Abstract
In this report we present a transgenic mouse model in which we targeted gene expression specifically to B-lymphocytes. Using the human CD19 promoter, we expressed major histocompatibility complex class II I-E molecules specifically on B cells of all tissues, but not on other cell types. If only B cells expressed I-E in a class II-deficient background, positive selection of CD4+ T cells could not be observed. A comparison of the frequencies of I-E reactive Vβ5+ and Vβ11+ T cells shows that I-E expression on thymic B cells is sufficient to negatively select I-E reactive CD4+ T cells partially, but not CD8+ T cells. Thus partial negative but no positive selection events can be induced by B-lymphocytes in vivo.
The fate and development of thymocytes are determined by cellular interactions within their thymic environment. Depending on the thymic cell type presenting the peptide/major histocompatibility complex (MHC) and the developmental stage of the thymocyte, MHC restriction is imprinted (positive selection) or autoreactive thymocytes are deleted (negative selection) (see Jameson et al1 and Anderson et al2). The role of various thymic cell types in thymic T-cell education has been studied with different experimental approaches. Experiments with radiation bone marrow (BM) chimeras originally identified epithelial cells as mediators of positive selection and bone marrow-derived cells as responsible for negative selection of autoreactive thymocytes.3-5
The efficiency of negative selection events varied according to the in vitro experimental system used. In suspension cultures, BM-derived cells were as efficient as epithelial cells in deleting self-reactive thymocytes,6,7 whereas in reaggregation cultures, they were shown to be much better at deleting than epithelial cells.8,9 The precise function of each BM-derived cell type (dendritic cells [DC], macrophages, or B cells), however, remained relatively unclear for sometime. Macrophages are probably unable to delete self-reactive thymocytes clonally10,11 but seem instead to have scavenger functions.12 In contrast, it had been postulated that thymic DC could negatively select only in concert with B cells.13 Although it was demonstrated that DC have a strong capacity to induce negative selection in vitro with various experimental setups and DC from different sources,8,11,13-16 only recently could we demonstrate the capacity of unmanipulated DC to negatively select T cells in vivo.17
The role of thymic B cells as yet another BM-derived antigen-presenting cell (APC) component of the thymus remains unclear. In experiments with IgM-suppressed animals, an effect of thymic B cells on autoreactive thymocytes was described.18 It has further been proposed that thymic B cells could have the function of negatively selecting APC because of their capacity to present trinitrophenyl19 or Mls antigens18,20-22 intrathymically. In vitro deletion models13,23 and the neonatal injection of B cells20,22 have suggested that B cells from spleen and thymus have the capacity clonally to eliminate self-reactive thymocytes. Some researchers even came to the conclusion that inside the thymus exclusively B cells would present Mls antigens, leading to negative selection of Mls-reactive thymocytes.24,25 In thymus-grafting experiments into SCID mice, Frey et al26observed a restricted negative selection effect of thymic B cells on autoreactive thymocytes expressing specific T-cell receptor TCR Vβ segments only. This was in contrast to studies with B-cell deficient μMT mice, in which the absence of B cells in the thymus had no measurable impact on thymic negative selection.27
The involvement of BM-derived cells in thymic positive selection has remained controversial (reviewed in 1). Several experiments showed that many different cell types, such as fibroblasts28,29 or BM-derived cells,30 were able to mediate positive selection in some but not all cases4 when injected intrathymically or when used in BM reconstitution experiments. In a recent study, Zinkernagel et al31 reinvestigate this question and demonstrate that indeed BM-derived cells can induce positive selection. We have reported that targeted expression of MHC class II to thymic DC does not lead to positive selection of CD4-positive T cells, and we therefore excluded thymic DC from the pool of potential BM-derived thymic components able to positively select T cells.17
To clarify the role of thymic B cells in an unmanipulated, noninvasive in vivo system, we developed a transgenic model in which transgene expression was directed exclusively to B cells using an appropriate promoter. We selected the human CD19 promoter because it has been demonstrated that this promoter, when introduced into the germline of transgenic mice, directs the expression of transgenes exclusively to the B cell lineage.32-34
Here we present our findings obtained with such a B cell-specific expression system. We use the MHC class II I-Eα gene as a transgene in the I-E negative C57Bl/6 background and describe a B cell-specific MHC class II I-E transgene expression pattern. We could not detect transgene expression on thymic epithelium or DC, and we describe in this model how thymic B cells were able to negatively select self–I-E reactive CD4+ T cells but not CD8+ T cells. This finding was a clear contrast to transgenic mice expressing the same I-E transgene exclusively on DC because DC also negatively selected CD8+ thymocytes. Further, when mice in the MHC class II-deficient background were analyzed, CD4+ T cells were not positively selected, establishing that thymic B cells in vivo are unable to induce positive selection.
Materials and methods
Mice
All mice were bred in the animal facility of the Max–Planck–Institute for Immunobiology (Freiburg, Germany). The AD10 TCR transgenic line35 was a kind gift of Dr S. Hedrick.
Isolation of CD19 promoter region, DNA constructs, and microinjection
The 4.2-kilobase (kb) 5′ region of the human CD19 gene was isolated and cloned from cosmid DNA, kindly provided by M. Busslinger (Vienna, Austria) in the following way. A 3-kbHindIII–KpnI fragment from cosmid CD1,36containing a 1.3-kb 5′ sequence and exons 1-4 of the CD19 gene, as well as a 350-bp vector sequence, was subcloned in pBS II vector (Stratagene, La Jolla, CA) generating pCD19s. A 1-kbBamHI–XbaI fragment from pCD19s, containing the 5′ region of the CD19 gene, was used for hybridization ofEcoRI–BglII digests of DNA from different cosmids containing parts of the CD19 gene (Busslinger M, personal communication). One cosmid, CD3, showed hybridization of 2-kb and 3.2-kb fragments as expected from the known restriction map of the human CD19 5′ region.34 The 3.2-kbEcoRI–BglII fragment was inserted intoEcoRI–BamHI-digested pCD19s, generating pCD19L. From pCD19L the 4.3-kb 5′ region of the human CD19 gene was amplified using the 5′ primer, 5′-CAGTCTCGAGGCTGAGGCTGCAGTGAGC (containing aXhoI site), and the 3′ primer 5′-CATGGCGGCCGCACTCTCCGGGGCACCCAG (containing aNotI site). The amplified sequence was cloned in PCR2.1 vector (Invitrogen, Carlsbad, CA) as a XhoI–NotI fragment, and approximately 500 bp 5′ region of the NotI site was sequenced and compared with the published sequence. This construct was linearized by an EcoRV/NotI double digest and was blunt ended by treatment with Klenow fragment. In parallel a cDNA expression cassette containing the I-E cDNA17 and splice donor–acceptor sites, as well as a polyA site from rabbit β-globin, was isolated from vector pDOI537 byXhoI/BamHI double digest and consecutive blunt ending with Klenow enzyme. This fragment was ligated to the above-described linearized CD19 promoter containing plasmid, and the orientation was determined by BglII digestion. After digestion withXhoI and SpeI, the transgene DNA was separated from the vector sequences by electrophoresis, extracted from agarose slices, and resuspended in Tris-EDTA buffer (10 mmol/L Tris, pH 7.4; 0.1 mmol/L EDTA). Fertilized oocytes were obtained from C57Bl/6 mice, and DNA injection was performed as previously described.38
Monoclonal antibodies and reagents
The monoclonal antibodies (mAbs) specific for CD4 (no. 09,005), CD8 (no. 01,044), Vβ5.1/5.2 TCR (no. 01,354), Vβ8.1/8.2 TCR (no. 01,344), Vβ11 TCR (no. 01,374), I-Eα (no. 09,625), I-Ab, CD11c (no. 09,705), CD19 (no. 09,655), and B220 (no. 01,124) were purchased from PharMingen (San Diego, CA). With these mAbs flow cytometry was performed on a FACScalibur (Becton Dickinson, Mountain View, CA) instrument. Single-cell preparation, staining, and FACS analysis were performed according to standard procedures.
Generation of DC from BM cultures
Total BM was cultured at 5 × 105 cells/mL in culture medium containing 25 ng/mL granulocyte macrophage–colony stimulating factor (GM-CSF). Cells were cultured in a total volume of 10 mL in 90-mm tissue culture-treated Petri dishes. Maximal yield of DC was obtained between days 6 and 8 of culture.
T-cell proliferation analysis
T cells from AD10 TCR transgenic animals were purified by passing them over a CD4 T-cell purification column (R&D Systems, Minneapolis, MN) and prestimulated by incubation with plastic-coated anti-CD3 (10 μg/mL) in the presence of anti-CD28 (5 μg/mL in solution) in 75-mL flasks (Costar, Cambridge, MA) for 3 days.
B cells were purified by sorting B220-positive cells from spleen cell suspensions on a FACSvantage (Becton Dickinson) instrument. Then 1 × 105 irradiated (30 Gy) B cells and 5 × 104 T cells were cocultured in the presence of varying concentrations of the peptide MCC 88-103 in 250 μL complete culture medium (IMDM, 10% fetal calf serum) for 2 days and in the presence of 1 μCi [3H] thymidine for the last 10 hours. Incorporation of radioactivity was measured by harvesting the cells and measuring their radioactivity content on a Betaplate system (1205; Wallacoy, Turku, Finland).
Flow cytometry
Expression of cell surface proteins was assayed by indirect immunofluorescence. Organs were teased through a mesh, and cell suspensions of 1 × 105 viable cells were stained with 20 μg/mL mAb that was directly labeled. After washing, cells were analyzed using a FACScalibur (Becton Dickinson).
Results
Transgene design
To investigate the role of thymic B-lymphocytes in the negative and positive selection of thymocytes in vivo, we targeted MHC class II expression in a transgenic approach exclusively to B cells. In vivo studies with transgenic mice carrying the human CD19 locus have demonstrated that the human CD19 promoter drives transgene expression in mice in a B-lineage–specific fashion.32-34 We therefore isolated the human CD19 promoter by polymerase chain reaction (PCR) from a cosmid clone (see “Materials and methods”) and designed the CD19-IE transgene, as shown in Figure1. Approximately 4.2 kb of the huCD19 5′ UT region was used as the huCD19 promoter-containing region with the intention to drive the expression of cDNA encoding for the alpha chain of the MHC class II molecule I-Ed (see “Material and methods”). The CD19-IE transgenic construct (Figure1) was introduced into oocytes from C57BL/6-mice because this strain lacks expression of a functional I-E alpha chain as a result of a mutation in the I-Eαb promoter region39 and is, consequently, negative for MHC class II I-E expression.
Restriction map of the transgenic construct CD19-IE.
MHC class II I-Eαd cDNA (striped box) was placed under the control of a human CD19 promoter containing DNA segment (gray box). The rabbit β-globin gene fragment providing the cDNA with an intron and a polyadenylation signal (A(n)) is displayed as a white box. X, XhoI; N, NotI; B,BamHI; E, EcoRI; S, SpeI; restriction sites in parenthesis () have been destroyed by blunt-end cloning.
Restriction map of the transgenic construct CD19-IE.
MHC class II I-Eαd cDNA (striped box) was placed under the control of a human CD19 promoter containing DNA segment (gray box). The rabbit β-globin gene fragment providing the cDNA with an intron and a polyadenylation signal (A(n)) is displayed as a white box. X, XhoI; N, NotI; B,BamHI; E, EcoRI; S, SpeI; restriction sites in parenthesis () have been destroyed by blunt-end cloning.
Characterization of CD19-IE transgenic C57BL/6-mice
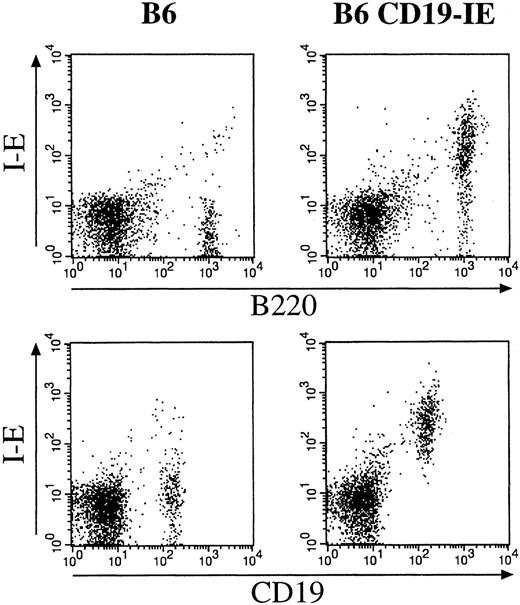
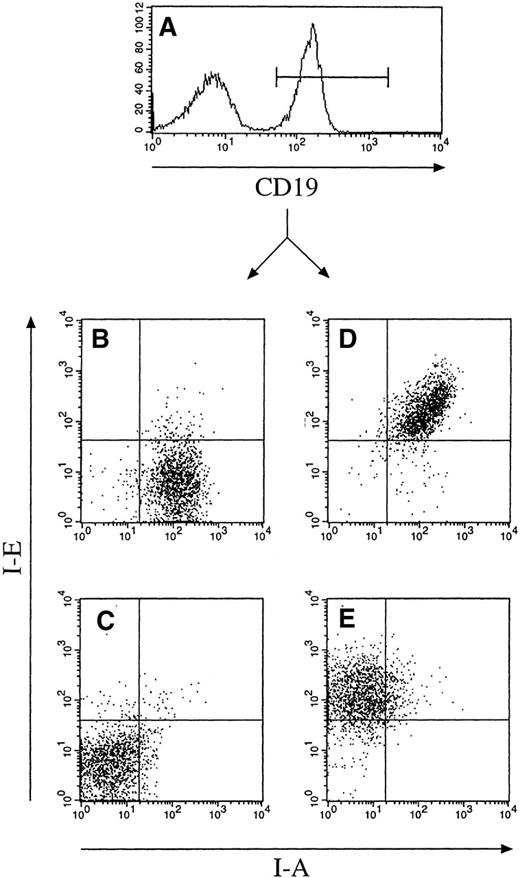
Mice carrying the CD19-IE transgene were initially screened by PCR using oligonucleotides specific for the CD19 promoter and the I-E cDNA (data not shown). The transgene-positive founders were then further analyzed by flow cytometry of blood leukocytes isolated by a Ficoll gradient and stained with mAbs for MHC class II I-A and I-E (data not shown). For further characterization of transgene expression specificity, we performed flow cytometric analysis of cell suspensions from lymph nodes of CD19-IE transgenic mice and their nontransgenic littermates (Figure 2). The double stainings with mAbs specific for the B-lineage markers B220 or CD19 with an MHC class II I-E–specific mAb indicate that, as expected, nontransgenic littermates (Figure 2, 6) do not express I-E on B cells. In contrast, in CD19-IE transgenic B6-mice, B220+ and CD19+ lymph node B cells do express I-E (Figure 2, 6 CD19-IE). A minor fraction of B220+ cells in CD19-IE mice does not show transgenic I-E expression (Figure 2, 6 CD19-IE). This B220+I-E− cell fraction can eventually be explained by B220-positive non-B cells because it has been described before.40,41 CD19 is reported to be more restricted to the B-cell lineage than B220,42 and, because all CD19+ B cells are I-E+, we conclude that all B cells in the lymph nodes do express the transgene. This has been verified by 3-color flow cytometric analysis on spleen cell suspensions (Figure 3). We first analyzed spleen cells through an FSC/SSC gate according to lymphocyte criteria (data not shown) and then further for the expression of the indicated surface markers. We gated on CD19+ cells (Figure 3A) and analyzed the CD19+ cell fraction further for their expression of endogenous I-A and transgenic I-E. CD19+ cells from B6 mice expressed I-A, but they were as expected negative for I-E (Figure 3B). In contrast, in B6 CD19-IE transgenic mice, CD19+ B cells were double positive and did therefore express MHC class I-A and transgenic I-E (Figure 3D). The level of transgenic I-E was identical to the expression level of endogenous MHC class II I-A in all mice analyzed (Figure 3D and data not shown). When mice in the MHC class II-deficient background were analyzed, as expected, expression of MHC class I-A could be detected neither on CD19+ B cells of transgene-negative littermates (Figure 3C) nor on B cells from CD19-IE transgene-positive mice (Figure 3E). In contrast, the latter expressed MHC class II I-E (Figure 3E), while in the transgene-negative animals B cells were I-E− (Figure 3C). These data further indicate that expression of the transgenic I-E follows stringently the expression of CD19 and is therefore restricted to B cells.
Flow cytometric analysis of cell suspensions from (pooled axillary, inguinal, and brachial) lymph nodes of CD19-IE transgenic mice (B6 CD19-IE) and their nontransgenic littermates (B6).
The double stainings were performed with mAbs specific for the B-lineage markers B220 (FITC) or CD19 (FITC) and an MHC class II I-E specific mAb (PE). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Flow cytometric analysis of cell suspensions from (pooled axillary, inguinal, and brachial) lymph nodes of CD19-IE transgenic mice (B6 CD19-IE) and their nontransgenic littermates (B6).
The double stainings were performed with mAbs specific for the B-lineage markers B220 (FITC) or CD19 (FITC) and an MHC class II I-E specific mAb (PE). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Flow cytometric analysis of spleen cell suspensions
from nontransgenic B6 animals (Figure 3B), transgenic B6 CD19-IE mice (Figure 3D), nontransgenic I-A–deficient B6/I-A−/− littermates (Figure 3C) and their transgenic B6 CD19-IE/I-A−/− counterparts (Figure 3E). Suspensions were stained with mAbs specific for CD19 (FITC), I-E (PE), and I-A–biotin plus Streptavidin APC. In all 3 color analyses, living cells were first gated according to their FSC/SSC properties and then for their expression of CD19 (Figure 3A). CD19+ cells were then further analyzed for expression of I-A and I-E (Figures 3B–E).
Flow cytometric analysis of spleen cell suspensions
from nontransgenic B6 animals (Figure 3B), transgenic B6 CD19-IE mice (Figure 3D), nontransgenic I-A–deficient B6/I-A−/− littermates (Figure 3C) and their transgenic B6 CD19-IE/I-A−/− counterparts (Figure 3E). Suspensions were stained with mAbs specific for CD19 (FITC), I-E (PE), and I-A–biotin plus Streptavidin APC. In all 3 color analyses, living cells were first gated according to their FSC/SSC properties and then for their expression of CD19 (Figure 3A). CD19+ cells were then further analyzed for expression of I-A and I-E (Figures 3B–E).
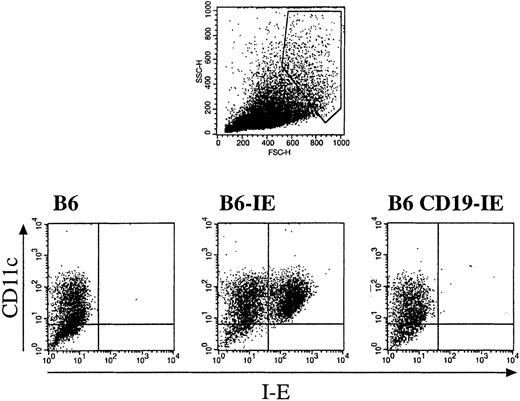
Because we intended to compare the role B cells and DC in thymocyte selection, it was important to demonstrate that only B cells but not DC would express the I-E transgene in CD19-IE mice. To this end, we isolated BM from various mice, cultured DC in the presence of GM-CSF, and analyzed them for expression of I-E by flow cytometry. We first gated on large cells that fulfilled the size criterion for DC (Figure4 top, dot histogram) and analyzed them further for the expression of MHC class II I-E and the DC-marker CD11c. As shown in Figure 4, GM-CSF–cultured CD11c+ DC from nontransgenic B6 mice did not express MHC class I-E (Figure 4, 6). In contrast, DC from B6-IE mice, which express I-E under the control of the MHC class II promoter, were I-E+ (Figure 4, 6-IE). When GM-CSF–cultured DC from B6 CD19-IE mice were analyzed, we could not detect any I-E expression, indicating the absence of CD19-promoter–driven I-E expression in BM-derived DC from CD19-IE mice.
Flow cytometry analysis of nonadherent cells from bone marrow GM-CSF cultures derived from either nontransgenic mice (B6) or mice expressing transgenic MHC class II I-E under the control of the MHC class II promoter (B6-IE56) or the CD19 promoter (B6 CD19-IE).
Gates were set on large cells according to FSC/SSC criteria (top panel), and cells fulfilling these criteria were further analyzed for expression of the DC marker CD11c (PE) and MHC class II I-E (FITC).
Flow cytometry analysis of nonadherent cells from bone marrow GM-CSF cultures derived from either nontransgenic mice (B6) or mice expressing transgenic MHC class II I-E under the control of the MHC class II promoter (B6-IE56) or the CD19 promoter (B6 CD19-IE).
Gates were set on large cells according to FSC/SSC criteria (top panel), and cells fulfilling these criteria were further analyzed for expression of the DC marker CD11c (PE) and MHC class II I-E (FITC).
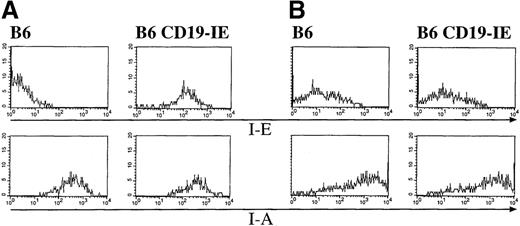
To further characterize thymic B cells and DC for expression of the I-E transgene, we performed flow cytometric analysis of thymic cell suspensions. By gating on thymic CD19+ B cells, they were analyzed for the expression of I-E and I-A. As shown in Figure5 (Figure 5A, 6), thymic B cells from nontransgenic B6 mice express I-A but are negative for I-E. In contrast, thymic B cells from B6 CD19-IE mice do express the transgenic I-E in addition to I-A (Figure 5A, 6 CD19-IE). Therefore, thymic B cells do resemble their splenic counterparts with respect to expression of the I-E transgene.
Expression of the CD19-IE transgene leads to I-E expression on thymic B cells but not on thymic dendritic cells.
(A) B cells. Thymi of B6 and B6 CD19-IE mice were teased, and cell suspensions were stained with mAbs specific for the pan B-cell antigen CD19 (FITC) (data not shown) and further analyzed for expression of I-E or I-A (PE), respectively (upper panels). (B) Dendritic cells. Thymi of B6 and B6 CD19-IE mice were collagenase digested, and DC were analyzed according to their FSC/SSC criteria (data not shown). Cells were stained with mAbs specific for CD11c (FITC) (data not shown), and CD11c-positive cells were further analyzed for I-E or I-A (PE) expression (lower panels).
Expression of the CD19-IE transgene leads to I-E expression on thymic B cells but not on thymic dendritic cells.
(A) B cells. Thymi of B6 and B6 CD19-IE mice were teased, and cell suspensions were stained with mAbs specific for the pan B-cell antigen CD19 (FITC) (data not shown) and further analyzed for expression of I-E or I-A (PE), respectively (upper panels). (B) Dendritic cells. Thymi of B6 and B6 CD19-IE mice were collagenase digested, and DC were analyzed according to their FSC/SSC criteria (data not shown). Cells were stained with mAbs specific for CD11c (FITC) (data not shown), and CD11c-positive cells were further analyzed for I-E or I-A (PE) expression (lower panels).
To exclude the expression of I-E on thymic DC in CD19-IE mice, we collagenase-digested thymi and performed flow cytometric analysis. Only cells showing high forward scatter and intermediate side scatter signals were included in this analysis (data not shown). These cells were further analyzed for expression of the DC-marker CD11c and MHC class II I-A or I-E. Although the CD11c-positive cells from C57Bl/6 mice stained brightly for MHC class II I-A molecules, they were negative for I-E (Figure 5B, 6). When the B6CD19-IE mice were analyzed (Figure 5B, 6 CD19-IE), we found the same MHC class II expression pattern as for B6 mice: thymic DC from CD19-IE mice did express I-A but were negative for the transgenic I-E (Figure 5B, 6 CD19-IE).
To test whether the expressed MHC class II I-E molecules were expressed functionally, we performed T-cell stimulation assays, with I-E as the restricting element. As shown in Figure 6, purified B cells from B6 CD19-IE animals, but not from their nontransgenic counterparts, were able to present the specific MCC-peptide to preactivated T cell blasts from AD10 transgenic mice in an antigen- and an I-E-dependent fashion. This demonstrated that the transgenic I-E molecules were functionally expressed.
Functional expression of the IE transgene on B cells from B6 CD19-IE mice induces antigen-specific proliferation in I-E restricted CD4 T cells.
B220-positive splenocytes from B6 and B6 CD19-IE animals were sorted, irradiated, and used as APC for preactivated T-cell blasts from AD10 TCR transgenic mice. The coculture was performed in the presence of graded amounts of antigenic peptide (MCC88-103), and proliferation was measured by [3H] thymidine incorporation.
Functional expression of the IE transgene on B cells from B6 CD19-IE mice induces antigen-specific proliferation in I-E restricted CD4 T cells.
B220-positive splenocytes from B6 and B6 CD19-IE animals were sorted, irradiated, and used as APC for preactivated T-cell blasts from AD10 TCR transgenic mice. The coculture was performed in the presence of graded amounts of antigenic peptide (MCC88-103), and proliferation was measured by [3H] thymidine incorporation.
Taken together, these data indicate that the human CD19- promoter–driven transgene is tightly restricted to the B lineage but is not expressed on DC, and it confirms previous studies that used the human CD19-promoter for B-lineage–specific transgene expression in mice.32-34 Histologic analysis of frozen sections from thymic tissue revealed further that neither cortical nor medullary epithelium express the CD19-IE transgene (data not shown). The expression pattern of an I-E transgene driven by the CD11c-promoter has already been extensively characterized.17 43 In CD11c-IE mice, I-E is exclusively expressed on DC but not on B cells or thymic epithelium. Therefore, these 2 mouse strains are well suited to compare the function of B cells versus DC during thymocyte development in vivo because they show reciprocal I-E expression on either B cells or DC.
Thymic B cells are unable to mediate positive selection in vivo
In earlier reports,44 targeted expression of class II molecules in transgenic mice showed that positive selection occurred only if the appropriate MHC molecules were present on cortical epithelium. If class II was only expressed on medullary epithelium and medullary BM-derived cells, no45 or little46positive selection was observed.
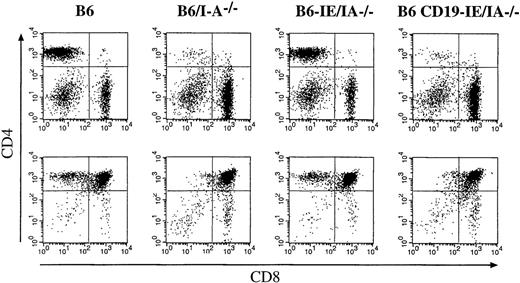
To this end we crossed the above-named mouse strains to the I-A−/− line described earlier.47When the I-A–deficient background was combined with the natural defect of I-E expression in C57BL/6 mice, this strain showed an absence of all MHC class II molecules and consequently an absence of CD4+mature T cells in thymus and periphery47 (Figure7, 6/IA−). When the I-E gene was reintroduced into these mice under the control of the class II promoter, the reexpression of class II (I-E) on thymic epithelium and BM-derived cells led to the full restoration of the mature CD4+ T cell compartment (Figure 7, 6-IE/IA−/−) as has been described before.46 In contrast, when I-E expression was restricted to thymic B cells (Figure 7, 6CD19-IE/IA−/−), restoration of the CD4+ compartment was not observed; the percentages of CD4+ T cells were identical to those of B6I-A−/− mice (Figure 7).
Influence of the different I-E transgenes on the number of peripheral (top row) and thymic (bottom row) CD4+ T cells.
Lymph node and thymus cell suspensions were stained with mAbs specific for CD4 (PE) and CD8 (FITC). The CD4/CD8 ratios in the LN T lymphocyte populations were determined from 4 mice per group, and the following values were obtained: B6, 8.2; B6/I-A−/−, 0.09; B6-IE/I-A−/−, 4.8; B6 CD19IE/I-A−/−, 0.06. The percentages of CD4+CD8− thymocytes in the thymi of the same animals were B6, 6.08%; B6/I-A−/−, 1.26%; B6-EαdI-A−/−, 8.16%; B6CD19-IE/I-A−/−, 1.26%.
Influence of the different I-E transgenes on the number of peripheral (top row) and thymic (bottom row) CD4+ T cells.
Lymph node and thymus cell suspensions were stained with mAbs specific for CD4 (PE) and CD8 (FITC). The CD4/CD8 ratios in the LN T lymphocyte populations were determined from 4 mice per group, and the following values were obtained: B6, 8.2; B6/I-A−/−, 0.09; B6-IE/I-A−/−, 4.8; B6 CD19IE/I-A−/−, 0.06. The percentages of CD4+CD8− thymocytes in the thymi of the same animals were B6, 6.08%; B6/I-A−/−, 1.26%; B6-EαdI-A−/−, 8.16%; B6CD19-IE/I-A−/−, 1.26%.
I-E expression restricted to thymic B cells mediates clonal deletion of Vβ5+ and Vβ11+ T cells in vivo
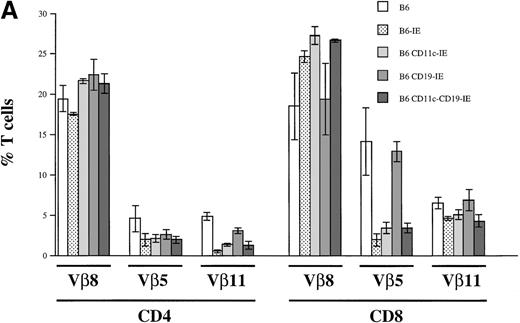
To assess in vivo clonal deletion, we measured the frequency of I-E reactive Vβ5+ and Vβ11+ T cells in B6, B6-IE, B6 CD11c-IE, and B6 CD19-IE mice. T cells expressing Vβ5 and Vβ11 genes are deleted in certain mouse strains that present retrovirally encoded superantigens48 in the context of I-E49,50 in the thymus. B6 mice do not express functional I-E molecules, and therefore the frequency of both Vβ5 and Vβ11-expressing CD4+ T cells among total CD4+cells is approximately 4% to 6% and approximately 15% and 6%, respectively, in the CD8+ population (Figure8a). In I-E transgenic mice, the efficiency of clonal deletion was reported to be maximal when the transgene was expressed on both thymic epithelium and BM-derived cells.51,52 This situation was reflected in the case of the B6-IE mouse. In comparison with B6 mice, and as reported earlier53 in this mouse strain, 57% of CD4+Vβ5+ T cells and more than 89% of Vβ11+CD4+ T cells were deleted (Figure 8). In the CD8 compartment, a strong but less complete deletion was observed (86% deletion of Vβ5+ and 29% deletion of Vβ11+ T cells). In T cells from the B6 CD11c-IE strain, we found a comparable degree of deletion of potentially autoreactive T cells in the CD4 subset and significant, but less complete, deletion in the CD8 subset as reported earlier17 (Figure 8A). When thymic B cells were the only source for negative selection, a more incomplete deletion was observed (Figure 8A, CD19-IE). In this case 42% of Vβ5+CD4+ and 36% of Vβ11+CD4+ T cells were deleted by thymic B cells. In the CD8 compartment, no deletion of Vβ11+CD8+ T cells (Figure 8A; 5.7% more Vβ11+CD8+ T cells compared with B6 animals) and only a marginal deletion of Vβ5+CD8+ T cells (8.6% deletion) could be detected. When CD19-IE mice were crossed into the CD11c-IE background to create double-transgenic mice expressing I-E on both thymic APC types, B cells, and DC, we could observe a deletion that was in general comparable to the deletion in B6 CD11c-IE mice (Figure 8A, 6 CD11c-CD19-IE). Only in Vβ11+CD8+ T cells was deletion stronger than it was in B6 CD11c-IE mice, and it even exceeded slightly the deletion in B6-IE mice (Figure 8A, 6 CD11c-CD19-IE).
Clonal deletion of T cells as a consequence of I-E transgene expression.
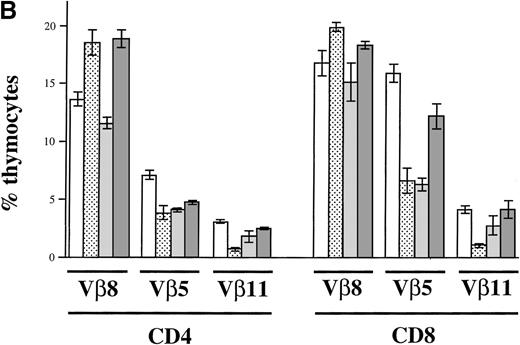
(A) Flow cytometric analysis of Vβ8, Vβ5, and Vβ11 expression among CD4+ and CD8+ LN T cells. LN cells of the 5 indicated mouse strains (n = 3 for B6-IE and B6 CD11c-IE; n = 6 for B6, B6 CD19-IE, B6 CD11c-CD19-IE) were triple stained with anti–CD4-PE, anti–CD8-CyChrome, and either anti-Vβ8.1,8.2-FITC, Vβ5.1, 5.2-FITC, or Vβ11-FITC, respectively. Results are expressed as a percentage of Vβ+CD4+ and Vβ+CD8+ cells. (B) Flow cytometric analysis of Vβ8, Vβ5, and Vβ11 expression among thymocytes. Total thymocytes were triple stained as described in (A). The percentages of positive cells among CD4+ or CD8+ thymocytes were determined (n = 3 for each type of transgenic mouse).
Clonal deletion of T cells as a consequence of I-E transgene expression.
(A) Flow cytometric analysis of Vβ8, Vβ5, and Vβ11 expression among CD4+ and CD8+ LN T cells. LN cells of the 5 indicated mouse strains (n = 3 for B6-IE and B6 CD11c-IE; n = 6 for B6, B6 CD19-IE, B6 CD11c-CD19-IE) were triple stained with anti–CD4-PE, anti–CD8-CyChrome, and either anti-Vβ8.1,8.2-FITC, Vβ5.1, 5.2-FITC, or Vβ11-FITC, respectively. Results are expressed as a percentage of Vβ+CD4+ and Vβ+CD8+ cells. (B) Flow cytometric analysis of Vβ8, Vβ5, and Vβ11 expression among thymocytes. Total thymocytes were triple stained as described in (A). The percentages of positive cells among CD4+ or CD8+ thymocytes were determined (n = 3 for each type of transgenic mouse).
To confirm that the observed negative selection took place in the thymus and was not a postthymic event, we performed the same analysis as described earlier in cell suspensions derived from thymi of the described animals (Figure 8B). Comparable to peripheral organs, a strong deletion of Vβ5+ and Vβ11+thymocytes could be observed in B6-IE and B6 CD11c-IE mice (Figure 8B). Similarly, a deletion could also be seen in thymi of CD19-IE animals (Figure 8B), where CD4+Vβ5+, CD4+Vβ11+, and CD8+Vβ5+ thymocytes show decreased percentages. Again, CD8+Vβ11+ thymocytes seem not to be deleted by I-E+ thymic B cells. These data confirm that the negative selection process of autoreactive T cells takes place in the thymus.
Taken together these data demonstrate a restricted role for B cells in thymic negative selection. Although in the compartment of CD4+ T cells a clear deletion was observed, in the CD8 compartment only a marginal (Vβ5+CD8+ T cells) or no deletion at all (Vβ11+CD8+ T cells) could be detected.
Discussion
By introduction of an MHC class II I-E transgene under the control of the human CD19 promoter, we expressed I-E exclusively on B cells. We could demonstrate that thymic B cells were able to induce negative selection but not positive selection of autoreactive thymocytes in vivo. With this skewing of function, they greatly resembled thymic DC; with a similar transgenic approach, we recently demonstrated negative but not positive selection functions for DC in vivo.17 This clearly differentiates DC and B cells from thymic macrophages. Despite the fact that all 3 cell types are BM-derived cells with potential APC capacities, only B cells and DC were able to negatively select CD4+ thymocytes. In contrast, macrophages cannot induce negative selection by clonal deletion, but they seem to induce tolerance in autoreactive thymocytes in vivo.10 One reason for this functional difference might be based on the geographic distribution of the various APC types within the thymus; though macrophages are scattered sparsely and equally throughout the thymic cortex and medulla,12,54 DC and B cells are concentrated in the medulla and the cortical–medullary junction.54 Because it has been demonstrated that Mls-reactive Vβ5+thymocytes in I-E positive mice are deleted in the medulla,12 the sparse appearance of macrophages in this region might explain their inability to negatively select by clonal deletion.10 In contrast, the nearly exclusive appearance of DC and B cells in the medulla54 might be why they negatively select by deletion. In this report we have further demonstrated that I-E–positive thymic B cells have a less strong capacity for thymic deletion of autoreactive thymocytes than thymic DC. These differential deletion capacities are comparable to the APC capacities of B cells and DC in peripheral lymphoid organs. In addition, DC have stronger stimulatory capacities to activate T cells than B cells.55 The incomplete ability of B cells to induce negative selection in the CD8 compartment might be a consequence of their weaker APC capacities. I-E reactive thymocytes are deleted in the medulla,12 which may explain why CD8 single, positive Vβ5+ and Vβ11+ thymocytes are not selected by B cells. The affinity of their MHC class I-specific TCR is in the absence of CD4 too low for MHC class II I-E on B cells, but additional interactions with costimulatory or accessory molecules on thymic DC could nevertheless lead to the observed deletion.
The ability to positively select thymocytes has been attributed to MHC-expressing cortical epithelial cells.1 The complete absence of positive selection capacities in B cells (this report) and DC17 in vivo was therefore confirmatory but contradicts a few studies in which positive selection of CD8 thymocytes by MHC class I-positive BM-derived cells was observed.30,31 Because neither DC nor B cells, nor both together (in B6 CD11c-IE × B6 CD19-IE/I-A−/− mice; data not shown) can induce positive selection in MHC class II-restricted CD4+ thymocytes in vivo, the observed positive selection events in other studies30,31 are difficult to explain. Either the macrophages, which have not been included in this study, induce this positive selection, or the described phenomena30 31 are unique for MHC class-I restricted CD8+ thymocytes. In this case 1 cannot exclude that MHC class I, which is expressed by thymocytes themselves, is responsible for positive selection.
Although all MHC class II-positive APC in the thymus are probably able to more or less efficiently induce negative selection of CD4+ thymocytes either by deletion (DC, B cells) or functional inactivation (macrophages), B cells and DC cannot induce the positive selection of CD4+ T cells.
Acknowledgments
We thank U. Mueller for microinjections and M. Riedinger for expert technical assistance, and we thank Drs M. Reth and H. Pircher for carefully reading the manuscript.
Supported by the Deutsche Forschungsgemeinschaft Leibniz–Program to M. Reth (T.B.) and by Deutsche Forschungsgemeinschaft grants Br1889/2-1 and Br1889/1-1 (T.B., P.K.). The Basel Institute for Immunology was founded and is supported by Hoffmann–La Roche Ltd, Basel, Switzerland.
Reprints:Thomas Brocker, Max–Planck–Institute for Immunobiology, Stuebeweg 51, D-79,108 Freiburg, Germany; e-mail:brocker@mm11.ukl.uni-freiburg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 6. Functional expression of the IE transgene on B cells from B6 CD19-IE mice induces antigen-specific proliferation in I-E restricted CD4 T cells. / B220-positive splenocytes from B6 and B6 CD19-IE animals were sorted, irradiated, and used as APC for preactivated T-cell blasts from AD10 TCR transgenic mice. The coculture was performed in the presence of graded amounts of antigenic peptide (MCC88-103), and proliferation was measured by [3H] thymidine incorporation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2610/4/m_bloo00811006x.jpeg?Expires=1769444437&Signature=mTgEqnzPqmWzbMFj0IxWtn8WnZ--vM8DB41GaS5R77VE30FEEyH9Le6pDIHyj6ciJNON0USPHTjWMgl7esRME8OXl4SqTBFqEZ6fhMDSuWXWiIAZMUvL~HsYxn63rKG7flpwnoROrHiHk4XhKy~Rr3NM3P1muU5802QTxuXFYJhDbKtYHMDfw770DcN2tO8kMNvAES9hMoJBoLOplep4iduEQKwhEHa7FyqN-4h3b5tJml5QD373JTyi~XiUz4YFsNOVQfqKgPl~91Ct57JZ8aYAl4y4D~Mn7UZaelp3Ubkc49I5SEckEtIwLraT7ebf3XpMpSVUT6IyN-pCf40FNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal