Abstract
Vascular endothelium plays an important role in regulating the transendothelial migration of polymorphonuclear leukocytes (PMNs). In this study, the intracellular calcium ion ([Ca2+]i) signaling of endothelial cells (ECs) during PMN transmigration was examined at the single-cell level. Human umbilical vein ECs were cultured on a thin layer of collagen gel. The ECs were labeled with fura-2, immersed in formyl-Met-Leu-Phe, and subsequently perfused with fresh buffer to establish a gradient of chemoattractant across the EC monolayer. The entire process of PMN rolling on, adhering to, and transmigrating across the EC monolayer was recorded under both phase-contrast and fluorescence optics. The data showed the following: (1) At high concentration (approximately 3 × 106/mL), both PMN suspension and its supernatant stimulated frequent EC [Ca2+]i elevations across the monolayer; (2) when used at lower concentration (approximately 5 × 105/mL) to avoid the interference of soluble factors, PMN transmigration, but not rolling or adhesion, was accompanied by EC [Ca2+]i elevation; (3) the latter EC [Ca2+]i elevation occurred simultaneously in ECs adjacent to the transmigration site, but not in those that were not in direct contact with the transmigrating PMNs; (4) this EC [Ca2+]i elevation was an initial and required event for PMN transmigration; and (5) PMNs pretreated with 5,5′-dimethyl-1,2-bis(2-aminophenoxy)ethane-N, N, N′, N′-tetraacetic acid transmigrated with the accompanying EC [Ca2+]i elevation, but they became elongated in the collagen gel. In conclusion, PMNs induce adjacent EC [Ca2+]i signaling, which apparently mediates the “gating” step for their subsequent transmigration.
Introduction
Intercellular junctions connecting a monolayer of vascular endothelial cells (ECs) occur as a complex network of transmembrane proteins. Presumably, they not only determine endothelial permeability, but also control leukocyte transmigration.1 2 One striking characteristic of the endothelial junctions is their dynamic organization, which is mostly quick and reversible; that is, the endothelium is able to disorganize and reorganize its intercellular junctions within minutes. It is conceivable that inflammatory agents or leukocytes bind to specific receptors on the EC surface and generate intracellular signals, which in turn cause cytoskeletal reorganization and opening of intercellular junctions. However, the mechanism that governs these processes is largely unknown at present.
One of the most intriguing features of EC–leukocyte interactions is the endothelial intracellular cytosolic free calcium concentration ([Ca2+]i) signaling that influences transmigration. When polymorphonuclear leukocytes (PMNs) are placed on human umbilical vein EC monolayers cultured on an amnion that separates chemoattractant, a [Ca2+]i rise occurs in single ECs.3 This EC [Ca2+]irise is associated with PMN–EC interactions, including PMN adhesion and transmigration. Moreover, ECs remain relatively quiescent when exposed to PMNs in the absence of chemoattractant. According to another report, a PMN adhesion-associated EC [Ca2+]ielevation is observed when a chemoattractant-free PMN suspension flows through a glass tube covered with a layer of human aortic ECs.4 In either study, large numbers of PMNs as well as possible bioactive soluble factors encountered individual ECs within a short time. However, neither the exact PMN–EC contact time nor the sequence of postcontact cellular events could be recorded.
In general, leukocyte adhesion and transmigration across the endothelial monolayer is an important physiologic process that probably involves EC [Ca2+]i signaling and the opening of EC junctions. However, the detailed steps of leukocyte transendothelial migration remain obscure, mainly because of technical difficulties. We report the development of a suitable model system to monitor this complex process step by step and to investigate the possible role of EC [Ca2+]i signaling in PMN transmigration. We cultured human umbilical vein ECs on a thin layer of collagen gel until confluence. The EC monolayer was labeled with fura-2, immersed in formyl-Met-Leu-Phe (fMLP), and subsequently perfused with fresh buffer to establish a gradient of chemoattractant across this EC monolayer. The entire process of PMN rolling on, adhering to, and transmigrating across the EC monolayer was examined under phase-contrast optics. The fluorescence ratio images were simultaneously recorded to monitor the EC [Ca2+]i level. At the end of experimentation, the specimen was fixed and further processed for observation under either a confocal or a scanning electron microscope.
Materials and methods
Materials
Collagenase, endothelial cell growth supplement, fMLP, Ficoll-Hypaque, fura-2 AM, heparin, and ionomycin were purchased from Sigma (St Louis, MO). Alexa Fluor 488–conjugated phalloidin and dimethyl-5,5′-dimethyl-1,2-bis(2-aminophenoxy)ethane-N, N, N′, N′-tetraacetic acid (BAPTA)-AM were purchased from Molecular Probes (Eugene, OR). Fetal bovine serum and medium M199 were from Gibco (Gaithersburg, MD).
EC isolation and culture
ECs were isolated from human umbilical vein by collagenase (0.02%) digestion and were grown to confluence on a plastic dish in medium M199 containing 10% fetal bovine serum, 10 U/mL heparin, and 25 μg/mL endothelial cell growth supplement. The EC monolayer was trypsinized and resuspended in M199, and thereafter seeded on a 0.2% collagen gel–coated cover glass. The first-passage cells reached confluence within 2 days and were subsequently used between 3 to 4 days.
PMN isolation
PMNs were isolated from human peripheral blood by centrifugation on a discontinuous Ficoll-Hypaque gradient, according to a previous report.5 Purified PMNs were resuspended in Krebs-Ringer HEPES (KRH) buffer (125 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L KH2PO4, 1 mmol/L MgSO4, 2 mmol/L CaCl2, 25 mmol/L HEPES, 6 mmol/L glucose, and 10% fetal bovine serum, pH 7.4) and held on ice for no more than 3 hours before use. In some experiments, PMN sedimentation was carried out for 1 hour at 4°C under gravitational force, and thereafter the supernatant was used to test the possible effect of soluble factors in our transmigration model system.
Chemoattractant gradient formation and endothelial [Ca2+]i measurements
The confluent EC monolayer grown on collagen gel was replenished with KRH buffer and allowed to stand overnight. The specimen was then immersed in 1 μmol/L fMLP for 1 hour. After this step, EC-treating reagents (fura-2 AM and BAPTA-AM) were prepared in the same fMLP-containing buffer. ECs were labeled for 40 minutes at room temperature with 5 μmol/L fura-2 AM, the fluorescent Ca2+indicator. Extracellular fura-2 AM was washed away, and the EC monolayer was incubated for an additional 20 minutes at 37°C in fura-2–free buffer. In some experiments, the specimen was incubated for 25 minutes with 50 μmol/L BAPTA-AM at 37°C to block the EC [Ca2+]i elevation. The cover glass containing the labeled specimen was assembled on a flow chamber6 7that was slightly modified to accommodate the extra thickness of collagen gel. The chamber was mounted on an inverted microscope (Diaphot 300; Nikon, Tokyo, Japan) and was continuously perfused with fMLP-free KRH buffer (0.05 mL/min, 37°C) to wash away fMLP from the EC apical surface. Thus, a gradient of fMLP was established across the EC monolayer.
The experimental setup for endothelial [Ca2+]i imaging was similar to the one used for [Ca2+]i measurements in single platelets and single ECs.8 9 After the flow chamber had been perfused with fMLP-free KRH buffer for 5 minutes (0.05 mL/min), PMNs were added to the flow chamber at the same flow rate. Phase-contrast and fluorescence images were recorded intermittently to monitor the PMN transmigration process and the accompanying EC [Ca2+]i changes, respectively. All experiments were performed at 37°C.
At the end of some experiments, the calcium concentration was calibrated by applying ionomycin (5 μmol/L) in the presence of 5 mmol/L EGTA, followed by 10 mmol/L CaCl2. All signals were corrected for autofluorescence determined by exposing the tissue to 5 mmol/L manganese to quench cytosol fura-2 fluorescence. After subtracting the background and the autofluorescence, endothelial [Ca2+]i was estimated according to an established formula.10 Because this calibration procedure damages the cell morphology, it was performed only in some experiments to demonstrate the magnitude of the EC [Ca2+]i elevation. At the end of most experiments, the specimens were fixed with glutaraldehyde (2.5%) and stained with silver nitrate11 to visualize the endothelial boundary.
Confocal microscopy and scanning electron microscopy
For confocal microscopy, fixed cells were permeabilized with 0.5% Triton X-100 and fluorescently labeled with Alexa Fluor-488–conjugated phalloidin. A field containing the PMN transmigration site was focused under fluorescence optics, and the optic sections of it were examined under the confocal microscope (MRC 1000; BioRad, Hemel Hempstead, Hertfordshire, England). For scanning electron microscopy, nonpermeabilized specimens were further fixed in 1% OsO4, 1% tannic acid, and saturated uranyl acetate to minimize handling artifacts.12 The specimens were subsequently dehydrated in increasing concentrations of acetone, critical-point dried, and coated with gold. Transmigrating PMNs that were previously recorded under a light microscope were identified and were further examined under a scanning electron microscope (S 2500; Hitachi, Tokyo, Japan).
Results
Our preliminary experiments showed that PMNs could migrate into a fMLP-treated collagen gel even in the absence of the EC monolayer (data not shown). When the EC/collagen layer was not pretreated with fMLP, PMNs migrated on the EC monolayer and maintained a relatively round shape for long periods, and were easily flushed away under flow. In contrast, they underwent rapid transendothelial migration through an fMLP-pretreated EC/collagen layer. However, this chemoattractant gradient was present only when fMLP-free buffer was continuously flowing through the chamber. Upon cessation of flow, PMN transmigration soon stopped, which indicates that the chemoattractant gradient was abolished. When the flow was reinitiated, PMNs resumed their transmigration. This process could be repeated several times until the ultimate depletion of fMLP.
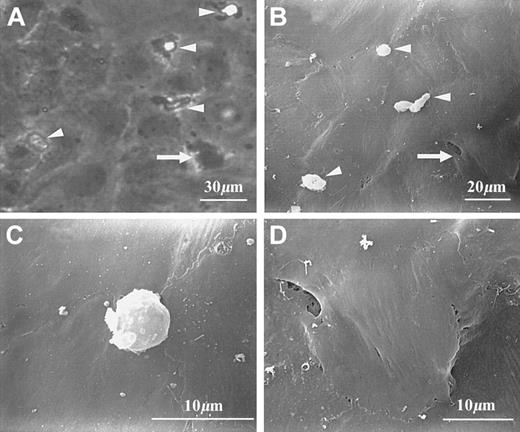
The morphology of transmigrating and transmigrated PMNs is shown in Figure 1. Under phase-contrast light microscopy, transmigrating cells usually showed bright cell bodies and dark, irregular protrusions (Figure 1A). Their cell bodies, but not protrusions, were also visible under the scanning electron microscope (Figure 1B-C). Visibility of both PMN protrusions and collagen fibers underneath the EC layer was noted only when an occasional gap was present near the transmigration site (scanning electron micrograph not shown). Whereas transmigrated cells were dark and irregular under phase contrast (Figure 1A), they were almost invisible in scanning electron micrographs (Figure 1B). When scanning electron micrographs and corresponding phase-contrast images were carefully examined, the locations of PMN transmigrated sites appeared as slightly raised areas (Figure 1B,D).
Morphology of PMNs at different stages of transendothelial migration.
PMNs can be identified under either phase-contrast (A) or scanning electron microscopy (B-D). (A) and (B) show several transmigrating PMNs (arrowheads) and a transmigrated PMN (arrow). Transmigrating PMNs are characterized by both clearly visible cell bodies and extended protrusions; the latter are visible under phase-contrast optics (A), but not under scanning electron microscopy (B,C). A transmigrated PMN is almost invisible from the top but appeared as a slightly raised area (B,D).
Morphology of PMNs at different stages of transendothelial migration.
PMNs can be identified under either phase-contrast (A) or scanning electron microscopy (B-D). (A) and (B) show several transmigrating PMNs (arrowheads) and a transmigrated PMN (arrow). Transmigrating PMNs are characterized by both clearly visible cell bodies and extended protrusions; the latter are visible under phase-contrast optics (A), but not under scanning electron microscopy (B,C). A transmigrated PMN is almost invisible from the top but appeared as a slightly raised area (B,D).
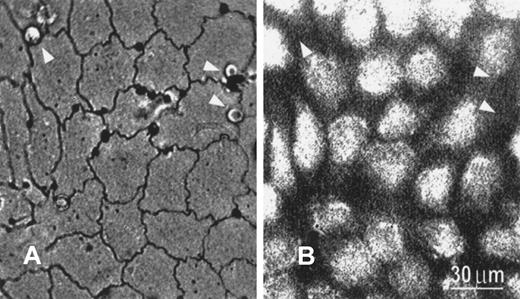
With silver staining, the EC boundary became clearly visible under bright-field optics (Figure 2A). PMNs were found to migrate preferentially to the tricellular corners, confirming previously published results.11 Moreover, compared with PMNs located elsewhere, PMNs located at the tricellular corners were more resistant to flow-induced detachment. Although PMNs were not fluorescently labeled and hence were invisible under fluorescence microscopy, their locations could be identified by mapping the fluorescent image with the corresponding silver-stained image (Figure 2B).
Silver-stained image and corresponding fluorescent image showing transmigrating PMNs.
(A) Transmigrating PMNs preferentially locate near tricellular corners, as shown in silver-stained micrograph viewed under bright-field optics. The endothelial borders as well as the locations of PMNs (arrowheads) are clearly visible under these conditions. (B) The fluorescence image (380 nm) shows the location of fura-2–stained endothelial cells. Because neither unstained PMNs nor the EC boundary is visible under fluorescence optics, their locations are identified by direct comparison between (A) and (B). Bar equals 30 μm.
Silver-stained image and corresponding fluorescent image showing transmigrating PMNs.
(A) Transmigrating PMNs preferentially locate near tricellular corners, as shown in silver-stained micrograph viewed under bright-field optics. The endothelial borders as well as the locations of PMNs (arrowheads) are clearly visible under these conditions. (B) The fluorescence image (380 nm) shows the location of fura-2–stained endothelial cells. Because neither unstained PMNs nor the EC boundary is visible under fluorescence optics, their locations are identified by direct comparison between (A) and (B). Bar equals 30 μm.
To examine the possible effect of soluble factors under our experimental conditions, we compared PMN suspension-evoked and supernatant-evoked EC [Ca2+]i signaling. At a concentration of 3 × 106 cells/mL, both the suspension and its supernatant were able to induce EC [Ca2+]i elevation (Figure3A-B). Although the average [Ca2+]i elevation of these ECs was low, many individual ECs showed [Ca2+]i spikes as high as 500 nmol/L. However, it was difficult to distinguish the cell-mediated effects from those mediated by soluble factors. To overcome this problem, we reduced the concentration of the PMN suspension to 5 × 105 cells/mL. ECs were relatively quiescent when exposed to the resulting supernatant (Figure 3C). Therefore, a PMN concentration of 5 × 105 cells/mL was used for further studies.
Endothelial [Ca2+]i elevations evoked by a suspension or supernatant of PMNs.
When a concentrated PMN suspension (3 × 106 cells/mL; A) or its cell-free supernatant (B) was perfused through the EC monolayer, EC [Ca2+]i elevations were clearly visible in either case. In contrast, the supernatant from a diluted PMN suspension (5 × 105 cells/mL) induced little EC [Ca2+]i elevation (C). The arrows mark the time at which the PMN suspension or supernatant reached the EC monolayer.
Endothelial [Ca2+]i elevations evoked by a suspension or supernatant of PMNs.
When a concentrated PMN suspension (3 × 106 cells/mL; A) or its cell-free supernatant (B) was perfused through the EC monolayer, EC [Ca2+]i elevations were clearly visible in either case. In contrast, the supernatant from a diluted PMN suspension (5 × 105 cells/mL) induced little EC [Ca2+]i elevation (C). The arrows mark the time at which the PMN suspension or supernatant reached the EC monolayer.
The PMN transmigration and EC [Ca2+]isignaling were monitored simultaneously under phase-contrast video recording and ratio imaging of fura-2 fluorescence, respectively. When signals from an area covering more than 50 ECs were averaged, there was no observable EC [Ca2+]i elevation upon the exposure of a PMN suspension. At the single-cell level, although occasional [Ca2+]i spikes were observed in some ECs, the [Ca2+]i level in most ECs remained quiescent (usually less than 100 nmol/L) throughout the experiment. Moreover, EC [Ca2+]i elevation did not occur when PMNs were in contact with, rolling on, or adhering to the ECs, which all happened in the couple of minutes between PMN arrival and transmigration (Figure 4). On the other hand, the [Ca2+]i elevation of ECs adjacent to the PMNs occurred simultaneously with PMN transmigration. Concomitant [Ca2+]i elevations in ECs that were one cell away from the PMN transmigration site were never observed. Thus, the [Ca2+]i elevation did not occur in any other EC that was not in direct contact with the transmigrating PMN. Consistent with the fact that PMNs preferentially located at tricellular corners in the EC monolayer (Figure 2), the majority of PMNs transmigrated with more than one concomitant EC [Ca2+]i signal. Among 79 transmigrated PMNs, 15 were accompanied by no concomitant EC [Ca2+]i elevation, 6 by 1 EC [Ca2+]i elevation, 20 by 2 EC [Ca2+]i elevations, 34 by 3 EC [Ca2+]i elevations, and 4 by 4 EC [Ca2+]i elevations. Those PMNs that transmigrated without accompanying EC [Ca2+]ielevations probably went through the EC monolayer via interendothelial gaps, which would be present in cultured cell monolayers.
PMN transmigration and concomitant [Ca2+]ielevation in adjacent ECs.
The standard concentration of PMN suspensions used in the rest of this study was 5 × 105 cell/mL. The upper panel shows morphologic changes of a PMN during various stages of transmigration. The lower panel shows [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. The insert shows the fluorescence image of these ECs with the transmigrating PMN marked by a circle (flow direction from top to bottom). While ECs 1, 2, and 3 were located right next to the transmigration site, another cell located one cell away from this site was selected as the control. The PMN arrives at 370 seconds (arrow). Contact, rolling, and adherence (all happening within a couple of minutes of PMN arrival) caused no observable EC [Ca2+]i elevation. In contrast, PMN transmigration was accompanied by sharp [Ca2+]i elevations in ECs 1, 2, and 3, but not in the control EC.
PMN transmigration and concomitant [Ca2+]ielevation in adjacent ECs.
The standard concentration of PMN suspensions used in the rest of this study was 5 × 105 cell/mL. The upper panel shows morphologic changes of a PMN during various stages of transmigration. The lower panel shows [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. The insert shows the fluorescence image of these ECs with the transmigrating PMN marked by a circle (flow direction from top to bottom). While ECs 1, 2, and 3 were located right next to the transmigration site, another cell located one cell away from this site was selected as the control. The PMN arrives at 370 seconds (arrow). Contact, rolling, and adherence (all happening within a couple of minutes of PMN arrival) caused no observable EC [Ca2+]i elevation. In contrast, PMN transmigration was accompanied by sharp [Ca2+]i elevations in ECs 1, 2, and 3, but not in the control EC.
It is interesting to note that only ECs adjacent to the transmigration site, but not the ECs even one cell away, showed [Ca2+]i elevation (Figure 4). Transmigration-induced EC [Ca2+]i elevation was thus a local phenomenon that did not spread to other ECs of the same monolayer. However, this EC [Ca2+]ielevation did not seem to be subcellularly localized, ie, it happened throughout the entire cell. When images of these transmigration-affected ECs were divided into halves, one being adjacent to the transmigration site (proximal half) and the other being away from it (distal half), the responses were identical between the halves (data not shown). Because of the limited time resolution (a few seconds) of our imaging setup, we were unable to detect the possible [Ca2+]i wave propagation within single endothelial cells.
To investigate at which stage of PMN transmigration the EC [Ca2+]i signaling actually happens, we fixed the specimens immediately after the occurrence of EC [Ca2+]i elevations. Figure5 shows an example. The fixative was in contact with the ECs within 20 seconds after the initiation of EC [Ca2+]i signaling, and the major portion of the cell body of this transmigrating PMN was still above the EC monolayer (confocal images in Figure 5). Thus, it is clear that EC [Ca2+]i signaling happens at an early stage of PMN transmigration.
Elevation of endothelial [Ca2+]i at an early stage of PMN transmigration.
A PMN arrives at 375 seconds (arrow). The transmigration-associated EC [Ca2+]i elevation happens at about 430 seconds, and the fixative arrives shortly afterward at about 450 seconds. The top panel shows confocal images of the fixed specimen. The positions of these optical sections relative to the apical surface of the EC monolayer (0 μm) are labeled underneath each image. Bar equals 20 μm.
Elevation of endothelial [Ca2+]i at an early stage of PMN transmigration.
A PMN arrives at 375 seconds (arrow). The transmigration-associated EC [Ca2+]i elevation happens at about 430 seconds, and the fixative arrives shortly afterward at about 450 seconds. The top panel shows confocal images of the fixed specimen. The positions of these optical sections relative to the apical surface of the EC monolayer (0 μm) are labeled underneath each image. Bar equals 20 μm.
Whether EC [Ca2+]i signaling is required for PMN transmigration or is merely an affiliated effect is an interesting question. The EC [Ca2+]i signaling could be blocked by pretreatment with the cell-permeable [Ca2+]i chelator BAPTA-AM, as indicated by the complete absence of ratio changes in fura-2 fluorescence (data not shown). Treatment of ECs with 50 μmol/L BAPTA-AM was sufficient to block both EC [Ca2+]i elevation and PMN transmigration under a fMLP gradient. Under these conditions, PMNs formed protrusions that extended underneath the EC monolayer (Figure6). Although these protrusions continuously changed their shape and direction, the cell bodies remained round above the monolayer for about 1 hour. Treatment of ECs with higher concentrations of BAPTA-AM caused obvious gaps in the monolayer without blocking PMN transmigration. These results confirm the previous finding that PMNs remain on top of a monolayer of ECs whose [Ca2+]i has been clamped with intracellular calcium chelators.3
Blockage of PMN transmigration by [Ca2+]i-buffered ECs.
ECs were pretreated with 50 μmol/L BAPTA-AM for 25 minutes. Both phase-contrast dynamic images (top panel) and confocal optic sections at the end (bottom panel) are shown. When EC [Ca2+]i elevation is prevented with BAPTA treatment (curve not shown), the PMN forms protrusions that extend underneath the EC monolayer. Although the protrusions repeatedly change shape and direction, the cell body is unable to pass through the monolayer. Bars equal 20 μm.
Blockage of PMN transmigration by [Ca2+]i-buffered ECs.
ECs were pretreated with 50 μmol/L BAPTA-AM for 25 minutes. Both phase-contrast dynamic images (top panel) and confocal optic sections at the end (bottom panel) are shown. When EC [Ca2+]i elevation is prevented with BAPTA treatment (curve not shown), the PMN forms protrusions that extend underneath the EC monolayer. Although the protrusions repeatedly change shape and direction, the cell body is unable to pass through the monolayer. Bars equal 20 μm.
PMN [Ca2+]i elevations have been demonstrated during their migration.13 To determine whether PMN [Ca2+]i elevation was a prerequisite for EC [Ca2+]i signaling and their own transendothelial migration, we pretreated PMNs with 50 μmol/L BAPTA-AM. Treatment with this concentration of BAPTA was enough to prevent even fMLP-evoked PMN [Ca2+]ielevation (data not shown). [Ca2+]i-buffered PMNs were capable of migrating on the EC monolayer and inducing normal EC [Ca2+]i signaling, but their morphology was drastically altered after transendothelial migration (Figure7). Among 23 transmigrated PMNs, 5 were accompanied by no concomitant EC [Ca2+]ielevation, 2 by 1 EC [Ca2+]i elevation, 6 by 2 EC [Ca2+]i elevations, and 10 by 3 EC [Ca2+]i elevations. Although entire cell bodies penetrated the collagen gel, these [Ca2+]i-buffered PMNs often stretched more than 30 μm, with rear ends located just underneath the EC monolayer. As a comparison, 377 of 388 transmigrated normal PMNs were shorter than 25 μm, but 295 of 346 BAPTA-treated PMNs that had already transmigrated were longer than 25 μm.
Transmigration of [Ca2+]i-buffered PMN and concomitant EC [Ca2+]i elevation.
The PMN was pretreated with 50 μmol/L BAPTA-AM for 25 minutes. The transmigration process appears normal at the early stages (top panel, up to 410 seconds), but the transmigrated PMN becomes elongated at the later stages (top panel, 910 seconds). The confocal microscopy images (middle panel) show a 3-dimensional view of this elongated PMN that was fixed after the last phase-contrast image was taken (star indicates rear end; arrow indicates front end). Bars equal 20 μm. The bottom panel shows the [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. This PMN arrives at 220 seconds.
Transmigration of [Ca2+]i-buffered PMN and concomitant EC [Ca2+]i elevation.
The PMN was pretreated with 50 μmol/L BAPTA-AM for 25 minutes. The transmigration process appears normal at the early stages (top panel, up to 410 seconds), but the transmigrated PMN becomes elongated at the later stages (top panel, 910 seconds). The confocal microscopy images (middle panel) show a 3-dimensional view of this elongated PMN that was fixed after the last phase-contrast image was taken (star indicates rear end; arrow indicates front end). Bars equal 20 μm. The bottom panel shows the [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. This PMN arrives at 220 seconds.
Discussion
In this study, an in vitro flow system has been developed to examine the entire process of PMN rolling, adhesion, and transendothelial migration, along with the accompanying EC [Ca2+]i signaling events. Our results demonstrate that PMN transmigration across the EC monolayer, but not rolling or adhesion, is accompanied by [Ca2+]i signaling in the ECs adjacent to the transmigration site. Furthermore, this EC [Ca2+]i signaling happens at an early stage of PMN transmigration and is essential for the PMN cell body to pass through.
Although the underlying molecular mechanism that triggers the EC [Ca2+]i signaling is unknown at present, it seems that direct contact between the transmigrating PMN and the adjacent ECs is needed for this triggering event. There are several reasons that support this point of view. First, EC [Ca2+]i signaling happens only in those ECs in contact with a transmigrating PMN. Second, if some soluble factors served as the trigger instead, one would observe [Ca2+]i elevation preferentially happening in the particular EC downstream of the PMN transmigration site. However, we did not observe such a phenomenon. Finally, PMNs pretreated with BAPTA, presumably with their secretion machinery blocked, were able to induce normal EC [Ca2+]i signaling. This is consistent with previous findings that neither the rapid rise in PMN [Ca2+]i nor specific granule fusion with the plasma membrane constitutes a prerequisite for PMN migration across the EC monolayer.14
We have tested a more concentrated PMN suspension (3 × 106 cells/mL) in our flow chamber system. Arrival of the PMN suspension caused random [Ca2+]ielevations in many ECs and resulting significant [Ca2+]i elevation of the entire EC monolayer (Figure 3A), which is similar to a previous report in which approximately 5 to 10 PMNs per EC in the monolayer were used.4 [Ca2+]i elevation in the EC monolayer under the latter experimental conditions happened at the moment of suspension arrival and was not restricted to ECs adjacent to the transmigration sites. Our data showed that similar EC [Ca2+]i elevations could be generated by the supernatant isolated from 3 × 106 cells/mL PMN suspension (Figure 3B). In our hands, the supernatant from a fMLP-pretreated PMN suspension induced an EC [Ca2+]i elevation whose cross-monolayer average value could reach 500 nmol/L (data not shown). Therefore, some soluble factors released from concentrated PMNs, regardless of whether these cells have been treated with chemoattractant, may be responsible for the immediate cross-monolayer EC [Ca2+]ielevation that is independent of PMN transmigration.3 4
As a comparison, clear adhesion-associated EC [Ca2+]i signaling has been established when natural killer (NK) cells, but not other lymphocytes, adhere to cultured human umbilical vein ECs.15 In one example reported in that study, simultaneous recording of NK cells and ECs at the single-cell level revealed that EC [Ca2+]i elevation happened shortly (50 seconds) after [Ca2+]i elevation in adherent NK cells. Moreover, the NK [Ca2+]i elevation was a prerequisite for the adhesion-dependent EC signaling. Although it is possible that some secreted products may be involved, blockage of the NK secretion pathway was unable to prevent [Ca2+]i elevation in either cell type. Together with our current results, the data indicate that contact-mediated outside-in signaling in ECs may be present in PMN transmigration across an EC monolayer as well as in NK cell EC adhesion.
Because the PMN transmigration-associated [Ca2+]i signaling spread across the entire EC cytoplasm, some other unidentified signaling/response components must be localized subcellularly to serve as a relay between [Ca2+]i signaling and intercellular junction opening. Phosphorylation of myosin light chains could serve as a [Ca2+]i-dependent relay because it appears to be a critical event in PMN-induced EC cytoskeletal alterations that accompany EC contractility.16 Activation of myosin light chain kinase (MLCK) is a well-known Ca2+/calmodulin-dependent event. MLCK can also regulate agonist- and flow-stimulated Ca2+ influx in ECs.17 Leukocytes may regulate this enzyme activity in ECs through the induction of postcontact EC [Ca2+]i signaling, thereby promoting their transmigration. It has also been shown that either Ca2+chelators or calmodulin/MLCK inhibitors effectively block PMN transmigration without inhibiting PMN adhesion.18 This is consistent with our observation that EC [Ca2+]i signaling appears to be related to PMN transmigration but not to adhesion. It would be interesting to examine the subcellular localization of MLCK in ECs.
Of the several types of junctional complexes identified at the EC boundary, the adherens junction seems to be the primary candidate in mediating transendothelial migration. Whether transendothelial migration requires the disassembly of adherens junctions appears to depend on the type of transmigrating cells. For example, ECs have been reported to actively participate in the transmigration of melanoma cells by disassembling and reassembling VE-cadherin–rich adherens junctions.19 However, adherens junctions remain intact during diapedesis of monocytes.20 Tight junctions, on the other hand, are unlikely to be involved because they are absent at tricellular corners.11
Whether PMN transmigration is accompanied by the breakdown of endothelial barrier function is an important issue. Although PMNs may stay on the EC monolayer for minutes, the transmigration process of individual PMNs is completed in less than a minute, as indicated by video images recorded under phase-contrast optics. The present results are consistent with those reported previously.21 We have never observed any physical gaps between a transmigrating PMN and the ECs in contact with it. This indicates that PMNs probably find their way through the transmigration sites that are preferentially located at tricellular corners. In contrast, interendothelial gaps caused by histamine treatment, which mostly occurred between 2 adjacent ECs, have been regarded as sites for plasma leakage in inflammation.22 These gaps last for hours and show complex morphology, such as fingerlike cell processes.
The current consensus is that leukocyte transmigration requires mechanisms that open EC junctions to allow leukocyte passage. However, it is controversial whether the leukocyte adhesion step alone can account for such mechanisms. Recently, it has been suggested that PMN adhesion, not the subsequent transmigration, triggers the disorganization of endothelial junctions.23,24 This point of view has been challenged by evidence that some methodologic artifacts may cause disappearance of the EC adherens junctional complex.25 PMN constituents, especially proteolytic enzymes, are released during detergent lysis of these cells. A high local concentration of these proteolytic enzymes could lead to specific degradation of the EC junctional complex, even with protease inhibitors in the bulk phase.
PMN [Ca2+]i elevation seems to be required for PMN detachment from the collagen gel, but not from the EC surface. In this study, [Ca2+]i-buffered PMNs showed elongated shapes after transendothelial migration, apparently because of “sticky” rear ends (Figure 7). Close observation reveals that these rear ends were not on the EC apical surface. Instead, they were located in the collagen gel underneath the ECs adjacent to PMN transmigration sites. It has been reported that repeated transient increases in PMN [Ca2+]i are required for PMN migration either on 2-dimensional surfaces (coated with fibronectin or vitronectin) or through 3-dimensional matrices (human amnion).26 27 Moreover, RGD-containing peptides and antibodies that block integrin receptors are able to rescue the migration of [Ca2+]i-buffered PMNs. Therefore, these repeated [Ca2+]i increases are required for integrin-mediated PMN detachment from these surfaces or matrices. Because we observed that [Ca2+]i-buffered PMNs adhered to and migrated normally on ECs, RGD-dependent integrins are probably not involved in these PMN–EC interactions.
In conclusion, we have provided additional evidence in support of the following model for PMN transmigration. Initially, PMNs form proximal protrusions that send out contact-mediated signals to activate adjacent EC [Ca2+]i signaling, which is required for subsequent opening of the intercellular junctions. The protrusions then migrate into the collagen gel and eventually pull the cell body through. Once initiated, the whole process usually takes less than l minute to complete. This model is consistent with previously reported results.3 21
Acknowledgments
We are grateful to Dr S. T. Jiang for his help in the development of the chemoattractant gradient system and to Dr H. Essackjee for his critical reading of this manuscript.
Supported by grants from The National Science Council and The National Health Research Institute, Taiwan, R.O.C. (grant numbers NSC 89-2320-B-006-034, NSC 89-2320-B-006-045, and NHRI-GT-EX89S834L).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Chauying J. Jen, Department of Physiology, College of Medicine, National Cheng-Kung University, Tainan 701, Taiwan, Republic of China; e-mail: jen@mail.ncku.edu.tw.

![Fig. 3. Endothelial [Ca2+]i elevations evoked by a suspension or supernatant of PMNs. / When a concentrated PMN suspension (3 × 106 cells/mL; A) or its cell-free supernatant (B) was perfused through the EC monolayer, EC [Ca2+]i elevations were clearly visible in either case. In contrast, the supernatant from a diluted PMN suspension (5 × 105 cells/mL) induced little EC [Ca2+]i elevation (C). The arrows mark the time at which the PMN suspension or supernatant reached the EC monolayer.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3816/5/m_h82300408003.jpeg?Expires=1769195213&Signature=XCAppTaIRFW-mJKNJpUhTRaahcAAHP9xDNPmD952v4V7uhHs0XUyPTOvZikjRz3Vhy3~9KjLPHgTjzIb~2JTEkO~CQ9t7kG-KVkc3XXgTxV0WfoI1zNQFeTO9bp8szlmhTpVF03L1kCUlE-dJPlmFADKUPTlpDKDX34wk~SE2P1cIDMxC4wtZeZG86Bly-qIAVJll~E9rKQIE4Q2UULn8jXuXNFhe2EfffJmNm~bl6PGzEhBQHaX075s2rv1Rham4gxjxg8s6efE4dnAxOZ6j2T3YpSd9QKGm1g6QnaBhhYnjPTBwW8sLcWqyQJoK1sV4xtwSTQ37fSdksYkfwgICw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. PMN transmigration and concomitant [Ca2+]ielevation in adjacent ECs. / The standard concentration of PMN suspensions used in the rest of this study was 5 × 105 cell/mL. The upper panel shows morphologic changes of a PMN during various stages of transmigration. The lower panel shows [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. The insert shows the fluorescence image of these ECs with the transmigrating PMN marked by a circle (flow direction from top to bottom). While ECs 1, 2, and 3 were located right next to the transmigration site, another cell located one cell away from this site was selected as the control. The PMN arrives at 370 seconds (arrow). Contact, rolling, and adherence (all happening within a couple of minutes of PMN arrival) caused no observable EC [Ca2+]i elevation. In contrast, PMN transmigration was accompanied by sharp [Ca2+]i elevations in ECs 1, 2, and 3, but not in the control EC.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3816/5/m_h82300408004.jpeg?Expires=1769195213&Signature=uqF7dWyXLTU9JDaBHF9GDRQ3jmkl30zVnKlIS7UfwrHd7Uq92qZWRG0u9MmhjooXqCI2dDTiXvShAoT~-7vFlaB7rJt6U2qI0~5fUXUg6JXItwdsL9JFvLOlnpN3AUJVlrkrqPfcJRwWhqgbbwWNbHhBSs0etnZEvugYOXJIcry~DrTBVNhxP4JOzUayh80GhvkzYD2wTN8-jim~H1qYpMm4Ge-18K9b2x6~8YRg7d7l0qBEMWD4L9fV~kYNy-utvKmSw-C02kVJvtpX6ar1Kw5fMvhtL1f8nev0fba3Q6yBcWF~6Ar6buFLlGr5HwhBUNm-5z2hCYgnbu-SRgHuyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Elevation of endothelial [Ca2+]i at an early stage of PMN transmigration. / A PMN arrives at 375 seconds (arrow). The transmigration-associated EC [Ca2+]i elevation happens at about 430 seconds, and the fixative arrives shortly afterward at about 450 seconds. The top panel shows confocal images of the fixed specimen. The positions of these optical sections relative to the apical surface of the EC monolayer (0 μm) are labeled underneath each image. Bar equals 20 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3816/5/m_h82300408005.jpeg?Expires=1769195213&Signature=ffNz2vg1FtKaiizVpsr6EgJEGgBn2GlT8hS77uEfQhNi4bV1Ml2CiyvrNrLoO54iC92II15smBZmlepZlt5-Mk~PaCSc2jOAQEh8HLwbGqkQDq4tgxeHOXvd1qb2BcoHaJFnsWEKcxCz69RnQ67RKlpSrMhrNLGhqXLrshqehH3CmbXLtOkzK94u58DxGeUvBDrd5qZuD69MxNkmS~sQPXhaB8I862tq-VGRPFIRG0FGsMocA0U1UlcDlg24j0i4eB-b7l3AAaEA8aTzy2y~~9Bo~rtHZb8I85kSfe0tUG~Q0B8UQ-7ywyBrjct~qsGqaLtp2ZZD0GKeYAD11L0H5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Blockage of PMN transmigration by [Ca2+]i-buffered ECs. / ECs were pretreated with 50 μmol/L BAPTA-AM for 25 minutes. Both phase-contrast dynamic images (top panel) and confocal optic sections at the end (bottom panel) are shown. When EC [Ca2+]i elevation is prevented with BAPTA treatment (curve not shown), the PMN forms protrusions that extend underneath the EC monolayer. Although the protrusions repeatedly change shape and direction, the cell body is unable to pass through the monolayer. Bars equal 20 μm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3816/5/m_h82300408006.jpeg?Expires=1769195213&Signature=jEFAHhYntbmRvLnqoxEB~JA6vKJXtMAYY~NPUCNNw2ynIEbrSjbjidUXKwTRHSInCMI6fCc9cXbVzT4EJ0VluynjrMPH86v2bF7N~I~1pABPVXqGRpBXw416pydAuS2R59pFA9N~DQ3oze6zjH1IG1mCKhIxv-7tu0Kk3QY-4UA3nOE4OZ9mJt2gWN8Cd7tNfHzz-eXZwky0Q7YQftkIV3-EXCNUJQStJvy3l1qkOR00DXMZK7NwxoZkvvGuSwbtPmuYKj67FRxWfvoyK4O1c7Opto55AqMp2dNcvhCMHrzS9g5JCdy8fTyQN3XOqo8g1bSMdoL6PMio6nFrOHfkQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Transmigration of [Ca2+]i-buffered PMN and concomitant EC [Ca2+]i elevation. / The PMN was pretreated with 50 μmol/L BAPTA-AM for 25 minutes. The transmigration process appears normal at the early stages (top panel, up to 410 seconds), but the transmigrated PMN becomes elongated at the later stages (top panel, 910 seconds). The confocal microscopy images (middle panel) show a 3-dimensional view of this elongated PMN that was fixed after the last phase-contrast image was taken (star indicates rear end; arrow indicates front end). Bars equal 20 μm. The bottom panel shows the [Ca2+]i elevation in ECs adjacent to the PMN transmigration site. This PMN arrives at 220 seconds.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3816/5/m_h82300408007.jpeg?Expires=1769195213&Signature=QM2ri2Zvtdqky7huqHASZHkd6c29u1bsdW-IxEfbtR4eokNNgqS2iRMjkE~O9DjY~UMrBMCeAB4qaGOHvRC7hsBgal4Cfa-K0gIrxxs68UEPLFwAUrMMpNO52U6mGHZRSFFl~Rq0tksjPG1FFZqjBO5nma3elFmonzOfaJG2BAcokSY1GxMZKxqRkZqZMZ7rPWdgOMdIT3-vQB9hU~SnaxnTbfxo2jhMq8kcIbol6nMH81H8JCN-zYAmvjRWhKvw7o0GR00NAaHU-WyuNhu344MDKLsHd5TPStdASbDNcXY8L9mIsGrnxUyP4lfZbpk0mnOXX4GHL5JQjYxc8idUMA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal