Abstract
Telomere shortening is associated with disease evolution in chronic myelogenous leukemia (CML). We have examined the relationship between diagnostic telomere length and outcome in 59 patients with CML who entered into the MRC CMLIII Trial by Southern blot hybridization using the (TTAGGG)4 probe. Age-adjusted telomere repeat array (TRA) reduction was found to significantly correlate with time from diagnosis to acceleration, such that patients with a larger TRA reduction entered the accelerated phase more rapidly (r = −0.50; P = .008). Cox-regression analysis for this group was suggestive of a relationship between a greater TRA-reduction and a shorter time to acceleration (P = .054). Age-adjusted TRA reduction did not significantly affect either the time to blast crisis or overall survival. Our results show that telomere shortening observed at the time of diagnosis in CML significantly influences the time to progress to the accelerated phase. The measurement of diagnostic TRA may prove to be clinically important in the selection of patients at high risk of disease transformation in CML.
Telomeres are the termini of eukaryotic chromosomes and are composed of TTAGGG repeats.1 A reduction in telomere length has been described in a wide range of human cancers, including both solid tumors and leukemias.2,3 Indeed, progressive telomere shortening has been shown to contribute to genomic instability.4 Most late-stage human tumors express telomerase and this may contribute to the relative immortality of these cells.5,6 Reduced telomere length has been shown to be associated with the evolution of certain hematologic malignancies, including chronic myelogenous leukemia (CML).2,7-13 We have shown that telomere length in the accelerated and/or blast phase was reduced compared with serial samples taken at the chronic phase in patients with CML.11 Similarly, Ohyashiki et al8 have also shown that telomere length was significantly shortened in the blast phase compared with the chronic phase in CML. We have previously shown that leukocytes from patients with CML have shorter telomere length values than healthy controls and that patients display heterogeneity of telomere length in the chronic phase,11 which may reflect the relative variation in time-to-disease progression in CML. These data lead to the hypothesis that the mean telomere repeat array (TRA) at the time of diagnosis may be of prognostic importance.
Materials and methods
Patients and controls
Diagnostic DNA samples were available from 59 patients with CML who had entered the UK Medical Research Council CMLIII Trial between 1989 and 1994.14 Patients in this study were randomized to receive either interferon-alfa or chemotherapy without interferon-alfa for maintenance. The protocol and results of this study have been previously reported.14 All patients were positive for the Philadelphia chromosome or showed molecular evidence of the BCR-ABL rearrangement.15 Patients were defined as being in the accelerated phase according to criteria outlined in Table1. Blast crisis was defined as more than 20% blasts in the peripheral blood or more than 30% blasts in the bone marrow. Patients not fulfilling these criteria were classified as being in the chronic phase.15 Peripheral blood samples were collected from 75 healthy individuals aged between 20 and 60 years.
Criteria defining patients as being in accelerated phase
| Trial no. . | Resistant to therapy . | Spleen size increasing . | Major cytogenetic evolution . | Blasts >10% . | Platelets >1000 × 109/L . | Other (specified) . |
|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | N | N | |
| 2 | Y | Y | NK | N | N | |
| 3 | Y | N | N | N | N | |
| 4 | N | N | N | Y | Y | Anemia |
| 5 | Y | Y | Y | N | N | |
| 6 | Y | Y | N | Y | N | |
| 7 | Y | NK | N | Y | N | |
| 8 | N | Y | Y | N | N | BM fibrosis |
| 9 | Y | Y | N | Y | N | |
| 10 | Y | Y | N | N | N | Anemia |
| 11 | Y | Y | Y | N | N | |
| 12 | Y | N | N | N | Y | Basophils >20% |
| 13 | Y | Y | NK | N | N | |
| 14 | Y | Y | N | N | N | Fever and bone pain |
| 15 | Y | Y | Y | Y | N | |
| 16 | N | N | N | N | Y | |
| 17 | Y | Y | Y | N | N | |
| 18 | N | N | N | N | N | Rising blast count (7.5%) |
| 19 | Y | Y | N | N | Y | |
| 20 | Y | Y | N | Y | N | |
| 21 | Y | Y | N | N | Y | |
| 22 | NK | NK | Y | N | N | Anemia and falling platelet count |
| Trial no. . | Resistant to therapy . | Spleen size increasing . | Major cytogenetic evolution . | Blasts >10% . | Platelets >1000 × 109/L . | Other (specified) . |
|---|---|---|---|---|---|---|
| 1 | Y | Y | Y | N | N | |
| 2 | Y | Y | NK | N | N | |
| 3 | Y | N | N | N | N | |
| 4 | N | N | N | Y | Y | Anemia |
| 5 | Y | Y | Y | N | N | |
| 6 | Y | Y | N | Y | N | |
| 7 | Y | NK | N | Y | N | |
| 8 | N | Y | Y | N | N | BM fibrosis |
| 9 | Y | Y | N | Y | N | |
| 10 | Y | Y | N | N | N | Anemia |
| 11 | Y | Y | Y | N | N | |
| 12 | Y | N | N | N | Y | Basophils >20% |
| 13 | Y | Y | NK | N | N | |
| 14 | Y | Y | N | N | N | Fever and bone pain |
| 15 | Y | Y | Y | Y | N | |
| 16 | N | N | N | N | Y | |
| 17 | Y | Y | Y | N | N | |
| 18 | N | N | N | N | N | Rising blast count (7.5%) |
| 19 | Y | Y | N | N | Y | |
| 20 | Y | Y | N | Y | N | |
| 21 | Y | Y | N | N | Y | |
| 22 | NK | NK | Y | N | N | Anemia and falling platelet count |
Y, yes; N, no; NK, not known; BM, bone marrow.
Mean telomere repeat array measurement
High-molecular weight DNA was obtained using standard methods from diagnostic peripheral blood leukocyte samples taken from the patients with CML and from the peripheral blood leukocyte samples taken from healthy individuals.16 Total white blood counts at diagnosis were very high (median 210 × 109/L [range 50-780]). This means that the proportion of DNA derived from nonclonal (Philadelphia negative) lymphoid cells was very minor and insufficient to alter the overall Southern blot results. Ten micrograms of DNA digested with the restriction enzyme HinfI was size fractionated by electrophoresis through 0.8% agarose gels. The DNA was transferred to Hybond N (Amersham Int, Amersham, UK), according to standard procedures for Southern blotting.16 The filters were hybridized to a 3′-32p–labeled (TTAGGG)4telomeric probe as previously described.11,17 Telomere length was measured with an LKB Ultrascan XL densitometer (LKB-Bromma, CO), with the peak of telomere length in kilobases (peak TRA) taken as the average telomere length in each patient.7The TRA was performed with each DNA sample on 2 separate occasions and the mean value determined.
Analysis of TRA influence on patient outcome
The relationship between the diagnostic age-adjusted TRA loss and patient outcome were examined. The 3 outcome measures studied were time to (1) accelerated phase, (2) blast crisis, and (3) death. Linear regression and Cox-regression analyses, assuming proportional hazards, were carried out in STATAv6.0 (Stata Corp, College Station, TX). Univariate, multivariate, and backward stepwise Cox regression analysis was used to investigate the affect of age, sex, white blood count, percentage blast count in peripheral blood, platelet count, spleen size, treatment at trial randomization, and age-adjusted TRA loss on survival. Variable addition was set at P = .25 and removal atP = .3.
Results and discussion
To test the hypothesis that telomere length is a significant prognostic factor in CML, we have analyzed samples taken from 59 patients who entered into the MRC CML III Trial. As a result of the therapeutic randomization, 34 patients received interferon-alfa and 24 patients received cytotoxic therapy (hydroxyurea or busulphan). One patient died before the therapy could be started. The average age at entry was 48 years (SD 15). At the time of analysis (August 1999), there had been 51 deaths, with a median follow-up of 3.72 years.
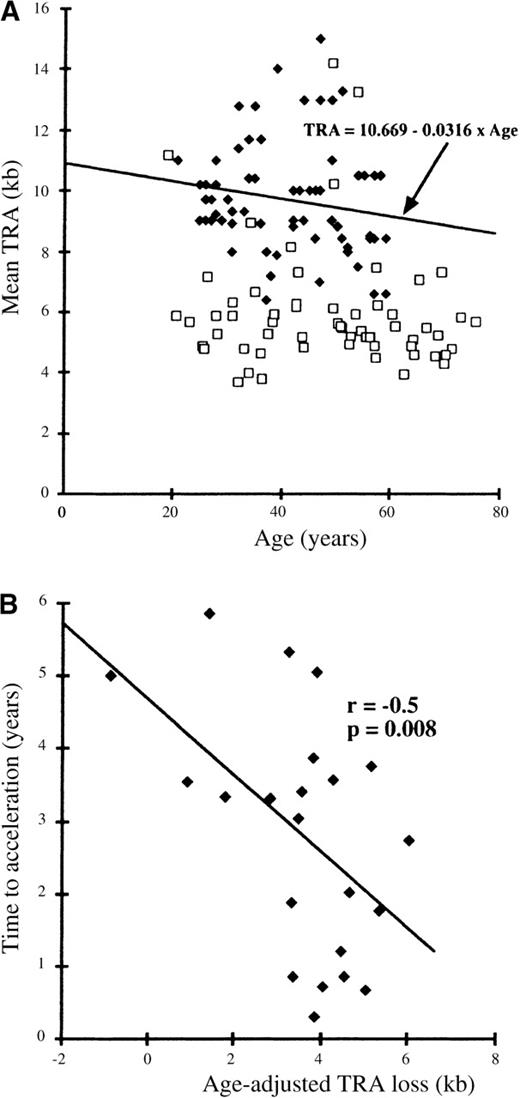
To allow for age-associated telomere shortening, regression analysis was performed on the observed TRA values for a control group consisting of 75 healthy individuals aged between 20 and 60 years. This regression analysis confirmed TRA shortening with increasing age such that T = 10.669-0.0316 × A (T = TRA in kilobases and A = age in years) (Figure 1A). Age-related telomere shortening was hence equivalent to 31.6 base pairs (bp) per year, similar to that described by other groups.12,18 19
Relationships between mean TRA and age and between TRA loss and time to acceleration.
(A) Relationship between mean TRA and age. Controls: solid diamonds. Patients: unfilled squares. (B) Relationship between age-adjusted TRA loss and time to acceleration.
Relationships between mean TRA and age and between TRA loss and time to acceleration.
(A) Relationship between mean TRA and age. Controls: solid diamonds. Patients: unfilled squares. (B) Relationship between age-adjusted TRA loss and time to acceleration.
The mean TRA at the time of diagnosis for patients with CML was 6.0 kb (SD = 2.0 kb), which was significantly lower than that for the control group (mean TRA = 9.7 kb; SD = 1.8 kb) (P < .001) (Figure 1A). To determine the prognostic significance of this TRA reduction, the observed TRA loss was adjusted for age: The age-adjusted TRA reduction was expressed as the difference between the observed TRA and the regression estimate for TRA for age in healthy individuals: TE-O = Expected TRA − Observed TRA. TE-O represents the difference in kilobases between the measured TRA and the age-appropriate point on the normal regression line (Figure 1A).
The age-adjusted TRA (TE-O) was found to correlate with the time from diagnosis to acceleration (r = −0.502;P = .008) (Figure 1B). Cox-regression analysis, according to tertile of TRA reduction, showed a relationship with time to acceleration, although this just failed to attain statistical significance; HR3v1 = 4.43 (1.28-15.3); probability of TE-O effect = 0.054. A date of acceleration was available for only 22 patients, reflecting the clinical difficulty in defining the onset of this phase of CML in the remaining patients. The criteria defining these 22 patients as having entered the accelerated phase are shown in Table 1. The most common criteria are the development of resistance to therapy (17 patients), increasing spleen size (15 patients), major cytogenetic evolution (7 patients), blast percentage more than 10% (6 patients), and platelet count more than 1000 × 109/L (5 patients).
We also examined the influence of TE-O on the time to blast crisis. There was no correlation between TE-O and time to blast crisis (r = −0.30; P = .18; n = 21) and regression analysis, according to tertile, failed to show significant relationship (HR2v1 = 1.22 [0.42-3.55]; HR3v1 = 1.97 [0.64-5.99]; probability of a trend effect = 0.49).
All diagnostic characteristics, including TRA, were investigated to identify important prognostic factors influencing survival. In a full multivariate model, percentage blast count (HR = 2.40, CI 1.13-5.09,P = .02) and white blood count (HR = 0.96; CI 0.93-0.99;P = .03) were significant. Blast count was also significant in a univariate model. The stepwise multivariate model did not suggest any strong prognostic factors, TRA and TE-O included. The lack of influence of diagnostic TRA on outcome was confirmed via tertiles of TE-O.
Eighteen patients within this patient cohort at some point underwent allogeneic bone marrow transplantation. These patients were excluded from the same analyses in case the allogeneic procedure had a confounding impact on survival. A repeat analysis on the 41 remaining patients again failed to show an impact of TE-O on survival.
The finding that patients with greatly reduced telomere length at the time of diagnosis enter the accelerated phase relatively early suggests the existence of a high-risk subgroup of patients with CML who lack an efficient mechanism of telomere maintenance early on, during disease onset. In general, telomerase levels have been shown to be normal or only slightly raised in the chronic phase, but significantly increase in the blast crisis.8 It would be interesting to measure telomerase levels in patients with a large telomere length reduction at diagnosis, as this subgroup might be expected to be just beginning to show higher levels of telomerase activation than most patients in the chronic phase with average or long telomeres.
Our observations support the findings of Iwama et al,19 who have shown that in a group of patients treated with interferon-alfa, those with normal TRA values in the chronic phase had a significantly lower frequency of blast crisis, a significantly higher incidence of cytogenetic responses, and a favorable prognosis compared with those with shortened TRA values. Most recently, Brummendorf et al20 have shown that patients with CML, in whom the blast phase subsequently developed within 2 years, had significantly shorter telomeres than those in whom the blast phase did not develop for at least 2 years.
The lack of impact of TRA loss on time to blast crisis and overall survival shown in this study is somewhat surprising. It is possible that, up until the point of acceleration, the patients were treated in a fairly uniform manner according to the trial protocol, either receiving interferon-alfa or chemotherapy (with hydroxyurea or busulphan). Once the patients entered the accelerated phase, then therapy varied considerably, and it is possible this may have altered subsequent survival. It may be that, by increasing the number of patients included in the study, a prognostic impact of TRA loss could be detected. Alternatively it may be that progressive telomere shortening throughout the chronic phase eventually terminates when a critical threshold is reached and the accelerated phase commences. After this point, factors independent of TRA may determine outcome. This situation is analogous to that in SV40-transfected fibroblasts: Progressive telomere shortening occurs until a critical threshold, after which telomerase levels rise within some cells that become immortalized. The threshold telomere length in these fibroblasts is associated with chromosomal instability.4
In conclusion, diagnostic TRA loss is significantly related to the time to onset of the accelerated phase. The measurement of diagnostic TRA may therefore eventually prove to be of clinical importance in the selection of patients at high risk of disease transformation.
Acknowledgments
Many thanks to Sarah Cullip at the CTSU, Oxford, for assistance in providing patient data.
Supported by the Leukaemia Research Fund of the United Kingdom. The CMLIII Trial was supported by the UK Medical Research Council.
Reprints:J Boultwood, Leukaemia Research Fund Molecular Haematology Unit, Department of Cellular Science, John Radcliffe Hospital, Oxford, 0X3 9DU; e-mail: jboultwo@worf.molbiol.ox.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal