Abstract
Human T lymphotropic virus type I (HTLV-I) is the etiological agent of adult T-cell lymphocytic leukemia (ATLL), whereas HTLV-II has not been associated with hematopoietic malignancies. The control of apoptotic pathways has emerged as a critical step in the development of many cancer types. As a result, the underlying mechanism of long-term survival of HTLV-I and HTLV-II was studied in infected T cells in vitro and in ex vivo ATLL samples. Results indicate that HTLV-I– and HTLV-II–infected T cells in vitro express high levels of the antiapoptotic protein Bcl compared with other human leukemic T cell lines or uninfected peripheral blood mononuclear cells. The levels of proapoptotic proteins Bax, BAD, and Bak were not significantly altered. HTLV-I and HTLV-II viral transactivators, Tax1 and Tax2, are known to increase expression of cellular genes. These proteins were tested for increased transcription from the human Bcl2 and Bcl-XL promoters. Whereas no effect was observed on the Bcl2 promoter, both Tax1 and Tax2 increased transcription of the Bcl-XL promoter in T cells, although Tax1 appeared to be more efficient than Tax2. The biological significance of these observations was validated by the finding of an increased expression of Bcl-XL in ex vivo ATLL cells, especially from patients unresponsive to various chemotherapy regimens. Altogether, these data suggest that overexpression of Bcl-XL in vivomay be in part responsible for the resistance of ATLL cells to chemotherapy. In addition, inefficient activation of the Bcl-XL promoter by Tax2 may result in a shorter survival time of HTLV-II–infected cells in vivo and a diminished risk of leukemia development.
Programmed cell death is a normal physiological process essential to tissue remodeling and is characterized by an active physiological mechanism, apoptosis, that eliminates DNA-damaged, senescent, cancer, or virus-infected cells. A wide variety of human diseases, including cancers and degenerative diseases, appears to be associated with dysregulation of the apoptotic pathways.1-4Bcl2 and related proteins are part of an expanding family involved in the regulation of the apoptotic signaling.5-7 This family is generally divided into 2 groups: the death antagonists (eg, Bcl2, Bcl-XL, and BAG-1) and the death agonists (eg, Bax, Bak, and BAD). Bcl2 and Bcl-XL are associated mainly with mitochondria membranes, where they exert part of their protective functions by preventing the release of cytochrome C to the cytosol and the subsequent activation of caspases.8 The defined mechanism by which Bcl2 and Bcl-XL exert their antiapoptotic functions remains uncertain.
A model, whereby homodimers of death agonists induce apoptosis, while heterodimers or homodimers of death antagonists prevent apoptosis,3,9 has been proposed. Alternatively, both agonist homodimers and heterodimers may be active, and the stoichiometry of heterodimers to homodimers may dictate the fate of a cell.10 Other studies indicated that in some instances, Bcl2, Bcl-XL, and Bax might function independently and without the formation of heterodimers.11-13
To counter host apoptotic responses, many viruses have evolved strategies that interfere with key steps of the apoptotic pathway. Some viruses, such as H saimiri, Epstein-Barr virus (EBV), human herpes virus 8 (HHV8), equine herpes virus (EHV)-2, bovine herpes virus (BHV)-4, avian herpes virus (AHV)-1, and the African swine fever virus, carry a cellular homologue of the antiapoptotic protein Bcl2 within their genome.14 Other strategies include the production of viral inhibitors of caspases (cowpox virus, murine herpes virus (MHV) 68, vaccinia virus, and the African swine fever virus); the secretion of soluble cytokine receptors (myxoma virus and EBV); the inhibition of cellular stress responses (Papillomavirus,Polyomavirus, and adenovirus); and the inhibition of death receptor–mediated apoptosis (H saimiri, HHV-8, EHV-2, and BHV-4).14
HTLV-I–mediated T-cell transformation presumably arises from a multistep oncogenic process in which the virus induces chronic T-cell proliferation resulting in an accumulation of genetic defects and the dysregulated growth of infected cells.15 The viral transactivator Tax plays an essential role during the oncogenic process, and its expression is sufficient to immortalize primary T cells and transform rat fibroblasts in vitro.16,17 Although some aspects of viral transformation remain elusive, the Tax effect on cell cycle regulatory proteins, such as p53, p15INK,4 p16INK,4p21WAF1, and MAD1,18-22 are the key to the viral-induced growth dysregulation of T cells. In addition, Tax also represses the expression of β-polymerase and interferes with the DNA repair machinery, thereby increasing the mutation rate in virus-infected cells.23 24 In contrast, HTLV-II, which also immortalizes and transforms T cells in vitro, although it induces a life-long infection, is not associated with hematopoietic malignancies.
The poor prognosis in HTLV-I–induced ATLL is associated with the resistance of neoplastic T cells to the conventional combination of high-dose chemotherapy and radiotherapy. The disease is invariably fatal, and generally, survival from onset of the acute disease does not exceed 6-8 months. Treatment with the antiretroviral agent zidovudine (AZT), interferon-α (IFN-α), all transretinoic acid (ATRA), or arsenic trioxide appears to prolong the survival of some patients, who ultimately relapse in conjunction with the reemergence of drug-resistant neoplastic cells.25-28
We demonstrate that cells infected in vitro by HTLV-I and the related virus HTLV-II have elevated expression of antiapoptotic proteins Bcl2 and Bcl-XL. Because the expression of the proapoptotic proteins Bax, Bak, and BAD were not significantly increased, the ratio of Bcl2 and Bcl-XL homodimers to heterodimers was increased in HTLV-I–infected T cells. Interestingly, whereas both HTLV-I and HTLV-II Tax transactivated the Bcl-XL promoter in human T cells, HTLV-II Tax had a reduced activity. Importantly, the Bcl-XL expression was also markedly increased in uncultured leukemic cells in 6 of 6 ATLL patients tested, and its up-regulated expression appears to correlate with the severity of the disease. As in the case of other histological types of human cancers, these data support the notion that aberrant expression of Bcl-XL may increase the survival of virus-infected T cells as well as their resistance to apoptotic signals, thereby contributing to HTLV-I–induced leukemogenesis.
Materials and methods
Cell lines
Human leukemic T cell lines (Jurkat and Molt-4), HTLV-I–transformed T cell lines (MT-2, MT-4, C8166, and C91/PL), and HTLV-II–transformed T cell lines (C19 and 344Mo) were cultured in Roswell Park Memorial Institute medium (RPMI 1640) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Life Technologies, Gaithersburg, MD), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. HTLV-I–immortalized cell lines 1185 and 1996 and HTLV-II–immortalized cell line c96II were cultured in RPMI 1640 supplemented with 20% heat-inactivated FCS, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in the presence of 50 U/mL interleukin-2 (IL-2) (Boehringer Mannheim, Mannheim, Germany). Two weeks prior to analysis, peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient (Hyclone, San Francisco, CA); washed with phosphate-buffered saline (PBS); activated with 5 μg/mL phytohemagglutinin (PHA) for 48 hours; washed in serum-free medium; and then cultured in RPMI 1640 supplemented with 20% serum and 50 U/mL IL-2.
Patient samples
Lymphocytes from the peripheral blood of healthy volunteers or ATLL patients were purified by Ficoll-Hypaque gradient, washed in PBS, and lysed in radioimmunoprecipitation assay (RIPA) buffer. All samples were obtained after informed consent was received.
Plasmids and transfections
Expression vectors for HTLV-I Tax, HTLV-II Tax, and mutants M47 and M22 were previously described.29,30 We used luciferase reporter constructs for the human Bcl2 promoters P1 and P2 (Dr C. Paya, Mayo Foundation, Rochester, MN)31 and reporter constructs for the human Bcl-XL promoter luciferase and control vectors (Dr K.E. Boulukos, Faculté de Nice, France).32 Transient transfections were carried out in Jurkat T cells using the Superfect reagent (Quiagen, Madison, WI) according to the manufacturer's instructions. This was done using 5 × 106 cells, 4 μg reporter construct, 1 μg Tax vectors, and 100 ng CMV-RL (renilla) to control transfection efficiency. After 48 hours, transfected cells were collected by centrifugation, washed with PBS, and lysed in reporter lysis buffer (Promega, Madison, WI) and assay using the Dual Luciferase Assay System (Promega). Luciferase activity, measured with a Bertholdt luminometer (EGNG, Gaithersburg, MD), was normalized for transfection efficiency, and standard deviations were calculated from 3 independent transfections.
Immunoblots and antibodies
Exponentially growing cells were collected by centrifugation, washed with PBS, and lysed in RIPA buffer containing protease inhibitors and 1 mmol/L sodium orthovanadate. Protein concentration was determined by the Bradford assay (Bio Rad Laboratories, Hercules, CA). Protein (50 μg) was resolved on SDS-polyacrylamide Tris-glycine gels (sodium dodecyl sulfate–polyacrylamide tris[hydroxymethyl] aminomethane–glycine gels) (Novex, Bedford, PA) and transferred onto a PVDF membrane (Millipore, San Diego, CA). Nonspecific sites were blocked for 30 minutes at room temperature in a 5% PBS-milk combination, and a primary antibody was diluted in 1% PBS-milk and incubated overnight at 4°C; BAD was incubated overnight at room temperature. A secondary antibody, horseradish peroxidase conjugate diluted in 1% PBS-milk, was incubated for 2 hours at room temperature. Immunoblots were washed 5 times for 15 minutes with TNE (50 mmol/L Tris [pH 7.5], 2 mmol/L ethylenediamine tetraacetic acid [EDTA], 100 mmol/L sodium chloride [NaCl]) 0.05% Tween. The immunoblots were then developed using the chemiluminescence West-Dura (Pierce, Barcelona, Spain). Comparable loading of protein was confirmed by reprobing the membrane with a specific antibody for the housekeeping gene product β-tubulin. Each immunoblot is representative of 2 separate experiments using uninfected PBMCs from 2 different donors as a control.
The following products were used in the study (brand names in parentheses): primary antibodies Bcl2 (100), Bcl-XS/L (S-18), Bcl-XL (H-62), Bax (P-19), and Bad (K-17) (Santa Cruz Biotechnology, Santa Cruz, CA); secondary antibodies (Santa Cruz Biotechnology); Bak (Ab-1; Calbiochem Oncogene Research Products); and β-tubulin (Ab-1; Boehringer-Mannheim). Membrane stripping was performed by immersion in Tris (pH 8), 1% SDS, and 100 mmol/L NaCl for 30 minutes at 62°C; membranes were then washed extensively with 0.1% TNE-Tween and PBS.
Immunoprecipitation
Total protein extracts (200 μg) were incubated with 4 μg each of Bax, Bak, and BAD antibodies for 4 hours at 4°C, and depletion was further verified by Western blot analysis. The remaining supernatants were incubated with 4 μg Bcl2 or Bcl-XL for 4 hours at 4°C. Immune complexes were captured by adding 30 μL protein alanine/glycine agarose (A/G agarose) (Life Technologies), which was collected by centrifugation and washed twice with 5 volumes of 0.05% PBS-Tween 20. Immunoprecipitates were resuspended in 1 times the loading buffer, boiled for 5 minutes, and resolved on 12.5% SDS-PAGE (polyacrylamide gel electrophoresis). After this was completed, electrotransfer immunoblots were incubated with antibodies specific for Bcl2 or Bcl-XL. Quantifications were realized by densitometry using the Image Quant software (Molecular Dynamics) from 2 independent experiments. The average values, which were calculated from the total intensity of signals corresponding to the homodimers and heterodimers, were expressed as the ratio of homodimers to heterodimers.
Results
Increased expression of antiapoptotic Bcl2 and Bcl-XLproteins in HTLV- immortalized and HTLV-transformed T cells in vitro
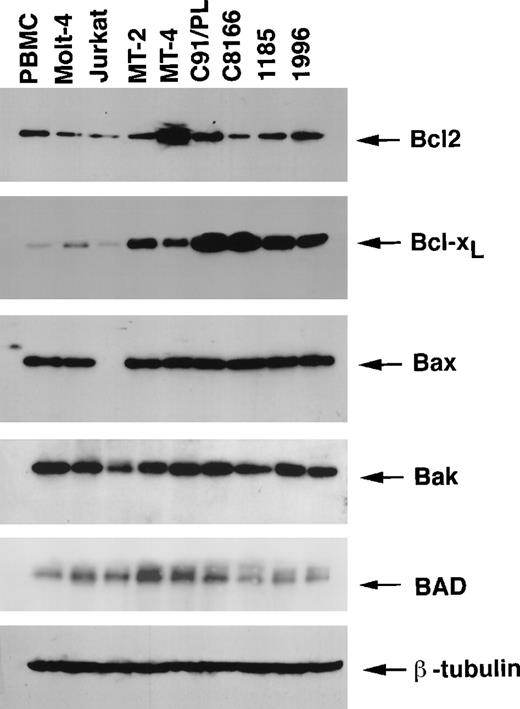
Control of the apoptotic pathways has emerged as a critical step in the development of many cancer types. We have previously shown that (1) replication of HTLV-I in human endothelial cells resulted in an increased expression of Bcl233 and (2) HTLV-I –infected T cells in vitro are resistant to apoptosis-inducing treatments.34 To gain further insight into the cellular components involved in the survival of HTLV-I–infected T cells, the levels of apoptotic and antiapoptotic protein expression were studied in the HTLV-I–transformed cell lines (MT-2, MT-4, C8166, and C91/PL) and in the 2 IL-2–dependent HTLV-I–immortalized cell lines (1185 and 1996). The human leukemic T cell lines, Jurkat and Molt-4, as well as normal PBMCs from 2 uninfected donors, were used for comparison. The levels of the Bcl2 protein expression in normal PBMCs were similar to the levels in most HTLV-I–infected T cell lines (MT-2, C91/PL, 1185, and 1996) except the MT-4, C8166, Jurkat, and Molt-4 T cell lines (Figure 1). Markedly, the Bcl2-related antiapoptotic protein Bcl-XL was expressed at much higher levels in all HTLV-I–infected cell lines as compared with the levels in PBMCs and the Molt-4 and Jurkat cells (Figure 1).
Expression of the Bcl2 family proteins in HTLV-I–transformed and HTLV-I–immortalized cell lines.
Total proteins (50 μg) were resolved on 12.5% SDS-PAGE and transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, Bax, Bak, and BAD. Comparable protein loading was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent immunoblots using uninfected PBMCs from 2 separate donors.
Expression of the Bcl2 family proteins in HTLV-I–transformed and HTLV-I–immortalized cell lines.
Total proteins (50 μg) were resolved on 12.5% SDS-PAGE and transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, Bax, Bak, and BAD. Comparable protein loading was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent immunoblots using uninfected PBMCs from 2 separate donors.
The alternatively spliced proapoptotic Bcl-XS protein was not detected by Western blot, even when an antibody that recognizes both forms of Bcl-X was used (data not shown). Despite a previous report proposing that Tax transrepresses a Bax promoter reporter construct in a transient transfection assay,35 Bax protein expression was not affected in HTLV-I–infected cell lines and was comparable to the expression in PBMCs and Molt-4 cells (Figure 1). This finding is consistent with a recent study that showed no effect of Tax on the endogenous Bax promoter in mouse T cells expressing Tax.36 The absence of Bax expression in the Jurkat cell line has previously been attributed to the presence of a termination codon mutation.37 The expression of proapoptotic Bak was similar in all the cell lines investigated, although there were reduced levels in the Jurkat T cell line. The proapoptotic BAD protein was expressed at similar levels in the MT-2, MT-4, C91/PL, Jurkat, and Molt-4 cell lines, and lower levels were present in the C8166, 1185, and 1996 cell lines and in PBMCs. A comparable loading of protein was confirmed using a specific antibody for the housekeeping gene product β-tubulin (Figure 1).
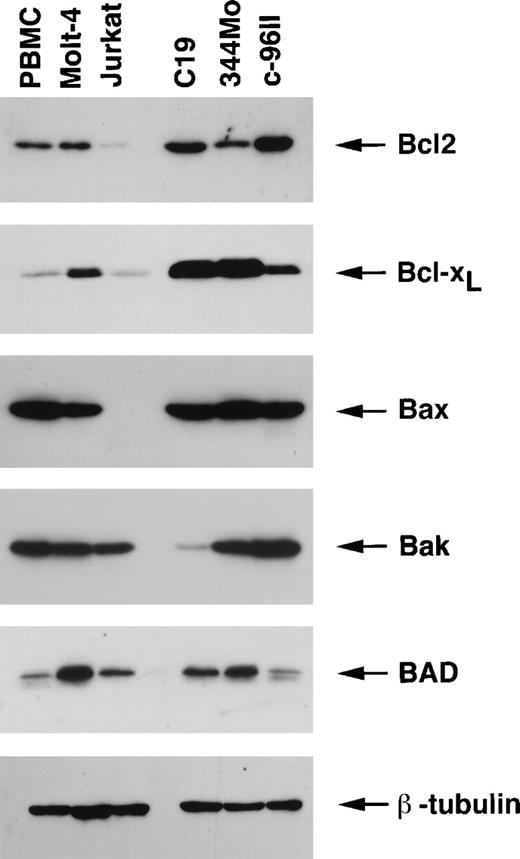
A similar analysis used the HTLV-II–transformed 344Mo and C19 cell lines and the HTLV-II–immortalized IL-2–dependent c96II cell line. This study revealed that both the antiapoptotic Bcl2 and Bcl-XL proteins were expressed at higher levels in HTLV-II–infected cell lines than in PBMCs and Jurkat and Molt-4 cell lines (Figure 2). Similar to HTLV-I–infected cells, the levels of the proapoptotic gene product Bax were not increased in HTLV-II–infected cells, and the expressions of Bak and BAD were lower in C19 and c-96II, respectively. Comparable loading of protein was confirmed using a specific antibody for the housekeeping gene product β-tubulin (Figure 1). Together these results indicate that both HTLV-I– and HTLV-II–infected cells in vitro have acquired altered cellular signaling pathways that result in high levels of expression of antiapoptotic proteins, mainly Bcl-XL.
Expression of the Bcl2 family proteins in HTLV-II–transformed and HTLV-II–immortalized cell lines.
Total proteins (50 μg) were resolved on 12.5% SDS-PAGE and transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, Bax, Bak, and BAD. Comparable protein loading was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent experiments using uninfected PBMCs from 2 separate donors.
Expression of the Bcl2 family proteins in HTLV-II–transformed and HTLV-II–immortalized cell lines.
Total proteins (50 μg) were resolved on 12.5% SDS-PAGE and transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, Bax, Bak, and BAD. Comparable protein loading was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent experiments using uninfected PBMCs from 2 separate donors.
Tax1 and Tax2 differential transactivation of the human Bcl-XL promoter in Jurkat T cells
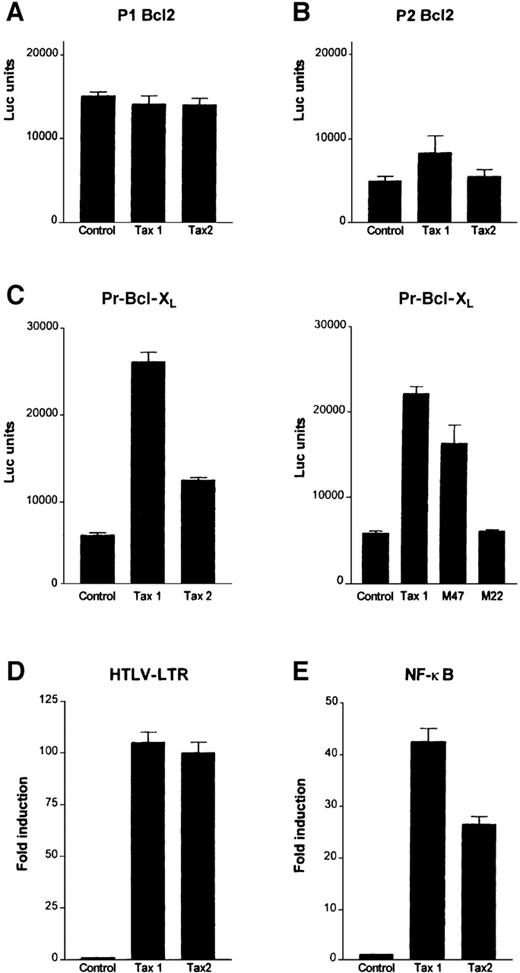
Tax1 and Tax2, the virus-encoded transactivator Tax proteins of HTLV-I and HTLV-II, respectively, stimulate transcription through distinct cellular pathways including CREB/ATF; NF-κB, and serum responsive factor (SRF). To investigate their potential effect, Tax1 and Tax2 were transiently transfected together with human Bcl2 and Bcl-XL promoter reporter constructs in Jurkat T cells. In agreement with a previous report,32 Tax1 was unable to stimulate transcription from either one of the Bcl2 promoters, the P1 Bcl2-Luc or P2 Bcl2-Luc reporter constructs; similarly Tax2 had no significant effect (Figure3A). Tax1 increased transcription from Bcl-XL promoter more than 4-fold. Although Tax2 had a reduced activity compared to Tax1, it still activated the Bcl-XL promoter approximately 2-fold (Figure 3B).
Tax transactivates the Bcl-XL promoter through the NF-κB pathway in T cells.
Jurkat cells were transfected with HTLV-I Tax (Tax1), HTLV-II Tax (Tax2), M22, M47, or pCMV4 vector and (A) P1 Bcl2, (B) P2 Bcl2, (C) Pr Bcl-XL, (D) HTLV-LTR, or (E) NF-κB luciferase reporter constructs. Results are representative of 3 independent transfections.
Tax transactivates the Bcl-XL promoter through the NF-κB pathway in T cells.
Jurkat cells were transfected with HTLV-I Tax (Tax1), HTLV-II Tax (Tax2), M22, M47, or pCMV4 vector and (A) P1 Bcl2, (B) P2 Bcl2, (C) Pr Bcl-XL, (D) HTLV-LTR, or (E) NF-κB luciferase reporter constructs. Results are representative of 3 independent transfections.
The NF-κB pathway has previously been shown to stimulate the Bcl-XL promoter.38 As a result, 2 additional Tax mutants that retain the ability to activate the CREB/ATF (M22) or NF-κB pathway (M47) were tested. Whereas the M47 mutant increased the Bcl-XL promoter reporter activity, no significant activation was observed with the M22 mutant (Figure 3C). Consistent with these observations, Tax2, which is partially defective in transactivation of Bcl-XL, had a reduced ability to transactivate an NF-κB reporter construct in Jurkat T cells. This occurred even though both Tax1 and Tax2 transactivated the HTLV-LTR (long terminal repeat sequence) to a similar extent (Figure 3D). These observations underscore the importance of Tax activation of the NF-κB pathway in T-cell survival and/or transformation.
Increased antiapoptotic homodimers in HTLV-I–infected T cell lines
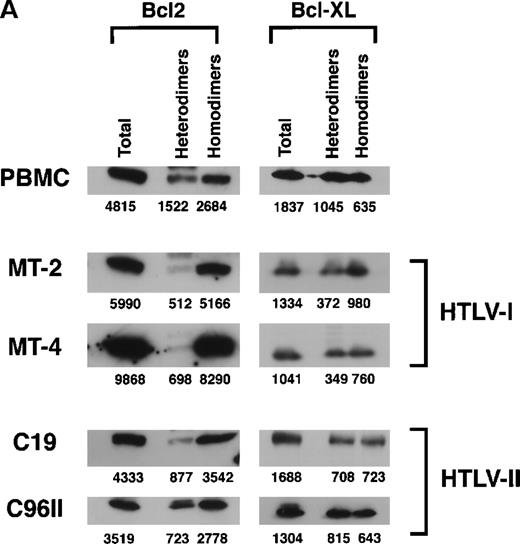
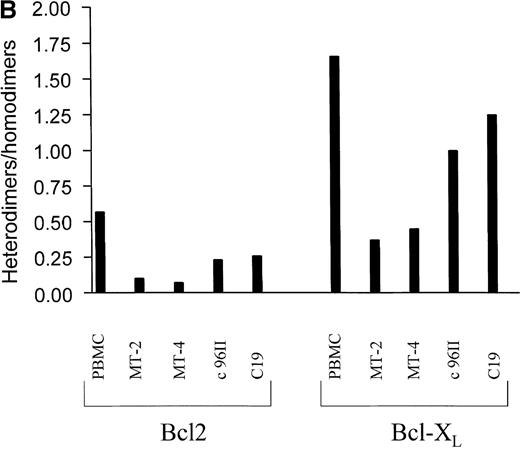
The mechanism by which Bcl2 and Bcl-XL exert their antiapoptotic functions is unclear. Part of the Bcl2 and Bcl-XL protective effect may occur through heterodimerization with the proapoptotic proteins Bax, Bak, and BAD.3,9,10,39 Thus, a cell's resistance to apoptotic signals may be modulated by the amount of antiapoptotic homodimers that can potentially antagonize increased amounts of proapoptotic proteins through heterodimerization.40 We therefore investigated the intracellular levels of unbound Bcl2 and Bcl-XL as well as the levels bound to the Bax, Bak, and BAD proteins. Protein lysates from HTLV-I (MT-2 and MT-4) or HTLV-II (C19 and c96II) cell lines and uninfected PBMCs were simultaneously immunoprecipitated with antibodies directed against Bax, Bak, and BAD. The immunocomplexes were captured using protein A/G agarose and analyzed for the presence of Bcl2 or Bcl-XL proteins (bound fractions or heterodimers). Proteins remaining in the supernatant were then immunoprecipitated with either Bcl2 or Bcl-XL in order to evaluate the unbound fractions or homodimers (Figure 4A). The relative ratio of homodimers to heterodimers, as quantified by densitometry, indicated an increased ratio in HTLV-I–transformed cells compared with PBMCs. HTLV-II–infected cells also presented an increase of antiapoptotic homodimers, albeit to a lower extent than when compared with HTLV-I–transformed cells.
Increased antiapoptotic homodimers to heterodimers in HTLV-I–transformed cells.
(A) Protein extracts from uninfected PBMCs, HTLV-I cell lines (MT-2 and MT-4), and HTLV-II cell lines (C19 and C96II) were immunoprecipitated with mixed antibodies against Bax, Bak, and BAD, and the immunocomplexes were probed with either Bcl2 or Bcl-XL(heterodimers). Depletion of Bax, BAD, and Bak was confirmed by Western blot, and the proteins in the depleted fraction were immunoprecipitated with antibodies directed against either Bcl2 or Bcl-XL (homodimers). The relative intensity of each band was calculated by densitometry and is presented as an arbitrary unit. (B) The ratio of heterodimers to homodimers was calculated from 2 independent immunoprecipitations.
Increased antiapoptotic homodimers to heterodimers in HTLV-I–transformed cells.
(A) Protein extracts from uninfected PBMCs, HTLV-I cell lines (MT-2 and MT-4), and HTLV-II cell lines (C19 and C96II) were immunoprecipitated with mixed antibodies against Bax, Bak, and BAD, and the immunocomplexes were probed with either Bcl2 or Bcl-XL(heterodimers). Depletion of Bax, BAD, and Bak was confirmed by Western blot, and the proteins in the depleted fraction were immunoprecipitated with antibodies directed against either Bcl2 or Bcl-XL (homodimers). The relative intensity of each band was calculated by densitometry and is presented as an arbitrary unit. (B) The ratio of heterodimers to homodimers was calculated from 2 independent immunoprecipitations.
High levels of Bcl-XL expression in leukemic cells from ATLL patients
ATLL cells in vivo are resistant to radiation and chemotherapy treatments.27 28 To assess the relevance of our findings in vitro to the ATLL cells ex vivo, we analyzed the levels of antiapoptotic proteins in uncultured blood cells from ATLL patients. The clinical status of the ATLL patients at the time of sample collection is summarized in Table1. Western blot analysis of ATLL cell lysates revealed levels of Bcl2 expression in ATLL cells comparable to levels of normal PBMCs, as observed in HTLV-I–infected cells in vitro (Figures 1 and 5). BAD expression was not detected in nonactivated PBMCs and patient ATLL cells (data not shown). Importantly, similar to HTLV-I–infected T cells in vitro, all ATLL patient cells analyzed expressed higher levels of Bcl-XL compared with levels from uninfected PBMCs (Figure5). The only exception noted was patient No. 3; at the time of collection, this patient had very few cells that displayed a neoplastic phenotype (Table 1).
Clinical features of ATLL patients at the time of sample collection
| Patient no. . | WBC count, mm3 . | NBCs, % . |
|---|---|---|
| 1 | 74 000 | 83 |
| 2 | 13 800 | 92 |
| 3 | 5800 | na |
| 4 | 56 000 | 64 |
| 5 | 19 000 | 94 |
| 6 | 11 000 | 53 |
| Patient no. . | WBC count, mm3 . | NBCs, % . |
|---|---|---|
| 1 | 74 000 | 83 |
| 2 | 13 800 | 92 |
| 3 | 5800 | na |
| 4 | 56 000 | 64 |
| 5 | 19 000 | 94 |
| 6 | 11 000 | 53 |
WBC indicates white blood cell; NBCs indicates neoplastic blast cells; and na indicates not applicable.
Overexpression of Bcl-XL in uncultured acute T-cell lymphocytes from ATLL patients.
Lymphocytes from uninfected donors and ATLL patients were isolated by Ficoll-Hypaque gradient, washed, and lysed in RIPA buffer. Total proteins (50 μg) were separated on 12.5% SDS-PAGE transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, and Bax. Comparable protein loading in each lane was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent experiments using 2 separate donors for PBMCs.
Overexpression of Bcl-XL in uncultured acute T-cell lymphocytes from ATLL patients.
Lymphocytes from uninfected donors and ATLL patients were isolated by Ficoll-Hypaque gradient, washed, and lysed in RIPA buffer. Total proteins (50 μg) were separated on 12.5% SDS-PAGE transferred onto PVDF membranes and probed with specific antibodies for Bcl2, Bcl-XL, and Bax. Comparable protein loading in each lane was verified using an antibody specific for the housekeeping gene product β-tubulin. Results are representative of 2 independent experiments using 2 separate donors for PBMCs.
With the exception of patient No. 5, the overall levels of the proapoptotic Bax protein were not significantly different among ATLL cells and normal PBMCs. Interestingly, the sample from patient No. 5 contained high levels of Bcl-XL. The patient failed all therapies and succumbed to the disease a few days after sample collection. Although Bax is generally considered a proapoptotic protein, high levels may indicate a poor prognosis, as recently evidenced by the finding that an increased Bax expression is associated with the high probability of relapse in childhood ALL.41
Discussion
Accumulating evidence indicates that dysregulation of the physiological cell death suicide program, apoptosis, often leads to uncontrolled accumulation of cells that carry genetic defects. Thus, alteration in apoptotic pathways is frequently associated with the development of cancers and autoimmune and neurodegenerative diseases.1-4 Indeed, overexpression of either Bcl2 or Bcl-XL is found in roughly half of all human cancers, and it is associated with both an increased mutation rate and a resistance of tumor cells to radiotherapy and chemotherapy.33-35 Overexpression of the Bcl-XL protein found in ATLL may explain this malignancy's resistance to chemotherapy. A previous investigation of HTLV-I–infected T-cell resistance to damaging agents in vitro revealed an alteration in cell cycle regulatory proteins.22 34 However, there has not been a thorough study of the Bcl2 protein family in HTLV-I– and HTLV-II–infected T cell lines in vitro and in ex vivo ATLL.
In this study we investigated the expression level of some of the Bcl2 family members in various HTLV-I and HTLV-II cell lines as well as in uncultured leukemic cells isolated from ATLL patients. Here we demonstrate that the levels of Bcl2 protein are constitutively higher in most HTLV-I and HTLV-II T cell lines in vitro when compared with noninfected leukemic cells such as Jurkat or Molt-4. However, there were no significant differences observed between cultured PBMCs and HTLV-infected cells in vitro or uncultured PBMCs and ex vivo ATLL cells. Notably, all HTLV-I and HTLV-II cell lines investigated in this study had elevated expression of the antiapoptotic protein Bcl-XLwhen compared with leukemic T cell lines Jurkat and Molt-4 or with uninfected PBMCs from different donors.
In our study, the expressions of proapoptotic Bax, BAD, and Bak proteins were not significantly affected. In addition, the ratio of antiapoptotic Bcl2 and Bcl-XL homodimers to heterodimers was increased in HTLV-I–infected cells compared with the ratio in uninfected PBMCs. Our results also suggest that elevated Bcl-XL expression may preferentially sequester the death agonists and thereby increase the formation of Bcl2 homodimers. Interestingly, HTLV-II–infected cells presented a lower increase of antiapoptotic homodimers compared with HTLV-I–transformed cells. We observed that in vitro Tax, through the NF-κB pathway, up-regulates Bcl-XL. This finding parallels the in vivo finding that in ATLL, the NF-κB pathway is constitutively activated despite the low level of Tax expression in these cells.42,43 NF-κB activation may be the key to increasing the survival of HTLV-I–infected T cells in vitro and in vivo. In support of this theory is the observation that in Tax transgenic mice, treatment with NF-κB antisense oligonucleotides is associated with massive apoptotic cell death.44 In addition antisense inhibition of NF-κB p65 has been shown to block tumorigenicity and cause tumor regression. Therefore NF-κB–induced expression of Bcl-XL may contribute to HTLV-I leukemogenesis. Indeed, increasing evidence indicates that up-regulation of Bcl-XL is a critical step for tumor progression in many cancers such as breast and colorectal cancer, multiple myelomas, ovarian carcinomas, gastric adenomas, non-Hodgkin lymphomas, and Hodgkin disease.46-51
The IL-2/IL-2R pathway is constitutively activated in most HTLV-I–transformed cells in vitro as well as in most ATLL cells in vivo.52,53 However, ATLL cells in vivo likely need several signals to survive and replicate. The inability of HTLV-II to activate this pathway,54 coupled with the lower efficiency of Tax2 activation of the Bcl-XL promoter, may affect the survival of HTLV-II–infected T cells in vivo.
In most cases, up-regulation of Bcl2 and/or Bcl-XL is not sufficient for tumor expansion in vivo, and leukemia may result when Bcl2 and/or Bcl-XL cooperate with other oncogenes, such asc-Myc or Ras.55,56 Interestingly, HTLV-I Tax has been shown to increase expression ofc-Myc,57 which correlates with an increased neoplastic potential of HTLV-I Tax in transgenic mice.58 In addition, it has also been demonstrated that HTLV-I Tax cooperates withRas in fibroblast transformation in vitro.59 60Further studies are needed to determine whether HTLV-II Tax is also able to increase expression and/or cooperate with these oncogenes.
The finding that ex vivo ATLL cells from HTLV-I–infected patients also have higher levels of Bcl-XL expression than PBMCs from uninfected donors validates the observations made in HTLV-I–infected T cells in vitro. In the case of HTLV-II, however, it is unfeasible to validate in vitro observations because very few HTLV-II–infected cells are present in the blood of HTLV-II–infected individuals. Bcl-XL also increases the mutagenesis rate61and promotes resistance of tumor cells to chemotherapy or radiotherapy.62 63 As a result, Bcl-XL may increase the survival of infected T cells with consequent accumulation of genetic mutations that may predispose HTLV-I–infected individuals to the development of ATLL. In addition, it is tempting to speculate that the reduced activity of Tax2 on the Bcl-XL promoter may be associated with a lower long-term survival of HTLV-II–infected cells in vivo and, consequently, a reduced probability to develop leukemia.
Reprints:Christophe Nicot, Basic Research Laboratory, National Cancer Institute, 9000 Rockville Pike, Bldg 41, Rm C303, Bethesda, MD 20892; e-mail: cbeben@helix.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal