Abstract
Newly diagnosed patients with acute myeloid leukemia (AML) were randomized to receive either 2.5 or 5 μg/kg/day of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) or a placebo administered subcutaneously after completion of chemotherapy. The study evaluated the toxicity of PEG-rHuMGDF and any effect on the duration of thrombocytopenia. Each of 35 patients under 60 years of age received the following therapy: 45 mg/m2 daunorubicin on days 1-3, 100 mg/m2cytarabine (ARA-C) for 7 days, and 2 gm/m2 high-dose ARA-C (HIDAC) for 6 doses on days 8-10. The 22 patients 60 years or older received standard daunorubicin and ARA-C without HIDAC. PEG-rHuMGDF was well tolerated, and no specific toxicities could be attributed to its use. There was no difference in the time to achieve a platelet count of at least 20 × 109/L among the 3 groups (median 28-30 days for patients less than 60 years old and 21-23 days for patients 60 years or older). Patients receiving PEG-rHuMGDF achieved higher platelet counts after remission. However there was no significant difference in the number of days on which platelet transfusions were administered among the 3 groups. The complete remission rate was 71% for patients less than 60 years and 64% for those 60 years or older, with no significant difference among the 3 groups. Postremission consolidation chemotherapy with either placebo or PEG-rHuMGDF was given to 28 patients beginning the day after completion of chemotherapy. There was no apparent difference in the time that was necessary to reach a platelet count of at least 20 or 50 × 109/L or more platelets or in the number of platelet transfusions received. In summary, PEG-rHuMGDF was well tolerated by patients receiving induction and consolidation therapy for AML; however, there was no effect on the duration of severe thrombocytopenia or the platelet transfusion requirement.
Lineage-specific preparations of thrombopoietin have been known to produce marked increases in the megakaryocyte mass and the platelet count after subcutaneous dosing.1,2 After promising results in preclinical murine3 and primate models,4-6 early trials in humans demonstrated the following changes: a dose-dependent rise in the platelet count, beginning a few days after administration of the thrombopoietin and peaking at 10-14 days, without changes in the red blood cell or neutrophil counts7-11; the production of morphologically and functionally normal platelets9,11; mobilization of hematopoietic colony-forming units into the peripheral blood12; attenuation of the mild thrombocytopenia that occurs after moderate doses of chemotherapy8 10; and an absence of significant side effects in recipients.
Demonstration of these marked thrombopoietic effects is not, however, the same as proving clinically meaningful benefit, and studies were therefore begun in patient populations receiving more intensive therapies and requiring repetitive transfusions. Clinical trials evaluating pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) were initiated in patients with acute myeloid leukemia (AML) and following myeloablative therapy because of the predictable need for platelet transfusions in these patients. We describe the results of a trial evaluating newly diagnosed adults receiving chemotherapy for AML. PEG-rHuMGDF is a truncated form of the mpl ligand expressed in Escherichia coli that has been modified by the addition of a polyethyleneglycol moiety to increase its circulating half-life. PEG-rHuMGDF has been shown to be a potent thrombopoietic agent, as outlined above.
Patients and methods
Eligible patients were 18 years of age or older with newly diagnosed, previously untreated de novo AML with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-3. Patients with FAB M3 (acute progranulocytic leukemia) and FAB M7 (acute megakaryoblastic leukemia) were excluded from the study. The former were treated with all trans retinoic acid–based therapy, while the latter were excluded because of concern about stimulation of leukemia cell growth with a thrombopoietic agent.13 14 Additional exclusion criteria included blast transformation of chronic myeloid leukemia, AML secondary to a known preexisting myelodysplastic syndrome or other bone marrow disorders, known allergy to an E coli–derived pharmaceutical, or history of a clinically relevant coagulation disorder unrelated to AML (including deep vein thrombosis, pulmonary embolism, stroke, myocardial infarction, or unstable angina) within the last 6 months. All patients provided written informed consent according to protocol guidelines approved by the institutional review boards at their individual institutions.
Study design
Patients received initial induction chemotherapy with daunorubicin and ARA-C as outlined in Table1. Patients at least 60 years of age or older received standard induction therapy with 3 days of daunorubicin and 7 days of ARA-C by continuous infusion. For patients not achieving remission with the first course of therapy, there was the possibility of a second course of induction therapy that included 2 days of daunorubicin and 5 days of ARA-C.15 Patients less than 60 years of age received the same therapy with the addition of 6 doses of HIDAC administered on days 8, 9, and 10 of treatment. This regimen was adopted from experience at the Brigham and Women's Hospital and the New England Medical Center; these institutions reported a very high complete response rate in younger patients using this approach.16 There was no provision for a second course of induction therapy for the younger patients. Patients who failed to achieve remission were removed from the study, whereas patients in complete remission were to receive consolidation chemotherapy as outlined in Table 1.
Chemotherapy
| . | Dose . | Schedule . |
|---|---|---|
| Age < 60 y | ||
| Induction | Daunorubicin 45 mg/m2 IV | d 1-3 |
| ARA-C 100 mg/m2 CI | d 1-7 | |
| HIDAC 2 g/m2 IV over 90 min | 6 doses, every 12 h; d 8-10 | |
| Consolidation (3 cycles) | ARA-C 3 g/m2 over 3 h | 6 doses, every 12 h; d 1, 3, 5 |
| Age ≥ 60 y | ||
| Induction | Daunorubicin 45 mg/m2 IV | d 1-3 |
| ARA-C 200 mg/m2 CI | d 1-7 | |
| Consolidation | ARA-C 1.5 g/m2 over 2 h | 8 doses, every 12 h |
| . | Dose . | Schedule . |
|---|---|---|
| Age < 60 y | ||
| Induction | Daunorubicin 45 mg/m2 IV | d 1-3 |
| ARA-C 100 mg/m2 CI | d 1-7 | |
| HIDAC 2 g/m2 IV over 90 min | 6 doses, every 12 h; d 8-10 | |
| Consolidation (3 cycles) | ARA-C 3 g/m2 over 3 h | 6 doses, every 12 h; d 1, 3, 5 |
| Age ≥ 60 y | ||
| Induction | Daunorubicin 45 mg/m2 IV | d 1-3 |
| ARA-C 200 mg/m2 CI | d 1-7 | |
| Consolidation | ARA-C 1.5 g/m2 over 2 h | 8 doses, every 12 h |
IV indicates intravenous; CI indicates continuous infusion.
On the last day of the first induction chemotherapy, patients were randomized to receive either 2.5 or 5 μg/kg/day of PEG-rHuMGDF or a placebo administered subcutaneously beginning the day after completion of induction chemotherapy. Administration of PEG-rHuMGDF or a placebo was continued until the platelet count was at least 50 × 109/L or for a maximum of 28 days. Patients receiving a second course of induction chemotherapy because of persistence of leukemia had the study drug discontinued prior to administration of the second course of chemotherapy and restarted after the completion of the second course. Beginning the day after completion of each consolidation course, patients in remission received either a placebo or the same dose of the study drug they had received during induction. Participating physicians and patients were blinded as to the study drug assignment.
Platelet transfusions and supportive care
Platelet transfusions were administered prophylactically to patients each day on which the platelet count was less than or equal to 20 × 109/L. Transfusions were given to patients with higher platelet counts if they had a clinically significant hemorrhage, were undergoing invasive surgical procedures, or had other clinical conditions requiring transfusion (based on the discretion of the patient's physician). Pooled platelet concentrates or single donor platelets obtained by apheresis were used according to individual institutional policies or availability. Red blood cell transfusions were given to keep the hemoglobin level at 80 mmol/L or greater or when clinically indicated for bleeding. Empiric antibiotic therapy was used in febrile patients, and antifungal therapy was initiated for patients with a persistent fever while on antibiotics or with documented fungal infections. No cytokines were permitted other than the randomized study drug.
Statistical considerations
Sample size.
The size of the cohorts was calculated so that there was a high probability (greater than 90%) of detecting the proportion of patients experiencing dose limiting toxicity (DLT) with an incidence rate of 40% or greater. Because this was an early pilot study, it was difficult to detect a significant difference in efficacy. For example, there was only a 10% chance of detecting a significant difference at the 5% level if the median time to platelet recovery, measured as a platelet count of 20 × 109/L or greater, was reduced by 4 days by the PEG-rHuMGDF treatment.
Definitions.
For each patient, the end of the study was defined as the day of withdrawal from the study, the day of loss to follow-up or day of death, or 35 days after the last dose of chemotherapy. The time to transfusion-independent platelet recovery was defined as the number of days from the first day of chemotherapy until the first of 5 consecutive days with platelet counts equal to or greater than 20 × 109/L without a platelet transfusion. For patients who did not achieve platelet recovery before the end of the study, the time to platelet recovery was censored on the last day of the study drug administration. In patients for whom no platelet counts were available, the time to platelet recovery was censored on the day of the last available recorded platelet value.
The number of days of platelet transfusion was defined as the total number of days in the cycle, beginning with the first day of the study drug administration, in which the patient received a platelet transfusion. The total number of days the platelet count was less than or equal to 20 × 109/L was counted beginning with the first day of the study drug administration. Data for absolute neutrophil counts were calculated in a similar fashion. Survival was calculated from the day of study registration until the last follow-up or death. Disease-free survival was calculated from the date of complete remission until relapse, the date of last follow-up, or death.
Analysis.
All tests of statistical significance were assessed against a 2-sided alternative hypothesis using a nominal type 1 error rate of 5% (ie, alpha = .05). Cumulative probability distributions for time to platelet recovery were generated by the Kaplan-Meier product limit method. The treatment groups were compared using the log-rank test. An estimate of the hazard ratio and 95% confidence intervals was calculated. Total days of platelet transfusion were compared between groups using the Wilcoxon signed rank test.
Results
A total of 60 patients were initially registered in the study; 3 patients either died, did not receive chemotherapy, or were withdrawn in the first week. As summarized in Table2, 57 patients were randomized in the study; 19 patients received a placebo, and 38 received PEG-rHuMGDF. Of the 57 total patients, 35 patients were less than 60 years of age, and 22 patients were 60 years of age or older. There was no significant difference in baseline clinical characteristics between the placebo and PEG-rHuMGDF patients.
Baseline demographics
| . | Placebo n = 19 . | PEG-rHuMGDF (μg/kg/d) . | |
|---|---|---|---|
| 2.5 (n = 19) . | 5 (n = 19) . | ||
| Age, below 60 y | 11 | 12 | 12 |
| Age, at least 60 y | 8 | 7 | 7 |
| Gender, male | 74% | 58% | 53% |
| ECOG PS 0 or 1 | 79% | 68% | 68% |
| Median platelets, × 109/L | 58 | 61 | 56 |
| Median ANC, × 109/L | 0.7 | 0.5 | 0.4 |
| Cytogenetics | |||
| Favorable | |||
| t(8;21) inv (16) | 0 | 2 (11%) | 1 (5%) |
| Unfavorable | |||
| −7, −5, del(5q), abnormalities of 3q, complex karyotypes | 3 (16%) | 3 (16%) | 3 (16%) |
| . | Placebo n = 19 . | PEG-rHuMGDF (μg/kg/d) . | |
|---|---|---|---|
| 2.5 (n = 19) . | 5 (n = 19) . | ||
| Age, below 60 y | 11 | 12 | 12 |
| Age, at least 60 y | 8 | 7 | 7 |
| Gender, male | 74% | 58% | 53% |
| ECOG PS 0 or 1 | 79% | 68% | 68% |
| Median platelets, × 109/L | 58 | 61 | 56 |
| Median ANC, × 109/L | 0.7 | 0.5 | 0.4 |
| Cytogenetics | |||
| Favorable | |||
| t(8;21) inv (16) | 0 | 2 (11%) | 1 (5%) |
| Unfavorable | |||
| −7, −5, del(5q), abnormalities of 3q, complex karyotypes | 3 (16%) | 3 (16%) | 3 (16%) |
Percentages are given based on total patient population. PS indicates performance status; ANC, absolute neutrophil count.
Remission induction therapy
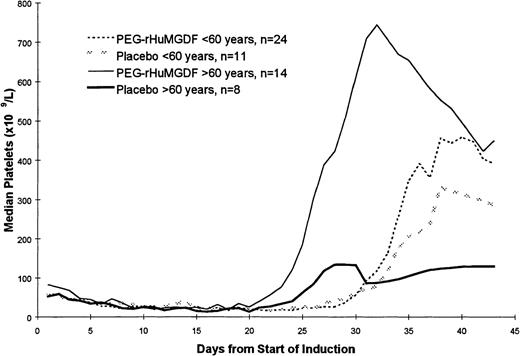
The pattern of platelet count recovery for all patients is shown in Figure 1. There was approximately a 1-week delay in platelet count recovery in the younger patients receiving more intensive induction chemotherapy compared with the patients who were 60 years of age or older who received standard daunorubicin and ARA C chemotherapy. Because the data for the 2 PEG-rHuMGDF doses were similar, these data have been pooled. Postrecovery platelet counts were higher in the patients receiving PEG-rHuMGDF, particularly in the older group of patients. Two of the older patients who received a placebo, and 1 patient in each of the PEG-rHuMGDF arms received 2 courses of induction therapy. Three patients receiving PEG-rHuMGDF had maximal platelet counts equal to or greater than 1000 × 109/L; none of the patients receiving a placebo developed thrombocytosis of this magnitude.
Pattern of platelet count recovery following induction chemotherapy. A 1-week delay in platelet count recovery was noted in the younger patients receiving more intensive induction chemotherapy, and postrecovery platelet counts were higher in the patients receiving PEG-rHuMGDF. Data for the 2 PEG-rHuMGDF doses were similar, and these data have been pooled together.
Pattern of platelet count recovery following induction chemotherapy. A 1-week delay in platelet count recovery was noted in the younger patients receiving more intensive induction chemotherapy, and postrecovery platelet counts were higher in the patients receiving PEG-rHuMGDF. Data for the 2 PEG-rHuMGDF doses were similar, and these data have been pooled together.
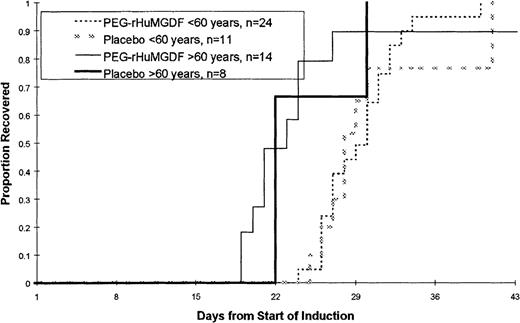
The time to platelet recovery following induction therapy is shown in Figure 2 and Table3. There were no statistically significant differences in the time to platelet recovery, measured as a platelet count of 20 × 109/L or greater, or in the number of days that patients received platelet transfusions among the 3 treatment groups in either patient age group. There were no significant differences in the time to recover to an absolute neutrophil count (ANC) of at least 0.5 × 109/L or in the number of red blood cell transfusions that patients received (data not shown). The overall complete response rate was 68% (71% in younger patients less than 60 years of age and 64% in patients 60 years of age or older). The complete remission (CR) rate in younger patients was lower than had been initially reported using this regimen.16 There was no statistically significant effect of PEG-rHuMGDF administration on CR rate.
Time to platelet count recovery to at least 20 × 109/L or greater following induction chemotherapy.
No statistically significant differences were noted in the time to platelet recovery (measured as a platelet count of 20 × 109/L or greater).
Time to platelet count recovery to at least 20 × 109/L or greater following induction chemotherapy.
No statistically significant differences were noted in the time to platelet recovery (measured as a platelet count of 20 × 109/L or greater).
Induction therapy results
| . | Placebo . | PEG-rHuMGDF (μg/kg/d) . | |
|---|---|---|---|
| 2.5 . | 5 . | ||
| Age, below 60 y | |||
| n | 11 | 12 | 12 |
| Complete remission, % of total | 9 (82%) | 7 (58%) | 9 (75%) |
| Median days to platelet recovery (≥ 20 × 109/L) | 28 | 30 | 30 |
| Median days of platelet transfusion | 8 | 7 | 10 |
| Age, at least 60 y | |||
| n | 8 | 7 | 7 |
| Complete remission, % of total | 4 (50%) | 5 (71%) | 5 (71%) |
| Median days to platelet recovery (≥ 20 × 109/L) | 22 | 23 | 21 |
| Median days of platelet transfusion | 5 | 5 | 6 |
| . | Placebo . | PEG-rHuMGDF (μg/kg/d) . | |
|---|---|---|---|
| 2.5 . | 5 . | ||
| Age, below 60 y | |||
| n | 11 | 12 | 12 |
| Complete remission, % of total | 9 (82%) | 7 (58%) | 9 (75%) |
| Median days to platelet recovery (≥ 20 × 109/L) | 28 | 30 | 30 |
| Median days of platelet transfusion | 8 | 7 | 10 |
| Age, at least 60 y | |||
| n | 8 | 7 | 7 |
| Complete remission, % of total | 4 (50%) | 5 (71%) | 5 (71%) |
| Median days to platelet recovery (≥ 20 × 109/L) | 22 | 23 | 21 |
| Median days of platelet transfusion | 5 | 5 | 6 |
Consolidation therapy
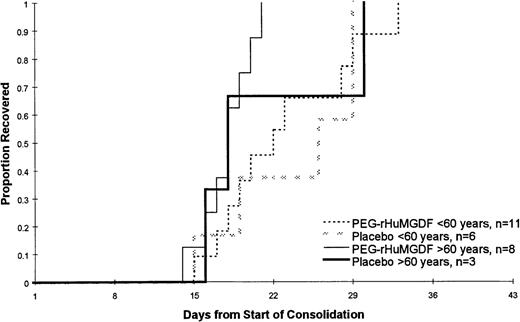
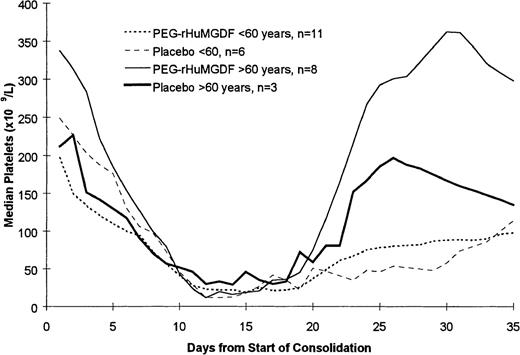
Of the 39 patients achieving CR, 28 patients went on to receive at least 1 course of consolidation chemotherapy including 17 of 25 patients who were less than 60 years of age and 11 of 14 patients who were 60 years of age or older. The data for the 2 PEG-rHuMGDF patient groups are pooled in these analyses because of the relatively small number of patients. As shown in Table 4 and Figure 3, which present the data following the first course of consolidation, there was no statistically significant difference in the time to platelet count recovery or the number of days on which patients received platelet transfusions. Of note is that younger patients receiving the more intensive consolidation therapy received only a median of 3 days of transfusions, while the older patients received a median of only 1 or 2 platelet transfusions. As was seen during induction therapy, the platelet counts following consolidation therapy were higher in the older patients (Figure 4). This is presumably a reflection of the greater intensity of the consolidation therapy used in patients less than 60 years of age as well as a possible lingering myelosuppressive effect from the induction therapy.
Consolidation therapy results (first course)
| . | Placebo . | PEG-rHuMGDF . |
|---|---|---|
| Age, below 60 y | ||
| n | 6 | 11 |
| Median days to platelet recovery (≥ 20 × 109/L) | 26 | 22 |
| Median days of platelet transfusions | 3 | 3 |
| Age, at least 60 y | ||
| n | 3 | 8 |
| Median days to platelet recovery (≥ 20 × 109/L) | 18 | 18 |
| Median days of platelet transfusions | 1 | 2 |
| . | Placebo . | PEG-rHuMGDF . |
|---|---|---|
| Age, below 60 y | ||
| n | 6 | 11 |
| Median days to platelet recovery (≥ 20 × 109/L) | 26 | 22 |
| Median days of platelet transfusions | 3 | 3 |
| Age, at least 60 y | ||
| n | 3 | 8 |
| Median days to platelet recovery (≥ 20 × 109/L) | 18 | 18 |
| Median days of platelet transfusions | 1 | 2 |
Time to platelet count recovery to at least 20 × 109/L or greater following the first course of consolidation chemotherapy. There was no statistically significant difference in the time to platelet count recovery or the number of days on which patients received platelet transfusions. That data for the 2 PEG-rHuMGDF patient groups are pooled because of the relatively small number of patients.
Time to platelet count recovery to at least 20 × 109/L or greater following the first course of consolidation chemotherapy. There was no statistically significant difference in the time to platelet count recovery or the number of days on which patients received platelet transfusions. That data for the 2 PEG-rHuMGDF patient groups are pooled because of the relatively small number of patients.
Pattern of platelet count recovery following the first course of consolidation chemotherapy. The platelet counts following consolidation therapy were higher in the older patients.
Pattern of platelet count recovery following the first course of consolidation chemotherapy. The platelet counts following consolidation therapy were higher in the older patients.
Of the younger patients, 28 patients went on to receive a second consolidation course, and 14 patients received a third consolidation course. Although the number of patients in each group is small, again there was no apparent difference in the time to recovery or the number of platelet transfusions received between the patients receiving the placebo and the patients receiving PEG-rHuMGDF.
Survival
There was no statistically significant difference in overall survival between patients receiving placebo (median survival: 8.3 months) and those receiving PEG-rHuMGDF (median survival: 12.3 months). It is projected that 38% of patients receiving a placebo and 38% of patients receiving PEG-rHuMGDF will be alive 18 months after randomization.
Adverse effects
There was no difference in the incidence of severe side effects reported in the placebo versus the PEG-rHuMGDF patient groups. As expected for intensive AML induction and consolidation therapy, there was universal pancytopenia, moderate nausea and vomiting, and a near-universal requirement for systemic antibiotic therapy. There were 4 thrombotic events in the placebo recipients including 1 episode of angina, 2 thromboses in central lines, and 1 splenic infarction. In contrast, 1 patient receiving PEG-rHuMGDF developed a proximal leg deep venous thrombosis (platelet count: 238 × 109/L) and recovered uneventfully after systemic anticoagulation. There was no significant difference in the incidence of grade 3-5 hemorrhagic events. All patients had serum samples assayed for antibodies against PEG-rHuMGDF, and no antibodies were detected by radioimmunoassay.17
Discussion
Because fatal or serious hemorrhage is uncommon even at very low platelet counts and because it is difficult to quantify more minor degrees of bleeding, the benefit of thrombopoietin use in patients with leukemia must be evaluated primarily by a shorter duration of severe thrombocytopenia, a reduction in the number of platelet transfusions, and by the inference of a decreased likelihood of some of the complications of transfusion.18 19 None of these parameters were improved by the use of 2 different doses of PEG-rHuMGDF in the current study, despite the fact that PEG-rHuMGDF did exhibit a considerable thrombopoietic effect, as evidenced by higher maximal platelet counts achieved at the time of bone marrow recovery.
These results are virtually identical to those noted in a study20 of a similar design conducted in Europe using the same doses of PEG-rHuMGDF. As was done in our study, 70 newly diagnosed patients with AML were randomized to receive PEG-rHuMGDF or a placebo daily until platelet count recovery. Following induction therapy with daunorubicin, ARA-C, and etoposide, an additional 38 patients received either a placebo, a single dose of 2.5 μg/kg PEG-rHuMGDF, or 7 daily doses of 2.5 μg/kg PEG-rHuMGDF. Patients receiving the larger number of doses of PEG-rHuMGDF had the highest posttreatment platelet counts following both induction and consolidation chemotherapy, but the time to platelet count recovery and the number of days of platelet transfusion remained the same. Although both the European study and our study are relatively small, the conclusions are remarkably consistent and indicate that this schedule of PEG-rHuMGDF administration is unlikely to be of clinical benefit despite its potent thrombopoietic effects in this and other clinical situations.
There are a number of possible explanations for this finding. Most transfusions given during induction treatment of AML are administered during the first few weeks of treatment, either during the period of chemotherapy administration or while the marrow is aplastic, and thus prior to any expected effect of thrombopoietin.21Therefore, as was apparent in trials of myeloid growth factors in this patient population, there is a relatively small interval during the last week of marrow regeneration in which a thrombopoietic factor can decrease the need for transfusion.22,23 Furthermore, thrombopoietin seems to have its major effect on early megakaryocytic progenitors and does not seem to promote accelerated release of platelets.1 As a result there may be a built-in delay due to the required time for megakaryocyte maturation, and it may be difficult to shorten the duration of thrombocytopenia following induction chemotherapy to a clinically meaningful extent. The lower peak platelet counts observed in the younger patients also suggest that there may be limits in the ability to stimulate megakaryocytopoiesis after more intensive marrow cytotoxic therapy. Finally, there is a marked rise in endogenous thrombopoietin during periods of severe thrombocytopenia24; therefore the marrow may already be significantly or maximally stimulated, and even pharmacologic doses of exogenous growth factor may have little added effect.
It was anticipated that the outcome would have been more favorable following postremission consolidation therapy because patients start with morphologically normal bone marrow and essentially always recover normal blood counts. In addition, it has been shown that myeloid growth factors can be of benefit in this situation.23,25 However, the duration of severe thrombocytopenia was only 8-10 days, and a median of only 1-3 platelet transfusions was administered per patient. It may therefore be difficult for a thrombopoietic agent to improve further on these already modest transfusion requirements. The European study20 evaluating PEG-rHuMGDF also failed to demonstrate shortening of the duration of thrombocytopenia or a reduction in the need for platelet transfusion following consolidation. Furthermore, 3 recent randomized trials in patients with acute leukemia have demonstrated the safety of a 10 × 109/L platelet “threshold” for prophylactic transfusion in stable patients, and most physicians are now comfortable in observing clinically stable patients at lower counts without platelet transfusion.26-28 It is likely that even fewer transfusions would have been administered had we used this lower transfusion threshold. Even if larger trials could be statistically powered to demonstrate a possible reduction of 1 or 2 platelet transfusions, this would be unlikely to have an important clinical impact and also would probably not be cost effective. Lastly, a small trial using PEG-rHuMGDF before and after myeloablative therapy with peripheral blood stem cell support did not show a reduction in the number of platelet transfusions or an accelerated platelet count recovery, presumably for many of the same reasons noted above.29
PEG-rHuMGDF was very well tolerated in this trial, and no untoward events were ascribed to its use. In particular, there was no increase in the incidence of thrombotic events, and there were no patients who developed neutralizing antibodies or sustained thrombocytopenia that could not be attributed to persistent leukemia. In contrast, there have been reports of neutralizing antibodies developing in healthy volunteers who received multiple doses of PEG-rHuMGDF, with some individuals developing moderate to severe thrombocytopenia, presumably due to antibody-mediated neutralization of endogenous thrombopoietin.30 As a consequence, development of PEG-rHuMGDF has been discontinued by Amgen, although this agent is still being evaluated by others, and a nonmodified recombinant thrombopoietin compound is still in active clinical trials. It is unknown whether antibody problems will develop using the nonmodified compound or whether antibody formation will always be attenuated, as was noted in this study, if higher dose chemotherapy is administered concurrently. In any event, the data reported here would suggest that future trials of thrombopoietins in patients with AML should consider different doses and scheduling strategies. Indeed, pilot trials using higher doses of PEG-rHuMGDF given before and after completion of chemotherapy have been recently completed by our group and in Europe. The results of these studies should be available in the near future.
Acknowledgments
The authors would like to thank Drs Alan Barge, Dora Menchaca, and William Sheridan for their important roles in the design and conduct of the study and the many nurses and data managers who were critical to the success of this clinical trial.
Supported by research grants from Amgen Inc, Thousand Oaks, CA.
Reprints:Charles A. Schiffer, Harper Hospital, Division of Hematology/Oncology, 505 Hudson, 3990 John Rd, Detroit, MI 48201; e-mail: schiffer@karmanos.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal