Abstract
In a randomized, placebo-controlled, double-blind trial, thalidomide or placebo together with glucocorticoids and either cyclosporine or tacrolimus was administered as initial therapy for clinical extensive chronic graft-versus-host disease (cGVHD). All patients had thrombocytopenia or cGVHD that evolved directly from acute GVHD as an indicator of a poor prognosis. The study drug (thalidomide or placebo) was administered initially at a dose of 200 mg orally per day, followed by a gradual increase to 800 mg/d if side effects were tolerable. Treatment with the study drug was discontinued before resolution of cGVHD in 23 (92%) of the 25 patients who received thalidomide and in 17 (65%) of the 26 patients who received placebo (P = .02). Neutropenia and neurologic symptoms were the most frequent reasons for early discontinuation of treatment with thalidomide. The duration of treatment with thalidomide was too short to assess its efficacy in controlling cGVHD.
Introduction
Case reports have described the use of thalidomide for treatment of chronic graft-versus-host disease (cGVHD),1-4 and preclinical studies demonstrated activity in a rat model of cGVHD.5 In a subsequent phase II clinical trial, 8 (38%) of 21 patients with high-risk cGVHD improved after primary treatment with thalidomide.6 In the same study, improvement was also observed after treatment with thalidomide in 18 (78%) of 23 patients with cGVHD who had not responded previously to other treatment. In a confirmatory study, 16 (20%) of 80 patients with refractory cGVHD showed improvement after treatment with thalidomide.7 To evaluate the efficacy of thalidomide as primary treatment for patients with high-risk cGVHD, we conducted a phase III, randomized trial comparing thalidomide versus placebo, given in combination with cyclosporine (or tacrolimus) and prednisone.
Study design
Fifty-two patients with clinical extensive cGVHD consented to participate. Twenty-six were assigned to receive thalidomide and 26 were assigned to receive placebo. One patient assigned to receive thalidomide withdrew from the study before beginning treatment and was excluded from further evaluation.
Prednisone was administered orally at a dose of 1.0 mg/kg per day for the first 2 weeks, followed by gradual reduction to 0.5 mg/kg every other day by week 22. Cyclosporine was administered orally at a dose of 12 mg/kg per day, if tolerated, and then gradually decreased after 22 weeks to 6 mg/kg per day. Alternatively, tacrolimus was administered orally at a dose of 0.12 mg/kg per day, if tolerated, and then gradually decreased after 22 weeks to 0.06 mg/kg per day.
The study drug (thalidomide or placebo with identical appearance) was supplied by Grünenthal GmbH (Stolberg, Germany), dispensed under IND 40758 and administered initially at 200 mg orally once daily (3 mg/kg once daily for children less than 12 years of age and less than 67 kg of body weight). The dose was gradually increased to reach a target dose of 200 mg 4 times daily (3 mg/kg 4 times daily for children), if sedation was tolerable. If sedation was intolerable, the dose of study drug was decreased by 25% to 50%. After sedation resolved, the dose was re-escalated by 25% to 50% increments as tolerated. A monthly patient self-assessment for symptoms of weakness, dysesthesias, or clumsiness was used for interim neurologic evaluations, and administration of the study drug was discontinued if neuropathy was documented at any time after starting treatment. Treatment with the study drug was suspended if the absolute neutrophil count was 500 to 1000/μL on 2 consecutive occasions or less than 500/μL on a single occasion. Treatment was resumed with a 50% reduction in dose when the absolute neutrophil count surpassed 1500/μL, and the dose was gradually increased as allowed by toxicity. If neutropenia recurred, treatment with study drug was discontinued permanently.
The original study design specified that the primary end point was death from any cause other than recurrent malignancy. Enrollment of 66 patients in each arm was projected to have 90% power and 95% confidence for detecting a decrease from 35% transplant-related mortality at 2 years among patients treated with placebo to 10% among patients treated with thalidomide. An interim analysis by an independent data and safety monitoring committee was planned after the first 15 transplant-related deaths had occurred, and the study was to be terminated if the P value for the difference between the 2 arms was less than .0051. Results of the interim analysis did not show a significant transplant-related mortality difference between the 2 arms. Moreover, the results indicated less than 42% probability of reaching statistical significance for this end point if the study was continued to its originally planned enrollment, assuming that the original hypothesis was correct for the remaining patients. Because it had taken nearly 5 years to enroll the first 51 patients, and because there was little chance of a positive result, the study was closed prematurely. In the analysis summarized below, survival was evaluated with the use of Kaplan-Meier estimates, and all other time-to-event end points were evaluated with the use of cumulative incidence estimates to account for competing risk events.8 Time-to-event data were compared by log-rank tests, and other outcomes were compared by Mann-Whitney tests, χ2 tests, or Fisher exact tests.
Results and discussion
The maximum administered dose of the study drug was significantly lower for patients who received thalidomide as compared to those who received placebo (P = .005) (Table1). Only 4 (16%) of 25 patients were able to tolerate thalidomide at the prescribed daily target dose, whereas 14 (54%) of 26 patients were able to tolerate placebo at the prescribed daily target dose. Thirteen patients (52%) in the thalidomide group and 2 (8%) in the placebo group received only 25% of the prescribed daily target dose. Neutropenia occurred in 64% of the patients treated with thalidomide and in 23% of those who received placebo (P = .003). Numbness occurred in 48% of the patients treated with thalidomide and in 23% of those who received placebo (P = .08). After treatment with thalidomide, 17 patients reported sedation, and 10 had constipation. After treatment with placebo, 5 patients reported sedation, and 2 had constipation (P = .001 and .009, respectively).
Maximum dose of study drug, according to treatment arm
| Percent of prescribed dose . | Thalidomide no. (%) . | Placebo no. (%) . |
|---|---|---|
| 25 | 13 (52) | 2 (8) |
| 50 | 4 (16) | 9 (35) |
| 75 | 1 (4) | 1 (4) |
| 100 | 7 (28) | 14 (54) |
| Percent of prescribed dose . | Thalidomide no. (%) . | Placebo no. (%) . |
|---|---|---|
| 25 | 13 (52) | 2 (8) |
| 50 | 4 (16) | 9 (35) |
| 75 | 1 (4) | 1 (4) |
| 100 | 7 (28) | 14 (54) |
The maximum administered daily dose at any time after randomization was calculated as a percentage of the prescribed daily target dose. Three of the 7 patients who reached 100% of the prescribed target dose of thalidomide later had to reduce the dose because of toxicity. None of the 14 patients who reached 100% of the prescribed target dose of placebo had toxicity that led to reduction of the dose. The P value for trend was .005.
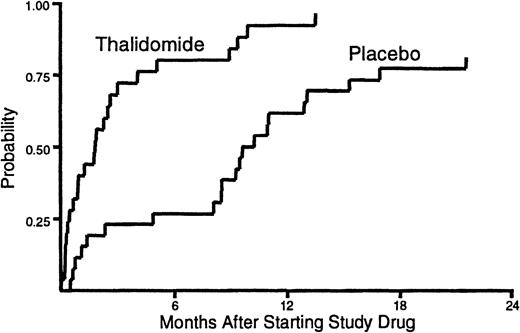
The median duration of treatment with thalidomide was 53 days (range, 1-411) compared to 245 days (range, 9-654) for placebo (Figure1). Administration of study drug was discontinued before resolution of cGVHD in 23 (92%) of the patients assigned to receive thalidomide and in 17 (65%) of those assigned to receive placebo (P = .02). Treatment with study drug was discontinued before resolution of cGVHD because of neutropenia in 14 patients who received thalidomide and in 4 patients who received placebo (P = .002). Treatment with study drug was discontinued because of neurologic symptoms in 11 patients who received thalidomide and in 3 patients who received placebo (P = .01). We suspect that patients who enrolled in previously published studies6 7 required considerable encouragement and support to sustain compliance with a regimen of thalidomide at doses of 200 mg or greater per day.
Duration of treatment.
The time to discontinuation of study drug was shorter for patients who received thalidomide than for those who received placebo. One patient who received thalidomide and 5 patients who received placebo continued treatment with study drug until the onset of their terminal illness. These patients were categorized as not having discontinued treatment with the study drug before death.
Duration of treatment.
The time to discontinuation of study drug was shorter for patients who received thalidomide than for those who received placebo. One patient who received thalidomide and 5 patients who received placebo continued treatment with study drug until the onset of their terminal illness. These patients were categorized as not having discontinued treatment with the study drug before death.
The cumulative incidence of secondary therapy for cGVHD is projected to reach 28% at 4 years for patients treated with thalidomide and 47% for those who received placebo (P = .35). The cumulative incidence of discontinuation of all immunosuppressive medications after resolution of cGVHD is projected to reach 39% at 4 years for patients who received thalidomide and 23% for those who received placebo (P = .12). These trends support previous results6 7 suggesting that thalidomide might have limited efficacy for treatment of cGVHD. At 3 years after enrollment in the study, the product limit estimate of survival was 49% for patients treated with thalidomide and 47% for those who received placebo (P = .87). The most frequent causes of death were infection, cGVHD, and recurrent malignancy.
The duration of treatment with thalidomide in our study was quite short. Treatment with thalidomide might promote the development of tolerance, thereby explaining how such a limited intervention might improve the longer-term prospects of resolving cGVHD during continued immunosuppressive treatment. Our results suggest that a regimen of 100 mg/d might be well tolerated, especially if given as a single dose at night. With this regimen, it would be possible to determine whether administration of thalidomide for 9 to 12 months has any benefit for patients with cGVHD.
Acknowledgments
We thank the physicians and nurses who cared for patients, Aurora Brandvold and Terese Ajer for help with data collection and data management, Alison Sell and Jennifer Brackensick for editorial assistance.
Supported by grants HL36444 and CA18221 from the National Institutes of Health, Department of Health and Human Services, and Grünenthal GmbH, Stolberg, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul J. Martin, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D2-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: pmartin@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal