Extranodal marginal zone B-cell lymphoma is a discrete clinicopathological entity arising in mucosa-associated lymphoid tissue (MALT) that has commanded increasing attention in recent years because of its unique pathogenetic, histologic, and clinical features.1-3 Two types of MALT can be identified in disparate organs that do not correspond to peripheral sites of the immune system. The native type consists of lymphoid tissue physiologically present in the gut (eg, Peyer's patches), whereas acquired MALT develops in sites of inflammation in response to either infectious conditions, such as Helicobacter pylori gastritis, or autoimmune processes, such as myoepithelial sialadenitis (MESA), associated with Sjögren's syndrome or Hashimoto's thyroiditis.4-6 In the context of these prolonged lymphoid reactive proliferations, the outgrowth of a pathological clone can progressively replace the normal lymphoid population, giving rise to a MALT lymphoma.7-11
The group of lymphomas classified as low-grade MALT lymphomas include a number of extranodal B-cell lymphomas, composed mostly of small cells, that share similar clinical, pathological, and molecular features; these lymphomas are defined as extranodal marginal zone B-cell lymphomas of MALT type in the Revised European-American Classification of Lymphoid neoplasms (REAL classification)12 and in the last World Health Organization (WHO) Classification of Neoplastic Diseases of the Hematopoietic and Lymphoid Tissue.13 The histologic features of low-grade B-cell lymphomas of MALT type are similar regardless of site of origin. In this review we will deal with gastric MALT lymphoma because the stomach is by far the most common and best-studied site.
Far from being rare, extranodal marginal zone B-cell lymphomas accounted for 7.6% of 1378 cases of non-Hodgkin lymphoma (NHL) in an international evaluation of the clinical significance of the REAL classification.14 The highest incidence of gastric MALT lymphoma has been reported in northeastern Italy (13.2 per 100 000 per year, 13 times higher than in corresponding communities in the United Kingdom), suggesting the existence of important geographic variations.15 It has been speculated that the extraordinarily high number of primary gastric lymphomas in northeastern Italy is related to the very high rate ofHelicobacter pylori infection observed in the examined population. In the United States, the incidence has been estimated as between 1:30 000 and 1:80 000 in the H pylori–infected population.16 However, additional unknown genetic, environmental, or dietary factors may also play a role. In fact, in some cases an infection from H pylori cannot be documented.
Most gastric lymphomas in historical series were of diffuse, large-cell type, and only a few cases of low-grade histology were reported. In more recent series of primary gastric lymphomas, however, the low-grade MALT lymphomas appear to account for approximately 40% of the cases.17-19 Indeed, in the past, most cases would have been misinterpreted as pseudolymphomas. However, many features contribute to its definition as a malignant condition: monoclonality is usually demonstrable,20-22 nonrandom chromosomal aberration23-29 can be detected, and histologic transformation into a high-grade lymphoma, with the possibility of dissemination to the regional lymph nodes and to the bone marrow, has also been described.30 31
Pathogenesis and molecular genetics of MALT lymphomas
Association between H pylori and gastric MALT lymphoma
The onset of MALT lymphoma in the stomach—where lymphocytes are not normally present—is preceded by the acquisition of MALT as a result ofH pylori infection. The microorganism can be found in the gastric mucosa in nearly all instances of gastric MALT lymphoma, with several lines of evidence suggesting a link between H pylori–chronic gastritis and the lymphoma.7,8,15,20,32-40 A close association has been reported in epidemiologic studies between H pylori infection and gastric malignancies, especially carcinomas41 but also lymphomas of either low-grade or high-grade histology.8,15,33,42 In vitro experiments have demonstrated that the neoplastic cells of low-grade gastric MALT lymphoma proliferate in a strain-specific response to H pylori and that this response is dependent on T-cell activation by the microorganism.32,39 The presence of the B-cell clone that would become predominant in the transformation to MALT lymphoma has been demonstrated in H pylori gastritis specimens taken several years before development of the lymphoma.7 A regression of gastric MALT lymphoma after antibiotic eradication of H pylorihas been reported in more than half of the treated patients.20,34,35,43,44 This close association of H pylori with gastric MALT lymphoma has led to the hypothesis that the microorganism may provide the antigenic stimulus for sustaining the growth of the lymphoma in the stomach.2 3
Genetic evidence for antigen-driven selection of MALT lymphoma clones
Naive B cells express unmutated immunoglobulin genes. During the physiologic B-cell development, somatic mutations in the immunoglobulin variable region (V) genes occur during the T-cell–dependent antibody response that takes place in the germinal center within secondary lymphoid follicles. Somatic hypermutations of the V genes and subsequent antigen selection of B cells result in generation of antibodies with increased ability to bind the antigen. These replacement mutations are clustered in the complementarity-determining regions of the V genes, which constitute the antigen binding site, and are typically found in the germinal center B cells and their progeny (memory B cells and differentiated germinal center–derived plasma cells).45
Sequence analysis of the immunoglobulin genes expressed by the gastric MALT lymphoma B cells shows somatic hypermutation with a high replacement-to-silence ratio and a distribution pattern that suggests that the tumor cell was positively selected through its antigen receptor in germinal centers. It has been speculated that these malignant cells have left the germinal center to give rise to the lymphoma. This is emphasized by the consistent presence in most cases of MALT lymphoma of a plasmacytoid differentiation in a subset of tumor cell.1-3 In addition, ongoing mutations (ie, intraclonal variation) of the immunoglobulin genes have been seen in some cases of low-grade lymphoma. This finding may suggest that clonal expansion of tumor cells continues to be antigen-dependent.36,37 The possibility that the antigen could directly stimulate the lymphoma growth is well in keeping with the observation that these lymphomas arise in sites of infectious or autoimmune processes. Further evidence for antigen-driven selection may come from the observation of unusual immunoglobulin gene rearrangements of the diversity (D) genes segments.36-38 These peculiar V-D-J rearrangements of the B cells have been postulated to be important mechanisms for the generation of antibody diversity and antigen-binding affinity. Remarkably, we observed that in 2 patients, despite different DNA sequences in the third complementarity determining region (CDR3), which is the most variable region of the immunoglobulin gene, the resultant amino acid sequences matched almost completely, suggesting the presence of common selecting antigens.38
The dependence on the stimulation provided by the antigen or by the associated local immune response may explain the tendency of MALT lymphoma to remain localized. However, cases of histologic transformation into an aggressive rapidly disseminating tumor have been described.7,30 The long-term antigenic stimulation gives the B-cell clones with increased affinity a growth advantage over those that cannot respond or that respond less efficiently to the antigen. Hence, due to antigenic selection and clonal expansion, such a B-cell clone could become predominant by a Darwinian mechanism. Because of the persistent antigenic stimulation, the clone may become more susceptible to genetic alterations that can result in neoplastic transformation and tumor progression.45
Genetic abnormalities in MALT lymphoma
Karyotype studies of gastric MALT lymphoma present technical difficulties, and only a few series have been published. Nonrandom chromosomal aberrations have been detected: the most common is the t(11;18)(q21;q21) present in at least a third of cases.24,46 The recent identification of the genes at the translocation breakpoints (the apoptosis inhibitor geneAPI2 and a novel 18q gene, MLT) suggests that this translocation may result in a survival advantage for MALT lymphoma B-cell clones.27,47 48
A second nonrandom translocation, much more rarely detected, the t(1;14)(p22;q32) might confer to the tumor an increased capacity of autonomous growth by means of inactivating mutations and overexpression of the BCL10 gene.28,29 However, the oncogenetic role of BCL10 is still controversial,49,50and BCL10 genomic mutations were almost absent in a recent large series of NHL that included several cases of extranodal marginal zone B-cell histology.50 51
Trisomy 3 had been previously reported as the most frequent abnormality, detectable in approximately 60% of cases.23However, it is also one of the most common numerical abnormalities, reported in several different subtypes of lymphoma,52 and a much lower incidence was shown in more recent studies of low-grade MALT lymphomas.53,54 The distinctive nature of low-grade MALT lymphoma also seems to be confirmed by the lack of BCL1 andBCL2 gene rearrangements.55,56BCL6rearrangements have been reported in only 3 of 34 studied cases of marginal zone B-cell lymphoma: all 3 cases were extranodal but not gastrointestinal and carried the t(3;14)(p27;q32) involving theBCL6 locus on 3p27.57
The c-MYC oncogene may be implicated in the development of MALT lymphomas58 but in a manner that is different from its involvement in nodal lymphomas and Burkitt's lymphoma, wherec-MYC is typically translocated.59 In contrast,c-MYC rearrangements are usually not detected in MALT lymphoma, but point mutations in the exon I /intron I regulatory region of this gene were found in 17% of 54 cases analyzed; those mutations could be related to the development of early MALT lymphoma lesions.58
In contrast to other types of NHL where it seems absent,60microsatellite instability was reported to be a common genetic feature of MALT lymphomas, detected in approximately 50% of cases.61 However, we and other groups were not able to reproduce these findings.62-65
Studies of the p53 gene showed a loss of heterozygosity in approximately 7% of low-grade and in 29% of high-grade (large-cell) MALT lymphomas.66 Mutations in p53 were observed in 19% and 33% of low-grade and high-grade MALT lymphoma, respectively. Moreover, only 1 of the 11 low-grade cases showed concomitantp53 allelic loss and mutation, whereas both aberrations were present in 6 of the 9 high-grade tumors. Therefore, it appears that partial inactivation of the gene may play an important role in the development of low-grade MALT lymphoma, whereas complete inactivation may be associated with high-grade transformation in at least some cases.66 Inactivation of the p16 gene—a cyclin-dependent kinase inhibitor and a main negative regulator of the cell cycle—has also been described as a possibly important event in the progression from low-grade to high-grade MALT lymphoma.67 68
Fas/CD95 is a receptor involved in the physiologic apoptosis pathway, regulating peripheral blood deletion of activated and autoreactive lymphocytes and the killing of virus-infected and cancer cells. Somatic mutations of this gene have been demonstrated in 3 of 5 MALT lymphomas, suggesting a possible role for its inactivation in the lymphoma pathogenesis.69 However, we could not detect the presence of Fas/CD95 somatic mutations in a series of 27 cases of marginal zone B-cell lymphomas (16 extranodal, 6 nodal, and 5 splenic) analyzed in our laboratory though, on the basis of our data, we cannot rule out that other genes coding for proteins involved in the Fas-induced apoptotic pathway might be altered.70
Hypothetical model for the pathogenesis of gastric MALT lymphoma
The precise significance of most of the abnormalities described above is not known, but it is likely that some of them can provide a selective growth advantage to malignant cells. A tentative explanation for the pathogenesis of gastric MALT lymphomas may be that B and T lymphocytes are recruited in the gastric mucosa as part of the immune response to H pylori. Proliferation of B cells is secondary to specific activation of reactive T cells by H pylori and cytokines. It is not clear whether the B-cell activation requires the continuing presence of H pylori as an antigenic source or is related to an indirect autoimmune mechanism.71 In fact, the neoplastic B cells often show antibody specificity for autoantigens71 and need contact-dependent help from intratumoral T cells to proliferate.32 The contact-dependent help is apparently mediated by CD40 and CD40 ligand (CD154) interactions.39,40 This immunologic drive mediated by mucosal T cells may explain the tendency of low-grade MALT lymphoma to remain localized and to regress after H pylori eradication. It is possible that genetic alterations can continue until a point is reached at which autonomous (ie, H pylori–independent) growth can occur, and additional alterations might ultimately result in transformation to a high-grade lymphoma. However, the exact mechanism of the transition from H pylori infection to low-grade MALT lymphoma is still unclear. Most patients with H pylorigastritis do not develop lymphoma; therefore, it is widely accepted that additional environmental and microbial or host genetic factors may play a role in gastric lymphomagenesis.72-74
H pylori strains expressing the CagA protein (cytotoxin-associated gene A) seem to be more aggressive, inducing either a more severe gastritis or peptic ulcerations, and have been associated with the development of gastric adenocarcinoma.75 The finding of anti-CagA antibodies in almost all cases of MALT lymphomas with a significantly higher rate than in active gastritis has led to the hypothesis that CagA+H pylori strains can be associated with the development of gastric MALT lymphoma.75 Another study found a significantly higher frequency of CagA+ strain infections in high-grade gastric lymphoma than in low-grade lymphomas or gastritis, suggesting a possible role in histologic transformation.76 However, additional studies were not able to find any correlation.77-80 Hence, the pathogenetic role of the CagA protein remains uncertain, as well as the one of several other H pylori or host proteins that have been suggested as possibly implicated in the MALT lymphoma pathogenesis.72,74 80-84
The observation in some series from Italy of an increased incidence of extranodal B-cell marginal zone lymphomas in hepatitis C virus (HCV)-positive populations85 suggested a possible pathogenetic role for the virus, but studies in other countries failed to confirm this association, and the possible pathogenetic link between HCV infection and certain histologic lymphoma subtypes remains a highly controversial issue.86,87 In a series of 180 patients with lymphoma studied at our institution, the percentage of HCV seropositive subjects was significantly higher than in a control population of blood donors; however, HCV infection was not specifically associated with lymphomas having MALT-type histology or with the gastric localization. On the contrary, in the same series, the presence of anti-Helicobacter antibodies was significantly associated with the development of primary lymphomas of the stomach, further supporting the key role of H pylori infection.87
Pathology
Histologic features
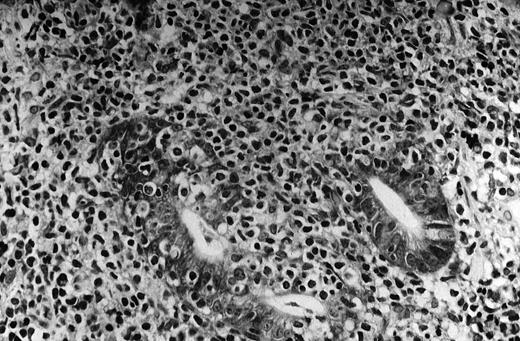
The pivotal feature of low-grade MALT lymphoma is the presence of a variable number of lymphoepithelial lesions that can be defined as unequivocal invasion and partial destruction of gastric glands or crypts by aggregates of tumor cells (Figure1). Lymphoepithelial lesions are of striking relevance for the diagnosis of low-grade gastric MALT lymphoma; however, they can sometimes be seen in the context of florid chronic gastritis and can also be present in other sites of both native and acquired MALT.88 89
MALT lymphoma of the stomach: histologic features.
The neoplastic lymphocytes infiltrate some residual gastric glands, giving rise to lymphoepithelial lesions (Giemsa staining, × 512, courtesy of Prof Stefano Pileri, University of Bologna, Italy).
MALT lymphoma of the stomach: histologic features.
The neoplastic lymphocytes infiltrate some residual gastric glands, giving rise to lymphoepithelial lesions (Giemsa staining, × 512, courtesy of Prof Stefano Pileri, University of Bologna, Italy).
The tumor cells are usually small- to medium-sized lymphocytes with moderately abundant cytoplasm and irregularly shaped nuclei resembling those of follicular center cells, or centrocytes, and have been designated centrocyte-like (CCL) cells.1,88 However, although resemblance to centrocytes is the general rule, the morphologic pattern of the neoplastic cells can cover quite a large spectrum. Some are similar to the so-called monocytoid cells (abundant pale cytoplasm and well-defined cell borders), and some are similar to small lymphocytes, sometimes with lymphoplasmacytic features. Any of these cytologic aspects can predominate, or they can coexist to various degrees in the same tumor. Scattered transformed blasts (large cells) can also be found. Some degree of plasma cell differentiation is often present; the plasma cells are sometimes so conspicuous as to suggest a diagnosis of extramedullary plasmacytoma.88 Plasma cells often present varying degrees of atypia, Dutcher bodies, or other intracellular inclusions.1,88,89 The lymphoma cells diffusely infiltrate the lamina propria and grow around reactive follicles. The preexisting germinal centers are selectively overrun, and sometimes extensively replaced, by the neoplastic cells, which often undergo striking plasma cell differentiation after invasion of the follicles. Less commonly, the complex interaction between the CCL cells within the follicles may undergo prominent blast transformation. The complex interaction between the CCL cells and the reactive B-cell follicles is known as follicular colonization.90
There is a continuous spectrum of lesions during the transition fromH pylori–associated gastritis to low-grade MALT lymphoma20 and, although the diagnosis in gastric biopsies is usually evident, early or borderline cases can be confused withH pylori–related follicular gastritis, and variations in the minimum histologic criteria have been found among expert pathologists.91-94 Indeed, in certain cases it can be very difficult to make a distinction purely on the basis of morphologic characteristics. Certain parameters have been reported as being very useful for diagnosing low-grade lymphoma in a gastric biopsy specimen: prominent lymphoepithelial lesions, moderate cytologic atypia of neoplastic lymphocytes, and plasma cells with Dutcher bodies. However, the absence of these factors does not necessarily exclude the diagnosis of lymphoma.88,89 Because lymphoma represents a clonal outgrowth of cells that have acquired certain genetic alterations, finding a monoclonal B-cell population might provide support for a diagnosis.20-22,95-98 A monoclonal population can be revealed by genotype investigations of MALT lymphomas that use the Southern blot or polymerase chain reaction (PCR) technique. The latter methodology is more sensitive and can be done on the paraffin-embedded archival tissues21,22,97 where, however, the technique may produce a relevant percentage of false negative results,3,98 thus raising questions on its validity and feasibility as a diagnostic tool. In chronic H pylori–associated gastritis, B-cell monoclonality has been reported to possibly precede the development of gastric MALT lymphoma.11 Indeed, the significance of PCR-detected monoclonality in absence of histologic evidence of lymphoma is still uncertain, and interpretation of the molecular results must always be done in the context of the histologic findings.2,3 95
Within the stomach, low-grade MALT lymphoma is often multifocal.31,99 Microscopic lymphomatous foci can be present at gastric sites distant from the main tumor and may explain the frequent report of relapses in the gastric stump after surgical excision. MALT lymphoma usually remains localized within the tissue of origin but can sometimes present with involvement of multiple mucosal sites; some cases with simultaneous gastric and intestinal involvement have been reported, and thyroid and salivary gland MALT lymphomas may also disseminate to the gastrointestinal tract.88,100 It has been postulated that this dissemination may be due to specific homing properties similar to those of the normal B cells of MALT.100-102
Some diagnostic problems can arise from the presence of an increased number of large cells, which may suggest histologic progression to a high-grade lymphoma.88,89 Only the “low-grade” MALT lymphoma, composed mostly of small cells, has been included as extranodal marginal zone B-cell lymphoma in the REAL/WHO classification,12,13 whereas “high-grade” MALT lymphomas should be defined as diffuse large B-cell lymphomas (with or without areas of marginal zone/MALT-type lymphoma).13Indeed, they are a distinct disease with aggressive clinicopathological features.12,17-19 102-104
Histologic grading of MALT lymphoma in gastric biopsies is often problematic; a small component of low-grade MALT lymphoma can be identified in a significant proportion of diffuse, large B-cell lymphomas and, conversely, foci of high-grade (large-cell) lymphoma can be seen in low-grade MALT lymphomas, suggesting the possible transition from one to the other, analogously to other low-grade lymphomas. The prevalence and time interval of histologic transformation are, however, unknown. It has been proposed by Chan and Isaacson105 that the presence of compact confluent clusters, or sheets of large cells, may indicate the emergence of new clones and can be used as the criterion for the histologic transformation. However, a general agreement has never been achieved.2,3,13 De Jong et al106 proposed the distinction of the following 4 groups, which in their series appeared to be of prognostic relevance: (a) pure low-grade MALT lymphoma, defined by a cluster of blast accounting for less than 5 cells and with no evidence of a diffuse blastic component; (b) low-grade MALT lymphoma with a high-grade component in which a cluster of blast of 5 to 20 cells (and occasionally single large clusters) can be found and with a diffuse blastic component below 10% of the tumor cells; (c) high-grade MALT lymphoma with a low-grade component showing large clusters of more than 20 cells or, also, a diffuse blastic component of more than 10%, with sporadic lymphoepithelial lesions that may still be present; and (d) high-grade lymphoma without a low-grade component with only a diffuse, blastic (large-cell) component. Whether this latter group should be categorized as MALT lymphoma remains controversial; at least some of these lymphomas seem to be derived from the MALT. The hypothesis of a clonal outgrowth from a preexisting low-grade lesion is strongly supported by the epidemiologic findings of Parsonnet et al,33 who found that H pylori infection precedes the growth of low-grade MALT lymphoma and is also strongly associated with the development of the diffuse, large-cell gastric lymphomas.
Diffuse, large B-cell lymphomas of the stomach may represent, in certain cases, clonal evolution from a previously low-grade MALT lymphoma.7,30,107 However, the t(11;18)(q21;q21) was detected in low-grade MALT lymphomas but neither in primary (de novo) or secondary (derived from low-grade MALT lesions) high-grade lesions,108 and trisomy 3 was found in some diffuse, large B-cell lymphomas with remnant foci of low-grade MALT lymphoma but not in those without a low-grade component.54 Nevertheless, the histologic and the clinical behavior of gastric diffuse, large B-cell lymphomas with or without areas of marginal zone/MALT-type lymphoma appear to be similar.17 88
Furthermore, the prognostic relevance of the histologic grading is still to be completely clarified. A retrospective clinicopathological study from Spain on a series of 56 patients with primary gastric lymphoma109 showed that the presence of limited areas of high-grade histology within a low-grade specimen did not change the survival pattern. However, in other studies the presence of an increased number of blasts was found to be predictive of a less favorable long-term outcome in gastric lymphoma patients but not in those with MALT lymphoma presenting in nongastrointestinal sites.106 110
Immunophenotype
Immunohistochemistry studies are relevant for many reasons. There is an almost complete homology between the phenotypes of CCL cells of MALT lymphoma and normal marginal zone B cells (in spleen, Peyer's patches, and lymph nodes). Both types of cells have positivity for surface immunoglobulins and pan-B antigens (CD19, CD20, and CD79a) and a lack of CD5, CD10, CD23, and cyclin D1 expression.88,89,111However, more detailed studies can contribute to the accuracy of a diagnosis. Immunostaining for CD21 (follicular dendritic cells), Ki-67, Bcl-2, and Bcl-6 may help identify residual reactive follicles; immunostaining for those proteins as well as for CD10 can also aid in distinguishing follicular colonization from the very rare extranodal follicular lymphomas.111 Moreover, immunostaining with the pan–B-cell CD20 antibody may help distinguish CCL cells from plasma cells and identify lymphoepithelial lesions. The latter can be further highlighted with antibodies to cytokeratin.
An analysis of surface immunoglobulins with effective antigen retrieval techniques may be essential for distinguishing between a suspicious reactive lymphoid infiltrate and a MALT lymphoma in cases with an equivocal morphologic pattern. In fact, CCL cells express surface and, to a lesser extent, cytoplasmic monotypic immunoglobulins (IgM in most cases, IgA in a few, and IgG rarely; CCL cells are usually negative for IgD) that show light-chain restriction. Therefore, demonstration of a clear-cut restriction of light chains (κ:λ ratio more than 10:1 or vice versa) strongly supports the diagnosis of B-cell lymphoma.
The degree and distribution of the T-cell component can be evaluated with a panel of T-cell antibodies (CD3, CD4, CD8, and CD45RO). An abundance of CD4+ T cells is sometimes associated with the neoplastic B cells in low-grade MALT lymphoma, supporting the theory that T-cell help is necessary for sustaining the initial tumor growth. In contrast, this infiltration of CD4+ T cells is not evident in the high-grade lesions.39,40 112
In addition to the presence of intratumoral T cells, other histologic features suggest that the lymphoma cells may be involved in an immune response,2 further indicating an antigen-driven pathogenesis: the presence of reactive lymphoid follicles and follicular colonization, the plasma cell differentiation, and the presence of scattered transformed cells in the cell cycle and CD30+ cells.
Clinical features
Diagnosis and staging
The most common presenting symptoms of low-grade gastric MALT lymphomas are nonspecific dyspepsia and epigastric pain. Constitutional B symptoms are exceedingly uncommon. Endoscopy usually reveals nonspecific gastritis or peptic ulcer, with mass lesions being unusual.19,31 Few patients present with elevated lactate dehydrogenase (LDH) or β2-microglobulin levels31 (Table 1).
Low-grade gastric MALT lymphoma: most common clinical features
| Age, median, y | 63 |
| Male:female ratio | 1:1 |
| Localized disease (stage I) | 88% |
| Bone marrow infiltration | 7% |
| Main symptoms at presentation | |
| Epigastric or abdominal pain | 53% |
| Dyspepsia | 32% |
| Nausea and vomiting | 8% |
| Gastric bleeding | 2% |
| B symptoms | 1% |
| Elevated LDH | 1% |
| Elevated β2-microglobulin | 4% |
| Main endoscopic findings | |
| Erythema | 30% |
| Erosions | 23% |
| Ulcers | 47% |
| Gastric localization | |
| Antrum | 41% |
| Body | 12% |
| Fundus | 11% |
| Multifocal | 33% |
| Gastric stump | 3% |
| Age, median, y | 63 |
| Male:female ratio | 1:1 |
| Localized disease (stage I) | 88% |
| Bone marrow infiltration | 7% |
| Main symptoms at presentation | |
| Epigastric or abdominal pain | 53% |
| Dyspepsia | 32% |
| Nausea and vomiting | 8% |
| Gastric bleeding | 2% |
| B symptoms | 1% |
| Elevated LDH | 1% |
| Elevated β2-microglobulin | 4% |
| Main endoscopic findings | |
| Erythema | 30% |
| Erosions | 23% |
| Ulcers | 47% |
| Gastric localization | |
| Antrum | 41% |
| Body | 12% |
| Fundus | 11% |
| Multifocal | 33% |
| Gastric stump | 3% |
The best staging system is still controversial. We currently adopt the revised version of the Blackledge staging system (Table2) that was recommended for general use by an international workshop held in Lugano, Switzerland, in 1993.113 When ultrasound endoscopy is available, the TNM system (using the criteria initially proposed for gastric carcinoma by the American Joint Committee on Cancer and Union International Contre le Cancer) can also be employed, based on the echoendoscopic extent of the gastric wall involvement44 (Table 2).
Staging of gastric MALT lymphoma: comparison of different systems
| . | Lugano Staging System for gastrointestinal lymphomas113 . | TNM Staging System adapted for gastric lymphoma44 . | Ann Arbor stage . | Tumor extension . |
|---|---|---|---|---|
| Stage I | Confined to GI tract (single primary or multiple, noncontiguous) | T1 N0 M0 | IE | Mucosa, submucosa |
| T2 N0 M0 | IE | Muscularis propria | ||
| T3 N0 M0 | IE | Serosa | ||
| Stage II | Extending into abdomen | |||
| II1 = local nodal involvement | T1-3 N1 M0 | IIE | Perigastric lymph nodes | |
| II2 = distant nodal involvement | T1-3 N2 M0 | IIE | More distant regional lymph nodes | |
| Stage IIE | Penetration of serosa to involve adjacent organs or tissues | T4 N0 M0 | IE | Invasion of adjacent structures |
| Stage IV | Disseminated extranodal involvement or concomitant supra | T1-4 N3 M0 | IIIE | Lymph nodes on both sides of the diaphragm/distant metas- |
| diaphragmatic nodal involvement | T1-4 N0-3 M1 | IVE | tases (eg, bone marrow or additional extranodal sites) |
| . | Lugano Staging System for gastrointestinal lymphomas113 . | TNM Staging System adapted for gastric lymphoma44 . | Ann Arbor stage . | Tumor extension . |
|---|---|---|---|---|
| Stage I | Confined to GI tract (single primary or multiple, noncontiguous) | T1 N0 M0 | IE | Mucosa, submucosa |
| T2 N0 M0 | IE | Muscularis propria | ||
| T3 N0 M0 | IE | Serosa | ||
| Stage II | Extending into abdomen | |||
| II1 = local nodal involvement | T1-3 N1 M0 | IIE | Perigastric lymph nodes | |
| II2 = distant nodal involvement | T1-3 N2 M0 | IIE | More distant regional lymph nodes | |
| Stage IIE | Penetration of serosa to involve adjacent organs or tissues | T4 N0 M0 | IE | Invasion of adjacent structures |
| Stage IV | Disseminated extranodal involvement or concomitant supra | T1-4 N3 M0 | IIIE | Lymph nodes on both sides of the diaphragm/distant metas- |
| diaphragmatic nodal involvement | T1-4 N0-3 M1 | IVE | tases (eg, bone marrow or additional extranodal sites) |
The initial staging should comprise a gastroduodenal endoscopy with multiple biopsies from each area of the gastric map and from all the abnormal sites. Upper airway examination is required as well as all of the usual procedures performed for nodal lymphomas, including bone marrow biopsy (Table 3). The presence of active H pylori infection114 must be always ruled out by histology (Genta stain or Warthin-Starry stain of antral biopsy specimen); serology studies are mandatory when results of histology are negative.
Recommended staging procedures for gastric lymphoma
| History (duration and presence of local or systemic symptoms) |
| Physical examination (careful evaluation of all lymph node regions, inspection of the upper airways and tonsils, clinical evaluation of the size of liver and spleen, detection of any palpable mass) |
| Laboratory tests, including complete blood counts and peripheral blood smear, LDH and β2-microglobulin levels, evaluation of renal and liver function |
| Bone marrow biopsy |
| Standard posteroanterior and lateral chest radiographs |
| Abdominal and pelvic computed tomography scan |
| Gastroduodenal endoscopy with multiple gastric biopsies from all the visible lesions and the noninvolved areas with a complete mapping of the organ |
| Gastric endoscopic ultrasound |
| History (duration and presence of local or systemic symptoms) |
| Physical examination (careful evaluation of all lymph node regions, inspection of the upper airways and tonsils, clinical evaluation of the size of liver and spleen, detection of any palpable mass) |
| Laboratory tests, including complete blood counts and peripheral blood smear, LDH and β2-microglobulin levels, evaluation of renal and liver function |
| Bone marrow biopsy |
| Standard posteroanterior and lateral chest radiographs |
| Abdominal and pelvic computed tomography scan |
| Gastroduodenal endoscopy with multiple gastric biopsies from all the visible lesions and the noninvolved areas with a complete mapping of the organ |
| Gastric endoscopic ultrasound |
Unlike most low-grade B-cell lymphomas of peripheral lymph nodes, low-grade MALT lymphoma is usually a very indolent disease, often remaining localized for a prolonged period; in some cases, no progression is seen during several years without treatment. Systemic dissemination and bone marrow involvement occur in a few patients; prognosis seems particularly poor in the few cases presenting with advanced stages17,103,115 or with an unfavorable International Prognostic Index score.14 Patients with primary gastrointestinal presentation might have a better survival than those with nongastrointestinal MALT lymphoma.110 A deep infiltration of the gastric wall by the lymphoma has been reported to be strongly associated with spread to the regional lymph nodes, analogous to findings in gastric carcinoma. It has therefore been recommended that the depth of infiltration be included in pathology reports concerning primary gastric lymphoma of the MALT.116Endoscopic ultrasound might be useful to evaluate the depth of infiltration and to distinguish benign lymphoid aggregate from lymphoma and should be included in the initial procedures whenever possible.117 118
Therapy
Despite abundant literature on histologic, clinical, and biological features of MALT lymphoma, results of controlled trials to define the optimal therapy have not yet been published, and only a few randomized studies are ongoing. Literature data are confusing: insufficient staging and outdated histologic classifications are a major problem of the older reports, and more recent studies often refer to retrospective series of patients not uniformly staged and treated.
Few published studies specifically report treatment outcome for localized gastric MALT lymphoma. The patients have been treated with a variety of combinations of surgery, radiotherapy, and chemotherapy, and the overall survival rates range from 80% to 95% at 5 years.17,19,31 103 Therefore, while prognosis of patients with MALT lymphoma seems excellent regardless of treatment, optimal therapy remains to be determined.
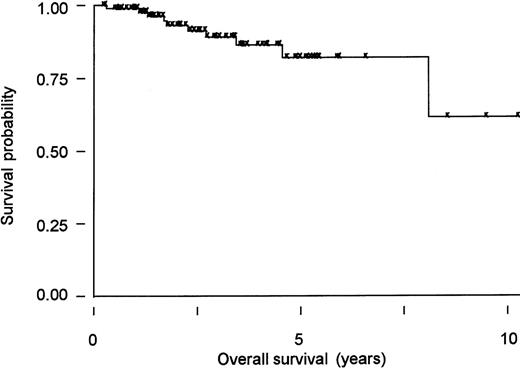
Increasing evidence indicates that eradication of H pylori with antibiotics can be effectively employed as the sole initial treatment.20,34,35,43,44,118-121 In a series of 93 patients from northern Italy and southern Switzerland with low-grade gastric MALT lymphoma, no statistically significant difference was apparent in overall survival or event-free survival between patients who received different initial treatments (chemotherapy alone, surgery alone, surgery with additional chemotherapy or radiation therapy, or antibiotics against H pylori).31 The actuarial 5-year overall survival was 82% (95% confidence interval [CI], 67%-91%) in the series as a whole (Figure2). At a median follow-up of 3 years, 10 of 93 patients had died, all but 1 from a second (solid) tumor. The unexpectedly high incidence of additional neoplasms was not treatment-related122 and has been described in other series, but its significance is controversial.85,109,123,124 In this series, 49 patients with stage I disease were given antibiotics alone as initial treatment; eradication of H pylori was achieved in 97% of patients (95% CI, 88.2%-99.9%), and histologic regression of the MALT lymphoma was documented in 67% of the patients (95% CI, 51%-80%) after the eradication.31 The median time required to achieve histologic regression was 5 months (range, 3 to 18 months).31 A first update125 of the German multicenter trial35 has also been published and confirms, with a median follow-up of 2 years, the efficacy of antibiotics in inducing apparently durable lymphoma remission. Moreover, this study demonstrated that patients in whom the lymphoma did not respond toH pylori eradication may have harbored high-grade lesions that had not initially been recognized.125 These findings support the hypothesis of a continuous clonal evolution from a low-grade B-cell MALT lymphoma whose growth is still dependent on T-cell help (ie, H pylori–dependent) to an autonomous low-grade, and eventually to an autonomous high-grade, lymphoma, neither requiring antigenic drive for survival and growth. An American uncontrolled trial of 34 patients with stage I-II disease showed that the antibiotic efficacy is higher in early lesions: 70% (95% CI, 35%-93%) of the cases with disease confined to the mucosa and submucosa achieved a complete remission (CR), whereas those with locally advanced disease infiltrating the muscularis mucosae, the serosa, or the perigastric lymph nodes had a significantly lower CR rate (38%; 95% CI, 17%-64%).44 A preliminary response evaluation has been performed in the first 170 patients with localized low-grade lymphoma of the stomach enrolled in the ongoing international controlled clinical trial LY03 of chlorambucil versus observation after antibiotic therapy; it confirmed that at least half of the treated cases can achieve a histologic CR.126 A subset of patients has undergone a molecular follow-up by the PCR assay for the detection of a monoclonal rearrangement of the immunoglobulin gene127: 6 of 13 patients with a histologic CR also had a molecular complete response that sometimes required a long time (up to 2 years) to be demonstrated.127,128 Neubauer and colleagues125 have reported that 22 of 31 initially monoclonal low-grade tumors treated with antibiotics remained monoclonal at a median posttreatment follow-up of 1 year despite the histologic CR. Steinbach and colleagues44 reported the disappearance of B-cell monoclonality in 5 of 10 histologic complete responders. These data demonstrate that PCR-detectable B-cell monoclonality may persist after the disappearance of histologic evidence of MALT lymphoma, suggesting that H pylorieradication suppresses but does not eradicate the lymphoma clones.2 Whether the persistence of PCR-detected B-cell monoclonality is associated with a higher risk of lymphoma relapse remains to be determined.44,120,125,127,128 This detectable monoclonal population might also be due to the presence of benign precursors of the B-cell clone that has given origin to the lymphoma, which share the same immunoglobulin receptor. Thus, careful histologic examination of multiple gastric biopsies remains the cornerstone for the follow-up of gastric MALT lymphoma patients.120
MALT lymphoma of the stomach: Kaplan-Meier curve of overall survival.
Overall survival is shown of a cohort of 93 patients diagnosed between 1986 and 1995 in 3 different institutions in northern Italy (Ospedale S Orsola, Brescia and Ospedale di Circolo, Varese) and southern Switzerland (Bellinzona).31 The median age was 63 years (range, 21-89 years), and most presented with localized disease (82 stage I, 4 stage II, and 7 stage IV). The type of treatment depended on the policy followed in the respective institutions at the time of diagnosis: 24 patients had gastrectomy with11 or without13 additional chemotherapy or irradiation, 49 had antibiotics, 12 chemotherapy, 1 radiotherapy, and 7 refused any treatment. There was no apparent difference in overall survival between patients who received different treatments. At a median follow-up of 3 years, 10 of 93 patients had died, only 1 because of tumor progression. The 5-year projected overall survival is 82% (95% CI, 67%-91%) in the series as a whole.31
MALT lymphoma of the stomach: Kaplan-Meier curve of overall survival.
Overall survival is shown of a cohort of 93 patients diagnosed between 1986 and 1995 in 3 different institutions in northern Italy (Ospedale S Orsola, Brescia and Ospedale di Circolo, Varese) and southern Switzerland (Bellinzona).31 The median age was 63 years (range, 21-89 years), and most presented with localized disease (82 stage I, 4 stage II, and 7 stage IV). The type of treatment depended on the policy followed in the respective institutions at the time of diagnosis: 24 patients had gastrectomy with11 or without13 additional chemotherapy or irradiation, 49 had antibiotics, 12 chemotherapy, 1 radiotherapy, and 7 refused any treatment. There was no apparent difference in overall survival between patients who received different treatments. At a median follow-up of 3 years, 10 of 93 patients had died, only 1 because of tumor progression. The 5-year projected overall survival is 82% (95% CI, 67%-91%) in the series as a whole.31
In our opinion, the indolent nature of the disease in most cases of MALT lymphoma makes a conservative approach advisable, with antibiotic therapy as the sole initial treatment provided that strict oncohematologic and endoscopic follow-up is carried out. The use of antibiotics as first-line therapy may avert or at least postpone the necessity for surgical resection in most patients, and we recommend eradication of H pylori before consideration of further therapeutic options.129,130 Any of the highly effective antibiotic regimens proposed131,132 can be used (Table4). A strict endoscopic follow-up is recommended, with multiple biopsies taken 2 months after treatment to document H pylori eradication and, subsequently, at least twice per year for 2 years to monitor the histologic regression of the lymphoma. In case of unsuccessful H pylori eradication, a second-line anti-Helicobacter therapy should be attempted with alternative triple- or quadruple-therapy regimens of proton-pump inhibitor plus antibiotics.131,132 However, it is still unknown whether H pylori eradication will definitely cure the lymphoma; therefore, long-term follow-up of antibiotic-treated patients is mandatory. Some cases of documented tumor recurrence following H pylori reinfection have been reported, suggesting that residual dormant tumor cells can be present despite clinical and histologic remission. Relapses have also been documented in the absence of H pylori reinfection, indicating the presence of B-cell lymphoma clones that have escaped the antigenic drive.125 The efficacy of antibiotic therapy is reduced in locally advanced disease, with bulky masses or deep infiltration of the gastric wall, and in disease associated with increased numbers of large cells. In our experience, however, eradication of H pylori is worthwhile even in these cases but usually cannot be the unique therapeutic approach. In addition to antibiotic therapy, chemotherapy (or radiotherapy) should be given to these patients as well as to those with regional nodal involvement.44
Regimens for the eradication of Helicobacter pylori
| Therapy and drug . | Dosage . | Frequency . |
|---|---|---|
| Triple therapy with bismuth subcitrate | ||
| Bismuth | 120 mg | qid for 7 days |
| Tetracycline | 500 mg | qid for 7 days |
| Metronidazole | 500 mg | tid for 7 days |
| Triple therapy with H2-receptor antagonists | ||
| Ranitidine | 300 mg | daily for 6-10 weeks |
| Metronidazole | 500 mg | tid for 12 days |
| Amoxicillin | 750 mg | tid for 12 days |
| Triple therapy with acid-pump inhibitors | ||
| Omeprazole | 20 mg | bid for 7 days |
| Amoxicillin | 1000 mg | bid for 7 days |
| Clarithromycin | 500 mg | bid for 7 days |
| Therapy and drug . | Dosage . | Frequency . |
|---|---|---|
| Triple therapy with bismuth subcitrate | ||
| Bismuth | 120 mg | qid for 7 days |
| Tetracycline | 500 mg | qid for 7 days |
| Metronidazole | 500 mg | tid for 7 days |
| Triple therapy with H2-receptor antagonists | ||
| Ranitidine | 300 mg | daily for 6-10 weeks |
| Metronidazole | 500 mg | tid for 12 days |
| Amoxicillin | 750 mg | tid for 12 days |
| Triple therapy with acid-pump inhibitors | ||
| Omeprazole | 20 mg | bid for 7 days |
| Amoxicillin | 1000 mg | bid for 7 days |
| Clarithromycin | 500 mg | bid for 7 days |
All of these schemes showed eradication rates of about 90%. Any antibiotic regimen that is effective in eradication of H pylorican be used in the initial treatment of MALT lymphoma. Thus far, no regimen has been proven to be superior in inducing lymphoma remissions. A second-line anti-Helicobacter therapy with different antibiotics may be needed in some instances. Patients allergic to penicillin may be given a tetracycline-based regimen.
No treatment guidelines exist for the management of patients after antibiotics failure and for the subset of cases in which no evidence ofH pylori can be found.91 It has been shownthat the chance of a response to antibiotics is dramatically reduced in the latter group.44 A choice can be made between conventional oncologic modalities, including chemotherapy, radiotherapy, and surgery, alone or in combination. Unfortunately, there are no published randomized studies to help the decision.
Chemotherapy has never been adequately evaluated in gastric MALT lymphomas because it was usually not administered or given after surgery or radiotherapy. Some scanty data suggesting the efficacy of chlorambucil in low-grade gastric lymphoma can be found in the older literature,133 but only 1 nonrandomized trial has thus far tested the activity of chemotherapy with single alkylating agents in MALT lymphomas.134 In this study, 24 patients, 17 with stage IE and 7 with stage IV were given continuous oral administration of cyclophosphamide, 100 mg/day, or chlorambucil, 6 mg/day (median treatment duration, 18 months; range, 8-24 months). A 75% CR rate was reported. Five patients relapsed (2 with stage I and 3 with stage IV) at 12 to 96 months, all in initial sites, and 1 with large-cell transformation. The projected 5-year event-free and overall survivals were 50% and 75%, respectively.134
Also, the efficacy of local radiotherapy has not been extensively studied in trials that take account of the MALT concept.106,135,136 In a recent study, 17 patients with stage I-II MALT lymphoma of the stomach without evidence of H pylori infection or with persistent lymphoma after antibiotics were treated with radiation alone (1.5-Gy fractions in 4 weeks to the stomach and the adjacent lymph nodes, with a median total dose of 30 Gy). The results are encouraging, with 100% biopsy-confirmed CR and 100% event-free survival (at a median follow-up of 27 months).136
Surgery has been widely used in the past. Cogliatti et al17reported a series of histologically reviewed cases of low-grade MALT lymphoma (48 patients with stage IE and 21 with stage IIE disease): 45 had surgery alone; 12 surgery and adjuvant chemotherapy; 11 surgery and irradiation; 1 surgery, chemotherapy, and radiotherapy. The 5-year overall survival was 91% (95% for stage IE and 82% for stage IIE) with no significantly different survival rates between gastrectomy alone versus additional treatment.
While the use of local treatment is evidently associated with an excellent disease control, the precise role for surgical resection must nowadays be redefined.130,137,138 Follow-up endoscopy may reveal the reappearance of lymphoepithelial lesions in the remaining gastric mucosa that can be responsible for local recurrence. Indeed, the fact that MALT lymphoma is often a multifocal disease99suggests that clear excision margins are not necessarily a guarantee of radical resection. If surgery is chosen, a total gastrectomy may offer greater chances of cure, but this operation carries a risk of mortality and may severely impair the patient's quality of life.130
The clinical significance of histologic grading has been discussed above in “Histologic features.” Only De Jong and colleagues found a prognostic relevance of the presence of a minor large-cell component in MALT lymphoma patients treated with local radiotherapy (plus chemotherapy in cases of advanced or bulky disease).106 Other authors found that cases with a minor large-cell component did not necessarily have a worse prognosis when treated with surgery (with or without chemotherapy),13,105 109 while their outcome with antibiotics alone has still to be precisely determined.
The best treatment for diffuse large B-cell lymphoma of the stomach remains very controversial, particularly with respect to the role of surgery.130,137,138 Despite anecdotal cases of regression of high-grade lesions after anti–H pyloritherapy,139,140 it is our opinion that gastric lymphoma with aggressive histologic features must be treated aggressively.130 However, eradication of H pylorishould be attempted (in addition to chemotherapy) even in the setting of large-cell lymphoma, because this approach may eliminate a residual or relapsed low-grade component that can be responsible for tumor recurrence following antigen stimulation.35 141
With the spreading popularity of stomach-conserving approaches, prospective studies evaluating the histologic grading and its prognostic significance will be fundamental. A further challenge to the ongoing research is the definition of the molecular events responsible for the escape of MALT lymphoma from a state of antigen-dependent growth to one of autonomous growth, as well as the molecular events that can cause histologic transformation. This knowledge will allow us to define appropriate therapeutic strategies for the individual patient.
Acknowledgments
We are indebted to Prof Oliver W. Press for helpful discussions and advice.
Supported in part by grants from the Swiss National Science Foundation (grant 32-45993.95) and the Schweizerische Krebsliga/Krebsforschung Schweiz (Swiss Cancer League/Cancer Research Switzerland) (grant AKT 623).
Reprints:Emanuele Zucca, Oncology Institute of Southern Switzerland, Department of Medical Oncology, Ospedale San Giovanni, 6500 Bellinzona, Switzerland; e-mail: oncosg@siak.ch.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal