Dendritic cells (DC) are professional antigen-presenting cells with a unique capacity to initiate and regulate immune responses. Immature CD1a+ DC can be cultured from CD14+monocytes in the presence of interleukin (IL)-4 and granulocyte macrophage colony-stimulating factor in vitro. Results of this study show that the nonsteroidal anti-estrogens toremifene and tamoxifen inhibit this differentiation. In the presence of anti-estrogens the cells lose CD14 expression, but remain CD1a− and clearly have less dendritic processes than immature DC. Functionally, anti-estrogen-treated cells are inferior to immature DC in inducing proliferation of allogeneic T cells and in producing IL-12 p70 protein after CD40 ligation. The expression of the costimulatory molecules CD80 and CD86 is differentially regulated by anti-estrogens during DC differentiation. Furthermore, anti-estrogens are also able to inhibit the terminal maturation of DC. By inhibiting the functional differentiation of DC, anti-estrogens may have a role in the treatment and prevention of autoimmune diseases.
Dendritic cells (DC) are antigen-presenting cells (APC) that differentiate from bone marrow-derived precursors.1,2Because of their capacity to stimulate naive T cells, DC have a central role in the initiation3 of primary immune responses and regulation of self-tolerance and autoimmunity.1,2 Immature DC reside in peripheral nonlymphoid tissues, where they efficiently capture and process antigens. Bacterial products, such as lipopolysaccharide (LPS), or inflammatory cytokines drive the maturation of DC, which is characterized by the up-regulation of the major histocompatibility complex (MHC) class II and costimulatory molecules CD80 (B7-1) and CD86 (B7-2). This results in an increased capacity to stimulate T cells.1,2 DC migrate into T-cell areas of secondary lymphoid tissues where they receive terminal maturation signals via CD40 on the interaction with CD154 (CD40L) on antigen-specific T cells.4,5 In response to CD40 ligation, DC produce high levels of interleukin (IL)-12,4,5 a key cytokine in the development of interferon (IFN)-γ-producing Th1 cells.6 Immature myeloid DC can be cultured in vitro from CD14+ peripheral blood monocytes in the presence of IL-4 and granulocyte macrophage colony-stimulating factor (GM-CSF). During this differentiation the cells lose CD14 expression, start to express CD1a, and acquire increased capacity to stimulate allogeneic T cells.7 Addition of IL-10 to the culture inhibits this differentiation and results in the generation of CD14+CD1alow macrophage-like cells.8 The terminal maturation of DC can be induced by inflammatory stimuli such as tumor necrosis factor (TNF)-α and LPS,7,9 after which the cells start to express CD83, a marker for functionally mature DC.10 11
Nonsteroidal anti-estrogens such as toremifene (TOR) and tamoxifen (TAM) have a variety of tissue-specific effects. Depending on the target cells, they can act as estrogen agonists or antagonists and are therefore also called selective estrogen receptor modulators. In breast tissue, anti-estrogens antagonize the effect of estrogen and are widely used in the treatment of breast cancer. On the other hand, they seem to mimic the beneficial effects of estrogen in cardiovascular tissue and in bone.12,13 TAM has also been reported to be beneficial in experimental models of autoimmune diseases such as collagen-induced arthritis,14 adjuvant-induced arthritis,15 and systemic lupus erythematosus (SLE).16,17 However, these studies have provided little insight into the immunomodulatory mechanisms of TAM. TAM has been shown to enhance the production of the anti-inflammatory cytokine transforming growth factor (TGF)-β1.18-21 It has also been reported that the estrogen receptor (ER) can interact with the transcription factor NF-κB,22 which is a key regulator of inflammatory responses.23 Both enhanced production of TGF-β1 and antagonism of NF-κB have been suggested as mechanisms of immunomodulation by TAM.14
The present study characterized the effect of nonsteroidal anti-estrogens on the functional differentiation of DC and demonstrated that anti-estrogens inhibit DC differentiation by mechanisms independent of ER or enhanced production of TGF-β1. These findings may be relevant for the treatment and prevention of autoimmune diseases by anti-estrogens.
Materials and methods
Reagents and antibodies
Toremifene and TAM as citrate salt were obtained from Orion-Farmos Corporation (Turku, Finland) and stored in 10−2 mol/L stock solution, diluted in 70% ethanol. The highest amount of ethanol in the final cultures was 0.07%. 17β-Estradiol was purchased from Steraloids (Croydon, UK). ICI 182780 was provided by Dr A.E. Wakeling (Zeneca Pharmaceuticals, Cheshire, UK). Purified recombinant human (r) IL-4 and rIL-10 were obtained from Schering-Plough Research Institute (Kenilworth, NJ), rGM-CSF (Leucomax) was purchased from Schering-Plough/Sandoz, rTNF-α from R&D Systems (Minneapolis, MN), and LPS (Escherichia coli serotype 0127:B8) from Sigma (St. Louis, MO). Phycoerythrin (PE)-conjugated antihuman CD14, HLA-DR, CD80, and mouse-IgG were purchased from Becton Dickinson (San Jose, CA). PE-conjugated antihuman CD86 and nonconjugated CD1a were purchased from Pharmingen (San Diego, CA). Nonconjugated antihuman CD40 (IgG1) was obtained from Schering-Plough Research Institute and anti-CD83 (IgG2b)10,11 was provided by Dr T.F. Tedder (Duke University Medical Center, Durham, NC). PE-conjugated goat antimouse IgG1 and IgG2b were purchased from Southern Biotechnology Associated (Birmingham, AL). Sp 2/0 (mouse hybridoma culture supernatant) was used as a negative control. Anti-CD14, rat-antimouse IgG1, and goat antimouse IgG-conjugated microbeads for magnetic cell sorting (MACS) were purchased from Miltenyi Biotec (Auburn, CA). CD40L-transfected J558L cells were provided by Dr P. Lane (Basel Institute for Immunology, Basel, Switzerland) and have been previously described.24
Cell isolation and culture
Buffy coats of blood were obtained from healthy human donors (Finnish Red Cross Blood Transfusion Service, Turku, Finland). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and incubated on cell culture dishes (Becton Dickinson) for 1 hour at 37°C. Nonadherent cells were removed by washing 6 times with culture medium containing 5% human AB-serum (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland). Alternatively, PBMC were sorted by MACS (Miltenyi Biotec) with MACS CD14 microbeads. The cells (0.5-1 × 106/well) were cultured in flat-bottom 24-well plates (Costar, Cambridge, MA) in Iscove's modified Dulbecco's medium (Gibco BRL, Grand Island, NY) with or without phenol red, supplemented with 10% heat-inactivated fetal calf serum (FCS) (Bioproducts for Science, Indianapolis, IN) or dextran-charcoal stripped FCS, 1 mmol/L HEPES, 0.1 μmol/L 2-ME, and 100 μg/mL gentamycin (Biological Industries, Kibbutz beit Haemek, Israel). Immature DC were obtained by culturing adherent PBMC or MACS-sorted CD14+ cells in IL-4 (500 U/mL) and GM-CSF (50 ng/mL) for 7 days. To obtain mature DC, the cells were further cultured with LPS (1 μg/mL) or TNF-α (10 ng/mL) for an additional 24 to 36 hours.
Flow cytometry, cytologic analysis, and immunofluorescence confocal microscopy (ICM)
For immunofluorescence staining, the cells were incubated with PE-conjugated monoclonal antibodies (mAbs) or with nonconjugated mAbs for 30 minutes on ice, followed by incubation with isotype-specific secondary mAb. Before and after each incubation, the cells were washed twice with phosphate-buffered saline ([PBS] + 2% FCS + 0.01% NaN3). The cells were analyzed with a FACScan flow cytometer (Becton Dickinson) using CellQuest (Becton Dickinson) software. The data are expressed as mean fluorescence intensity ratios (MFIRs) (mean fluorescence intensity with mAb of interest/mean fluorescence intensity with control mAb). For cytologic examination the cells were cytocentrifuged and stained using a Dade Diff-Quik staining set (DADE, Düdingen, Switzerland). For ICM, the cultured cells were washed with PBS, allowed to settle onto glass slides, and fixed with 4% paraformaldehyde and methanol. The cells were then stained with anti-CD86 mAb or an isotype control Ab and the secondary fluorescein isothiocyanate-conjugated goat-antimouse Ab (Southern Biotechnology Associated). Confocal microscopy was performed using a Leica TCS SP Spectral Confocal Microscope and analyzed using the Leica Lasertechnik GmbH software (Heidelberg, Germany).
Mixed lymphocyte reaction
Adherent PBMC were first cultured in the presence of IL-4 + GM-CSF with or without anti-estrogens or IL-10 (100 U/mL) for 7 days. Alternatively, immature DC were activated with LPS (1 μg/mL) in the presence or absence of TOR or TAM for 36 hours. The cells were then irradiated (30 Gy) and counted, and 5 to 20 × 103cells were cocultured with 2 × 105Ficoll-Paque-isolated allogeneic PBMC for 5 days in U-bottom 96-well plates (Nunclon, Roskilde, Denmark). To determine the proliferative activity, 1 mCi 3H-thymidine (DuPont, Boston, MA) was added to each well 16 to 18 hours before terminating the culture. The cells were harvested to glass fiber filters (Wallac, Turku, Finland) with a 96-well harvester (Tomtec, Orange, CT). The radioactivity was measured with a 1450 Microbeta Plus liquid scintillation counter (Wallac).
Detection of estrogen receptor- and -β messenger RNA by nested reverse transcriptase-polymerase chain reaction
Monocytes were sorted from Ficoll-Paque-isolated PBMC with MACS using CD14 microbeads. The purity of these cells was confirmed by flow cytometry and was more than 99%. To obtain immature and mature DC, MACS-sorted CD14+ cells were cultured as described and further sorted using anti-CD1a mAb and rat-antimouse IgG1microbeads and anti-CD83 mAb and goat antimouse IgG microbeads, respectively. Total RNA was isolated from 5 × 106cells using the Ultraspec-II RNA Kit (Biotecx Laboratories, Houston, TX). Oligo-p(dT)–primed complementary DNA (cDNA) was synthesized using avian myeloblastosis virus (AMV) reverse transcriptase in a 20-μL reaction volume (1st Strand cDNA Synthesis Kit for reverse transcriptase-polymerase chain reaction [RT-PCR], Boehringer Mannheim, Indianapolis, IN) according to the manufacturer's instructions. Two microliters of cDNA was used for PCR amplification in 50-μL reaction mixtures containing 0.2 mmol/L of each dNTP, 1 U of DynaZyme DNA polymerase, and 10× reaction buffer (Finnzymes OY, Espoo, Finland) and 15 pmol/μL of each primer set. Constitutively expressed β-actin was amplified from the same pool of cDNA. RT was omitted from the control RT reactions. PCR amplification conditions were 95°C for 1 minute, 55°C for 50 seconds, 72°C for 1 minute for a total of 35 cycles. The second PCR was performed by adding 1 μL of the primary PCR product in 50 μL reaction mixtures containing 15 pmol/μL of nested ER-α and ER-β primers, 0.2 mmol/L of each dNTP, 1 U of DynaZyme DNA polymerase, and 10× reaction buffer. Thirty-five cycles were performed for ER-α (95°C for 1 minute, 55°C for 50 seconds, 72°C for 1 minute) and for ER-β (95°C for 1 minute, 65°C for 50 seconds, 72°C for 1 minute). The PCR products were analyzed by electrophoretic separations on a SeaKerm 1.5% agarose gel (FMC Bioproducts. Rockland, ME) and transferred to Hybond-N+Nylon membrane (Amersham International, Amersham, United Kingdom) for hybridization with internal oligonucleotide probe. All the primer sets for ER-α and ER-β, including the internal probes, were from MedProbe (Oslo, Norway) and have been previously described.25 Primers for human β-actin were sense: 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′ and antisense: 5′-GGG AGG TAG CAG GTG GCG TTT ACG CCG ATC-3′.
Cytokine measurements
Levels of TGF-β1 were measured using Quantikine immunoassay kits obtained from R&D Systems and IL-10 levels using enzyme-linked immunosorbent assay (ELISA) kits obtained from CLB (Amsterdam, The Netherlands). To detect latent TGF-β1, the culture supernatant samples were activated with 1 N HCl for 10 minutes and neutralized by adding 1.2 N NaOH + 0.5 HEPES. The antibodies for 2-site sandwich ELISA detecting the biologically active IL-12 heterodimer p70 were from R&D Systems. All ELISAs were performed in duplicate according to the instructions of the manufacturer.
Statistical analysis
The paired Student t test was used for statistical analysis.
Results
The effect of anti-estrogens on the functional differentiation of monocyte-derived DC
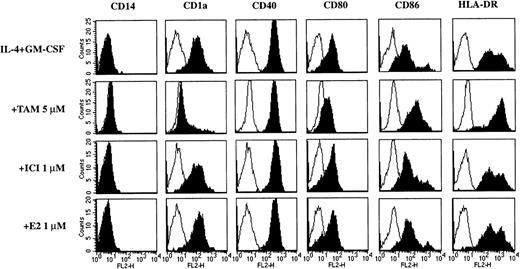
Adherent PBMC were cultured in the presence of IL-4 + GM-CSF, which resulted in a typical phenotype of immature DC. After 7 days of culture, the cells were CD14−CD1a+(Figure 1 and Table1) and at this stage of differentiation CD83low/− (not shown). In the presence of TOR the cultured cells lost CD14 expression, but remained mostly CD1a−, indicating that phenotypically the cells had not differentiated into immature DC (Figure 1 and Table 1). Both TOR and IL-10 inhibited the up-regulation of CD40 and CD80 during the DC differentiation. Interestingly, the expression of CD86 increased in the presence of TOR, whereas IL-10 had an opposite effect. The decrease in HLA-DR expression by IL-4 + GM-CSF was inhibited by 10 μmol/L TOR and, to some extent, by IL-10 (Figure 1 and Table 1). Morphologically, cells treated with TOR had clearly fewer dendritic processes than the cells cultured in IL-4 + GM-CSF alone, but they had lost the macrophage-like appearance that was completely retained in the presence of IL-10 (Figure 2). The effect of TOR on DC differentiation was also functionally significant, because TOR-treated cells were less potent than immature DC in inducing the proliferation of allogeneic T cells (Figure3). To reveal whether there are any differences between TOR and TAM, we compared their effects on the phenotype of monocyte-derived DC. As shown in Figure4, the effect of TOR and TAM on DC phenotype was identical.
The effect of TOR on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR. After 7 days of culture the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms from 5 individual experiments are shown.
The effect of TOR on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR. After 7 days of culture the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms from 5 individual experiments are shown.
The effect of TOR and IL-10 on the differentiation of monocyte-derived DC
| Treatment . | IL-4 + GM-CSF . | CD14 . | CD1a . | CD40 . | CD80 . | CD86 . | HLA-DR . |
|---|---|---|---|---|---|---|---|
| — | — | 150 ± 130 | 1.5 ± 0.4 | 13 ± 3.2 | 1.1 ± 0.1 | 21 ± 13 | 690 ± 410 |
| — | + | 2.6 ± 3.0 | 17 ± 12 | 180 ± 110 | 17 ± 10 | 10 ± 2.0 | 250 ± 180 |
| TOR 1 μmol/L | + | 1.2 ± 0.2 | 11 ± 6.9 | 140 ± 87 | 8.3 ± 1.6 | 9.0 ± 2.2 | 170 ± 87 |
| TOR 5 μmol/L | + | 1.3 ± 0.4 | 2.4 ± 1.2* | 87 ± 55* | 5.6 ± 4.0* | 26 ± 8.7† | 290 ± 160 |
| TOR 10 μmol/L | + | 2.9 ± 2.7 | 1.1 ± 0.1* | 69 ± 39* | 1.2 ± 1.2* | 190 ± 95* | 620 ± 440* |
| IL-10 | + | 74 ± 56* | 2.3 ± 1.6* | 33 ± 29* | 5.8 ± 3.2* | 6.8 ± 3.8 | 380 ± 220 |
| Treatment . | IL-4 + GM-CSF . | CD14 . | CD1a . | CD40 . | CD80 . | CD86 . | HLA-DR . |
|---|---|---|---|---|---|---|---|
| — | — | 150 ± 130 | 1.5 ± 0.4 | 13 ± 3.2 | 1.1 ± 0.1 | 21 ± 13 | 690 ± 410 |
| — | + | 2.6 ± 3.0 | 17 ± 12 | 180 ± 110 | 17 ± 10 | 10 ± 2.0 | 250 ± 180 |
| TOR 1 μmol/L | + | 1.2 ± 0.2 | 11 ± 6.9 | 140 ± 87 | 8.3 ± 1.6 | 9.0 ± 2.2 | 170 ± 87 |
| TOR 5 μmol/L | + | 1.3 ± 0.4 | 2.4 ± 1.2* | 87 ± 55* | 5.6 ± 4.0* | 26 ± 8.7† | 290 ± 160 |
| TOR 10 μmol/L | + | 2.9 ± 2.7 | 1.1 ± 0.1* | 69 ± 39* | 1.2 ± 1.2* | 190 ± 95* | 620 ± 440* |
| IL-10 | + | 74 ± 56* | 2.3 ± 1.6* | 33 ± 29* | 5.8 ± 3.2* | 6.8 ± 3.8 | 380 ± 220 |
Adherent PBMC were cultured with IL-4 (500 U/mL) + GM-CSF (50 ng/mL) in the presence or absence of toremifene (TOR) or IL-10 (100 U/mL). After 7 days in culture the cells were stained for various cell surface markers and analyzed with flow cytometry. The data represent MFIRs (see “Materials and methods”) of 5 individual experiments (mean ± SD).
P < .05 and
P < .01 comparing TOR and IL-4 + GM-CSF or IL-10 and IL-4 + GM-CSF versus IL-4 + GM-CSF.
The effect of TOR and IL-10 on the morphology of monocyte-derived DC.
(A) A macrophage-like appearance of adherent PBMC cultured in a medium for 7 days. (B) Immature DC cultured with IL-4 + GM-CSF for 7 days displaying dendrites and veils on the cell surface. (C) Mature DC cultured with IL-4 + GM-CSF followed by LPS (1 μg/mL) for 24 hours displaying a markedly dendritic surface morphology. (D) Cells cultured with IL-4 + GM-CSF and 5 μmol/L TOR or (E) IL-4 + GM-CSF and 10 μmol/L TOR with an intermediate morphology between macrophages and immature DC, with clearly fewer dendritic processes than immature DC. (F) Cells cultured with IL-4 + GM-CSF and IL-10 (100 U/mL) with a macrophage-like appearance. Magnification ×1000. The results are representative of 5 individual experiments.
The effect of TOR and IL-10 on the morphology of monocyte-derived DC.
(A) A macrophage-like appearance of adherent PBMC cultured in a medium for 7 days. (B) Immature DC cultured with IL-4 + GM-CSF for 7 days displaying dendrites and veils on the cell surface. (C) Mature DC cultured with IL-4 + GM-CSF followed by LPS (1 μg/mL) for 24 hours displaying a markedly dendritic surface morphology. (D) Cells cultured with IL-4 + GM-CSF and 5 μmol/L TOR or (E) IL-4 + GM-CSF and 10 μmol/L TOR with an intermediate morphology between macrophages and immature DC, with clearly fewer dendritic processes than immature DC. (F) Cells cultured with IL-4 + GM-CSF and IL-10 (100 U/mL) with a macrophage-like appearance. Magnification ×1000. The results are representative of 5 individual experiments.
TOR and IL-10 inhibit the function of monocyte-derived DC.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR or IL-10 (100 U/mL). After 7 days of culture, the cells were washed, irradiated (30 Gy), and counted. Five to 20 × 103 cells were then cocultured with 2 × 105 allogeneic PBMC for 5 days, after which the proliferative activity was measured. The results are expressed as mean counts per minute ([cpm] ± SD) of a representative experiment made in triplicate. Similar results were obtained in another 2 experiments.
TOR and IL-10 inhibit the function of monocyte-derived DC.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR or IL-10 (100 U/mL). After 7 days of culture, the cells were washed, irradiated (30 Gy), and counted. Five to 20 × 103 cells were then cocultured with 2 × 105 allogeneic PBMC for 5 days, after which the proliferative activity was measured. The results are expressed as mean counts per minute ([cpm] ± SD) of a representative experiment made in triplicate. Similar results were obtained in another 2 experiments.
TOR and TAM have identical effects on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of TOR or TAM. After 7 days of culture, the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms of 5 individual experiments are shown.
TOR and TAM have identical effects on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of TOR or TAM. After 7 days of culture, the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms of 5 individual experiments are shown.
Anti-estrogens inhibit the differentiation of DC by mechanisms not involving the ER
Next we wanted to detect whether ER transcripts are expressed in cells representing various stages of DC differentiation. Therefore, resting CD14+ monocytes, cultured immature CD1a+, and mature CD83+ DC were sorted by MACS. In RT-PCR analysis both ER-α and ER-β mRNA were expressed, although at low levels, in CD14+, CD1a+, and CD83+ cells (Figure 5). These results indicated that ER-α and ER-β are expressed at all stages of DC differentiation and that the effect of anti-estrogens could be mediated via the ER. TAM and TOR bind to the ER at similar affinity, which is about 3% of the binding affinity of the natural ER ligand 17β-estradiol (E2),26 whereas the binding affinity of a pure antagonist ICI 182780 is about 90% of that of E2.27However, E2 or ICI 182780 did not modulate the phenotype of immature DC, when administered at concentrations up to 1 μmol/L (Figure6). Furthermore, 1 μmol/L of E2 or ICI 182780 could not counteract the effect of 5 μmol/L TOR or TAM on the expression of CD1a (Figure 7), suggesting that TOR and TAM inhibit DC differentiation by ER-independent mechanisms. To exclude the effect of endogenous hormones in the culture medium, we also studied the effect of TOR, TAM, E2, and ICI 182780 on DC differentiation using dextran-charcoal stripped FCS and medium without phenol red, which has weak estrogen-like effects.28Under these culture conditions, 10% to 50% of the cells expressed CD1a and about 50% of the cells remained CD14+ after 7 days of culture with IL-4 + GM-CSF. However, only TOR and TAM, but not E2 or ICI 182780, had a significant effect on the expression of the phenotypic markers of DC (data not shown).
Monocytes, immature DC, and mature DC express mRNA for ER- and ER-β.
CD14+, CD1a+, and CD83+ cells were sorted with MACS as described in “Materials and methods.” The hybridized PCR products of ER-α and ER-β are shown. MCF-7 breast cancer cells were used as a positive control for the ER mRNA expression. RT was omitted from the control reactions. The constitutively expressed β-actin is shown in the bottom panel.
Monocytes, immature DC, and mature DC express mRNA for ER- and ER-β.
CD14+, CD1a+, and CD83+ cells were sorted with MACS as described in “Materials and methods.” The hybridized PCR products of ER-α and ER-β are shown. MCF-7 breast cancer cells were used as a positive control for the ER mRNA expression. RT was omitted from the control reactions. The constitutively expressed β-actin is shown in the bottom panel.
The effect of TAM, ICI 182780, and 17β-estradiol on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of tamoxifen (TAM), ICI 182780 (ICI), or 17β-estradiol (E2). After 7 days of culture the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms from 3 individual experiments are shown.
The effect of TAM, ICI 182780, and 17β-estradiol on the phenotype of monocyte-derived DC.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of tamoxifen (TAM), ICI 182780 (ICI), or 17β-estradiol (E2). After 7 days of culture the cells were stained for various cell surface markers and analyzed with flow cytometry. Representative histograms from 3 individual experiments are shown.
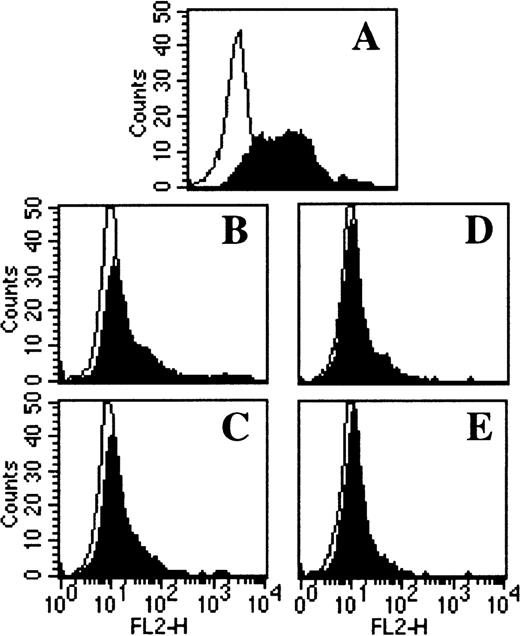
TOR and TAM inhibit DC differentiation by mechanisms not involving the ER.
Adherent PBMC were cultured with (A) IL-4 + GM-CSF alone or in combination with (B) 5 μmol/L TOR, (C) 5 μmol/L TOR + 1 μmol/L E2, (D) 5 μmol/L TAM, or (E) 5 μmol/L TAM + 1 μmol/L ICI 182780. After 7 days of culture the cells were stained for CD1a expression and analyzed with flow cytometry. Representative histograms from 3 individual experiments are shown.
TOR and TAM inhibit DC differentiation by mechanisms not involving the ER.
Adherent PBMC were cultured with (A) IL-4 + GM-CSF alone or in combination with (B) 5 μmol/L TOR, (C) 5 μmol/L TOR + 1 μmol/L E2, (D) 5 μmol/L TAM, or (E) 5 μmol/L TAM + 1 μmol/L ICI 182780. After 7 days of culture the cells were stained for CD1a expression and analyzed with flow cytometry. Representative histograms from 3 individual experiments are shown.
TOR-induced changes in the DC phenotype are not due to the enhanced production of TGF-β1 or IL-10
The enhanced production of TGF-β1 has been suggested to be a potential mechanism by which anti-estrogens have therapeutic effects in autoimmune diseases.14 When TGF-β1 is added into culture with IL-4 + GM-CSF, the monocyte-derived cells differentiate into Langerhans cells, which express E-cadherin.29 We observed that addition of TOR into culture with IL-4 + GM-CSF enhanced the expression of E-cadherin in monocyte-derived cells (unpublished data). Therefore, we studied the effect of TOR on the production of TGF-β1 in adherent PBMC cultured together with IL-4 + GM-CSF. After 1, 3, and 7 days, no detectable levels of the biologically active form of TGF-β1 were found in the culture supernatants. In contrast to active TGF-β1, high levels of latent TGF-β1 were observed in all cultures. Only a minority of the latent TGF-β1 was produced by monocytes/immature DC, because the FCS in the culture medium contained high amounts of latent TGF-β1 (Table 2). Addition of TOR to the culture with IL-4 + GM-CSF did not increase the levels of active or latent TGF-β1 (Table 2), indicating that TOR does not inhibit DC differentiation by enhancing the production of TGF-β1. To exclude the possibility that TOR inhibits DC differentiation by enhancing the production of IL-10, we also measured IL-10 levels after 1, 3, and 7 days in culture. However, IL-10 levels were undetectable or very low and were not found increased in the presence of TOR (data not shown).
The effect of TOR on the production of latent TGF-β1 by adherent PBMC cultured with IL-4 + GM-CSF
| Treatment . | Day 1 . | Day 3 . | Day 7 . |
|---|---|---|---|
| Control | 1.1 ± 0.18 | 1.1 ± 0.11 | 1.2 ± 0.43 |
| IL-4 + GM-CSF | 1.2 ± 0.24 | 1.2 ± 0.24 | 1.2 ± 0.43 |
| +TOR 10 μmol/L | 1.2 ± 0.21 | 1.2 ± 0.18 | 1.2 ± 0.51 |
| Treatment . | Day 1 . | Day 3 . | Day 7 . |
|---|---|---|---|
| Control | 1.1 ± 0.18 | 1.1 ± 0.11 | 1.2 ± 0.43 |
| IL-4 + GM-CSF | 1.2 ± 0.24 | 1.2 ± 0.24 | 1.2 ± 0.43 |
| +TOR 10 μmol/L | 1.2 ± 0.21 | 1.2 ± 0.18 | 1.2 ± 0.51 |
Adherent PBMC were cultured in a medium alone (control) or with IL-4 (500 U/mL) + GM-CSF (50 ng/mL) in the presence or absence of TOR. After 1, 3, and 7 days the latent TGF-β1 levels were measured from the culture supernatants by ELISA. The data represent TGF-β1 levels (ng/mL) of 4 individual experiments (mean ± SD). The amount of latent TGF-β1 in the culture medium without cells added was 0.9 ng/mL. The data were similar for 1-5 μmol/L TOR concentrations.
Anti-estrogens exert differential effects on the expression of costimulatory molecules CD80 and CD86
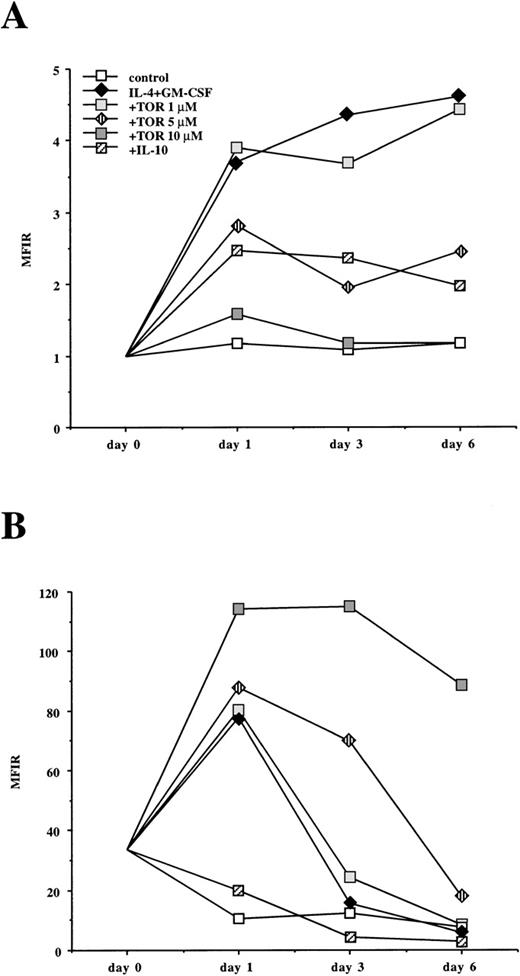
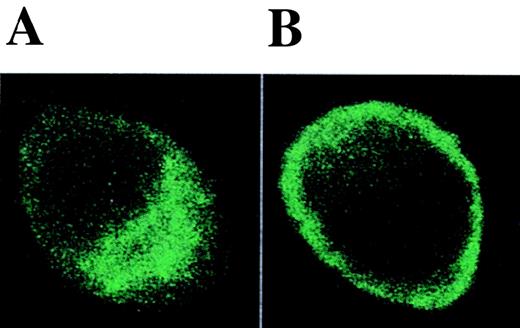
The expression of CD80 and CD86 on monocyte-derived cells seemed to be differentially regulated by anti-estrogens (Table 1 and Figures 1,4, and 6). A similar effect was also observed in macrophages from the synovial fluid of patients with rheumatoid arthritis (unpublished data). To characterize these findings in more detail, we studied the kinetics of CD80 and CD86 expression during DC differentiation. CD80 was not expressed on freshly isolated monocytes but it was up-regulated in the presence of IL-4 + GM-CSF. This up-regulation was inhibited by both TOR and IL-10 (Figure 8A). On the other hand, CD86 was already expressed on freshly isolated monocytes. In the presence of IL-4 + GM-CSF, CD86 was strongly up-regulated after 24 hours of culture and its expression decreased thereafter. IL-10 inhibited the up-regulation of CD86, whereas TOR clearly enhanced and prolonged its expression (Figure 8B). E2 or ICI 182780 did not enhance CD86 expression or modulate the TOR-induced up-regulation of CD86, suggesting that anti-estrogens regulate CD86 expression by ER-independent mechanisms (Figure 9). To reveal whether TOR affects the distribution of CD86 protein between the cell surface and the intracellular reservoir recently described in monocytes,30 we performed ICM. When monocytes were cultured with IL-4 + GM-CSF for 48 hours, CD86 protein was found to be localized both on the cell surface and in the intracellular compartment, whereas in the presence of TOR, CD86 protein was concentrated on the cell surface (Figure10).
The effect of TOR and IL-10 on the kinetics of CD80 and CD86 expression on adherent PBMC cultured with IL-4 + GM-CSF.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR or IL-10 (100 U/mL). After 1, 3, and 6 days of culture the cells were stained for (A) CD80 and (B) CD86 expression and analyzed with flow cytometry. The MFIRs (see “Materials and methods”) of a representative experiment from 3 are shown.
The effect of TOR and IL-10 on the kinetics of CD80 and CD86 expression on adherent PBMC cultured with IL-4 + GM-CSF.
Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR or IL-10 (100 U/mL). After 1, 3, and 6 days of culture the cells were stained for (A) CD80 and (B) CD86 expression and analyzed with flow cytometry. The MFIRs (see “Materials and methods”) of a representative experiment from 3 are shown.
TOR enhances CD86 expression by mechanisms not involving the ER.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of TOR, ICI 182780, and/or E2 in a culture medium containing dextran-charcoal stripped FCS without phenol red (see “Materials and methods”). After 48 hours the cells were stained for CD86 expression and analyzed with flow cytometry. The data represent MFIRs (see “Materials and methods”) of 3 individual experiments (mean ± SD).
TOR enhances CD86 expression by mechanisms not involving the ER.
Adherent PBMC were cultured with IL-4 + GM-CSF in the presence or absence of TOR, ICI 182780, and/or E2 in a culture medium containing dextran-charcoal stripped FCS without phenol red (see “Materials and methods”). After 48 hours the cells were stained for CD86 expression and analyzed with flow cytometry. The data represent MFIRs (see “Materials and methods”) of 3 individual experiments (mean ± SD).
The effect of TOR on the distribution of CD86 protein.
Adherent PBMC were cultured with (A) IL-4 + GM-CSF or (B) IL-4 + GM-CSF and 5 μmol/L TOR for 48 hours and immunostained with anti-CD86 mAb. Representative ICM images in the x-y plane are shown. Isotype-control mAb staining was negative (not shown).
The effect of TOR on the distribution of CD86 protein.
Adherent PBMC were cultured with (A) IL-4 + GM-CSF or (B) IL-4 + GM-CSF and 5 μmol/L TOR for 48 hours and immunostained with anti-CD86 mAb. Representative ICM images in the x-y plane are shown. Isotype-control mAb staining was negative (not shown).
The effect of anti-estrogens on the terminal maturation of DC
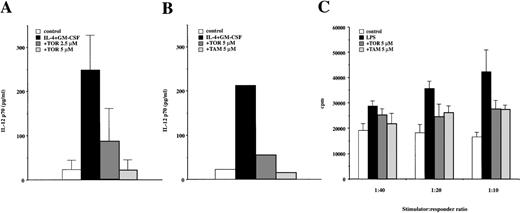
To evaluate the capacity of anti-estrogens to interfere the maturation of DC, adherent PBMC were cultured with IL-4 + GM-CSF with or without TOR and stimulated with CD40L. As shown in Figure11A, the TOR-treated cells were clearly impaired in producing the biologically active IL-12 p70 heterodimer when cocultured with CD40L-transfected cells, showing that they could not respond normally to the CD40 ligation. A similar effect was also observed with TAM (Figure 11B). Next, we stimulated immature DC with LPS or TNF-α. These cultures resulted in mature CD83+ DC (Table 3 and Figure 2) with high capacity to stimulate the proliferation of allogeneic T cells (Figure 11C). TOR significantly inhibited LPS- and TNF-α–induced up-regulation of CD1a, HLA-DR, and LPS-induced up-regulation of CD83, whereas the up-regulation of other molecules was inhibited to a lesser degree (Table 3). Similar findings were also observed with TAM (data not shown). The effect of TOR and TAM on DC maturation was also functionally significant, because anti-estrogen–treated cells were less capable in inducing the proliferation of allogeneic T cells than their fully matured counterparts (Figure 11C). Altogether, these data indicated that terminal maturation of DC can also be partially inhibited by anti-estrogens.
Anti-estrogens inhibit the terminal maturation of DC.
(A) Adherent PBMC were cultured for 7 days with IL-4 + GM-CSF in the presence or absence of TOR. On days 3 and 5, 200 μL fresh medium containing IL-4 (500 U/mL) + GM-CSF (50 ng/mL) was added with or without TOR (2.5 or 5 μmol/L). The cells were then cocultured with 2 × 105 CD40L-transfected J558L cells for 48 hours. The levels of biologically active IL-12 p70 were measured from culture supernatants by ELISA. The data represent mean cytokine levels ± SD of 3 individual experiments. The control J558L cells did not induce IL-12 production (not shown). (B) The cells were cultured as described in (A). A representative experiment of 3 experiments comparing TOR and TAM on the IL-12 p70 production after CD40 ligation is shown. (C) The immature DC were cultured in a medium alone (control) or activated with LPS (1 μg/mL) in the presence or absence of TOR or TAM. After 36 hours the cells were washed, irradiated (30 Gy), and counted. Five to 20 × 103 cells were then cocultured with 2 × 105 allogeneic PBMC for 5 days, after which the proliferative activity was measured. The results are expressed as mean cpm ± SD of a representative experiment made in triplicate. Similar results were obtained in 2 other experiments.
Anti-estrogens inhibit the terminal maturation of DC.
(A) Adherent PBMC were cultured for 7 days with IL-4 + GM-CSF in the presence or absence of TOR. On days 3 and 5, 200 μL fresh medium containing IL-4 (500 U/mL) + GM-CSF (50 ng/mL) was added with or without TOR (2.5 or 5 μmol/L). The cells were then cocultured with 2 × 105 CD40L-transfected J558L cells for 48 hours. The levels of biologically active IL-12 p70 were measured from culture supernatants by ELISA. The data represent mean cytokine levels ± SD of 3 individual experiments. The control J558L cells did not induce IL-12 production (not shown). (B) The cells were cultured as described in (A). A representative experiment of 3 experiments comparing TOR and TAM on the IL-12 p70 production after CD40 ligation is shown. (C) The immature DC were cultured in a medium alone (control) or activated with LPS (1 μg/mL) in the presence or absence of TOR or TAM. After 36 hours the cells were washed, irradiated (30 Gy), and counted. Five to 20 × 103 cells were then cocultured with 2 × 105 allogeneic PBMC for 5 days, after which the proliferative activity was measured. The results are expressed as mean cpm ± SD of a representative experiment made in triplicate. Similar results were obtained in 2 other experiments.
The effect of TOR on the terminal maturation of DC
| Treatment . | CD1a . | CD83 . | CD80 . | CD86 . | HLA-DR . |
|---|---|---|---|---|---|
| Control | 8.7 ± 5.8 | 1.7 ± 0.9 | 11 ± 7.6 | 11 ± 10 | 95 ± 92 |
| TOR | 6.5 ± 2.6 | 1.5 ± 0.4 | 7.9 ± 4.1 | 7.8 ± 6.4 | 51 ± 48 |
| LPS | 14 ± 5.1 | 13 ± 5.9 | 38 ± 26 | 89 ± 72 | 430 ± 350 |
| TOR + LPS | 10 ± 3.73-150 | 8.7 ± 4.03-150 | 30 ± 20 | 76 ± 61 | 290 ± 2903-150 |
| TNF-α | 9.4 ± 4.4 | 5.2 ± 3.4 | 15 ± 8.8 | 25 ± 19 | 210 ± 180 |
| TOR + TNF-α | 6.6 ± 3.63-150 | 4.6 ± 3.2 | 12 ± 11 | 34 ± 40 | 130 ± 1503-150 |
| Treatment . | CD1a . | CD83 . | CD80 . | CD86 . | HLA-DR . |
|---|---|---|---|---|---|
| Control | 8.7 ± 5.8 | 1.7 ± 0.9 | 11 ± 7.6 | 11 ± 10 | 95 ± 92 |
| TOR | 6.5 ± 2.6 | 1.5 ± 0.4 | 7.9 ± 4.1 | 7.8 ± 6.4 | 51 ± 48 |
| LPS | 14 ± 5.1 | 13 ± 5.9 | 38 ± 26 | 89 ± 72 | 430 ± 350 |
| TOR + LPS | 10 ± 3.73-150 | 8.7 ± 4.03-150 | 30 ± 20 | 76 ± 61 | 290 ± 2903-150 |
| TNF-α | 9.4 ± 4.4 | 5.2 ± 3.4 | 15 ± 8.8 | 25 ± 19 | 210 ± 180 |
| TOR + TNF-α | 6.6 ± 3.63-150 | 4.6 ± 3.2 | 12 ± 11 | 34 ± 40 | 130 ± 1503-150 |
First, adherent PBMC or sorted CD14+ cells were cultured with IL-4 + GM-CSF for 7 days. On days 3 and 5, 200 μL medium was replaced with new medium including IL-4 (500 U/mL) and GM-CSF (50 ng/mL). After 7 days of culture, LPS (1 μg/mL), TNF-α (10 ng/mL) and/or TOR (5 μmol/L) was added. After an additional 24 hours, the cells were stained for various cell surface markers and analyzed with flow cytometry. The data represent MFIRs (see “Materials and methods”) of 5 individual experiments (mean ± SD).
P < .05 comparing TOR versus control, LPS versus TOR + LPS, and TNF-α versus TOR + TNF-α.
Discussion
Nonsteroidal anti-estrogens have been suggested to have therapeutic potential in the treatment of autoimmune diseases. In experimental models TAM has been reported to inhibit the development of collagen-induced arthritis14 and adjuvant-induced polyarthritis,15 and to have beneficial effects in murine models of SLE.16,17 In the present study we provide phenotypical, morphologic, and functional evidence that anti-estrogens are able to inhibit the differentiation of monocyte-derived DC in vitro. DC have a central role in the initiation and regulation of immune responses1,2 and a unique ability to produce high levels of IL-12.4-6 IL-12 is the major cytokine responsible for the generation of inflammatory Th1 cells,4-6 which are the predominant cells in various autoimmune diseases.31 We found that cells treated with anti-estrogens were less potent than immature DC in inducing allogeneic T-cell proliferation and clearly impaired in producing the biologically active IL-12 p70 heterodimer after CD40 ligation. This suggests that by inhibiting the functional differentiation of DC, anti-estrogens may inhibit the development of inflammatory Th1 responses. It also implies that the beneficial effects of anti-estrogens in experimental models of autoimmune disease may occur at the level of APCs. We found no differences between the effects of TOR and TAM. Importantly, both anti-estrogens inhibited DC differentiation at concentrations that can be achieved in vivo. Administration of high-dose TAM and TOR can result in 4 to 6 μmol/L and 10 to 15 μmol/L plasma concentrations, respectively.32,33 Because TOR has been shown to be less genotoxic than TAM in animal studies,34-38 it could have some benefits over TAM when administered at high doses.
To reveal the mechanisms of anti-estrogen action, the expression of ER on monocyte-derived cells was studied. Interestingly, both ER-α and ER-β mRNA were expressed at all stages of DC differentiation. However, the natural ER ligand E2 or a pure antagonist ICI 182780, which binds to the ER at much higher affinity than TOR or TAM, did not affect DC differentiation. Neither did they modulate the inhibitory effects observed with TOR or TAM, suggesting that anti-estrogens inhibit DC differentiation by ER-independent mechanisms. Protein kinase C (PKC) is important for DC differentiation because activation of PKC with phorbol-12-myristate-13-acetate (PMA) can induce the differentiation of DC from CD34+precursors.39 TAM is a well-known inhibitor of PKC,40 suggesting a potential mechanism for the effects of anti-estrogens on DC differentiation. We have recently shown that in activated T cells TOR inhibits the PMA-induced activation of c-Jun amino terminal kinase (JNK).41 Because JNK is required for the development of Th1 cells,42 43anti-estrogens may also have direct PKC-mediated inhibitory effect on the activation of Th1 cells.
Although controversial, it has been suggested that CD80 and CD86 on the APC regulate the development of Th1 and Th2 responses by delivering differential signals to helper T cells.44 Evidence suggests that CD80 would preferentially drive the response into Th1 and CD86 into Th2 pathway.45-47 We found that TOR and TAM inhibited the up-regulation of CD80, but clearly enhanced the cell surface expression of CD86 during DC differentiation. How do the anti-estrogens modulate CD86 expression? TAM is known to activate transcription of genes from an AP-1 enhancer element via the ER48 and via the recently discovered raloxifene responsive element.49However, E2 or ICI 182780 did not modulate the anti-estrogen–induced up-regulation of CD86 expression, suggesting that CD86 expression is not controlled via the ER. In ICM experiments, CD86 protein was retained on the cell surface in the presence of TOR. TAM, independently of the ER, inhibits acidification of endosomes and lysosomes and decreases the rate of vesicular transport through the recycling and secretory pathways.50 Therefore, it is possible that anti-estrogens enhance the surface expression of CD86 by inhibiting the recycling of CD86 protein. Whether the differential effect of anti-estrogens on the expression of CD80 and CD86 has functional relevance in directing the T-cell response remains to be elucidated.
Being the most potent APCs, DC play a critical role in the regulation of immune responses and are considered a promising target for immunotherapy. The immunosuppressive effect of glucocorticoids is at least partly mediated by the inhibition of DC differentiation, maturation, and function.51 In addition, glucocorticoids and 1,25-dihydroxyvitamin have been shown to inhibit the production of IL-12 by DC.52 53 The present data provide evidence that nonsteroidal anti-estrogens also have inhibitory effects on DC differentiation, maturation, and function, suggesting that anti-estrogens could have a therapeutic potential in autoimmune diseases.
Acknowledgments
We thank Ms Marianne Laine for expert technical assistance. We thank Dr P. Lane for providing the CD40L transfected J558L cell line, Dr A. E. Wakeling for providing ICI 182780 and Dr T. F. Tedder for providing the anti-CD83 mAb. We acknowledge Dr J. Uksila, Dr J. Punnonen, Dr M. Möttönen, Dr D. Smith and Dr H. Arvilommi for their critical reading of the manuscript and Dr P. Lakkakorpi for assistance with the confocal microscopy.
Supported by the Academy of Finland, Turku Graduate School of Biomedical Sciences, Turku University Central Hospital special funds, and the South-Western Cancer Society of Finland.
Submitted May 24, 1999; accepted October 22, 1999.
Reprints:Janne Komi, Turku Immunology Centre, Department of Medical Microbiology, Turku University, Kiinamyllynkatu 13, 20520 Turku, Finland; e-mail: janne.komi@utu.fi.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 3. TOR and IL-10 inhibit the function of monocyte-derived DC. / Adherent PBMC were cultured in a medium alone (control) or with IL-4 + GM-CSF in the presence or absence of TOR or IL-10 (100 U/mL). After 7 days of culture, the cells were washed, irradiated (30 Gy), and counted. Five to 20 × 103 cells were then cocultured with 2 × 105 allogeneic PBMC for 5 days, after which the proliferative activity was measured. The results are expressed as mean counts per minute ([cpm] ± SD) of a representative experiment made in triplicate. Similar results were obtained in another 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/9/10.1182_blood.v95.9.2875.009k12_2875_2882/5/m_bloo00912003x.jpeg?Expires=1767750086&Signature=yVd1nrKggdhe75UokA~43i0oDsNNKXb0P3j0JV-CqXujqum0Ip3niAQsnKMNI75hHrWBpWlrACiQe-oK-M27jkvY2WlMrNvGaOJQDOikxbbZlroYv3Dmm7IJwNzxRhhD5Mc2OWDev5xm78zmkruKpQz3X8-FTF3CE651q1v~q9lVCS5wRcawmPZavowvjkqpmAoMWON0grH59JA1uh8Nj4fTi9EaXjOVemWh8gQT7aGDGPo5aR3cp4Iy5fExk8bJRFJiV85TQhOEH-YZMAQGyNe-v9kEt5xeHghGDagEIBBCWhzATlkLxt6hQJQyCLdqQaMZ3OUurs-0zGEshpz15A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal