.
Statistics
Data were analyzed by the Student t test (SigmaPlot), whereby a P of less than .05 indicated that the value of the test sample was significantly different from that of the relevant controls.
Results
Growth-inhibition effect of dendritic cells on human tumor lines in vitro
DCs were generated from adherent human PBMCs of healthy donors in the presence of GM-CSF and IL-4. The majority of cells in the culture at day 7 showed morphology of DC-like cells (Figure1) with the veils and dendritic processes.1 2 Examination of surface markers of the DC-like cells by FACS analysis with specific mAb demonstrates the expression of HLA-DR on 99% of cells, as well as moderate levels of costimulatory molecule CD80 (greater than 65%) and CD86 (greater than 35%). Fewer than 5% of cells were stained with antibodies to CD14, CD16, and CD19, indicating a low content of monocytes/macrophages and B cells, respectively. Approximately half of the DCs expressed CD4, but the majority of the cells did not express CD3 or CD8 T-cell markers (Figure2). Increased concentrations of IL-4 up to 50 ng/mL did not significantly change morphology or the levels of CD80 and CD86 expression on DCs (data not shown). Thus, our DC preparation represents a population of immature DCs with a low content of macrophages, B cells, and T cells.
Morphology of human PBMC-derived DCs.
Representative photographs of Wright-Giemsa–stained cytospins of adherent PBMCs from healthy donors cultured for 7 days in the presence of GM-CSF and IL-4 are presented. (A) ×400. (B) ×1000.
Morphology of human PBMC-derived DCs.
Representative photographs of Wright-Giemsa–stained cytospins of adherent PBMCs from healthy donors cultured for 7 days in the presence of GM-CSF and IL-4 are presented. (A) ×400. (B) ×1000.
The phenotype of human PBMC-derived DCs.
DCs were generated by culturing adherent PBMCs of healthy donors for 7 days in the presence of GM-CSF and IL-4. Expression of the indicated antigens was analyzed by means of 1 color staining. Dead cells were excluded by gating out propidium-iodide–positive cells. Similar results were obtained by staining DCs from 3 unrelated donors.
The phenotype of human PBMC-derived DCs.
DCs were generated by culturing adherent PBMCs of healthy donors for 7 days in the presence of GM-CSF and IL-4. Expression of the indicated antigens was analyzed by means of 1 color staining. Dead cells were excluded by gating out propidium-iodide–positive cells. Similar results were obtained by staining DCs from 3 unrelated donors.
To examine whether the DCs described above can affect the growth of tumor cells, we performed a 48-hour growth-inhibition assay by coculturing DCs with a panel of tumor cell lines. DCs exhibit significant growth-inhibition effects on 8 out of 13 tested tumor lines. These lines include tumors of hematopoietic origin, THP-1 and K562, and of epithelium origin, 293, SW620, HuTu80 and WiDr (Figure3A, 3B). Inclusion of 5 μg/mL of LPS in the cultures increased the antitumor effect (Figure 3A, 3B). The growth-inhibition effect of DCs could be observed at an effector-to-target (E-to-T) ratio as low as 1:1 for many tumor lines (data not shown). It is of interest that the DCs do not inhibit the growth of 2 B-lymphocyte origin lines, Raji and LCL, as well as HL60, even in the presence of LPS (Figure 3A). During our experiments, a notable variation in the antitumor activity of DC was observed among tested donors. Nevertheless, LPS-stimulated DC from these donors were tumoristatic against most tested tumor cell lines.
DC-mediated growth inhibition of human tumor lines in vitro.
DCs at 5 × 104 cells/well were cultured with or without 5 μg/mL of LPS in 96-well flat-bottom plates. At 24 hours, 100 μL of culture medium was removed, and indicated human tumor lines at 1 × 104cells were added to the wells. The cultures were pulsed with3H-TdR for the last 24 hours the DCs were cultured with tumor cells. The results are presented as the percentage of inhibition of tumor-cell proliferation. The results are presented as the mean ± SD of triplicate wells. 3H-TdR incorporation into DC was less than 1500 cpm; 2 (A and B) of 5 representative donors are shown. *P < .05 compared with DCs cultured with medium.
DC-mediated growth inhibition of human tumor lines in vitro.
DCs at 5 × 104 cells/well were cultured with or without 5 μg/mL of LPS in 96-well flat-bottom plates. At 24 hours, 100 μL of culture medium was removed, and indicated human tumor lines at 1 × 104cells were added to the wells. The cultures were pulsed with3H-TdR for the last 24 hours the DCs were cultured with tumor cells. The results are presented as the percentage of inhibition of tumor-cell proliferation. The results are presented as the mean ± SD of triplicate wells. 3H-TdR incorporation into DC was less than 1500 cpm; 2 (A and B) of 5 representative donors are shown. *P < .05 compared with DCs cultured with medium.
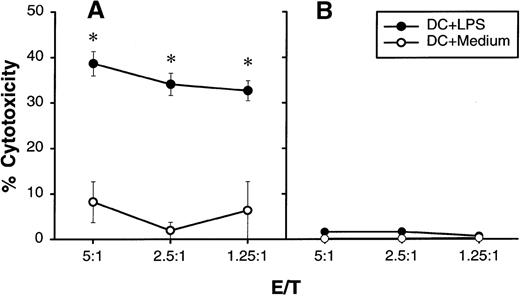
Recently it has been reported that rat DCs are cytolytic for natural-killer-cell–sensitive YAC-1 target cells,29suggesting that rat DCs may possess the machinery of cytotoxicity. In a preliminary experiment, we found that several tumor lines tested are not sensitive to human DCs in a standard 4-hour 51Cr release assay (data not shown). However, if the duration of LPS-stimulated DC and tumor-cell incubation was extended to 10 hours, U937 histocytic lymphoma could be lysed significantly whereas an NK-sensitive K562 line remained resistant (Figure4). In addition, propidium iodide staining and light microscopy analysis revealed no increase in cell death when LPS-activated DCs or their supernatants were cultured with various tumor targets for 48 hours, with the exception of U937 lymphoma, where massive DC-induced cell death was observed (data not shown). We concluded that human DCs are cytostatic rather than cytolytic for human tumor cells.
Cytolytic activity of human DCs on U937 histocytic lymphoma.
DCs were cultured for 24 hours with or without 5 μg/mL of LPS in a flat-bottom 96-well plate. After extensive washing,51Cr-labeled U937 (A) or K562 (B) at 1 × 104 cells/well was added into wells containing different numbers of DCs (shown as E/T ratio). Release of51Cr from target cells was measured 10 hours later. The results are presented as the mean ± SD of triplicate wells. The results of 2 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
Cytolytic activity of human DCs on U937 histocytic lymphoma.
DCs were cultured for 24 hours with or without 5 μg/mL of LPS in a flat-bottom 96-well plate. After extensive washing,51Cr-labeled U937 (A) or K562 (B) at 1 × 104 cells/well was added into wells containing different numbers of DCs (shown as E/T ratio). Release of51Cr from target cells was measured 10 hours later. The results are presented as the mean ± SD of triplicate wells. The results of 2 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
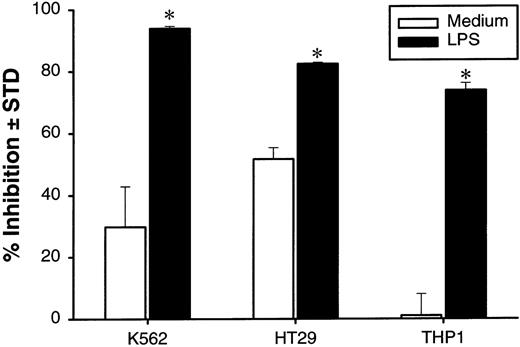
In the above assays, both DCs and tumor cells were in direct contact with LPS, and it is possible that LPS could directly affect the growth of tumor cells. In fact, we have found that LPS can partially decrease the 3H-TdR incorporation into 2 monocytic tumor lines, THP-1 and U937, but not the other tumor lines (data not shown). To exclude the direct effect of LPS on proliferation of tumor cells, the DCs were preincubated with LPS for 24 hours. After extensive washing with PBS, DCs were cocultured with target cells for 48 hours, and the growth-inhibition effect of the DCs was measured. Incubation with LPS for 24 hours is sufficient to induce growth-inhibition activity of DCs, and LPS is not required for the effector phase (Figure5). The addition of polymixin B, antibiotics that bind and neutralize activity of LPS,33into the culture at the effector stage did not abolish the tumoristatic function of LPS-stimulated DCs (data not shown). Taken together, our results indicate that activated DCs can inhibit the growth of a broad spectrum of human tumor cells despite their limited ability to lyse them directly.
Tumoristatic activity of human DCs pretreated with LPS.
DCs at 2 × 106 cells/mL were cultured in polypropylene tubes with or without LPS at 5 μg/mL. At 24 hours, DCs were washed 3 times and plated with indicated tumor cells at 1 × 104 cells/well in 96-well plates at an E-to-T ratio of 5:1. The cultures were pulsed with3H-TdR. Proliferation was measured at 48 hours after addition of tumor cells. The results are presented as the mean ± SD of triplicate wells. The results of 2 representative experiments using DC from different donors is shown. *P < .05 compared with DCs cultured with medium.
Tumoristatic activity of human DCs pretreated with LPS.
DCs at 2 × 106 cells/mL were cultured in polypropylene tubes with or without LPS at 5 μg/mL. At 24 hours, DCs were washed 3 times and plated with indicated tumor cells at 1 × 104 cells/well in 96-well plates at an E-to-T ratio of 5:1. The cultures were pulsed with3H-TdR. Proliferation was measured at 48 hours after addition of tumor cells. The results are presented as the mean ± SD of triplicate wells. The results of 2 representative experiments using DC from different donors is shown. *P < .05 compared with DCs cultured with medium.
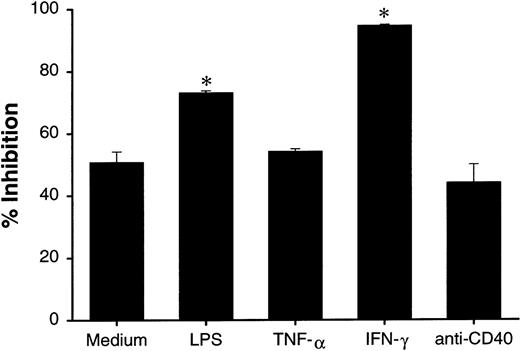
Increased growth-inhibition activity of dendritic cells by proinflammatory stimuli is not associated with their maturation status
Since LPS can induce maturation of DCs, it is possible that mature DCs are more active than immature DCs in growth inhibition of tumor cells. In addition to LPS, it has been reported that TNF-α, IFN-γ, and CD40 signaling are among the most potent maturating factors for DC.4-7 We examined whether these factors can further increase the growth-inhibition effect of DCs. Under microscope, the majority of cultured DCs in the presence of LPS and anti-CD40 mAb showed increased adherence to plastic plate and formation of cell clusters, suggesting matured DC morphology. Pretreatment of DCs by LPS and IFN-γ resulted in the increased growth inhibition of HT29 cells, whereas TNF-α or anti-CD40 mAb treatment did not change the activity (Figure 6). Our results suggest that the growth-inhibition effect of DCs is not associated with their maturation status.
Effect of maturation induction in DC-mediated growth inhibition.
DCs (2 × 106 cells/mL) were cultured in polypropylene tubes in the presence of medium, LPS (5 μg/mL), TNF-α (100 ng/mL), IFN-γ (1000 U/mL), or anti-CD40 mAb (10 μg/mL). At 24 hours, DCs were washed 3 times and cultured with HT29 (1 × 104 cells/well) in 96-well plates at an E-to-T ratio of 5:1. Proliferation was measured 48 hours after the start of the incubation of DCs with tumor targets. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors is shown. *P < .05 compared with DCs cultured with medium.
Effect of maturation induction in DC-mediated growth inhibition.
DCs (2 × 106 cells/mL) were cultured in polypropylene tubes in the presence of medium, LPS (5 μg/mL), TNF-α (100 ng/mL), IFN-γ (1000 U/mL), or anti-CD40 mAb (10 μg/mL). At 24 hours, DCs were washed 3 times and cultured with HT29 (1 × 104 cells/well) in 96-well plates at an E-to-T ratio of 5:1. Proliferation was measured 48 hours after the start of the incubation of DCs with tumor targets. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors is shown. *P < .05 compared with DCs cultured with medium.
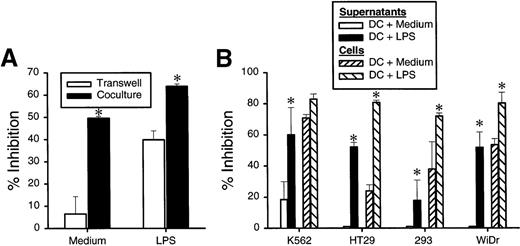
Dendritic-cell–mediated growth inhibition requires dendritic-cell–tumor-cell interaction
To determine the mechanism of the DC-mediated growth inhibition, we employed a transwell cell culture system, in which the DCs were cultured in an outer well separated by a membrane of 0.45 μm pore size from the tumor cells plated in an inner well. As shown in Figure7A, separation of DCs and HT29 cells diminished the growth inhibition whereas strong growth-inhibition activity remained if the DCs and tumor cells were in contact. These results indicate that tumor growth inhibition mediated by DCs is dependent on DC-tumor contact. In contrast, if LPS was included in the cultures, significant growth inhibition was still observed in the transwell. This result suggests that LPS-induced enhancement is mediated, at least in part, by soluble factors secreted from DCs. To directly test this possibility, the supernatants were collected at 24 hours from the DCs cultured in the presence or absence of LPS and were tested for their growth-inhibition activity on tumor cells. As shown in Figure 6B, supernatant from LPS-treated DCs had a significant inhibitory effect on the growth of K562, HT29, 293, and WiDr tumor lines (Figure 7B). These studies demonstrated that DC-tumor contact is required for inhibition of tumor-cell growth while LPS can further increase the activity by stimulating DCs to secrete growth inhibitors.
DC-mediated growth inhibition is mediated by DC-tumor contact while enhancement effect of LPS is caused mainly by soluble factors.
(A) DCs at 0.5 × 106 cell/well were incubated in 24-well plates with or without LPS. At 24 hours, HT29 cells (0.1 × 106 cells/well) were added to either outer well (with DCs) or inner well separated from DC by a membrane with 0.45 μm pore size. (B) DCs were cultured without or without 5 μg/mL of LPS in a flat-bottom 96-well plate. At 24 hours, 100 μL of undiluted supernatants was transferred to the well with indicated tumor cells at 1 × 104 cells/well. The plates were further incubated for 48 hours, and cell proliferation was measured by3H-TdR incorporation. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
DC-mediated growth inhibition is mediated by DC-tumor contact while enhancement effect of LPS is caused mainly by soluble factors.
(A) DCs at 0.5 × 106 cell/well were incubated in 24-well plates with or without LPS. At 24 hours, HT29 cells (0.1 × 106 cells/well) were added to either outer well (with DCs) or inner well separated from DC by a membrane with 0.45 μm pore size. (B) DCs were cultured without or without 5 μg/mL of LPS in a flat-bottom 96-well plate. At 24 hours, 100 μL of undiluted supernatants was transferred to the well with indicated tumor cells at 1 × 104 cells/well. The plates were further incubated for 48 hours, and cell proliferation was measured by3H-TdR incorporation. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
It has been reported that LPS induces DCs to release TNF-α, which can inhibit the growth of some tumor cells.17 To exclude this possibility, we first determined whether inclusion of anti–TNF-α antibody can neutralize the growth-inhibition effect of supernatant from LPS-treated DCs on HT29 cells. As shown in Figure8A, the activity of the supernatant was not inhibited by anti–TNF-α antibody in a concentration sufficient to neutralize more than 100 ng/mL of TNF (data not shown). This result demonstrates that the growth-inhibition effect of the supernatant from LPS-stimulated DC is not mediated by TNF-α. However, inclusion of anti–TNF-α antibody in DC tumor-cell cultures without transwell separation decreased approximately 30% of the growth-inhibition effect of the DCs on HT29 cells (Figure 8B), suggesting that TNF-α partially contributes to the inhibitory effect mediated by DC–tumor cell contact.
Effect of anti–TNF- serum on DC-mediated tumor growth inhibition.
DCs were cultured with or without LPS as described. At 24 hours, DC supernatants (A) or DCs themselves (B) were mixed with HT29 tumor cells and cultured in the presence or absence of anti–TNF-α serum at a final dilution of 1 to 400. The plates were further incubated for 48 hours, and tumor cell proliferation was measured by 3H-TdR incorporation. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
Effect of anti–TNF- serum on DC-mediated tumor growth inhibition.
DCs were cultured with or without LPS as described. At 24 hours, DC supernatants (A) or DCs themselves (B) were mixed with HT29 tumor cells and cultured in the presence or absence of anti–TNF-α serum at a final dilution of 1 to 400. The plates were further incubated for 48 hours, and tumor cell proliferation was measured by 3H-TdR incorporation. The results are presented as the mean ± SD of triplicate wells. The results of 3 representative experiments using DCs from different donors are shown. *P < .05 compared with DCs cultured with medium.
Discussion
We demonstrate that DCs derived from human PBMCs can directly inhibit proliferation of various human tumor lines in vitro and that the tumoristatic activity can be further enhanced by proinflammatory stimuli, such as LPS and IFN-γ, but not by TNF-α or CD40 signaling. DC-tumor contact is a prerequisite for the inhibition of tumor growth. Our observation suggests that DCs can be the effector cells in tumor immunity.
In this study, we used DCs derived from adherent human PBMCs by culturing with GM-CSF and IL-4 for 7 days. The results of FACS analysis indicate that DCs used in our experiments were in an immature stage of their differentiation as evidenced by the expression of moderate levels of HLA-DR and costimulatory molecules CD80 and CD86.34,35Coculture of these DCs with a wide spectrum of human tumor lines resulted in a profound decrease of proliferation. The low content of cells expressing T, B, NK, and macrophage markers implies that these cell populations are unlikely to contribute to DC-mediated growth-inhibition effect. The presence of CD4 marker in the DC population was not a surprising finding, since the expression of CD4 molecules on the surface of DCs generated from PBMCs in the presence of GM-CSF and IL-4 was described previously,36-38 but the functional significance of this molecule is not known. It is of interest that the tumor-growth–inhibition effect of DCs was significantly enhanced by LPS treatment since FACS analysis showed that DCs do not express CD14 (Figure 2), a principal LPS receptor.39 This controversy, however, can be explained by a recent finding that Toll-like receptor-2 mediates LPS-induced activation in CD14-negative cells,40 although it is not known whether human DCs express Toll-like receptors. Our results also indicate that the growth-inhibition effect of DCs is not associated with the maturation status of DCs. This conclusion is based on the findings that treatment of DCs by TNF-α and anti-CD40, 2 factors that are among the most potent inducers of DC maturation, failed to enhance the effect, whereas LPS and IFN-γ clearly increase the activity (Figure 5).
The question about the different sensitivity of various tumor cell lines to cytostatic activity of DCs cannot be answered without knowing the exact effector mechanisms of DC-mediated tumor growth inhibition. The fact that DCs did not inhibit and, in some cases, even stimulated proliferation of tumors of B-cell origin as well as HL60 can be explained by complicity of interaction between DCs and B cells. Several recent reports indicate that DCs directly augment the growth and differentiation of CD40-activated B lymphocytes.41,42 On the other hand, it was shown that IL-10 produced by normal and transformed B cells43,44 can down-regulate several functions of DCs.45 Thus, it is possible that cytokines produced by tumors of B-cell origin may block the antitumor function of DCs.
A recent study showed that DCs freshly isolated from rat spleen could effectively lyse mouse NK-sensitive target cells YAC-1, but not NK-resistant P815 cells.29 Our experiments, however, showed that human DCs were not cytolytic to NK-sensitive K562 target cells, but were able to inhibit proliferation of these cells. The tumor inhibition mediated by DCs was significantly abrogated (greater than 80%) by separation of DCs and tumor cells in a transwell (Figure 7A). In addition, the supernatant from DC culture did not transfer this activity (Figure 7B). These results indicate that DC–tumor cell contact is required for the activity. The fact that anti–TNF-α antibody partially decreased this activity (Figure 8A) further implies that membrane-bound, not the soluble form, of TNF-α partially contributes to the effect. It is of interest that the enhancement effect of LPS on the growth-inhibition function of DCs is mediated largely by TNF-α–independent soluble factors (Figures 7B, 8B). Although the nature of these factors is unknown, we have excluded several possible candidates, including Fas-FasL and nitric oxide. Our preliminary studies showed that DCs did not induce apoptosis of Jurkat, THP-1, or K562 cells. In addition, the inclusion of anti-FasL mAb in the cultures did not abrogate the DC-mediated inhibitory effect on Jurkat cells (data not shown). Furthermore, DC-mediated growth inhibition was not affected by inclusion of the nitric-oxide inhibitor L-NMMA46 in the culture (data not shown). The mechanisms of DC-mediated inhibition remain to be studied.
Our findings have important implications in host resistance to cancers. Since immature DCs are widely distributed in most tissues, they may be able to participate in the growth inhibition of tumors, especially metastases. By exploring approaches to further enhancing DC-mediated tumor growth inhibition in situ, we may find ways to strengthen antitumor immunity. Our study suggests that, in addition to their capacity for stimulating adoptive immunity by presenting tumor antigen to T and B cells, DCs could also serve as a component of innate immunity in control of tumor growth in vivo.
Acknowledgments
We thank Dr Svetlana Chapoval for help in morphological analysis of DCs, Dallas Flies for his expert technical assistance, and Kathy Jensen for editing the manuscript.
Supported in part by the Mayo Foundation and by grants from the National Institutes of Health to L.C.
Reprints:Lieping Chen, Department of Immunology, Mayo Clinic, Rochester, MN 55905; e-mail: chen.lieping@mayo.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal