Secondary neoplasms (SNs) represent serious late complications after successful treatment of malignant diseases. To evaluate the rate and type of SNs after Berlin-Frankfurt-Münster (BFM) treatment in children with acute lymphoblastic leukemia (ALL), we analyzed the data from the BFM database and the German Childhood Cancer Registry (GCCR). Between April 1979 and April 1995, 5006 children with B-precursor or T-ALL were enrolled in 5 ALL-BFM multicenter trials. The median follow-up time from diagnosis was 5.7 years (range 1.5-18 years). By December 1997, 52 SNs were documented, including 16 acute myeloid leukemias (AMLs), 13 neoplasms of the central nervous system (CNS), and 23 other neoplasms. Compared with the expected numbers estimated from incidence rates derived from the GCCR, this represented a 14-fold increase for all cancers and a 19-fold increase for CNS tumors. SNs developed 0.9 to 15 years (median: 6 years) after the diagnosis of ALL; 46 patients were in first complete remission (CR). The overall cumulative risk of SNs at 15 years was 3.3% (95% confidence interval [CI]: 1.6%-5.1%) and 2.9% (95% CI: 1.6%-4.2%) in first CR. The risk was 3.5% (95% CI: 1.5%-5.5%) after treatment, including cranial irradiation and significantly lower in nonirradiated patients: 1.2% (95% CI: 0.2%-2.3%;P = .048). The development of secondary AML was not associated with the use of any specific cytotoxic agent. Considering the high-survival rate of this large unselected ALL cohort, the risk of SN is relatively low, though higher, especially after cranial irradiation, than in the general population. Long-term follow-up is mandatory, and further SNs with longer latency periods are to be expected.
Intensive multimodal therapy has improved the survival of many patients with different types of neoplasms in childhood. The treatment of children with acute lymphoblastic leukemia (ALL) has become increasingly successful with an overall survival rate of almost 80% in the last Berlin-Frankfurt-Münster (BFM) study.1 However, the immunosuppressive and cytotoxic therapy necessary to achieve this improvement increases the risk of subsequent complications. One of the severe late effects is the occurrence of a second cancer. Compared with age-matched controls, individuals with a history of childhood cancer have been estimated to have a 10- to 20-fold greater risk of developing a secondary malignant neoplasm.2 Genetic predisposition, previously administered chemotherapy, and/or radiotherapy are considered the most important risk factors.
To determine the occurrence and type of secondary neoplasm (SN) among children with ALL previously treated by 1 of the ALL-BFM protocols since 1979 and to identify possible risk factors, we analyzed data from the BFM database and the German Childhood Cancer Registry (GCCR).
Patients and methods
Between April 1979 and April 1995, 5006 children up to 20 years of age with newly diagnosed B-precursor or T-cell ALL enrolled in 1 of the 5 BFM multicenter trials (ALL-BFM 79, 81, 83, 86, and 90) for previously untreated ALL were included in the current study. Of the patients, 57% were male, and the median age at diagnosis was 4.8 years (range: 5 days-20 years). Informed consent from the guardians was obtained for every patient. In all trials, patients were stratified and randomized into well-defined subgroups. In trial ALL-BFM 79, patients were stratified according to their risk index (RI), based on white blood cell count, central nervous system leukemia (CNS-L), mediastinal mass, age, and cytochemical reaction.3 In trial ALL-BFM 81 and 83, patients were stratified by leukemic cell burden (RF) only, based on peripheral blast count, liver, and spleen size at the time of diagnosis.4,5 In trials ALL-BFM 86 and 90, the initial response to prednisone ( = number of lymphoblasts in blood after a 7-day exposure to prednisone) was used as an overriding stratification factor in addition to the leukemic cell burden at diagnosis.5 6
Details of the treatment regimens have been previously published.3-7 The cumulative doses of the important treatment elements are listed in Table 1. Mean administered cyclophosphamide dose was 3000 mg/m2(range: 2000-5000 mg/m2), mean anthracycline dose was 240 mg/m2 (range: 120-280 mg/m2). Epipodophyllotoxins, only part of some ALL high-risk regimes, were administered up to 1.6 g/m2. Major treatment changes between the trials mainly concerned the high-risk regimen, except for the intravenous methotrexate (IV MTX) and cranial irradiation (CRT) dosages. Cyclophosphamide or ifosphamide and anthracyclines were part of the treatment for all patients. Treatment after relapse was taken into consideration when applicable. This included epipodophyllotoxins and alkylating agents for all relapsed patients.
Cumulative doses of selected chemotherapeutic agents and radiation of ALL-BFM trials 79 to 90 according to the risk groups
| Study Risk Group . | Patients* (%) . | DNR mg/m2 . | DOX mg/m2 . | CPM g/m2 . | MTX mg IT† . | MTX G/m2 IV . | CRT (Gy) by Age . | Others . |
|---|---|---|---|---|---|---|---|---|
| BFM 79 | < 1y-< 2y-≥ 2y | |||||||
| A (RI ≤ 2) | 62/4.2 | 100 (4) | 50 (2) | 3 (3) | 75 (6)+ | — | 12-15-18 | |
| B (RI > 2) | 38/2.6 | 100 (4) | 100 (4) | 4 (4) | 75 (6)+ | — | 16-20-24 | |
| BFM 81 | ||||||||
| SR (RF < 1.2) random | 30/3.7 | 120 (4) | 60 (2) | 2 (2) | 72 (6) | — | 12-15-18 | |
| 29/3.6 | 120 (4) | 60 (2) | 2 (2) | 120 (10) | 2 (4) | — | ||
| MR (RF < 1.7) | 34/4.1 | 120 (4) | 120 (4) | 3 (3) | 72 (6) | 16-20-24 | ||
| HR (RF > 1.7) | 7/0.9 | 120 (4) | 120 (4) | 3 (3) | 72 (6) | 16-20-24 | 0.66 g (4) VM26 | |
| BFM 83 | ||||||||
| SRL (RF < 0.8) random | 30/4.1 | 120 (4) | 0 | 2 (2) | 72 (6) | 2 (4) | — | |
| 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | — | |||
| SRH (RF < 1.2) random | 29/4.0 | 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | 12 | |
| 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | 12-15-18 | |||
| MR (RF < 1.7) | 33/4.6 | 120 (4) | 120 (4) | 3 (3) | 108 (9) | 2 (4) | 12-15-18 | |
| HR (RF ≥ 1.7) | 8/1.0 | 120 (4) | 120 (4) | 5 (13) | 132 (11) | 3 (6) | 16-20-24 | 0.33 g |
| (4) VM26 | ||||||||
| BFM 86 | ||||||||
| SRG (RF < 0.8) | 28/5.9 | 160 (4) | 0 | 2 (2) | 84 (7) | 20 (4) | — | |
| RG (RF ≥ 0.8) random (PGR) | 37/8.0 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | RF < 1.2:12 | 0.66 g (4) VM26 |
| 23/4.9 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | RF ≥ 1.2:18 | 12 mg (4) VDS | |
| < 1 y 0 | 4 g (4) IFO | |||||||
| EG (PPR) | 12/2.5 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | 0-12-18 | 40 mg (4) Mitox |
| 8 g (8) IFO | ||||||||
| BFM 90 | ||||||||
| SRG (RF < 0.8) | 28/13.1 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | — | |
| MR (RF ≥ 0.8) random (PGR) | 29/13.2 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | 0-12 | |
| 31/14.4 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | 0-12 | ||
| HRG (PPR) | 12/5.2 | 270 (7) | 0 | 0 | 144 (12)‡ | 30 (6) | 0-12 | 9 mg (3) VDS |
| 1.3 g (9) VP16 | ||||||||
| 6 g (15) IFO |
| Study Risk Group . | Patients* (%) . | DNR mg/m2 . | DOX mg/m2 . | CPM g/m2 . | MTX mg IT† . | MTX G/m2 IV . | CRT (Gy) by Age . | Others . |
|---|---|---|---|---|---|---|---|---|
| BFM 79 | < 1y-< 2y-≥ 2y | |||||||
| A (RI ≤ 2) | 62/4.2 | 100 (4) | 50 (2) | 3 (3) | 75 (6)+ | — | 12-15-18 | |
| B (RI > 2) | 38/2.6 | 100 (4) | 100 (4) | 4 (4) | 75 (6)+ | — | 16-20-24 | |
| BFM 81 | ||||||||
| SR (RF < 1.2) random | 30/3.7 | 120 (4) | 60 (2) | 2 (2) | 72 (6) | — | 12-15-18 | |
| 29/3.6 | 120 (4) | 60 (2) | 2 (2) | 120 (10) | 2 (4) | — | ||
| MR (RF < 1.7) | 34/4.1 | 120 (4) | 120 (4) | 3 (3) | 72 (6) | 16-20-24 | ||
| HR (RF > 1.7) | 7/0.9 | 120 (4) | 120 (4) | 3 (3) | 72 (6) | 16-20-24 | 0.66 g (4) VM26 | |
| BFM 83 | ||||||||
| SRL (RF < 0.8) random | 30/4.1 | 120 (4) | 0 | 2 (2) | 72 (6) | 2 (4) | — | |
| 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | — | |||
| SRH (RF < 1.2) random | 29/4.0 | 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | 12 | |
| 120 (4) | 60 (2) | 2 (2) | 96 (8) | 2 (4) | 12-15-18 | |||
| MR (RF < 1.7) | 33/4.6 | 120 (4) | 120 (4) | 3 (3) | 108 (9) | 2 (4) | 12-15-18 | |
| HR (RF ≥ 1.7) | 8/1.0 | 120 (4) | 120 (4) | 5 (13) | 132 (11) | 3 (6) | 16-20-24 | 0.33 g |
| (4) VM26 | ||||||||
| BFM 86 | ||||||||
| SRG (RF < 0.8) | 28/5.9 | 160 (4) | 0 | 2 (2) | 84 (7) | 20 (4) | — | |
| RG (RF ≥ 0.8) random (PGR) | 37/8.0 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | RF < 1.2:12 | 0.66 g (4) VM26 |
| 23/4.9 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | RF ≥ 1.2:18 | 12 mg (4) VDS | |
| < 1 y 0 | 4 g (4) IFO | |||||||
| EG (PPR) | 12/2.5 | 160 (4) | 120 (4) | 3 (3) | 108 (9) | 20 (4) | 0-12-18 | 40 mg (4) Mitox |
| 8 g (8) IFO | ||||||||
| BFM 90 | ||||||||
| SRG (RF < 0.8) | 28/13.1 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | — | |
| MR (RF ≥ 0.8) random (PGR) | 29/13.2 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | 0-12 | |
| 31/14.4 | 120 (4) | 120 (4) | 3 (3) | 132 (11) | 20 (4) | 0-12 | ||
| HRG (PPR) | 12/5.2 | 270 (7) | 0 | 0 | 144 (12)‡ | 30 (6) | 0-12 | 9 mg (3) VDS |
| 1.3 g (9) VP16 | ||||||||
| 6 g (15) IFO |
SR: standard risk; SRL: low standard risk; SRH: high standard risk; MR: medium risk; HR: high risk; RG: risk group; EG: experimental group; RF: risk factor; RI: risk index; PGR: prednisone good response; PPR: prednisone poor response; DOX: doxorubicin; CPM: cyclophosphamide; DNR: daunorubicin; IFO: ifosphamide; MTX: methotrexate; CRT: cranial radiotherapy; VM: teniposide; VP: etoposide.
In parentheses: number of dosages by which cumulative dose was administered.
Proportion of patients in percent of the trial, as well as of the whole study population.
Single dose 12 mg MTX intrathecally (IT), adjusted for children <3 y (MTX-dose: <1 y 6 mg; ≥1 y <2 y 8 mg; ≥2 and <3 y 10 mg; and ≥3 y 12 mg); +12.5 mg/m2 per dose.
Triple drug: MTX and cytarabin 144-270 mg and prednisolone 36-90 mg depending on age.
To determine the occurrence of late effects, the follow-up status was ascertained at least every 2 years (last update: December 1997). Standardized forms to be completed by the treating institution are used for follow-up, verifying the date of the most recent contact and the status of each patient. Additionally, the BFM study group was notified of all SNs after ALL, diagnosed in 1980 or later, registered by the GCCR.8 Supplemental information, including a verification of the diagnosis, surgical and pathology reports, records of radiation therapy, and patient's outcome, was obtained for all reported cases of SN.
Survival and event-free survival (EFS) were calculated from the time of initial diagnosis of ALL to death of any cause or first event (nonresponse, relapse, death of any cause) or last follow-up. The risk of SN was estimated by cumulative incidence functions9 for SN and death as causes of failure. Ninety-five percent confidence intervals (95% CI) were calculated using standard methods. Comparisons were performed using the 95% CI for the difference between 15-year point estimates for the incidence functions. In addition, standardized incidence rate ratios (SIRs), defined as the ratio of the number of observed divided by the number of expected cancers, were used to measure the difference in cancer occurrence between the study group and the general population.10 Confidence intervals were computed by the method of Byar.10 The number of SNs expected to occur was estimated from standardized incidence rates derived from the GCCR statistics between 1992 and 1996.11 12
The contribution of presenting features or treatment components to the development of SN was estimated with the Cox proportional-hazards model.13 The clinical and biologic features that were analyzed included age, sex, leukocyte count, CNS involvement, prednisone response, and immunophenotype.
Drugs specifically analyzed for impact on SN were epipodophyllotoxins, cyclophosphamide/ifosphamide, anthracyclines, and radiotherapy. The dosage of CRT was coded as follows: 0 for no RT, 1 for 12 Gy, and 2 for at least 18 Gy. Radiotherapy as part of the relapse strategy was included as a time-dependent variable in the model.
Results
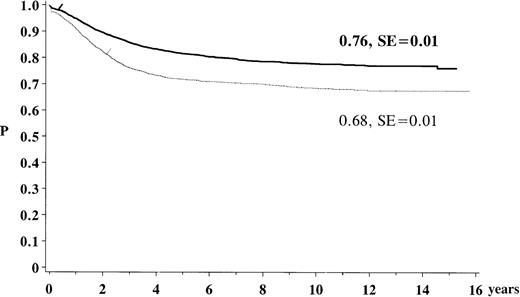
The estimated overall survival rate for children previously treated for ALL with 1 of the ALL-BFM 79 to 90 protocols was 76% (SE: 1%) after 15 years. The EFS was 68% (SE: 1%) (Figure1). Of the study population, 98% achieved complete remission and 23.2% of the children suffered a relapse. Of all the patients, 3% died before or while in remission and 1% developed an SN. Nine percent were reported as being lost to follow-up. At the time of analysis the median follow-up after diagnosis of ALL was 5.7 years (range 1.5-18 years), with a total of 28 605 person-years of follow-up.
Kaplan-Meier estimate of survival and event-free survival for the study population of the trials ALL-BFM 79 to 90. The solid line indicates survival (N = 5006; 969 events); the dotted line, event-free survival (N = 5006; 1371 events).
Kaplan-Meier estimate of survival and event-free survival for the study population of the trials ALL-BFM 79 to 90. The solid line indicates survival (N = 5006; 969 events); the dotted line, event-free survival (N = 5006; 1371 events).
As of December 1997, a total of 52 SNs had been observed. Most frequent, in decreasing order, were acute myeloid leukemias (AMLs) (31%), CNS tumors (25%), lymphomas (12%), and thyroid cancers (6%) (Table 2). The clinical characteristics of the patients, summarized in Table 3, showed no difference between all the patients and those who developed an SN with regard to sex, age, initial white blood count (WBC), tumor load (RF), and prednisone response, although boys and infants had a slightly (but not statistically significant) higher risk for developing an SN, as well as patients presented with T-cell and CNS leukemias.
Secondary neoplasms observed in the study population
| Leukemia and lymphoma n = 23 . | CNS tumors n = 13 . | Other neoplasm n = 16 . |
|---|---|---|
| Acute myeloid leukemia (16;1) (3.9 y) Chronic myeloid leukemia (1;1) (2.3 y) Hodgkin's disease (4;3) (2.4 y) Non-Hodgkin's lymphoma (2;2) (2.5 y) | Glioblastoma (4;2) (5.5 y) Astrocytoma (4;2) (8.5 y) CNS-PNET (3;–) (11.7 y) Meningioma (2;2) (9.5 y) | Thyroid cancer (3;3) (9.2 y) Malignant histiocytosis (2;–) (1.2 y) Basal cell carcinoma (2;2) (13.5 y) Epithelial carcinoma (1;–) Squamous cell carcinoma (1;1) Malignant teratoma (1;1) Mucoepidermoid carcinoma (1;1) Melanoma (1;1) Nephroblastoma (1;–) Osteosarcoma (1;1) PNET (1;1) Unknown secondary tumor with lung metastasis (1;?) |
| Leukemia and lymphoma n = 23 . | CNS tumors n = 13 . | Other neoplasm n = 16 . |
|---|---|---|
| Acute myeloid leukemia (16;1) (3.9 y) Chronic myeloid leukemia (1;1) (2.3 y) Hodgkin's disease (4;3) (2.4 y) Non-Hodgkin's lymphoma (2;2) (2.5 y) | Glioblastoma (4;2) (5.5 y) Astrocytoma (4;2) (8.5 y) CNS-PNET (3;–) (11.7 y) Meningioma (2;2) (9.5 y) | Thyroid cancer (3;3) (9.2 y) Malignant histiocytosis (2;–) (1.2 y) Basal cell carcinoma (2;2) (13.5 y) Epithelial carcinoma (1;–) Squamous cell carcinoma (1;1) Malignant teratoma (1;1) Mucoepidermoid carcinoma (1;1) Melanoma (1;1) Nephroblastoma (1;–) Osteosarcoma (1;1) PNET (1;1) Unknown secondary tumor with lung metastasis (1;?) |
In parentheses: number of patients and number of patients alive.
Median time span from diagnosis of ALL to the diagnosis of SN in years.
Characteristics of the study patients and association with the risk of a secondary neoplasm
| . | All patients (n = 5006) . | Patients with SN (n = 52) . | Cumulative incidence after 15 y (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 57% | 67% | 4.1% (1.3%-6.9%) |
| Female | 43% | 33% | 2.1% (0.4%-3.9%) P = .25 |
| Age (y) | |||
| < 1 | 2.7% | 1.9% | 5.1% (0%-24.7%) |
| ≥ 1-< 6 | 57.7% | 55.8% | 3.0% (1.3%-4.8%)P = .84 |
| ≥ 6 | 39.6% | 42.3% | 3.3% (0.2%-6.6%) P = .90 |
| Median age (y) | 4.8 | 5.2 | |
| Leukocyte count/μL | |||
| < 10 000 | 46.8% | 48.1% | 2.9% (1.2%-4.6%) |
| ≥ 10 000-< 50 000 | 31.8% | 26.9% | 4.2% (0.1%-9.0%) P = .61 |
| ≥ 50 000 | 21.4% | 25.0% | 2.9% (0.0%-6.3%) P = .65 |
| Median/μL | 11 500 | 11 750 | |
| Leukemia cell mass (RF) | |||
| Median | 1.03 | 1.1 | |
| Prednisone response | |||
| Good response | 90% | 93% | 2.8% (0.4%-5.6%) |
| Poor response | 10% | 7% | 0.7% (0%-2.9%) P = .29 |
| CNS-involvement | |||
| Negative ALL | 97.2% | 91.8% | 3.1% (1.4%-4.8%) |
| Positive ALL | 2.8% | 8.2% | 9.0% (0%-30.5%)P = .59 |
| Mediastinal mass | 9.1% | 14.3% | 9.6% (0%-27.8%) |
| Immunophenotype3-150 | (T vs non-T) P = .83 | ||
| Pro-B-ALL | 5% | 7% | 1.8% (0%-4.7%) |
| c-ALL | 67% | 58% | 4.5% (0.8%-8.2%) |
| Pre-B-ALL | 13% | 7% | 2.3% (0%-5.9%) |
| T-ALL | 14% | 26% | 5.8% (0%-17.2%) |
| . | All patients (n = 5006) . | Patients with SN (n = 52) . | Cumulative incidence after 15 y (95% CI) . |
|---|---|---|---|
| Sex | |||
| Male | 57% | 67% | 4.1% (1.3%-6.9%) |
| Female | 43% | 33% | 2.1% (0.4%-3.9%) P = .25 |
| Age (y) | |||
| < 1 | 2.7% | 1.9% | 5.1% (0%-24.7%) |
| ≥ 1-< 6 | 57.7% | 55.8% | 3.0% (1.3%-4.8%)P = .84 |
| ≥ 6 | 39.6% | 42.3% | 3.3% (0.2%-6.6%) P = .90 |
| Median age (y) | 4.8 | 5.2 | |
| Leukocyte count/μL | |||
| < 10 000 | 46.8% | 48.1% | 2.9% (1.2%-4.6%) |
| ≥ 10 000-< 50 000 | 31.8% | 26.9% | 4.2% (0.1%-9.0%) P = .61 |
| ≥ 50 000 | 21.4% | 25.0% | 2.9% (0.0%-6.3%) P = .65 |
| Median/μL | 11 500 | 11 750 | |
| Leukemia cell mass (RF) | |||
| Median | 1.03 | 1.1 | |
| Prednisone response | |||
| Good response | 90% | 93% | 2.8% (0.4%-5.6%) |
| Poor response | 10% | 7% | 0.7% (0%-2.9%) P = .29 |
| CNS-involvement | |||
| Negative ALL | 97.2% | 91.8% | 3.1% (1.4%-4.8%) |
| Positive ALL | 2.8% | 8.2% | 9.0% (0%-30.5%)P = .59 |
| Mediastinal mass | 9.1% | 14.3% | 9.6% (0%-27.8%) |
| Immunophenotype3-150 | (T vs non-T) P = .83 | ||
| Pro-B-ALL | 5% | 7% | 1.8% (0%-4.7%) |
| c-ALL | 67% | 58% | 4.5% (0.8%-8.2%) |
| Pre-B-ALL | 13% | 7% | 2.3% (0%-5.9%) |
| T-ALL | 14% | 26% | 5.8% (0%-17.2%) |
SN = secondary neoplasm; CNS = central nervous system; ALL = acute lymphoblastic leukemia.
Only patients with known immunophenotype (ALL: n = 4098, SN: n = 43).
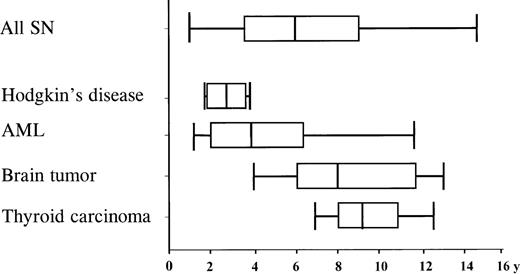
The median time from initiation of primary treatment to diagnosis of the SN was 6 years (range 11 months-15 years) and varied according to the type of SN (Figure 2). The median time span for AML was 3.8 years (range 1-11.5 years), and 7.9 years (range 4-13 years) for CNS tumors. Types of SN differed also, according to the age at diagnosis of ALL. Tumors of the CNS were the most common SNs among children below the age of 7 years at diagnosis of ALL. The cumulative probability that a CNS tumor would develop in these patients was 1.5% (95% CI: 0.2%-2.7%) after 15 years, significantly higher compared with the risk of 0.1% (95% CI: 0%-0.3%) in patients 7 years of age or older (P = .03). In contrast, incidence for AML was in the same range for these age groups.
Time interval from diagnosis of ALL to the diagnosis of SN . Median time interval, box: 25%-57% quartile; bars encompass the range
Time interval from diagnosis of ALL to the diagnosis of SN . Median time interval, box: 25%-57% quartile; bars encompass the range
SN developed in 46 patients during the first complete hematologic remission. Six patients had previously been treated for ALL relapse, of whom 4 patients had received bone marrow transplantation (BMT). Three patients had isolated bone marrow relapses, 2 had an isolated relapse in the testes, and 1 in the CNS. In the whole study population, a total of 291 BMTs had been performed during the observation time.
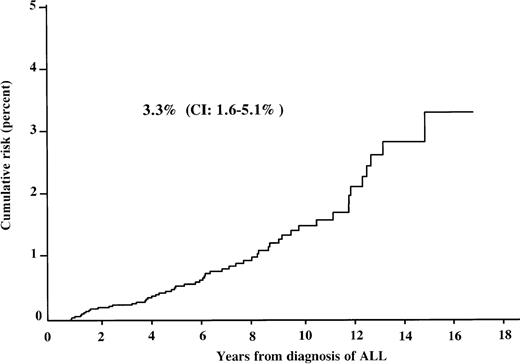
The overall cumulative incidence of developing an SN was 0.5% (95% CI: 0.4%-0.6%) after 5 years, 1.5% (95% CI: 1.3%-1.9%) after 10 years, and 3.3% (95% CI: 1.6%-5.1%) after 15 years from the diagnosis of ALL (Figure 3). The corresponding cumulative risk in patients during the first complete remission (CR) was 0.5%, 1.3%, and 2.9% (95% CI: 1.6%-4.2%), respectively. It was 3.9% (95% CI: 0.2%-10%) after 15 years in relapsed patients. If low-grade meningiomas and skin cancers were excluded from the analysis, the cumulative risk was 2.4% (95% CI: 1.7%-3.0%) at 15 years. Separate consideration of AML and CNS tumors, the 2 largest groups of SN in our cohort, showed a cumulative risk of 0.2% at 5 years, 0.4% at 10 years, and 0.6% (95% CI: 0.2%-1.1%) at 15 years for developing an AML, and 0.1%, 0.4%, and 1.0% (95% CI: 0.4%-1.8%) at 5, 10, and 15 years for developing a CNS tumor. Thus, after 10 years, the cumulative risk of secondary CNS tumors exceeds that of secondary AML, although the absolute number of CNS tumors is lower.
Cumulative risk of developing SN for patients after treatment for ALL. CI: 95% confidence interval.
Cumulative risk of developing SN for patients after treatment for ALL. CI: 95% confidence interval.
The comparison of the observed SNs with the expected cancer rates in the general population was carried out based on population rates derived from the GCCR.11 12 In a total of 28 605 person-years at risk, the expected incidence of new neoplasms would have been 3.7 (SIR: 14.1; 95% CI: 10.7-17.8), of new leukemia and lymphomas 1.8 (SIR: 12.8; 95% CI: 7.9-18.4), and of new CNS tumors 0.7 (SIR: 18.6; 95% CI: 9.8-29.4). This represents a 14-fold increase of all cancers and a 19-fold increase of neoplasms of the CNS.
In most cases of SN, there was no family history suggesting cancer predisposition: in 26 patients, the history was negative; in 16 cases, no report was received; and in 10, 1 first- or second-degree relative was suffering from a malignant disease. If all patients from the BFM database with a report on family history were considered in statistical analysis, a positive history is more frequent in patients with SN compared with all patients treated for ALL. Although the cumulative risk of developing an SN in patients with a positive family history is more than twice as high as in patients with a negative family history (1.2% vs 3.1%), there is no statistical significance.
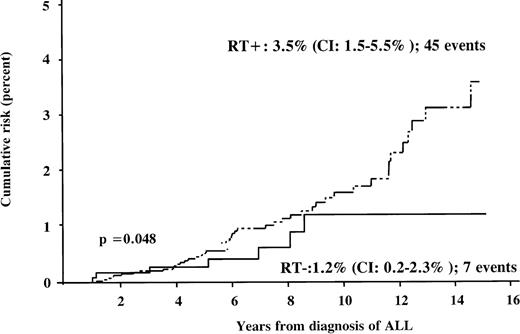
Forty-five (87%) SNs occurred in patients who had received CRT, either during frontline therapy or during relapse therapy. The median dose of irradiation was 12 Gy (range: 12-30 Gy). All patients with secondary CNS tumors, except 1 boy with a meningioma, and all patients with secondary thyroid cancer had undergone CRT. The estimated risk of developing an SN 15 years after the diagnosis of ALL was 3.5% (95% CI: 1.5%-5.5%) among patients who had received radiation therapy, significantly higher when compared with 1.2% (95% CI: 0.2%-2.3%) among nonirradiated patients (Table 4; Figure 4). For developing a CNS tumor, the relation was 1.3% versus 0.1%; for developing an AML, it was 0.6% in both groups. In the subgroup of patients during first CR, the cumulative risk after irradiation was 3.3% and 1.0% in nonirradiated patients. Among all irradiated patients (CRT performed either in frontline or in relapse therapy) the risk was 1.7% after cranial irradiation with 12 Gy, and 3.2% after irradiation with at least 18 Gy.
Risk of a secondary neoplasm associated with radiation therapy
| . | Number of patients (%) . | Number of SN . | Cumulative incidence after 15 y (95% CI) . |
|---|---|---|---|
| CRT (frontline therapy) | P = .044 | ||
| None | 1388 (27.7) | 7 | 1.0% (0%-2.1%) |
| ≥ 12 Gy | 3618 (72.3) | 39 | 3.3% (1.1%-5.6%) |
| 12 Gy | 2015 | 11 | 1.6% (0-3.4%) |
| ≥ 18 Gy | 1603 | 28 | 3.3% (0.9-5.6%) |
| CRT (+ relapse therapy) | P = .048 | ||
| None | 1140 (22.8) | 7 | 1.2% (0.2-2.3%) |
| ≥ 12 Gy | 3866 (77.2) | 45 | 3.5% (1.5%-5.5%) |
| 12 Gy | 1779 | 11 | 1.7% (0.1-3.4%) |
| ≥ 18 Gy | 2087 | 34 | 3.2% (1.1-5.3%) |
| . | Number of patients (%) . | Number of SN . | Cumulative incidence after 15 y (95% CI) . |
|---|---|---|---|
| CRT (frontline therapy) | P = .044 | ||
| None | 1388 (27.7) | 7 | 1.0% (0%-2.1%) |
| ≥ 12 Gy | 3618 (72.3) | 39 | 3.3% (1.1%-5.6%) |
| 12 Gy | 2015 | 11 | 1.6% (0-3.4%) |
| ≥ 18 Gy | 1603 | 28 | 3.3% (0.9-5.6%) |
| CRT (+ relapse therapy) | P = .048 | ||
| None | 1140 (22.8) | 7 | 1.2% (0.2-2.3%) |
| ≥ 12 Gy | 3866 (77.2) | 45 | 3.5% (1.5%-5.5%) |
| 12 Gy | 1779 | 11 | 1.7% (0.1-3.4%) |
| ≥ 18 Gy | 2087 | 34 | 3.2% (1.1-5.3%) |
SN = secondary neoplasm; CRT = cranial radiation therapy.
Frontline therapy: only patients with SN as first event.
Cumulative risk of developing SN according to radiation therapy (RT). The dotted line represents the irradiated subgroup, and the solid line nonirradiated patients.
Cumulative risk of developing SN according to radiation therapy (RT). The dotted line represents the irradiated subgroup, and the solid line nonirradiated patients.
By Cox stepwise regression, 3 parameters reached statistical significance: initial CRT, CNS involvement, and administration of epipodophyllotoxins in frontline therapy. Risk ratios (RR) and CIs are given here for the regression model, including only these variables: initial CRT (RR 1.5, CI: 1.01-2.3, P = .04), CNS involvement (RR 3.1, CI: 1.1-8.7, P = .03), and administration of epipodophyllotoxins in frontline therapy (RR: 2.6, CI: 1.3-5.4,P = .01). The risk ratios for these parameters were in the same range when a Cox model was calculated for each of these parameters alone (RT: 1.7, CI 1.1-2.5; CNS: 4.0, CI 1.5-11.2; and epipodophyllotoxins: 3.0, CI 1.4-6.2).
The analysis of treatment exposures failed to demonstrate a relationship between the different BFM trials and the cumulative risk, as well as between single chemotherapeutic agents (dose of cyclophosphamide/ifosphamide or anthracycline) and the cumulative incidence of SN after 15 years (Table 5), with the exception of epipodopyhyllotoxins. Comparing the cumulative incidences of SN for patients treated or not treated with epipodophyllotoxins, no difference was found. In Cox regression, however, the use of epipodopyhyllotoxins in frontline treatment had a significant impact on the incidence of second malignancies. When including the relapse therapy, this parameter is losing significance. Considering the patients with secondary AML, it is remarkable that 12 of 16 patients received no epipodophyllotoxins, a cumulative dose of less than or equal to 3000 mg/m2 cyclophosphamide, and less than or equal to 240 mg/m2 anthracyclines. Only 3 patients with secondary AML have been treated with epipodophyllotoxin (0.45-1.3 mg/m2). The other so-treated patients developed very different types of SN.
Risk of a secondary neoplasm associated with chemotherapeutic agents
| Therapeutic Elements . | Number of Patients (%) . | Number of SN . | Cumulative Incidence after 15 Years (95% CI) . |
|---|---|---|---|
| Anthracycline dose (f) | P = .11 | ||
| ≤ 240 mg/m2 | 3965 (79.2) | 37 | 3.0% (1.1%-4.9%) |
| > 240 mg/m2 | 1041 (20.8) | 9 | 1.2% (0%-2.5%) |
| Anthracycline dose (+r) | P = .38 | ||
| ≤ 240 mg/m2 | 3147 (62.9) | 3715 | 3.9% (2.0%-5.8%) |
| > 240 mg/m2 | 1859 (37.1) | 2.2% (0%-5.6%) | |
| Epipodophyllotoxin (f) | P = .99 | ||
| No | 4527 (90.4) | 37 | 2.8% (0.9%-4.7%) |
| Yes | 479 (9.6) | 9 | 2.8% (0%-6.6%) |
| Epipodophyllotoxin (+r) | P = .42 | ||
| No | 3569 (71.3) | 3715 | 3.7% (1.8%-5.5%) |
| Yes | 1437 (28.7) | 2.1% (0%-5.4%) | |
| Cyclophosphamide (f) | P = .45 | ||
| ≤ 3000 mg/m2 | 4584 (91.6) | 39 | 3.1% (0.9%-5.2%) |
| > 3000 mg/m2 | 422 (8.4) | 7 | 1.9% (0-4%.1%) |
| Cyclophosphamide (+r) | P = .14 | ||
| ≤ 3000 mg/m2 | 3560 (71.1) | 3913 | 4.1% (1.9%-6.2%) |
| > 3000 mg/m2 | 1446 (28.9) | 1.6% (0%-4.1%) |
| Therapeutic Elements . | Number of Patients (%) . | Number of SN . | Cumulative Incidence after 15 Years (95% CI) . |
|---|---|---|---|
| Anthracycline dose (f) | P = .11 | ||
| ≤ 240 mg/m2 | 3965 (79.2) | 37 | 3.0% (1.1%-4.9%) |
| > 240 mg/m2 | 1041 (20.8) | 9 | 1.2% (0%-2.5%) |
| Anthracycline dose (+r) | P = .38 | ||
| ≤ 240 mg/m2 | 3147 (62.9) | 3715 | 3.9% (2.0%-5.8%) |
| > 240 mg/m2 | 1859 (37.1) | 2.2% (0%-5.6%) | |
| Epipodophyllotoxin (f) | P = .99 | ||
| No | 4527 (90.4) | 37 | 2.8% (0.9%-4.7%) |
| Yes | 479 (9.6) | 9 | 2.8% (0%-6.6%) |
| Epipodophyllotoxin (+r) | P = .42 | ||
| No | 3569 (71.3) | 3715 | 3.7% (1.8%-5.5%) |
| Yes | 1437 (28.7) | 2.1% (0%-5.4%) | |
| Cyclophosphamide (f) | P = .45 | ||
| ≤ 3000 mg/m2 | 4584 (91.6) | 39 | 3.1% (0.9%-5.2%) |
| > 3000 mg/m2 | 422 (8.4) | 7 | 1.9% (0-4%.1%) |
| Cyclophosphamide (+r) | P = .14 | ||
| ≤ 3000 mg/m2 | 3560 (71.1) | 3913 | 4.1% (1.9%-6.2%) |
| > 3000 mg/m2 | 1446 (28.9) | 1.6% (0%-4.1%) |
SN: secondary neoplasm; f: frontline therapy, sek.mal. as first event; +r: frontline + relapse therapy.
At the time of the analysis, 26 patients (50%) with SN were alive. The clinical outcome of patients with AML has especially been dismal. Eleven of 16 children entered remission after receiving AML therapy, but only 3 patients have remained in remission so far. The median survival time was 6 months. Compared with that, approximately half of the patients with CNS tumors can be expected to be long-term survivors after surgical treatment and/or intensive chemotherapy. The median survival time for patients with CNS tumors was 14 months.
Discussion
The risk of an SN in childhood cancer survivors may be influenced by genetic and other predisposing factors, as well as by the treatment given for the primary disease. The risk to develop an SN has always to be viewed in the context of the survival probability provided by a given treatment protocol, as low survival will result in fewer SNs. Although the lifetime incidence of SNs has not yet been defined, within the first 20 years of initial diagnosis of childhood cancer it is in the order of 3% to 12%, according to the Late Effect Study Group (LESG).14 The nationwide GCCR observed (for a period of 15 years up to 1995) 127 children with secondary neoplasms.12For the first 15 years of life, the estimated cumulative risk of developing an SN within 10 years after the first malignancy was 1.9% (95% CI: 1.5%-2.3%), and the overall SIR for an SN was 12.5 (95% CI: 10.4-14.9). CNS tumors after ALL (13% of all SNs) and after other CNS tumors (9%), and leukemia after leukemia (10%) were the most frequently reported combinations.
A few studies have attempted to describe the overall risk of SN among children with ALL. In 1991, Nygaard et al15 from Norway found an overall cumulative risk of 2.9% (SE: 1.4%) by 20 years after diagnosis in a group of 895 patients, treated between 1958 and 1985. Neglia et al16 from the Children's Cancer Group (CCG) reviewed 9720 cases of ALL since 1972. They observed 43 SNs, after a median follow-up of 4.7 years, including 24 neoplasms of the CNS, 10 new leukemias and lymphomas, and 9 other neoplasms. The estimated cumulative risk was 2.5% (95% CI: 1.7%-3.4%) 15 years after diagnosis, whereas Pratt et al17 observed 20 SNs in a group of 1815 patients and estimated a cumulative risk of 5% during that interval. In view of a longer follow-up time, the cumulative risk of 3.3% (95% CI: 1.6%-5.1%) after intensive BFM therapy is comparable to these results. The cumulative incidence of an SN before another first event in our data (2.8%) was nearly the same as the one reported by Dalton et al (2.7%).18 The incidence of secondary brain tumors (1.0% at 15 years) is comparable to the results of Walter et al (1.39% at 20 years).19
Therapy-related SNs have been identified in patients receiving radiotherapy, chemotherapy, or combined modality therapy for a variety of primary neoplasms. Several studies have documented a clear relationship between prior therapeutic irradiation and the occurrence of CNS tumors,19-22 sarcomas of bone,23 and thyroid cancers.24 Our study identified radiation therapy as a significant risk factor, which was not found in the data of Dalton et al.18 Also in the nonirradiated group no SN was observed; however, a statistically significant difference in the cumulative risk, compared with the irradiated group, could not be demonstrated. This could be explained by the shorter follow-up of the nonirradiated cohort. As shown in Figure 4, there is no evidence that the cumulative risk of developing an SN has reached a plateau 15 years after diagnosis in the irradiated group of our cohort, suggesting a continuing effect of irradiation on the rates of SN. Even among nonirradiated patients, it is at this time unknown whether the reduced cumulative risk will remain constant, or whether SNs might arise after a longer latency period. The standard dose for preventive irradiation of 12 Gy was introduced in the BFM trials in the mid-1980s (Table 1). Analysis of the data demonstrates a relationship between the dose of cranial irradiation (12 Gy vs 18 Gy and more) and the incidence of SN (Table 4), although the difference between the single dosage groups was not statistically significant. Furthermore, the impact of CNS-targeted chemotherapy still remains unknown.
Multiagent chemotherapy as part of multimodality therapy for malignant disease has increased the difficulty of assessing which agents might play a causative role in the development of SNs. Alkylating agents, and more recently DNA-topoisomerase II inhibitors, have been linked to the development of secondary AML and myelodysplastic syndrome (MDS).25-28 Compared with the results of Neglia et al,16 using intensive nonepipodophyllotoxin-containing treatment regimens, we noted similar rates of SN, but a higher rate of AML, which on the other side was clearly below the rate reported by Pui et al.25 In that study, the estimated risk was 3.8% to develop acute nonlymphocytic leukemia, occurring within 6 years. In contrast to their conclusions and the results of other studies, this investigation could not demonstrate a clear relationship between the dose of cyclophosphamide/ifosphamide or anthracyclines and the occurrence of SNs or special types of SN. Epipodophyllotoxin in frontline therapy was administered only in high-risk patients (10% of all patients). There was no difference in cumulative incidence rates, but a significantly increased RR in a Cox model for patients treated with epipodophyllotoxins in frontline therapy. The interpretation of these data remains difficult because an explorative type of analysis has been used, the number of SNs after epipodophyllotoxin was small, and there are known (risk-adapted therapy) and possible unknown interactions between biologic features and different treatment modalities.
Other risk factors for inducing SN comprise genetic predisposition and possible combinations. According to the reports of the Late Effects Study Group,29 some combinations of tumors seem to be related to underlying genetic syndromes. It has been suggested that genetically determined susceptibility is involved in the association observed between brain tumors and leukemia/lymphoma. Increased incidence of leukemia in the first-degree relatives of patients with CNS tumors and a 2.8-fold increased risk was reported among siblings,30 as well as CNS tumors in relatives of patients with leukemia, are raising the possibility of a link between ALL and brain tumors.31,32 SNs may also result from a specific combination of age, genetics, first malignancy, and therapy. In retinoblastoma, for example, exposure to alkylating agents and radiation potentiates an already markedly elevated risk of SN.23 Furthermore, the biologic characteristics of the blast cells may be associated with the development of secondary malignancy. In 1989, Pui et al33 reported a substantial risk of secondary AML in patients with T-cell immunophenotype, but this correlation has disappeared with longer follow-up and the accrual of additional cases.25 Also, in our study, SN was more likely to occur in patients with T-cell leukemia, although the cumulative risk was not significantly increased.
In conclusion, we confirm that considering the high-survival rate of this large, unselected ALL cohort, the risk of developing an SN after ALL-BFM therapy is relatively low, although higher, especially after radiation therapy, than in the general population. In particular, young children are at increased risk when irradiation has been used. The intensive chemotherapy regimens used during these studies do not predict a higher risk as reported in other studies.15-19 25 AML and CNS tumors are the most common SNs.
In view of the long latency periods and long life expectancy of individuals treated in childhood, long and careful follow-up of these patients is warranted, especially for patients after irradiation and for those with T-cell phenotype, with a positive family history for cancer. This follow-up will also facilitate the evaluation of the role of low-dose cranial irradiation (12 Gy), other CNS-targeted chemotherapy, and genetic predisposition determining the risk of SN. Efforts to identify the causative cancerogenic factors should continue, and future treatment protocols should take these factors into account to maximize the chances of a long and healthy life, while preserving efficacy of treatment of the primary malignancy.
Acknowledgments
We thank U. Meyer and N. Götz for preparing the data of the ALL-BFM studies, and J. Meyers for proofreading the text. We also thank all the investigators and staff involved for their continuous cooperation with the BFM study center.
Supported in part by grants from the Deutsche Krebshilfe and the Madeleine Schickedanz Kinderkrebs-Stiftung.
Reprints:Martin Schrappe, Hannover Medical School, Department of Pediatric Hematology/Oncology, 30623 Hannover, Germany; e-mail:schrappe.martin@mh-hannover.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal