Abstract
Persistence of donor leukocytes in the circulation of recipients of intrauterine transfusion (IUT) has been observed up to 5 years after birth. The aim of this study was to determine whether transfusions with nonirradiated, nonleukocyte-depleted donor blood during the fetal period resulted in long-term immunomodulation of the recipient. Twenty-four surviving IUT recipients between 1966 and 1976 were tested for autoimmune disease and autoantibodies at follow-up. Ten had sex-mismatched donors and were therefore informative for chimerism studies using fluorescence in situ hybridization (FISH). Seven female recipients could be tested for chimerism using a Y- chromosome–specific polymerase chain reaction (PCR) because they received at least 1 IUT from a male donor. Nine recipients could be studied for cytotoxic T-lymphocyte precursor (CTLp) and helper T-lymphocyte precursor (HTLp) frequencies because the original donors were available for testing. All surviving IUT recipients were in good health at the time of the examination, and routine laboratory testing revealed no abnormalities. None of the IUT recipients were chimeric as determined by FISH analysis, but Y-chromosome–specific sequences were detected by PCR in 6 of the 7 women. However, the CTLp and HTLp frequencies of the IUT recipients against the donors were comparable to those of the controls. The current study provides evidence that IUT can result in the persistence of donor cells in the recipient for a period longer than 20 years but that it is not associated with immunotolerance or with signs of chronic antigenic stimulation.
Allogeneic bone-marrow transplantation can result in chronic graft-versus-host disease (GVHD) in long-term survivors. The symptoms of this condition mimics scleroderma, an autoimmune disease of unknown origin. In a large percentage of patients with chronic graft-versus-host disease, antinuclear (ANA), antismooth muscle, and antimitochondrial antibodies develop, which are considered autoantibodies.1 In a chimeric environment, this could be the result of an immune response of the donor cells against the ongoing allogeneic stimulus of the host. Recently, male cells, presumably derived from the fetus, have been isolated from peripheral blood in 40% to 60% of female patients with scleroderma, which is significantly higher than in the controls.2-4 This indicates that fetal–maternal microchimerism may be involved in the development of this disease. It has been suggested that microchimerism with fetal cells may also be associated with other autoimmune diseases.5 Pregnancy, twin–twin transfusion, solid organ transplantation, and (intrauterine) blood transfusion can result in the persistence of donor cells.
Because the induction of tolerance and chimerism is relatively easy to establish in the fetal and neonatal period,6 7 intrauterine transfusions (IUT) from unrelated donors provide a unique model for studying the immunomodulating effect of blood transfusion, including the persistence of donor cells in these otherwise healthy, unprimed persons.
The first successful intraperitoneal IUT was performed by Liley in 1963.8 In the first years after the introduction of this technique, it became apparent that IUT could result in transfusion-associated GVHD.9 10 This serious side-effect prompted obstetricians to transfuse only leukocyte-reduced and irradiated (25 Gy) blood.
Several investigators have observed a persistence of donor leukocytes in the circulation of IUT-treated patients up to 5 years after intraperitoneal IUT with nonfiltered, nonirradiated donor blood but without the development of GVHD.11-14 These studies revealed that approximately one third of the recipients of intraperitoneal IUT did not reject the transfused donor leukocytes, thus indicating that suppression of donor alloreactivity, induction of tolerance to donor antigens, or both must have taken place.
We sought to determine whether IUT with nonirradiated, nonfiltered donor blood resulted in the development of chimerism, long-term immunomodulation, alloantibodies, autoantibodies, or autoimmune disease in the recipient. Our results provide evidence that IUT can result in long-term microchimerism but that it is not associated with immunotolerance or with signs of chronic antigenic stimulation.
Materials and methods
Subjects
This study included 24 recipients of IUT therapy from 1966 to 1976. In this period, 89 fetuses were treated in our center with intraperitoneally administered IUTs for severe hemolytic disease. Of this group, 39 IUT recipients were born alive. Twelve died within 1 week of birth. Twenty-seven infants (9 girls, 18 boys) were eventually discharged from the hospital. Three of these surviving IUT recipients died before the initiation of this study. One died in a car accident at the age of 21 years. He was reported to be in good health before that. Two died as a result of complications after kidney transplantation. Both had urinary tract infections within 10 days of birth that progressed to terminal renal failure; they underwent transplantation at 4 and 20 years of age. All 24 remaining IUT recipients (9 women, 15 men) agreed to participate in the current study. The mean age of the subjects at the time of the examination was 24.3 years (range, 21 to 28 years). They had undergone a total of 65 IUTs (individual range, 1 to 5). The first IUT was administered at an average gestational age of 29.4 weeks (range, 26 to 31 weeks). The mean transfusion volume was 88 mL (range, 60 to 120 mL). The hematocrit of the transfused red cells was approximately 80%. The mean gestational age at delivery was 34 weeks (range, 31 to 37 weeks), and the mean birth weight was 2200 g (range, 1500 to 3170 g). All neonates received 1 or more exchange transfusions (average, 2; range, 1 to 3) and an occasional top-up transfusion after birth. All 24 subjects underwent transfusion only for the treatment of hemolytic disease. None of the female subjects had ever been pregnant. The medical histories of all 24 IUT recipients were taken, and physical examinations and routine laboratory tests were performed.
From this cohort of 24 subjects, 9 were selected on the basis that their first or second IUT donors were still available to be tested. Patients, IUT donors, and third-party controls were human lymphocyte antigen (HLA)-typed for class 1 and class 2 antigens by conventional serologic methods, based on complement-dependent cytotoxicity. Third-party controls were selected on the basis of HLA type and were matched for the HLA class 1 and 2 antigens shared between the original IUT donor and the patient and were mismatched for the nonshared HLA antigens whenever possible (Table1). This was done to mimic natural circumstances as closely as possible.
HLA typing of IUT patients, IUT donors, and third-party controls used for CTLp and HTLp frequency determination
| Patient . | HLA-A . | HLA-B . | HLA-DR . | Sex . | Additional Donor(s) . | FISH Analysis . | PCR Analysis . |
|---|---|---|---|---|---|---|---|
| 1 | 2, 11 | 35, 44 | 1, 12 | M | 1 (M) | No | No |
| IUT donor 2 | 2, 3 | 18, 35 | 1, 11 | M | |||
| third-party | 2, 24 | 8, 35 | 17 | ||||
| 2 | 2 | 60, 62 | 4, | F | 2 (2 M) | Yes | Yes |
| IUT donor 1 | 2, 32 | 8, 27 | 7, 17 | F | |||
| third-party | 2, 66 | 41, 44 | , 15 | ||||
| 3 | 2, 32 | 44, 57 | 11, 15 | F | 1 (F) | Yes | Yes |
| IUT donor 1 | 2, 31 | 51, 61 | 13 | M | |||
| IUT donor 2 | 2, 26 | 51, 60 | 4, 13 | F | |||
| third-party (1) | 2, 11 | 35, 62 | 4 | ||||
| third-party (2) | 2 | 62 | |||||
| 4 | 1, 26 | 8, 45 | 7, 17 | M | 1 (M) | No | No |
| IUT donor 2 | 2, 32 | 57, 60 | 15 | M | |||
| third-party | 3, 28 | 14, 39 | 13 | ||||
| 5 | 2 | 18 | 7, 11 | F | 2 (1 M, 1 F) | Yes | Yes |
| IUT donor 1 | 2, 31 | 27, 35 | 1 | M | |||
| third-party | 2, 24 | 7, 62 | 4 | ||||
| 6 | 2, 31 | 35, 60 | 1, 4 | F | 2 (2 M) | Yes | Yes |
| IUT donor 1 | 2 | 7 | 12, 15 | M | |||
| third-party | 2 | 62 | 13 | ||||
| 7 | 2, 24 | 7, 14 | 7, 15 | F | 2 (1 M, 1 F) | Yes | Yes |
| IUT donor 2 | 26, 32 | 38, 61 | 11, 13 | M | |||
| third-party | 1, 11 | 8, 57 | 17, 9 | ||||
| 8 | 11 | 55, 57 | 15, 17 | F | 3 (3 M) | Yes | Yes |
| IUT donor 1 | 25, 26 | 53, 57 | 11, 13 | M | |||
| third-party | 1, 23 | 57, 49 | 17, 8 | ||||
| 9 | 2, 3 | 7, 44 | 4, 15 | M | 0 | No | No |
| IUT donor 1 | 1, 2 | 8, 60 | 7, 17 | M | |||
| third-party | 2 | 27, 49 | 1, 14 |
| Patient . | HLA-A . | HLA-B . | HLA-DR . | Sex . | Additional Donor(s) . | FISH Analysis . | PCR Analysis . |
|---|---|---|---|---|---|---|---|
| 1 | 2, 11 | 35, 44 | 1, 12 | M | 1 (M) | No | No |
| IUT donor 2 | 2, 3 | 18, 35 | 1, 11 | M | |||
| third-party | 2, 24 | 8, 35 | 17 | ||||
| 2 | 2 | 60, 62 | 4, | F | 2 (2 M) | Yes | Yes |
| IUT donor 1 | 2, 32 | 8, 27 | 7, 17 | F | |||
| third-party | 2, 66 | 41, 44 | , 15 | ||||
| 3 | 2, 32 | 44, 57 | 11, 15 | F | 1 (F) | Yes | Yes |
| IUT donor 1 | 2, 31 | 51, 61 | 13 | M | |||
| IUT donor 2 | 2, 26 | 51, 60 | 4, 13 | F | |||
| third-party (1) | 2, 11 | 35, 62 | 4 | ||||
| third-party (2) | 2 | 62 | |||||
| 4 | 1, 26 | 8, 45 | 7, 17 | M | 1 (M) | No | No |
| IUT donor 2 | 2, 32 | 57, 60 | 15 | M | |||
| third-party | 3, 28 | 14, 39 | 13 | ||||
| 5 | 2 | 18 | 7, 11 | F | 2 (1 M, 1 F) | Yes | Yes |
| IUT donor 1 | 2, 31 | 27, 35 | 1 | M | |||
| third-party | 2, 24 | 7, 62 | 4 | ||||
| 6 | 2, 31 | 35, 60 | 1, 4 | F | 2 (2 M) | Yes | Yes |
| IUT donor 1 | 2 | 7 | 12, 15 | M | |||
| third-party | 2 | 62 | 13 | ||||
| 7 | 2, 24 | 7, 14 | 7, 15 | F | 2 (1 M, 1 F) | Yes | Yes |
| IUT donor 2 | 26, 32 | 38, 61 | 11, 13 | M | |||
| third-party | 1, 11 | 8, 57 | 17, 9 | ||||
| 8 | 11 | 55, 57 | 15, 17 | F | 3 (3 M) | Yes | Yes |
| IUT donor 1 | 25, 26 | 53, 57 | 11, 13 | M | |||
| third-party | 1, 23 | 57, 49 | 17, 8 | ||||
| 9 | 2, 3 | 7, 44 | 4, 15 | M | 0 | No | No |
| IUT donor 1 | 1, 2 | 8, 60 | 7, 17 | M | |||
| third-party | 2 | 27, 49 | 1, 14 |
The HLA-DR types that are underlined do not completely match the HLA selection strategy outlined in “Materials and methods.” M, male; F, female.
Fluorescence in situ hybridization (XY) of peripheral blood cells
A modified fluorescence in situ hybridization (FISH) analysis was performed as described by Price et al.15 Cytospin preparations containing 1 × 105peripheral blood mononuclear cells per slide were fixed using methanol and formaldehyde. After dehydration, FISH analysis was performed with fluorochrome-labeled X (pBAM × 5, fluorescein isothiocyanate labeled) and Y (pY 3.4, Cy-3 labeled) chromosome-specific probes. Intracellular DNA and the DNA probes were denatured by heating the slides for 4 minutes at 80°C. Hybridization was performed overnight at 37°C using 60% (vol/vol) formamide in 2 × SSC (pH 7.0). After washing with 2 × SSC (pH 7.0) and dehydration, the slides were mounted with antifade Vectashield mounting solution (Vector Laboratories, Utrecht, the Netherlands) containing 4′6-diamidino-2-phenylindole dihydrochloride (DAPI; 40 ng/mL) to counterstain the nuclei. Cells were examined for DAPI staining of DNA and fluorescent chromosomal staining using a Zeiss (Oberkochen, Germany) Axioscope 20 epifluorescence microscope. Three thousand cells were counted per slide to determine whether any expressed the phenotype of the opposite sex. Only cells with 2 chromosomal signals (XX or XY) were counted.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells were isolated using Ficoll– amidotrizoaat density gradient sedimentation directly after collection. They were frozen in RPMI medium (GIBCO, Paisley, UK) containing 20% fetal calf serum (Bodinco, Alkmaar, The Netherlands) and a final concentration of 10% dimethyl sulfoxide (Fluka, Buchs, Switzerland). The cells were cryopreserved at −70°C and then stored in liquid nitrogen until further use.
Polymerase chain reaction analysis for Y chromosome–specific sequences on peripheral blood
We studied 7 female IUT recipients treated with at least 1 intrauterine transfusion from a male blood donor for the presence of male-specific sequences in peripheral blood. Measures were taken to prevent contamination, including handling by a female investigator only, use of a dedicated room, dedicated equipment, dedicated reagents, and a laminar flow hood dedicated for this purpose only. Mononuclear cells from 20-mL blood samples were separated by Ficoll centrifugation. Cells were resuspended in phosphate-buffered saline with a final volume of 2 mL containing 10 × 106 cells. DNA was extracted from each subject's 2-mL suspension of mononuclear cells in phosphate-buffered saline according to the manufacturer's instructions (Qiagen, Westburg, The Netherlands). Because the long arm of the human Y chromosome contains 800 to 5000 copies of the 3.56-kb EcoRII fragment16 and eachEcoRII fragment contains 1 specific sequence (sequence accession number X06228) that can be amplified in PCR, total genomic DNA was digested with EcoRII (Boehringer Mannheim, Mannheim, Germany) for 2 hours at 37°C, thereby maximizing the amount of Y-specific sequences and reducing the possibility of sampling error. Subsequently, the digested DNA was precipitated overnight at −20°. The precipitated DNA was resuspended in distilled water. A specific 237-bp Y-chromosome sequence was detected by amplifying DNA in PCR with primers designed by our laboratory. The forward primer had the following sequence 5′ACA TTC CTT TTG GTT CCC TGC C3′, and the reverse primer had the sequence 5′GGA ATG GAA CAG AGA GCA ATG G3′. PCR reactions were performed in a total volume of 50 μL containing 5 μL 10 × PCR buffer (Life Technologies, Breda, The Netherlands), 10 mmol/L each dNTP (Promega, Leiden, the Netherlands), 10 pmoL each primer, 1 mmol/L MgCl2 (Life Technologies), 2 U Taqpolymerase (Perkin Elmer Cetus, Nieuwekerk aan de Ijssel, The Netherlands) and 1 μg digested DNA in 5 μL. Sixty amplification cycles were performed under the following conditions in a Perkin Elmer DNA Thermal Cycler: denaturation at 94°C (1 minute), annealing at 61°C (1 minute), and extension at 72°C (2 minutes). The PCR analysis contained a negative control (without DNA), a known positive sample for the Y sequence (male DNA), and sample DNA of nulligravid women who had never received a blood transfusion. The resultant Y chromosome–specific fragment was identified by ethidium bromide staining after electrophoresis on a 2% agarose gel using ultraviolet light. Serial dilutions showed that the DNA equivalent of 1 cell could be detected in a background of at least 1 × 106 female cells. We observed no amplifications from nulligravid women (n = 3) who had never undergone blood transfusion.
Limiting dilution analysis for determination of the CTLp and HTLp frequencies
To assess CTLp and HTLp frequencies of the IUT recipients, the original donors, and the third-party controls against each other, a single combined limiting dilution assay (LDA) was used as described previously.17 Responder lymphocytes were serial 2-fold diluted (from 40 000 cells/well to 625 cells/well) in 24 replicates using 96-well V-bottom plates (Greiner, Frickenhauzen, Germany). Responder cells were excluded from the last 24 wells. To each well, 50 000 irradiated (25 Gy) stimulator lymphocytes were added in a total volume of 100 μL RPMI 1640 medium (GIBCO) containing 10% heat-inactivated pooled human serum supplemented with 3 mmol/L L-glutamine (referred to as complete medium). After 3 days of culture, 80 μL supernatant was harvested and used for determination of the HTLp frequencies. The remaining cells were transferred to U-bottom plates (Costar, Cambridge, MA). Fresh complete medium (200 μL) containing 20 U/mL rIL-2 (Cetus, Amsterdam, The Netherlands) was added to each well on day 3. After 7 days of culture, half the medium was replaced with fresh complete medium containing 20 U/mL rIL-2. On day 10 of culture, a split-well CML analysis was performed. Half the medium was tested against 5000 europium-labeled phytohemagglutinin blasts prepared from the original stimulator to serve as targets. The other half was incubated with a noncytotoxic anti-CD8 monoclonal antibody (for 1 hour at room temperature) before 5000 target cells were added. After an incubation period of 4 hours, cells were pelleted by centrifugation, and 20 μL supernatant was harvested and transferred to 96-well flat-bottom with a low background fluorescence (Fluoroimmunoplate, Nunc, Roskilde, Denmark). Release of europium was measured using a time-resolved fluorometer (Fluoroimmunoplate). As controls, the spontaneous release (only target cells and medium) and maximum release (target cells and 1% Triton X-100; Fluka, Buchs, Switzerland) were also determined.
For measurement of HTLp frequencies, supernatant from the LDA culture (harvested at day 3) was tested against the IL-2–dependent CTLL-2 cell line as described.18 Briefly, supernatants were thawed and added to 3000 CTLL-2 cells that were cultured for 24 hours in IL-2–free medium. After incubation for 72 hours, lysing–staining–quenching medium containing Triton X-100 (Fluka), ink (Leitz, Wetzlar, Germany), and propidium iodide (Sigma, St. Louis, MO) in EDTA buffer was added. The plates were then read using an automated fluorescence microscope (Leica-Patimed, Wetzlar, Germany), which measures photometer values (mV) to determine the number of proliferating propidium iodine-stained nucleated cells and thus the proliferation of CTLL-2 cells. Because proliferation of the CTLL-2 cells is dependent on the amount of IL-2 present in the supernatants, this LDA can be used as a measure of the number of IL-2–secreting cells in the responder fraction. Because the T-helper cells are mainly considered responsible for IL-2 production, the HTLp frequencies can be estimated.
Frequencies of CTLp and HTLp were calculated as described by Strijbosch et al.19 In the CTLp assay, the goodness of fit was in some cases greater than 15, indicating that single-hit kinetics could not always be guaranteed (indicated by an asterisk in Table 1). In all HTLp assays, the goodness of fit was always less than 15. Statistical analysis was performed using the 2-tailed Mann-Whitney U test. P < .05 was considered significant.
Antibody screening test
We tested for the presence of auto-antibodies and HLA class 1 antibodies in 24 IUT recipients. Detection of antinuclear antibodies (ANA), smooth muscle antibodies, antimitochondrial antibodies, and parietal cell antibodies was performed using an indirect immunofluorescence assay on appropriate substrates as previously described.20 We screened for the presence of extractable nuclear antibodies by counterimmunoelectrophoresis with reference sera from a panel of selected patients.21 Thyroglobulin antibodies (TGA) and microsomal antibodies (MA) were detected by passive hemagglutination using commercially available kits (Murex, Burwedel, Germany). Screening for antibodies against HLA class 1 determinants was performed using the complement-dependent lymphocytotoxicity test.22
Results
Physical examination and routine laboratory blood testing
Medical history, physical examination, and routine laboratory blood testing of the 24 IUT recipients revealed no abnormalities. There was no evidence of active autoimmune disease, cancer, or signs and symptoms of immunodeficiency.
Most (70%) IUT recipients died within the fetal period or shortly after birth. Autopsy was performed on most deceased fetuses and babies. Because transfusion-associated GVHD was relatively unknown at that time, extensive searches for signs of this disease were not performed. Based on clinical records, personal communication with the obstetricians who performed the IUTs, and autopsy reports, there were no indications that any of these infants had symptoms or signs of active graft-versus-host disease.
2 IUT recipients died as a result of complications after kidney transplantation. One of these, severely hydropic, was born in poor condition. He had kidney failure (of unknown cause) from birth. Another was born in good condition, but a severe urinary tract infection, possibly caused by the insertion of a urinary catheter, developed 8 days after birth, and he responded poorly to standard antibiotic treatment. Despite specific antibiotic treatment, his renal function deteriorated. He underwent kidney transplantation at the age of 4. Unfortunately, he died at 18 years of age as a result of complications after a shunt operation.
Fluorescence in situ hybridization analysis on peripheral blood
Ten IUT recipients (7 females, 3 males) had sex-mismatched donors and could therefore be tested for chimerism using FISH analysis with X and Y chromosome-specific probes. The remaining 14 recipients (2 females, 12 males) were not tested because they received transfusions from donors of the same sex. For each IUT recipient tested, 3000 cells were examined, and none of these cells revealed a phenotype of the opposite sex. Therefore, the percentage of chimerism with donor cells did not exceed 0.1% (95% confidence interval). Six of these 10 IUT recipients, who were tested for the persistence of donor cells using FISH, were also included in the CTLp and HTLp studies (case numbers 2, 3, 5, 6, 7, and 8).
Polymerase chain reaction analysis for Y chromosome–specific sequences in peripheral blood
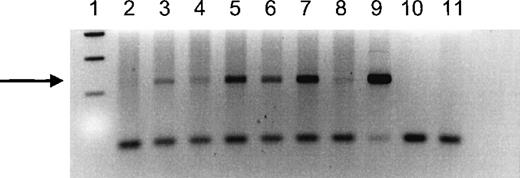
Seven female IUT recipients received at least 1 IUT from a male donor and were therefore informative for chimerism tested using a sensitive Y chromosome–specific PCR. We were able to detect Y chromosome–specific DNA in 6 of the 7 women tested (Figure1). The positive signal ranged from weak to strongly positive, but it was clearly visible in all positive women. In control experiments using DNA from nulligravid women without histories of transfusion, the Y-specific band could not be detected.
PCR analysis of a Y-specific sequence in DNA extracted from peripheral blood of female IUT recipients who received at least 1 transfusion from a male donor.
Recipient numbers correspond to recipient numbers in Table 1. Lane 1 shows 100-bp size markers; lane 2 shows DNA from recipient 5; lane 3 shows DNA from recipient 8; lane 4 shows DNA from recipient 6; lane 5 shows DNA from recipient 3; lane 6 shows DNA from recipient 2; lane 7 shows DNA from recipient 7; lane 8 shows DNA from an IUT recipient not tested by the CTLp/HTLp assay; lane 9 shows DNA from a normal male; lane 10 shows a blank; lane 11 shows DNA from a nulligravid woman. The samples in lanes 3 to 9 show a band corresponding to the 237-bp product amplified from Y-chromosome DNA (indicated by the arrow).
PCR analysis of a Y-specific sequence in DNA extracted from peripheral blood of female IUT recipients who received at least 1 transfusion from a male donor.
Recipient numbers correspond to recipient numbers in Table 1. Lane 1 shows 100-bp size markers; lane 2 shows DNA from recipient 5; lane 3 shows DNA from recipient 8; lane 4 shows DNA from recipient 6; lane 5 shows DNA from recipient 3; lane 6 shows DNA from recipient 2; lane 7 shows DNA from recipient 7; lane 8 shows DNA from an IUT recipient not tested by the CTLp/HTLp assay; lane 9 shows DNA from a normal male; lane 10 shows a blank; lane 11 shows DNA from a nulligravid woman. The samples in lanes 3 to 9 show a band corresponding to the 237-bp product amplified from Y-chromosome DNA (indicated by the arrow).
Cytotoxic T-lymphocyte precursor (CTLp) frequencies
Nine patients could be studied for CTLp and HTLp frequencies because the IUT donors were also available for testing. The CTLp frequencies of the IUT recipients against the first or second original IUT donor and against a mismatched third-party control were compared (Table2 and Figure2). In 1 case (IUT recipient 3) the CTLp was determined against the first and second donors. The CTLp frequencies of the IUT recipients against the original IUT donors (mean = 152/106) were similar to the responses of the third-party controls against the IUT donors (mean = 144/106) and slightly higher than the responses of the IUT recipients against the third-party controls (mean = 98/106; P = .176). The CTLp frequencies of the IUT recipients against the IUT donors and third-party controls decreased significantly when anti-CD8 monoclonal antibody was added 1 hour before target cells were added in the cell-mediated lympholysis phase of the test (inhibition 89% and 85%, respectively; Table 2), which is indicative for low-avidity (naive) cytotoxic T cells.23
CTLp and HTLp frequencies of IUT patients against 1 or more IUT donors
| Recipient . | No. IUTs {IUT tested} . | CTLp/1 × 106 . | HTLp/1 × 106 . | ||
|---|---|---|---|---|---|
| Donor (+FK18) . | 3dp (+FK18) . | Donor . | 3dp . | ||
| 1 | 2 {2} | 49 (14) | 58 (20) | 73 | 31 |
| 2 | 3 {1} | 370 (38) | 162 (28) | 1475 | 171 |
| 3 | 3 {1} | 141 (25) | 138 (15)* | 127 | 194 |
| 3 {2} | 40 (6) | 64 (12) | 180 | 215 | |
| 4 | 2 {2} | 71* | 99* | 175 | 886 |
| 5 | 2 {1} | 417 (20) | 88 (7)* | 969 | 357 |
| 6 | 1 {1} | 32 (2) | 43 (4) | 621 | 499 |
| 7 | 4 {2} | 134 (28) | 140 (19)* | 350 | 110 |
| 8 | 4 {1} | 226 (20) | 82 (11) | 40 | 64 |
| 9 | 1 {1} | 38 (0) | 111 (15) | 566 | 1175 |
| Recipient . | No. IUTs {IUT tested} . | CTLp/1 × 106 . | HTLp/1 × 106 . | ||
|---|---|---|---|---|---|
| Donor (+FK18) . | 3dp (+FK18) . | Donor . | 3dp . | ||
| 1 | 2 {2} | 49 (14) | 58 (20) | 73 | 31 |
| 2 | 3 {1} | 370 (38) | 162 (28) | 1475 | 171 |
| 3 | 3 {1} | 141 (25) | 138 (15)* | 127 | 194 |
| 3 {2} | 40 (6) | 64 (12) | 180 | 215 | |
| 4 | 2 {2} | 71* | 99* | 175 | 886 |
| 5 | 2 {1} | 417 (20) | 88 (7)* | 969 | 357 |
| 6 | 1 {1} | 32 (2) | 43 (4) | 621 | 499 |
| 7 | 4 {2} | 134 (28) | 140 (19)* | 350 | 110 |
| 8 | 4 {1} | 226 (20) | 82 (11) | 40 | 64 |
| 9 | 1 {1} | 38 (0) | 111 (15) | 566 | 1175 |
Precursor frequencies are displayed as number of precursors per 1 × 106 nucleated cells. No IUTs refers to the total number of IUTs; number between brackets ({ }) refers to the specific IUT donor tested. CTLp frequencies determined after addition of anti-CD8 antibody (FK 18) to effector cells are displayed in parentheses.
Frequencies with a goodness of fit > 15. 3dp, third-party controls.
CTLp and HTLp frequencies of IUT recipients, IUT donors, and third-party controls against each other.
Individual CTLp • and HTLp ○ frequencies of IUT patients, IUT donors, and third-party controls (3dp) tested against each other using different stimulator and responder combinations. Horizontal bars represent the mean value of the group.
CTLp and HTLp frequencies of IUT recipients, IUT donors, and third-party controls against each other.
Individual CTLp • and HTLp ○ frequencies of IUT patients, IUT donors, and third-party controls (3dp) tested against each other using different stimulator and responder combinations. Horizontal bars represent the mean value of the group.
Helper T-lymphocyte precursor frequencies
HTLp frequencies of the 9 IUT recipients against the 10 original IUT donor and the third-party controls are displayed in Table 2 and Figure2. Fetal exposure to donor antigens did not result in significantly higher antidonor-specific HTLp frequencies (mean, 457/106) when compared to the responses elicited by third-party controls (mean, 370/106; P = .641). The stimulatory capacities of IUT recipients, IUT donors, and third-party controls were comparable in the HTLp test.
Presence of antibodies
All 24 IUT recipients were tested for the presence of autoantibodies. These antibodies could be detected only in the serum of 1 male IUT recipient, in whom a weak reaction in the indirect immunofluorescence test with ANA and a low titer against TGA (1:320; cut-off value for normal controls, 1:80) was found. None of the IUT recipients had HLA class 1 alloantibodies or autoantibodies.
Discussion
Several investigators have observed the persistence of donor leukocytes in the circulation of IUT recipients up to 5 years after transfusion.11-14,24 Despite the use of standard cytogenetic analysis with low detection levels, Turner et al11 observed chromosomal chimerism in 23 of 65 IUT recipients at birth. Jones14 reported that the number of leukocytes from donor origin could exceed 50% but slowly decreased in the following years. Fowler et al25 revealed that newborns treated with exchange transfusions had prolonged skin graft acceptance from the same donor. However, this induction of tolerance was not sustained.
The long-term immunologic consequences of IUT are unknown. The current study was performed to determine the long-term influence of intraperitoneal IUT therapy on the health of IUT recipients in general and the immune system in particular. The survivors of IUT treatment could be studied for transfusion-induced sequelae such as immunization, autoimmune reactions, tolerance, and persistence of donor cells in their circulation after an interval of more than 20 years.
All patients were in good health at the time of examination. Their medical histories did not reveal any major illnesses, and routine laboratory testing did not show any abnormalities. In a 24-year-old male IUT recipient, a weak positive ANA and a low titer against TGA was observed. However, the prevalence of these autoantibodies in the general population is high (approximately 6%).26-28 We cannot exclude that the occurrence of terminal renal failure in 2 IUT patients was related to immunomodulatory effects of IUT treatment. Neither can we rule out immunomodulatory explanations for intrauterine or perinatal death of the IUT recipients ineligible for this study.
The results of this study indicate that IUT treatment with nonirradiated, nonfiltered blood from unrelated donors can result in long-term microchimerism. This state of chimerism could only be detected with the use of a sensitive Y chromosome–specific sequence PCR. Because microchimerism was detected in 6 of 7 women tested, it can be assumed that a low level of chimerism is present in most IUT patients who receive nonirradiated, nonleukocyte-depleted donor blood. The level of chimerism is low because these cells could not be detected with FISH analysis using X and Y chromosome–specific probes and could have been left unnoticed if techniques with low detection levels were used. The level of chimerism after more than 20 years is considerably lower when compared to the levels reported in the literature investigating the first few months to years after transfusion, when it could exceed 50%.14
Persistence of donor leukocytes in the circulation of the recipient has not only been observed after fetal or neonatal transfusions. Recently, Lee et al29 observed long-term survival of donor cells in immunocompetent patients who underwent severe trauma. These patients received multiple transfusions from several male or female donors, or both. In most female patients, Y-chromosome sequences were detectable after 0.5 year or more. In 2 patients, a mixed lymphocyte reaction (with the donors who provided the transfusions) was performed, revealing no or very low responses of the recipient against 1 male donor but none of the other male donors. Cells from these donors were presumed to be the source of microchimerism.
To investigate whether the persistence of donor cells in IUT recipients was the result of modulation of the alloimmune response against the original IUT donor, functional in vitro tests were used to determine the alloreactive capacity of the recipient against the original IUT donor. For this purpose, CTLp and HTLp frequencies of 9 IUT recipients were determined against 10 original IUT donors. Although there was considerable variation in the extent of these responses, normal HLA-mismatched pairs have a range of CTLp frequencies that varies from 1:4000 to 1:50 000.30,31 The frequencies correlate with the degree of HLA disparity between individuals. In general, the less the degree of mismatches between 2 persons, the lower the CTLp frequencies. If exposure to alloantigens during the fetal period resulted in tolerance induction against donor leukocytes, this would have been reflected in a decrease of CTLp and HTLp frequencies. Surprisingly, this was not the case because the CTLp and HTLp frequencies against the IUT donors were comparable to those against third-party controls. In 3 cases, CTLp frequencies against the IUT donors were even substantially higher when compared to third-party controls. To evaluate whether these high frequencies were the result of the establishment of a memory response, we investigated whether these CTL responses could be blocked by the addition of anti-CD8 monoclonal antibodies in the cytotoxic phase of the LDA. When blocking is observed, this indicates that T-cell receptors with a low avidity for the allo-antigen are involved. Generally, these are present on naive T cells, whereas committed CTLs cannot be blocked by anti-CD8 antibodies.32 33 In our experiments, all responses could easily be blocked by anti-CD8, both against the IUT donors and the third-party controls. This indicates that the high responses were not the result of previous priming.
It is intriguing that the persistence of donor cells in vivo is not associated with tolerance induction in vitro. In the first place, it could be that the persistent cells were not derived from the IUT donor studied but from a subsequent IUT transfusion(s) or exchange transfusion donor after birth. The observations of Lee et al29suggest that persistent donor cells are associated with nonresponsiveness against 1 of multiple donors. However, the nonresponsiveness is not explained by a high degree of HLA sharing. We hypothesized that the donors used at the earliest gestational age were the most likely candidates to induce nonresponsiveness. We realize that this selection criterion is arbitrary. Secondly, this state of chimerism does not result in the (central) deletion of alloreactive, donor-specific T cells of the IUT recipients. Presumably other (peripheral) mechanisms account for the escape of these cells from normal immune surveillance, indicating selective tolerance for the surviving donor cells. This is in accordance with the observation that fetal cells can survive in the maternal circulation for more than 27 years.34 Persistent HLA nonidentical cells might evade the normal alloimmune response by down-regulation of molecules essential for immune recognition. Similar mechanisms have been observed in the immune response against tumor cells. Therefore, further studies will be carried out to determine the nature of the chimeric cells and to determine which immuno-regulatory molecules are expressed to explain the escape from rejection. Because recent studies indicate that microchimerism with fetal cells in the maternal circulation might be involved in the pathogenesis of systemic sclerosis,2,3 IUT therapy with nonirradiated, nonleukocyte-depleted donor blood might create serious potential side effects. However, more than 25 years have passed after IUT treatment, and all 24 participants in the current study are in good health. A major difference with studies on systemic sclerosis is that HLA compatibility between a child born to a mother with systemic sclerosis was strongly associated with disease risk,2 whereas in our studies the IUT donor and recipient were extensively HLA mismatched. The current study indicates that the persistence of allogeneic donor cells is not per se associated with any major illness.
Acknowledgments
We thank Dr D. L. Roelen and Dr G. C. Beverstock for critically reading the manuscript, and Monique van Lint, Jaqueline Waaijer, and Patrick Zeeuwen for their expert technical work.
Supported by a grant from the Macropa Foundation.
Reprints:Henk E. Viëtor, Department of Human Genetics 417, University Hospital Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: h.vietor@antrg.azn.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal