Abstract
The BCL6 gene, isolated from the breakpoints of 3q27-associated chromosomal translocations, has been implicated in diffuse large B-cell lymphomas (DLBL). Here we describe the molecular characterization of novel t(3;7)(q27;p12) translocations in 2 patients with DLBL. Molecular genetic analysis of the breakpoint area involving BCL6 revealed the presence of the Ikaros gene, a central regulator of lymphoid differentiation that had been mapped to human chromosome 7 band p13-p11.1. As a molecular consequence of the translocation, the 5′ regulatory region of the BCL6 gene was replaced by the putative 5′ regulatory region of theIkaros gene, probably leading to deregulated expression of theBCL6 gene throughout B-cell differentiation. Reverse transcription-polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) analyses of a patient sample established that the t(3;7)(q27;p12) results in fusion of the Ikaros andBCL6 genes. This study provides the first evidence that the Ikaros gene is rearranged in human hematopoietic malignant disorders.
The BCL6 gene has been implicated in the etiology of some non-Hodgkin's lymphomas (NHLs), especially diffuse large B-cell lymphomas (DLBLs).1-5 The BCL6 gene is located at 3q27 and has been isolated from recurrent breakpoint sites of chromosomal translocations in DLBLs. Rearrangement of theBCL6 gene was found in as many as 40% of DLBLs and in 10% of a subset of follicular lymphoma (FCL).6,7 These alterations are thought to cause deregulated expression of the BCL6 gene by means of a novel mechanism, promoter substitution—that is, the juxtaposition of heterologus promoters from different chromosome loci to the intact BCL6 coding region.8
The chromosomal translocations involving BCL6 are variable.9 In addition to the immunoglobulin (Ig) loci, 14q32 (IgH), 2p11 (IgLκ), and 22q11 (IgLλ), they also include 1q21, 2q21, 4p11, 5p13, 9p13, 11q23, 12p11, 12q13, and 15q21.7,9Recently, the small G protein TTF on 4p11, a B-cell–specific transcriptional activator BOB1 on 11q23, and histone H4 on 6p21 have been isolated from cases or cell lines with 3q27 translocations.10-12 Each gene of these translocations formed a fusion transcript with BCL6 by removing the first BCL6 noncoding exon, thus leaving this oncoprotein intact. We recently reported on 2 patients with DLBL with novel t(3;7)(q27;p12) translocations.13 We report here the case of a patient with DLBL whose Ikaros gene, a central regulator of lymphoid differentiation, fused to the BCL6 gene as a result of t(3;7)(q27;p12) translocation. Study design
5′-rapid amplification of complementary DNA (cDNA) ends (RACE)
First strand cDNA was generated by reverse transcription (RT) using the antisense primer 1 in BCL6 exon 3: (5′-TGTCGACTCCGGAGACGATTAAGGT-3′), followed by the addition of dCTPs to the 3′ end of the cDNAs and polymerase chain reaction (PCR) using 5′ RACE abridge anchor primer (5′-GGCCACGCGTCGACTAGTACGGGGG GGGGG) and the nest antisense primer 2 in exon 2 (5′-ACTGGATACAGCTGTCAG CC GGCGA-3′). The diluted PCR products were further amplified with the abridged universal amplification primer (5′-GGCCACGCGTCGACTAGTAC-3′) and the nest antisense primer 3 from exon 2 (5′-CCAAAATTTTGCTCAAAC-3′). The final PCR product was sequenced by using an automated 372 DNA sequencer (Applied Biosystems, Foster City, CA).
Genomic DNA and cDNA library construction
The genomic DNA library from patient 2's lymphoma samples and the cDNA library from a human B-cell lymphoma cell line, SP49, were constructed as described elsewhere.14 The genomic library (3 × 105 plaque- forming units) was screened with a 4.0-kilobase (kb) BCL6 major translocation cluster probe (Figure2).3
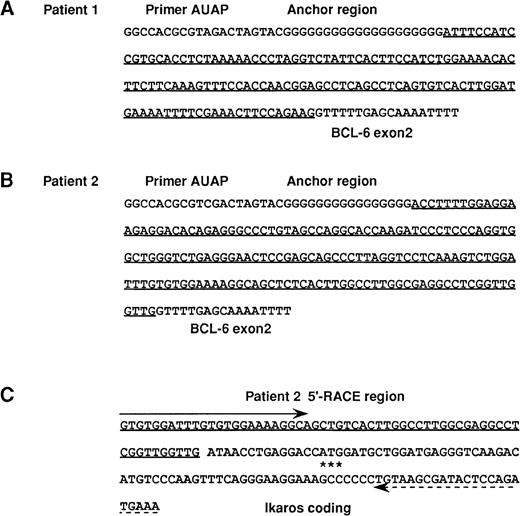
5′ RACE assay of patients 1 and 2 and RT-PCR analysis of 5′ noncoding region of Ikaros gene.
(A, B) The foreign sequences identified with the 5′-RACE method in 2 patients with DLBL with t(3;7)(q27;p12). (A) patient 1, (B) patient 2. The foreign sequences fused to BCL6sequence are underlined. GenBank accession no. AF180297. (C) Sequence of the same RT-PCR products of 4 B-cell lines obtained with a sense primer (shown by arrow) for the foreign sequence identified in patient 2 and an antisense primer (shown by dashed arrow) for the Ikaros coding region. The foreign sequence (underlined) in this patient was found to correspond to a part of the 5′ noncoding region of theIkaros gene. The translation initiation ATG codon of theIkaros gene is indicated with 3 asterisks.
5′ RACE assay of patients 1 and 2 and RT-PCR analysis of 5′ noncoding region of Ikaros gene.
(A, B) The foreign sequences identified with the 5′-RACE method in 2 patients with DLBL with t(3;7)(q27;p12). (A) patient 1, (B) patient 2. The foreign sequences fused to BCL6sequence are underlined. GenBank accession no. AF180297. (C) Sequence of the same RT-PCR products of 4 B-cell lines obtained with a sense primer (shown by arrow) for the foreign sequence identified in patient 2 and an antisense primer (shown by dashed arrow) for the Ikaros coding region. The foreign sequence (underlined) in this patient was found to correspond to a part of the 5′ noncoding region of theIkaros gene. The translation initiation ATG codon of theIkaros gene is indicated with 3 asterisks.
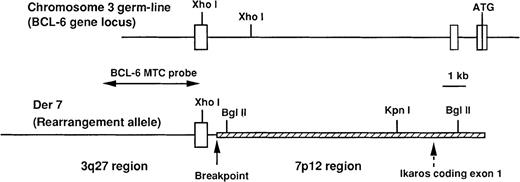
Schematic representation and restriction enzyme map of the chromosome 3 germ-line configuration (BCL6 gene locus) and the chromosome 7 derivative (der 7).
The 3q27 regions are shown by lines, and the 7p12 region by hatched bars. The breakpoint is indicated with a vertical arrow, and the presence of the Ikaros coding-exon 1 with a dashed vertical arrow. Exons I to III of the BCL6 gene are boxed. A BCL6 major translocation cluster (MTC) probe was used to screen a genomic DNA library constructed from patient 2.
Schematic representation and restriction enzyme map of the chromosome 3 germ-line configuration (BCL6 gene locus) and the chromosome 7 derivative (der 7).
The 3q27 regions are shown by lines, and the 7p12 region by hatched bars. The breakpoint is indicated with a vertical arrow, and the presence of the Ikaros coding-exon 1 with a dashed vertical arrow. Exons I to III of the BCL6 gene are boxed. A BCL6 major translocation cluster (MTC) probe was used to screen a genomic DNA library constructed from patient 2.
Fluorescence in situ hybridization (FISH) analysis
Results and discussion
To determine the molecular genetic basis for the 2 DLBL cases with t(3;7)(q27;p12) translocation,13 we first performed the 5′ RACE assay of the total RNA samples from patients 1 and 2. As expected, sequence analysis of the resultant PCR products revealed that 5′-foreign sequences were fused to the BCL6 exon 2 region, as shown in Figure 1A and B. However, a BLAST homology search revealed that these sequences did not bear any significant homology with any known sequences. We confirmed the presence of a specific-fusion transcript in each case by performing RT-PCR analysis with a sense primer for the foreign sequence identified by RACE and an antisense primer for the BCL6 exon 3 region (data not shown).
To clone the genomic DNA fragments that contain the chromosomal breakpoint of t(3;7)(q27;p12), we next constructed patient 2's lymphoma sample–specific genomic DNA library in a λDASH II phage vector. This screening allowed us to isolate 2 different genomic clones with insert sizes of 19 and 23 kb, as schematically illustrated in Figure 2. Restriction enzyme mapping and Southern blot analyses showed that the clone of 19 kb represented the BCL6 germ-line allele and the clone of 23 kb represented the rearranged allele.
The 2 fragments (7-kb Bgl II-Kpn I and 2.5-kb Kpn I-Bgl II in Figure 2) derived from the 7p12 locus of the rearranged allele were used for Northern blot analysis to examine whether this region included transcribed exons. At least 2 hybridization bands of approximately 5 to 8 kb were clearly detected by Northern blot analysis of a B-lymphoma cell line, SP-49 (data not shown). To isolate the BCL6 partner transcript, the genomic Kpn I-Bgl II fragment of 2.5 kb was then used to screen an SP-49 random-primed cDNA library constructed in λgt 10. BLAST homology search disclosed that the sequences of the 2 isolated cDNAs completely matched that of the human Ikaros, which was shown to play a central role in lymphoid differentiation.17 18
To confirm whether the foreign sequence fused to the BCL6transcript was derived from the Ikaros gene, we performed RT-PCR analysis of the total RNA samples from 4 B-cell lines (NALM6, SP-49, Raji, and KMS-12-BM) without BCL6 gene rearrangement at various differentiation stages by using a sense primer for the 5′-foreign sequence derived from patient 2 and an antisense primer for the Ikaros-coding exon 2 sequence. Sequence analysis of the amplified PCR products of the same size from these 4 cell lines led to identification of the fusion of the foreign sequence to the Ikaros region, thus establishing that the foreign sequence was indeed derived from the 5′ noncoding region of the Ikaros gene (Figure1C). Our observation is in agreement with an earlier report by Molnar et al19 that the human Ikaros gene is extremely large and complex, and it seems likely that as yet unidentifed 5′ noncoding exons are present in the humanIkaros gene. On the other hand, no apparent RT-PCR product could be amplified from any of the 4 above mentioned B-cell lines when a sense primer was used for the 5′-foreign sequence derived from patient 1 and an antisense primer for the Ikaros-coding exon 2 sequence. Thus, the origin of the foreign sequence (Figure 1A) from patient 1 remains unclear.
Finally, FISH analysis with a YAC clone 19GA1015 upstream of the BCL6 locus and a BAC clone, including the C-terminal portion of the Ikaros gene, provided further evidence for our conclusion that the Ikaros and BCL6 genes are fused as a result of the t(3;7) translocation in patient 2 (data not shown).
The Ikaros gene was previously assigned to chromosome band 7p13-p11.1,17 which is in agreement with our finding that this gene is rearranged in a human DLBL with t(3;7)(q27;p12). Because only 3 cases with t(3;7) have been reported in the literature,4,13 the frequency of involvement of theIkaros gene in DLBL appears to be relatively low. Given the importance of the Ikaros gene in the ontogeny of the murine lymphopoietic system, it has been presumed that it may be involved in some forms of human severe combined immunodeficiency, leukemia, and lymphoma.18,19 In fact, it has recently been found that acute infant leukemia often express dysfunctional dominant negative Ikaros isoforms, implicating Ikaros in leukemogenesis.20 This study provides the first evidence for the involvement of Ikaros gene rearrangement in human hematopoietic malignant disorders. Another group has recently performed the 5′-RACE analysis of 18 patients with DLBL carrying the BCL6 gene rearrangement, and one of theBCL6 partner genes was found to represent the Ikarosgene (Dr Moriyama, personal communication, June 1999). Together with the results of others, the chromosomal translocation between the Ikaros and BCL6 genes has now been shown to recurrently occur in human DLBLs.
Acknowledgments
We wish to thank Mr T. Yamaguchi and Y. Onizuka for their valuable technical assistance. We also thank Dr L. A. James (University of Manchester) for his kind donation of the YAC clone 19GA10.
Supported in part by a Grant-in-Aid for the Second-Term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan; a Grant-in-Aid for Science on Primary Areas (Cancer Research) from the Ministry of Education, Science and Culture, Japan; and a Bristol-Myers Squibb Unrestricted Biomedical Research Grant.
Reprints:Yoshitaka Hosokawa, Laboratory of Molecular Medicine, Aichi Cancer Center Research Institute, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681, Japan; e-mail:yhosokaw@aichi-cc.pref.aichi.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal