We read with interest the paper of Dördelmann et al1 on prednisone (PDN) response as the strongest predictor value of outcome in infant acute lymphoblastic leukemia (ALL).
Between June 1989 and November 1998, 60 (3.1%) infants, among 1963 children less than 18 years of age, were registered and treated according to the Children Leukemia Cooperative Group-European Organization for Research and Treatment of Cancer (CLCG-EORTC) protocol 58881. Patients were stratified according to the same risk factor (RF) as defined in Dördelmann et als paper:1 RF < 0.8 assigned them to the low risk (LR) group, and RF ≥ 0.8 to the standard risk (SR) group. Patients with prednisolone poor response (PPR), with t(9;22) or t(4;11) and/or not in remission (marrow blasts < 5%) on day 35 were assigned to the very high risk group (VHR).
Protocol CLCG-EORTC 58881 was very similar to the protocol ALL-BFM86 except for the following points: (1) prednisolone instead of prednisone and additional It MTX were used during induction, (2) protocol II was given to all LR and SR patients, (3) VHR patients, after induction, received 9 blocks of multiagents chemotherapy, followed by conventional maintenance for 1 year. In addition, a proportion of the patients were randomized to receive the following: (1) eitherErwinia or E coli asparaginase (all ALL patients in induction and consolidation) (2) HD MTX versus Ara-C plus HD MTX during interval therapy (standard risk patients) (3) pulses of 6 MP IV during maintenance therapy (all patients). In contrast with Berlin-Frankfurt-Münster (BFM) protocol, no patient received prophylactic or therapeutic cranial irradiation.
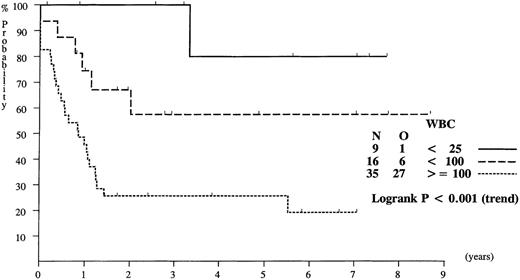
The overall event free survival (EFS) rate at 4 years in the 60 ALL infants treated with the same protocol was 41%. Univariate analysis of prognostic factors is given in the Table. Age below 6 months had no significant influence on EFS, but white blood cell (WBC) counts of at least 100 × 103/μL, PPR, presence of very high risk features, immunophenotype (CD10− or CD34+) had a significative negative influence on EFS. The leucocyte count had the most important impact. The EFS of infants with initial leucocyte count lower than 100 × 103/μL was 65%, which is comparable to that of older children with initial leucocyte count between 10 and 100 × 103μL (Figure 1).
Outcome according to presenting features in infant ALL treated with EORTC protocol #58881
| . | n . | EFS (%)* . | HR† . | P value . |
|---|---|---|---|---|
| Age | ||||
| Less than 6 mo | 34 | 30 | 1.7 | .13 |
| At least 6 mo | 26 | 56 | 1 | |
| WBC (×103/μL) | ||||
| Lower than 25 | 9 | 80 | 1 | |
| Lower than 100 | 16 | 57 | 4.1 | <.001 |
| At least 100 | 35 | 26 | 11.6 | |
| Prednisolone response | ||||
| Good (<1000/μL) | 38 | 48 | 1 | .02 |
| Poor (≥1000/μL) | 21 | 29 | 2.1 | |
| VHR feature | ||||
| No | 19 | 64 | 1 | .01 |
| Yes | 41 | 27 | 2.8 | |
| Immunophenotype | ||||
| CD10− | 39 | 29 | 3.7 | .02 |
| CD10+ | 11 | 79 | 1 | |
| CD34+ | 18 | 15 | 2.3 | .03 |
| CD34− | 21 | 56 | 1 | |
| Cytogenetics | ||||
| T(4;11) Yes | 26 | 23 | 2.5 | .01 |
| No | 23 | 63 | 1 |
| . | n . | EFS (%)* . | HR† . | P value . |
|---|---|---|---|---|
| Age | ||||
| Less than 6 mo | 34 | 30 | 1.7 | .13 |
| At least 6 mo | 26 | 56 | 1 | |
| WBC (×103/μL) | ||||
| Lower than 25 | 9 | 80 | 1 | |
| Lower than 100 | 16 | 57 | 4.1 | <.001 |
| At least 100 | 35 | 26 | 11.6 | |
| Prednisolone response | ||||
| Good (<1000/μL) | 38 | 48 | 1 | .02 |
| Poor (≥1000/μL) | 21 | 29 | 2.1 | |
| VHR feature | ||||
| No | 19 | 64 | 1 | .01 |
| Yes | 41 | 27 | 2.8 | |
| Immunophenotype | ||||
| CD10− | 39 | 29 | 3.7 | .02 |
| CD10+ | 11 | 79 | 1 | |
| CD34+ | 18 | 15 | 2.3 | .03 |
| CD34− | 21 | 56 | 1 | |
| Cytogenetics | ||||
| T(4;11) Yes | 26 | 23 | 2.5 | .01 |
| No | 23 | 63 | 1 |
Event free survival rate at 4 years.
Hazard ratio completed via the logrank O/E ratio method.
Probability of EFS for infants with ALL, according to white blood cell (WBC) counts at diagnosis.
Probability of EFS for infants with ALL, according to white blood cell (WBC) counts at diagnosis.
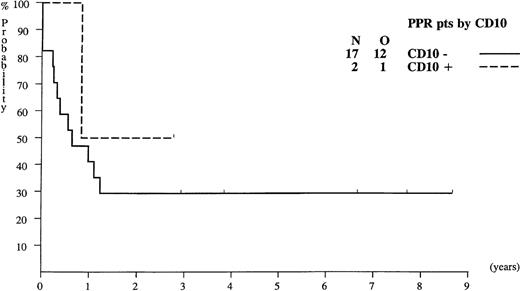
Probability of EFS for infants with ALL presenting poor prednisolone response (PPR), according to their CD10 expression.
Probability of EFS for infants with ALL presenting poor prednisolone response (PPR), according to their CD10 expression.
In contrast with the ALL-BFM results, the EFS of the 17 PPR infants with CD10− ALL was 29% (Figure 2), whereas in the Germany study, none of these infants survived. The difference between the 2 studies might be treatment-related.
Nevertheless, the overall results are similar with a global EFS of 41%. Of note is that the proportion of infants with CD10+ and steroid good response (PGR) respectively was higher in the BFM study than in the EORTC 58881 trial: 40% versus 22% for CD10+ and 74% versus 64% for PGR. Probably, the percentage of VHR patients is higher in the EORTC series, as the proportion of PPR was higher, due to the late administration of the It MTX.2
The number of patients was small in both studies (although twice as high in the BFM study), and conclusions with regard to the relation weight of different prognostic features should not be accepted without qualification. Results of the ongoing international protocol Interfant 99 will be essential to a refined ranking of the most important prognostic factors and to further progress in the treatment of this high-risk group.3-6
Early response in infant ALL determines prognosis
Department of Pediatric Hematology and Oncology
Hannover, Germany
Department of Pediatric Hematology and Oncology
Hannover, Germany
We are pleased that our study on prognostic factors in infant acute lymphoblastic leukemia (ALL) during BFM trials1-1provoked some discussion as indicated by Ferster et al's letter. We think their data may give some important additional information regarding the prognostic value of certain clinical and biologic features in infant ALL. But in our opinion, their results are neither new nor inconsistent with our results. They also do not substantially question the findings and conclusions of our study.
In contrast to Ferster et al's statement, we think that the EORTC used both different treatment stratification and different treatment for a substantial proportion of infants. Because antileukemic treatment itself is a well-known prognostic factor, this may significantly influence the prognostic value of clinical or biologic features.1-2 On the one hand, patients treated under the CLCG-EORTC protocol 58881 were stratified according to the same risk features, namely, tumor cell burden (BFM risk factor) and prednisone response. But in contrast to BFM, the translocation t(4;11) qualified for treatment in the highest-risk group (VHR in EORTC), and this may explain the much higher percentage of VHR patients in the EORTC series as compared to our study also (68% vs 26%). Among infants, the proportion of patients carrying this specific translocation is relatively high and ranged between 30 and 50% in several reports,1-3-1-7 including the EORTC (53%) and the BFM (31%) studies. Because of carrying t(4;11) alone, almost half of all infants in the EORTC trial (26 of 49) received the very intensive high-risk treatment regimen, as compared to 12% of infants in our series, who presented with a prednisone poor response (PPR) and the t(4;11). According to our data, more than half of the infants with t(4;11) had a prednisone good response (PGR) and were treated with standard BFM therapy (medium-risk group). In addition, application of cytarabine during interval therapy for EORTC-SR patients (corresponding to BFM-MR) may be an additional significant treatment difference because cytarabine is considered to be potent drug for treatment for AML, hybrid leukemias, and infant ALL. Whether the higher percentage of PPR in this EORTC trial (35%), as compared to our study (26%), was caused by the early administration of 1 intrathecal dose of methotrexate (on day 1) under BFM protocols remains speculative. It could also be explained by the small number of patients.
We agree, that most, if not all, differences between both studies are caused by both the smaller number of patients evaluated (EORTC, n = 60; BFM, n = 106) and, possibly, the type of treatment. We certainly agree that ”conclusions with regard to relation weight of different prognostic features should not be accepted without qualification.” But definition of independent parameters predicting outcome in infant ALL has been difficult so far. Besides our study, only 2 investigations have reported independent prognostic features in infant ALL: MLL gene rearrangement1-8 and/or cytogenetic 11q23 rearrangement.1-9
Regarding analysis of prognostic factors in infant ALL, Ferster et al basically demonstrated similar results to those of most other investigations, including our group's, with 2 exceptions: (1) that age less than 6 months was no significant prognostic factor and (2) that WBC was the most important prognostic feature in their study. The pEFS for infants older or younger than 6 months was almost exactly the same as in the respective subsets of our study, but for unknown reasons their outcome difference was not significantly different. Nevertheless, in accordance with other reports,1-3 1-7 age was the second most important prognostic feature in our series, even by multivariate analysis. By univariate analysis, we also noted both WBCs of at least 100 000/μL and the lack of CD10 expression as poor prognostic features. The pEFSs for these subgroups were almost comparable in both trials (EORTC vs BFM: 26% vs 37% respectively, and 29% vs 35%, respectively). But both features lost their significance in multivariate anlysis. Because the EORTC analysis was performed by univariate analysis only, the independent value of the reported prognostic features remains unclear. Finally, the analyses of EORTC protocol 58881 could be hampered by the relatively short observation time. The last patient evaluated entered the trial in November 1998 and was probably just off treatment by the time of data analysis.
Based on the BFM infant data, an ongoing international trial for treatment of infant ALL (INTERFANT 99) uses the prednisone response as the only stratification factor. Among many others, the EORTC-CLCG also takes part in this trial and has agreed to its treatment stratification. The value of prognostic features should be proved by prospective studies and multivariate analysis. Therefore, we may need to wait for studies by other investigators and/or the results of the INTERFANT 99 trial.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal