Abstract
Proteolytic cleavage of single-chain high molecular weight kininogen (HK) by kallikrein releases the short-lived vasodilator bradykinin and leaves behind 2-chain high molecular weight kininogen (HKa) that has been previously reported to exert antiadhesive properties as well as to bind to the urokinase receptor (uPAR) on endothelial cells. In this study we defined the molecular mechanisms for the antiadhesive effects of HKa related to disruption of integrin- and uPAR-mediated cellular interactions. Vitronectin (VN) but not fibrinogen or fibronectin-dependent vβ3 integrin–mediated adhesion of endothelial cells was blocked by HKa or its isolated domain 5. In a purified system, HKa but not HK competed for the interaction of VN with vβ3 integrin, because HKa and the isolated domain 5 but not HK bound to both multimeric and native VN in a Zn2+-dependent manner. The interaction between HKa or domain 5 with VN was prevented by heparin, plasminogen activator inhibitor-1, and a recombinant glutathione-S-transferase (GST)-fusion peptide GST-VN (1-77) consisting of the amino terminal portion of VN (amino acids 1-77), but not by a cyclic arginyl-glycyl-aspartyl peptide, indicating that HKa interacts with the amino terminal portion of VN (“somatomedin B region”). Furthermore, we have confirmed that HKa but not HK bound to uPAR and to the truncated 2-domain form of uPAR lacking domain 1 in a Zn2+-dependent manner. Through these interactions, HKa or its recombinant His-Gly-Lys–rich domain 5 completely inhibited the uPAR-dependent adhesion of myelomonocytic U937 cells and uPAR-transfected BAF-3 cells to VN and thereby promoted cell detachment. By immunogold electron microscopy, both VN and HK/HKa were found to be colocalized in sections from human atherosclerotic coronary artery, indicating that the described interactions are likely to take place in vivo. Taken together, HK and HKa inhibit different VN-responsive adhesion receptor systems and may thereby influence endothelial cell- or leukocyte-related interactions in the vasculature, particularly under inflammatory conditions.
Cell-to-cell and cell-to-extracellular matrix interactions determine morphogenetic processes during development, vascular remodeling, or inflammation. The dynamics of cellular contacts require the presence of pro- and anti- or counter-adhesive components as well as different proteolytic systems that need to be regulated in a spatiotemporal manner.1,2 In particular, the pattern of expression of adhesion receptors such as selectins or integrins in the vasculature as well as changes in the composition of the extracellular matrix are directly related to alterations of vascular cell interactions and relate to the pathophysiology of cardiovascular diseases.3
The multifunctional proteins fibrinogen (FBG)/fibrin and vitronectin (VN) accumulate at extracellular matrix sites associated with wound healing,4,5 malignant tumors,6 or angiogenesis.7,8 These and other adhesive glycoproteins are recognized by integrins of the αv subfamily, which are prominently expressed on migrating and proliferating vascular cells and whose functions can effectively be blocked by synthetic antagonists such as cyclic arginyl-glycyl-aspartyl (cRGD) peptides.9,10Moreover, the plasminogen activation system contributes to cell invasion by mediating not only pericellular proteolysis but also by participating in the modulation of cell adhesion in a nonproteolytic fashion.11,12 Through direct interactions particularly expressed at vitronectin-rich matrix sites, urokinase (uPA) acts as proadhesive factor, whereas plasminogen activator inhibitor-1 (PAI-1) abrogates both integrin- and urokinase receptor (uPAR)-dependent cell adhesion.13-17 In the latter system, uPAR also serves as high-affinity cell surface–associated binding protein for VN.18,19 A third ligand for uPAR is high molecular weight kininogen (HK)20; however, the functional relationships among these components in cellular contacts remain to be defined.
HK is composed of 6 domains and is present in plasma at a concentration of 0.67 μmol/L. It serves a nonenzymatic cofactor role in the initiation of the contact phase,21-23 associated with vascular injury, inflammation, or activation of complement in humoral immune defense. In particular, kallikrein can liberate the short-lived vasodilator peptide bradykinin from HK,24 thereby generating HKa (2-chain kinin-free HK), which lacks most of its domain 4. Domain 5 in HKa is rich in His, Gly, and Lys, which enables HKa to bind to anionic surfaces, zinc, or heparin.25-28 Moreover, HK/HKa binding to cells is mediated by domains 3 and 5,29,30 contributing to the regulation of pericellular plasmin generation by modulating plasma kallikrein-dependent formation of uPA,31 a reaction dependent on the binding of plasma prekallikrein to HK domain 6.22 On granulocytes, HK and FBG compete for binding to the integrin αMβ2,32,33 whereas on endothelial cells the binding proteins for globular C1q (denoted gC1qR) as well as cytokeratin-134-36 were identified as binding proteins for HK. This role for gC1qR has been disputed in light of the recent finding that gC1qR appears to be a mitochondrial protein.37 On endothelial cells, HKa is recognized by uPAR, and this Zn2+-dependent binding can be inhibited by VN.20
These diverse observations, together with a recent report on antiadhesive properties of HKa,38 39 prompted us to investigate the underlying mechanisms for the contribution of kininogens in adhesive interactions involving different adhesion receptors on blood and vessel wall cells. Our results indicate that direct binding of HKa to matrix-associated VN competes for integrin- and uPAR-dependent cell adhesion uncovering a plausible mechanism for the antiadhesive properties of HKa in tissue remodeling.
Materials and methods
Reagents
Recombinant Gly158scuPA (noncleavable mutant of high molecular weight uPA) was produced in Chinese hamster ovary cells40 and was provided by Dr H. Roger Lijnen (Leuven, Belgium). uPA was from Medac (Hamburg, Germany); VN was purified from human plasma and converted to the multimeric form as previously described41,42; FBG was purchased from Kabivitrum (Munich, Germany); fibronectin was from Sigma (Munich, Germany); cRGD-containing peptide (cRGDfV) was from Bachem (Heidelberg, Germany); and recombinant αvβ3 integrin was kindly provided by Dr Simon Goodman (Merck KGaA, Darmstadt, Germany). Recombinant soluble uPAR (suPAR) was obtained from Dr Niels Behrendt (Finsen Laboratory, Copenhagen, Denmark). Single- and 2-chain high molecular weight kininogen (HK and HKa) were purchased from Enzyme Research Laboratories (South Bend, IN). The purified HK and HKa (more than 95%) appeared as a major band of 140 kd and 110 kd, respectively, on nonreduced sodium dodecyl sulfate gels. HK had been digested with plasma kallikrein (HK to kallikrein = 100:1, mol/mol) for 20 minutes at 37°C. The resulting HKa was composed of 2 bands of 62 and 46 kDa when analyzed by reduced sodium dodecyl sulfate gel electrophoresis. Glutathione-S-transferase (GST) fused to domains 3, 5, and 6 of HK were produced as previously described.43,26 GST was N-terminally attached to the following sequences of HK: G235 to M357 (domain 3), K420 to S513 (domain 5), and T503 to S626 (domain 6). Likewise in GST-VN (1-77), GST was fused to the amino terminal portion (amino acids 1-77) of VN and produced as previously described.44 Active PAI-1 was from Astra Hässle AB (Mölndal, Sweden). Murine monoclonal antibody (mAb) 13H1 against human VN42,45 was kindly provided by Dr Paul Declerck (Leuven, Belgium); anti-uPAR mAb (R3)46 was provided by Dr Gunilla Hoyer-Hansen (Finsen Laboratory). ZnCl2 was from Sigma; vitamin D3- was from Biomol (Hamburg, Germany); transforming growth factor-β was from R & D Systems (Boston, MA); and interleukin-3 was from PBH (Hannover, Germany). The characteristics of mAb HKH13 against domain 3 and HKH18 against domain 1 of HK were previously described.47
Cell culture
Monocytic cells (U937) and MG63 human osteosarcoma cells were from American Type Culture Collection (ATCC, Rockville, MD) and cultured as described by the supplier in RPMI-1640 medium or Dulbecco's modified Eagle's medium (DMEM), respectively, containing 10% (vol/vol) fetal calf serum. BAF-3 cells were from ATCC and cultured in RPMI-1640 medium containing 10% fetal calf serum and 2 ng/mL interleukin-3. Bovine retinal endothelial cells (BRECs) were kindly provided by Dr Sigrid Zink (Diabetes Research Institute, University of Düsseldorf, Germany) and cultivated as described.48Bovine adrenocortical endothelial cells (BAECs) were kindly provided by Dr Mathias Clauss (Max-Planck-Institute, Bad Nauheim, Germany) and cultivated in DMEM containing 10% (vol/vol) fetal calf serum. All culture media were from Gibco (Eggenstein, Germany).
Construction of uPAR-transfected BAF-3 cells
BAF-3 cells (interleukin-3–dependent mouse B-cell line) were transfected by electroporation with uPAR complementary DNA in the sense and antisense orientation using the expression vector pCDNA3. Cells were selected in the presence of G418 (1.2 mg/mL) and found to express uPAR by fluorescence-activated cell sorter analysis, Northern blotting, and uPAR enzyme-linked immunosorbent assay (ELISA) (details to be published elsewhere).
Radiolabeling of vitronectin
Multimeric VN (100 μg) was labeled with 18.5 MBq Na125I (Amersham, Braunschweig, Germany) using Iodogen (Pierce, Oud Beijerland, The Netherlands) according to a previously outlined procedure.49 50 After separation on a Sephadex G-25 column (Pharmacia, Freiburg, Germany) suspended in Tris-buffered saline (TBS) (20 mmol/L Tris base; 0.15-mol/L NaCl, pH 7.4; TBS) containing 0.1% (wt/vol) bovine serum albumin (BSA; Sigma), the labeled protein was dialyzed against TBS. The specific activity was 185-370 kBq/μg VN.
Binding interactions in a purified system
Polystyrene microtiter wells (high binding, type I, Costar, Badhoevedorp, The Netherlands) were coated at a concentration of 5 μg/mL with purified uPAR in 15 mmol/L Na2CO3, 35 mmol/L NaHCO3 (pH 9.6), with 5 μg/mL αvβ3 integrin in TBS containing 1 mmol/L CaCl2 and 1 mmol/L MgCl2, or with 5 μg/mL of HK or its isoforms in TBS, and subsequently blocked with 3% (wt/vol) BSA. Binding of 125I-VN to the immobilized components was performed at 4°C for 18 hours in a final volume of 50 μL in the absence or presence of competitors as indicated in the figure legends: Binding to αvβ3 integrin was carried out in TBS containing 0.05% (wt/vol) Tween 20, 0.3% (wt/vol) BSA, 1 mmol/L CaCl2, and 1 mmol/L MgCl2, while binding to uPAR or HK/HKa was performed in the absence of added divalent cations. Thereafter, the wells were washed and counted in a γ counter. Nonspecific binding was measured in the presence of more than 100-fold molar excess of unlabeled VN to estimate specific binding. Nonspecific binding of 125I-VN to BSA-coated wells (in the absence of the immobilized receptor) was used as an additional control in all experiments and was subtracted in calculating the specific binding.
ELISA for ligand-receptor interactions
Maxisorp plates (high binding capacity; Nunc, Roskilde, Denmark) were coated with uPAR (5 μg/mL) or different forms and fragments of HK (HKa, D3, D5, D6; each at 5 μg/mL), respectively. After blocking with 3% (wt/vol) BSA in TBS, 2 μg/mL native or multimeric VN in TBS containing 0.3% BSA and 0.05% Tween 20 was added to the wells. After incubation for 2 hours at 22°C, mAb VN-745 against VN at a concentration of 125 ng/mL was added, followed by addition of a secondary goat antimouse immunoglobulin G (Dako, Hamburg, Germany) and the substrate ABTS, and binding was quantitated at 405 nm in a Thermomax Reader (Molecular Devices, Menlo Park, CA). The identical protocol was used when the binding of HK/HKa in the presence or absence of 50 μmol/L ZnCl2 to immobilized multimeric or native VN, uPAR, or αvβ3 integrin (each at 5 μg/mL) was tested, except that mAb HKH13 or HKH18 were used for the detection of bound kininogen. Nonspecific binding to BSA-coated wells was used as blank and was subtracted to calculate the specific binding. In case of binding of HK/HKa to immobilized VN, uPAR, or αvβ3 integrin, binding to noncoated wells and to BSA-coated wells in the absence or presence of Zn2+ was also estimated to calculate nonspecific binding. These values were also subtracted to estimate the specific binding of HK/HKa.
Cell adhesion assays
Cell adhesion to VN-, FBG-, or fibronectin-coated plates (and to BSA-coated wells as control) was tested according to a previously described protocol.19,51 Briefly, multiwell plates were coated with 2 μg/mL native or multimeric VN or 10 μg/mL FBG or fibronectin (dissolved in bicarbonate buffer, pH 9.6), respectively, and blocked with 3% (wt/vol) BSA. BAF-3 or U937 cells, which had been differentiated for 24 hours with vitamin D3 (100 nmol/L) and transforming growth factor-β (2 ng/mL), were washed in serum-free RPMI and plated onto the precoated wells for 60 to 90 minutes at 37°C in the absence or presence of competitors in serum-free RPMI. Proteolytic conversion of HK to HKa in the absence and the presence of monocytic cells as described in the same adhesion assay was performed and, after the incubation period, the supernatant was collected and analyzed for HK or HKa in a Western blot using the antibody HKH18.47
Confluent BREC, BAEC, or MG63 osteosarcoma cells were detached with trypsin, which was subsequently neutralized with soybean trypsin inhibitor (Sigma), washed, and plated onto precoated wells as described above. After the incubation period for the adhesion assay in serum-free DMEM, the wells were washed and the number of adherent cells were quantified by crystal violet staining at 590 nm.
Electron microscopy
Secondary antibodies conjugated to 15 nm colloidal gold were from BioCell Research Labs (Boston, MA), and protein A coupled to 10 nm gold was from the Department of Cell Biology, University of Utrecht, The Netherlands. All other reagents used for electron microscopy were from TAAB Laboratory Equipment Ltd, Reading, England.
Sections of human atherosclerotic coronary arteries were obtained within 2 hours of surgery, cut into approximately 1-μm pieces, and fixed in 4% (wt/vol) paraformaldehyde/phosphate-buffered saline (PBS) on ice for 30 minutes. Specimens were dehydrated through a graded series of ethanol solutions with progressive lowering of temperature for 1 hour each (30% at 0°C, 50% at −20°C, 70% at −30°C, 90% at −30°C, 100% ethanol at −30°C) and then infiltrated with 50% (vol/vol) K4M Lowicryl (TAAB) in ethanol at −30°C for 16 hours followed by 100% K4M Lowicryl at −30°C for 2 × 12 hours. Finally, tissues were embedded in K4M Lowicryl at −30°C for 16 hours under UV light, and polymerization was completed at room temperature under UV light. Blocks were cut on an Ultracut microtome, and 80-nm–thick sections were taken onto 400 mesh copper/rhodium electron microscopy grids (TAAB).
Double immunogold labeling of VN and kininogen was performed by sequentially staining both sides of tissue sections. These were initially treated with blocking buffer (10% [vol/vol] fetal calf serum/0.02 mol/L glycine or PBS) for 30 minutes to quench the unspecific binding sites. For detection of VN, the sections were incubated with rabbit antihuman VN antibodies41 diluted 1:50 in blocking buffer for 1 hour at room temperature. Thereafter, the sections were washed in PBS for 3 × 5 minutes and subsequently treated with protein A conjugated to 10 nm gold particles diluted 1:50 in blocking buffer for 1 hour.52 Grids were then washed in PBS for 3 × 5 minutes to remove any unbound protein A gold, fixed in 2.5% glutaraldehyde or PBS for 5 minutes, and finally washed in double-distilled water for 30 minutes. For the detection of the second antigen kininogen, the grids were turned over and incubated in blocking buffer as before. Kininogen antigen was detected by incubation for 1 hour with sheep antihuman kininogen antiserum (I107, 10 μg/mL) diluted 1:25 in blocking buffer. Unbound antibodies were washed off as mentioned above. Specifically bound antibodies were detected by incubation for 1 hour with rabbit antigoat immunoglobulin G coupled to 15 nm gold particles diluted 1:50 in blocking buffer. As negative controls, tissue sections were used that were similarly treated except that the primary antibodies were omitted. After immunostaining, the grids were coated with Formvar film, and sections were contrasted using uranyl acetate and lead citrate. Sections were analyzed using a Philips 201 transmission electron microscope.
Results
Inhibition of vβ3 integrin–dependent adhesion to VN by kininogen
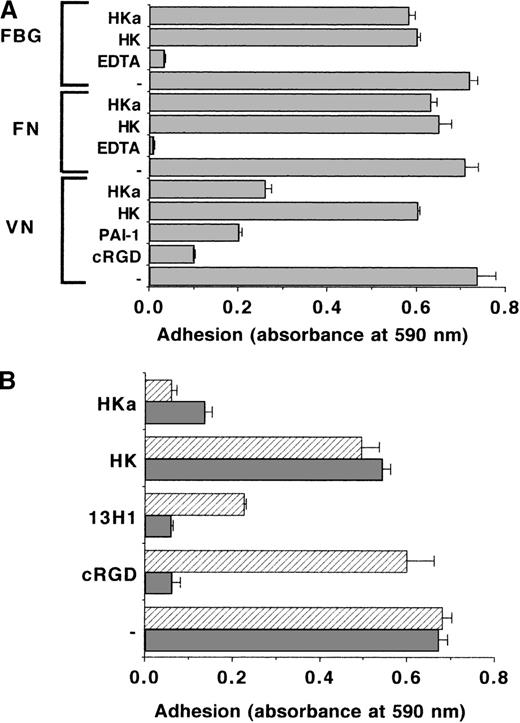
Endothelial cells adhere via different integrins to matrix proteins such as VN, FBG, or fibronectin. The effect of HK or HKa on the adhesion of BREC, BAEC, and MG63 human osteosarcoma cells to these proteins was tested. Adhesion to VN was αvβ3integrin–mediated, as it was abolished by cRGDfV. Similar to PAI-1, which can block this αvβ3integrin–VN interaction, HKa reduced endothelial cell adhesion to VN by 70% to 80%, whereas HK had a much weaker effect. Neither HK nor HKa affected the adhesion of these cells to FBG or fibronectin (Figure1A). To further define the specificity of the antiadhesive properties of HKa for the VN substrate, 2 adhesion protocols were compared: (1) cells and competitors were added simultaneously to the VN-coated wells, or (2) prior to the adhesion assay, the VN-coated wells were incubated with the competitors for 1 hour, followed by extensive washing. In both cases, HKa inhibited cell adhesion in a pattern similar to PAI-1 or mAb 13H1 directed against VN, which both block VN-dependent adhesion due to direct binding to VN. In contrast, the cRGDfV inhibited adhesion when added simultaneously to the cells, but it had no effect when it was preincubated on the VN substrate. The latter was expected, because cRGDfV abolishes adhesion by binding specifically to the αvβ3 integrin but not by binding to VN (Figure 1B). Adhesion to FBG was not blocked even by high concentrations of kininogens, when added simultaneously to the seeded cells, whereas partial inhibition of cell adhesion was observed after preincubation of HKa with immobilized FBG prior to the cell adhesion assay. This reduction in cell adhesion was due to the Vroman effect,53 because HKa displaced FBG from the adhesion plate (data not shown), while VN was resistant to the Vroman effect38,39 54 (data not shown). When endothelial cell adhesion was compared with both multimeric VN or native VN, there was hardly any difference between both substrates. HKa but not HK could block cell adhesion to multimeric VN and native VN to the same extent (not shown).
The role of kininogen on integrin-mediated endothelial cell adhesion.
(A) The adhesion of BRECs to VN-, fibronectin (FN)- and FBG-coated wells was analyzed in the absence (−) or in the presence of the competitors HK, HKa, or cRGDfV (10 μg/mL each), 100 nmol/L PAI-1, or 10 mmol/L ethylenediaminetetraacetic acid as indicated. The extent of cell adhesion is presented as absorbance at 590 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in at least 3 separate experiments. (B) The effect of HK, HKa, cRGDfV (10 μg/mL each), or 20 μg/mL anti-VN mAb 13H1 on the adhesion of BREC to VN was studied according to 2 different protocols: (a) Cells and competitors were added simultaneously to the VN-coated wells (filled bars), and (b) VN-coated wells were blocked and then incubated for 1 hour with the different competitors followed by extensive washing and addition of cells (hatched bars). The extent of cell adhesion is presented as absorbance at 590 nm. Data are mean ± SEM (n = 3) of a typical experiment; similar results were obtained in at least 3 separate experiments.
The role of kininogen on integrin-mediated endothelial cell adhesion.
(A) The adhesion of BRECs to VN-, fibronectin (FN)- and FBG-coated wells was analyzed in the absence (−) or in the presence of the competitors HK, HKa, or cRGDfV (10 μg/mL each), 100 nmol/L PAI-1, or 10 mmol/L ethylenediaminetetraacetic acid as indicated. The extent of cell adhesion is presented as absorbance at 590 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in at least 3 separate experiments. (B) The effect of HK, HKa, cRGDfV (10 μg/mL each), or 20 μg/mL anti-VN mAb 13H1 on the adhesion of BREC to VN was studied according to 2 different protocols: (a) Cells and competitors were added simultaneously to the VN-coated wells (filled bars), and (b) VN-coated wells were blocked and then incubated for 1 hour with the different competitors followed by extensive washing and addition of cells (hatched bars). The extent of cell adhesion is presented as absorbance at 590 nm. Data are mean ± SEM (n = 3) of a typical experiment; similar results were obtained in at least 3 separate experiments.
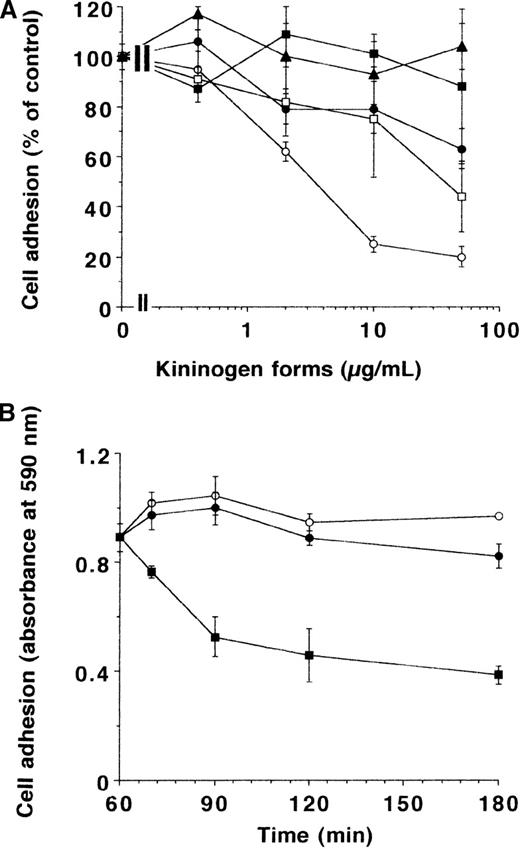
In subsequent experiments, the effect of different forms of kininogen on adhesion of endothelial cells to VN was investigated (Figure2A). HKa was by far the most effective component (inhibitory concentration of 50% = 25 nmol/L) and could completely block cell adhesion at a concentration of 10 μg/mL (about 85 nmol/L). While domain 5 but not 3 could also reproduce most of the HKa effect, higher concentrations of HK were needed to provide a maximal inhibition of 50%. In contrast to cRGDfV, which was able to reverse the adhesion process partially (Figure 2B), HK or HKa did not promote detachment of endothelial cells that had adhered onto the VN substrate for 1 hour. Similar results as with BREC and BAEC were also obtained with MG63 human osteosarcoma cells (not shown).
The effect of different kininogen forms on endothelial cell adhesion to VN.
(A) BRECs were allowed to adhere to VN in the absence or presence of various concentrations of HK (filled circles), HKa (open circles), domain 3 (filled squares), domain 5 (open squares), and domain 6 (filled triangles), and the extent of adhesion is presented as percentage of control. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were observed in at least 3 different experiments. (B) After cells had adhered to VN for 1 hour, HK (open circles), HKa (filled circles), or cRGDfV (filled squares) (10 μg/mL each) was added, and remaining adherent cells after various times were quantitated (presented as absorbance at 590 nm). Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
The effect of different kininogen forms on endothelial cell adhesion to VN.
(A) BRECs were allowed to adhere to VN in the absence or presence of various concentrations of HK (filled circles), HKa (open circles), domain 3 (filled squares), domain 5 (open squares), and domain 6 (filled triangles), and the extent of adhesion is presented as percentage of control. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were observed in at least 3 different experiments. (B) After cells had adhered to VN for 1 hour, HK (open circles), HKa (filled circles), or cRGDfV (filled squares) (10 μg/mL each) was added, and remaining adherent cells after various times were quantitated (presented as absorbance at 590 nm). Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of kininogen to VN
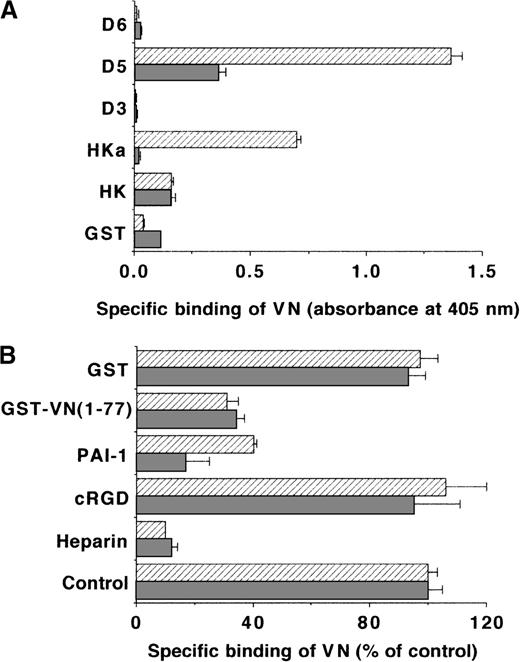
The specificity of the antiadhesive effect of HKa in the αvβ3 integrin–VN interaction can be explained by direct blockade of either the integrin αvβ3 or the substrate VN by HKa. The latter was demonstrated by the fact that preincubation of the VN substrate with HKa but not with HK could inhibit cell adhesion. HKa blocked the binding of 125I-VN to isolated αvβ3 integrin (Table1), whereas HK had minimal inhibitory activity. While HKa at 20 μg/mL (170 nmol/L) reduced specific binding by 60%, PAI-1 at 200 nmol/L diminished binding by 85%; however, these concentrations of HKa and PAI-1 induced a similar inhibition of endothelial cell adhesion to VN. Because both HKa and VN are Zn2+-binding proteins,51 the binding of different concentrations of VN to immobilized HK or HKa was tested in the absence or presence of ZnCl2 in an ELISA for direct ligand-receptor interactions. Zn2+-dependent specific binding of VN to only HKa but not to HK was observed. In addition to HKa, VN could specifically interact with immobilized domain 5 in a Zn2+-dependent manner, whereas no binding to domains 3 or 6 was found (Figure 3A). In the reverse situation, specific binding of soluble HKa and domain 5 but not HK to immobilized multimeric or native VN was noted, where more efficient binding was seen with immobilized multimeric VN (Table 2). Also, multimeric VN bound more efficiently to immobilized HKa or domain 5 than did native VN (Table 3). Heparin almost completely abolished the binding of multimeric VN to HKa or domain 5 but only partially interfered with the binding of native VN (Table 3). Because the interaction between VN and HKa or domain 5 was prevented by PAI-1 or by the fusion product GST-VN (1-77) (Figure 3B), kininogen forms can compete with PAI-1 for an overlapping binding site on VN, which is located within the amino terminal portion of the adhesive protein. In contrast, the cRGDfV had no effect on the kininogen-VN interaction (Figure 3B). No substantial differences in the binding pattern between VN and kininogen were observed regardless whether ELISA (Maxisorp) or cell adhesion plates were used (not shown).
Effect of different competitors on the binding of125I-VN to immobilized vβ3 integrin
| Additives . | Binding of 125I-VN (cpm/well) . | Specific binding (% of control) . |
|---|---|---|
| None | 3311 ± 122 | 100 ± 4 |
| “Cold” VN (nonspecific binding) | 254 ± 18 | — |
| cRGDfV | 763 ± 31 | 17 ± 1 |
| PAI-1 | 621 ± 31 | 12 ± 1 |
| HK | 2883 ± 61 | 86 ± 2 |
| HKa | 1246 ± 31 | 32 ± 1 |
| Additives . | Binding of 125I-VN (cpm/well) . | Specific binding (% of control) . |
|---|---|---|
| None | 3311 ± 122 | 100 ± 4 |
| “Cold” VN (nonspecific binding) | 254 ± 18 | — |
| cRGDfV | 763 ± 31 | 17 ± 1 |
| PAI-1 | 621 ± 31 | 12 ± 1 |
| HK | 2883 ± 61 | 86 ± 2 |
| HKa | 1246 ± 31 | 32 ± 1 |
The binding of 125I-VN to immobilized αvβ3 integrin was carried out in the absence (no additives) or presence of cRGDfV (10 μg/mL), PAI-1 (200 nmol/L), and HK or HKa (170 nmol/L each) in a TBS buffer containing 50 μmol/L ZnCl2. Nonspecific binding was counted in the presence of 100-fold excess “cold” VN. Data are expressed as counts per minute (cpm)/well. Moreover, specific binding was estimated (by subtracting the nonspecific binding from the total), and data are also expressed as percentage of control, which is represented by the specific binding in the absence of additives. Data are mean ± SEM (n = 3) of a typical experiment; similar results were obtained in 3 separate experiments.
Binding of VN to different immobilized kininogen isoforms.
(A) The binding of 2-μg/mL VN to immobilized HK, HKa, GST-D3, GST-D5, GST-D6, or GST (each 5 μg/mL) in the absence (filled bars) or the presence (hatched bars) of 50 μmol/L ZnCl2was carried out, and specific binding is presented as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments. (B) The binding of VN to immobilized HKa (filled bars) or domain 5 (hatched bars) was performed in the absence (−) or presence of 10 μg/mL heparin, 10 μg/mL cRGDfV, 200 nmol/L PAI-1, 10 μg/mL GST-VN (1-77), or 10 μg/mL GST in buffer containing 50 μmol/L ZnCl2. Specific binding of VN is presented as percentage of control (binding of VN to HKa or D5 in the absence of any competitor). Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of VN to different immobilized kininogen isoforms.
(A) The binding of 2-μg/mL VN to immobilized HK, HKa, GST-D3, GST-D5, GST-D6, or GST (each 5 μg/mL) in the absence (filled bars) or the presence (hatched bars) of 50 μmol/L ZnCl2was carried out, and specific binding is presented as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments. (B) The binding of VN to immobilized HKa (filled bars) or domain 5 (hatched bars) was performed in the absence (−) or presence of 10 μg/mL heparin, 10 μg/mL cRGDfV, 200 nmol/L PAI-1, 10 μg/mL GST-VN (1-77), or 10 μg/mL GST in buffer containing 50 μmol/L ZnCl2. Specific binding of VN is presented as percentage of control (binding of VN to HKa or D5 in the absence of any competitor). Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of HK or HKa to immobilized multimeric or native VN
| Additives . | HK binding to . | |
|---|---|---|
| VN multimeric . | VN native . | |
| None | 0.024 ± 0.030 | 0.002 ± 0.001 |
| Heparin | 0.005 ± 0.013 | 0.001 ± 0.001 |
| Zn2+ | 0.025 ± 0.012 | 0.026 ± 0.033 |
| Zn2+ plus heparin | 0.001 ± 0.016 | 0.003 ± 0.007 |
| Additives | HKa binding to | |
| VN multimeric | VN native | |
| None | 0.023 ± 0.011 | 0.041 ± 0.021 |
| Heparin | 0.001 ± 0.030 | 0.008 ± 0.013 |
| Zn2+ | 0.700 ± 0.061 | 0.324 ± 0.025 |
| Zn2+ plus heparin | 0.002 ± 0.005 | 0.149 ± 0.003 |
| Additives . | HK binding to . | |
|---|---|---|
| VN multimeric . | VN native . | |
| None | 0.024 ± 0.030 | 0.002 ± 0.001 |
| Heparin | 0.005 ± 0.013 | 0.001 ± 0.001 |
| Zn2+ | 0.025 ± 0.012 | 0.026 ± 0.033 |
| Zn2+ plus heparin | 0.001 ± 0.016 | 0.003 ± 0.007 |
| Additives | HKa binding to | |
| VN multimeric | VN native | |
| None | 0.023 ± 0.011 | 0.041 ± 0.021 |
| Heparin | 0.001 ± 0.030 | 0.008 ± 0.013 |
| Zn2+ | 0.700 ± 0.061 | 0.324 ± 0.025 |
| Zn2+ plus heparin | 0.002 ± 0.005 | 0.149 ± 0.003 |
The binding of 2 μg/mL HK or HKa to immobilized multimeric or native VN (each 5 μg/mL) is shown. Binding was studied in the absence or presence of Zn2+ (50 μmol/L) and heparin (10 μg/mL). Binding of HK to BSA in the absence or presence of Zn2+was 0.074 ± 0.001 and 0.182 ± 0.031, respectively. Binding of HKa to BSA in the absence or presence of Zn2+was 0.106 ± 0.026 and 0.194 ± 0.031, respectively. This nonspecific binding was subtracted to estimate specific binding. Binding is expressed as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of multimeric or native VN to immobilized kininogen
| Additives . | Multimeric VN binding to . | ||
|---|---|---|---|
| HK . | HKa . | D5 . | |
| None | 0.058 ± 0.007 | 0.087 ± 0.006 | 0.238 ± 0.001 |
| Heparin | 0.033 ± 0.001 | 0.001 ± 0.002 | 0.002 ± 0.001 |
| Zn2+ | 0.075 ± 0.002 | 0.701 ± 0.067 | 0.935 ± 0.088 |
| Zn2+ plus heparin | 0.066 ± 0.002 | 0.021 ± 0.007 | 0.003 ± 0.009 |
| Additives | Native VN binding to | ||
| HK | HKa | D5 | |
| None | 0.046 ± 0.006 | 0.082 ± 0.005 | 0.149 ± 0.004 |
| Heparin | 0.047 ± 0.004 | 0.018 ± 0.012 | 0.057 ± 0.004 |
| Zn2+ | 0.065 ± 0.022 | 0.349 ± 0.001 | 0.308 ± 0.021 |
| Zn2+ plus heparin | 0.074 ± 0.019 | 0.204 ± 0.010 | 0.181 ± 0.012 |
| Additives . | Multimeric VN binding to . | ||
|---|---|---|---|
| HK . | HKa . | D5 . | |
| None | 0.058 ± 0.007 | 0.087 ± 0.006 | 0.238 ± 0.001 |
| Heparin | 0.033 ± 0.001 | 0.001 ± 0.002 | 0.002 ± 0.001 |
| Zn2+ | 0.075 ± 0.002 | 0.701 ± 0.067 | 0.935 ± 0.088 |
| Zn2+ plus heparin | 0.066 ± 0.002 | 0.021 ± 0.007 | 0.003 ± 0.009 |
| Additives | Native VN binding to | ||
| HK | HKa | D5 | |
| None | 0.046 ± 0.006 | 0.082 ± 0.005 | 0.149 ± 0.004 |
| Heparin | 0.047 ± 0.004 | 0.018 ± 0.012 | 0.057 ± 0.004 |
| Zn2+ | 0.065 ± 0.022 | 0.349 ± 0.001 | 0.308 ± 0.021 |
| Zn2+ plus heparin | 0.074 ± 0.019 | 0.204 ± 0.010 | 0.181 ± 0.012 |
The binding of 2 μg/mL multimeric or native VN to immobilized HKa or D5 (each 5 μg/mL) is shown. Binding was studied in the absence or presence of Zn2+ (50 μmol/L) and heparin (10 μg/mL). Binding of multimeric VN to BSA in the absence or presence of Zn2+ was 0.163 ± 0.014 and 0.132 ± 0.006, respectively. Binding of native VN to BSA in the absence or presence of Zn2+ was 0.143 ± 0.005 and 0.142 ± 0.003, respectively. This nonspecific binding was subtracted to estimate specific binding. Binding is expressed as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Finally, no direct binding of HKa or HK to αvβ3integrin was observed (not shown), indicating that the interaction between HKa and VN prevented its ligation to the αvβ3integrin. This observation is consistent with the failure of a blocking antibody (7E3) to αvβ3 integrin or of FBG to inhibit the binding of HK/HKa to endothelial cells.20
Inhibition of leukocyte adhesion to VN by kininogen
As previously established, the adhesion of myelomonocytic U937 cells (differentiated with transforming growth factor-β [2 ng/mL] and vitamin D3 [100 nmol/L] for 24 hours) to immobilized VN is predominantly mediated by uPAR.18,55 Moreover, uPA augments this adhesion by increasing the affinity of the uPAR-VN interaction.19 The same characteristics were found for uPAR-transfected BAF-3 (sense-uPAR) but not for control cells (antisense-uPAR). The addition of HK or HKa resulted in a complete inhibition of adhesion of uPAR-transfected BAF-3 cells and U937 cells also in the presence of uPA, reminiscent of the blocking effect of PAI-1 in this system.16 An identical pattern of inhibition with HKa, HK, or PAI-1 was obtained for the adhesion of human peripheral blood monocytes (data not shown). HKa but not HK was also antiadhesive when these substances were preincubated onto the VN substrate prior to the cell adhesion step (data not shown).
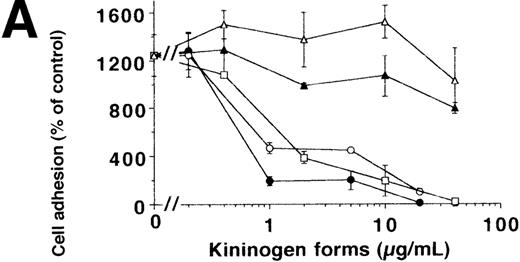
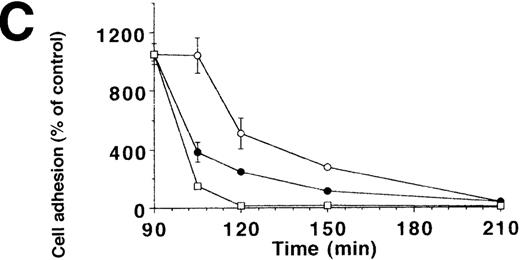
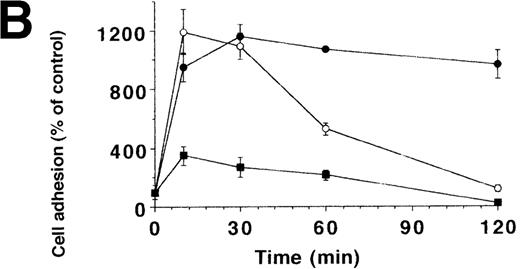
To characterize the involved domains of HKa responsible for antiadhesion, uPA-stimulated adhesion of U937 cells was tested in the presence of increasing concentrations of HK, HKa, and the recombinant GST-fusion domains 3, 5, or 6, respectively (Figure4A). HKa, HK, and domain 5 but not domain 3 or 6 abolished cell adhesion, indicating that domain 5 contains most of the antiadhesive activity also for the uPAR-dependent system. To differentiate between the varying antiadhesive activities of HK and HKa, the kinetics of cell adhesion in the presence of both kininogen forms was studied. When cells were plated in the presence of uPA and HK or HKa, respectively, HKa could immediately block the augmenting effect of uPA on cell adhesion, whereas only after 1 hour could HK significantly reduce adhesion (Figure 4B). In contrast to the αv integrin–dependent endothelial cell adhesion, HKa, similar to PAI-1, promoted the immediate detachment of U937 cells that had adhered to VN for 1.5 hours under the influence of uPA (Figure 4C), whereas about 20 minutes of lag-phase was needed for HK-induced cell dissociation. These findings indicate that HKa was directly interfering with the uPAR-VN interaction, whereas HK needed proteolytic activation by cell-derived proteases as deduced from Western blot analysis (Figure 4D).
Inhibition of uPA-induced cell adhesion by kininogen.
(A) uPA-induced U937 cell adhesion to VN was studied in the absence or presence of various concentrations of HK (open circles), HKa (filled circles), isolated domain 3 (filled triangles), domain 5 (open squares), or domain 6 (open triangles). (B) On a VN substrate, seeding of U937 cells together with uPA was performed in the absence (filled circles) and in the presence of 10 μg/mL HK (open circles) or 10 μg/mL HKa (filled squares), and the extent of cell adhesion was analyzed after various times as indicated. (C) After allowing the adhesion of U937 cells to VN in the presence of 50 nmol/L uPA for an incubation period of 90 minutes, 10 μg/mL HK (open circles), 10 μg/mL HKa (filled circles), or 100 nmol/L PAI-1 (open squares), respectively, was added, and the residual extent of cell adhesion was measured. Data are expressed as percentage of control, which is represented by the adhesion of cells in the absence of any stimulus or competitor. Data are mean ± SEM (n = 3) of a typical experiment; similar results were obtained in 3 separate experiments. (D) An adhesion assay with U937 myelomonocytic cells was performed in the absence (−) or presence of HK or HKa. After the incubation period for the adhesion assay (90 minutes), the supernatant was collected, centrifuged, and analyzed in a Western blot with the antibody HKH18, which is directed against domain 1 of kininogen and detects both HK and HKa. As a control, parallel wells without cells were incubated with the adhesion medium and HK or HKa. Molecular weight markers are indicated at the right margin. Similar results were observed in 3 separate experiments.
Inhibition of uPA-induced cell adhesion by kininogen.
(A) uPA-induced U937 cell adhesion to VN was studied in the absence or presence of various concentrations of HK (open circles), HKa (filled circles), isolated domain 3 (filled triangles), domain 5 (open squares), or domain 6 (open triangles). (B) On a VN substrate, seeding of U937 cells together with uPA was performed in the absence (filled circles) and in the presence of 10 μg/mL HK (open circles) or 10 μg/mL HKa (filled squares), and the extent of cell adhesion was analyzed after various times as indicated. (C) After allowing the adhesion of U937 cells to VN in the presence of 50 nmol/L uPA for an incubation period of 90 minutes, 10 μg/mL HK (open circles), 10 μg/mL HKa (filled circles), or 100 nmol/L PAI-1 (open squares), respectively, was added, and the residual extent of cell adhesion was measured. Data are expressed as percentage of control, which is represented by the adhesion of cells in the absence of any stimulus or competitor. Data are mean ± SEM (n = 3) of a typical experiment; similar results were obtained in 3 separate experiments. (D) An adhesion assay with U937 myelomonocytic cells was performed in the absence (−) or presence of HK or HKa. After the incubation period for the adhesion assay (90 minutes), the supernatant was collected, centrifuged, and analyzed in a Western blot with the antibody HKH18, which is directed against domain 1 of kininogen and detects both HK and HKa. As a control, parallel wells without cells were incubated with the adhesion medium and HK or HKa. Molecular weight markers are indicated at the right margin. Similar results were observed in 3 separate experiments.
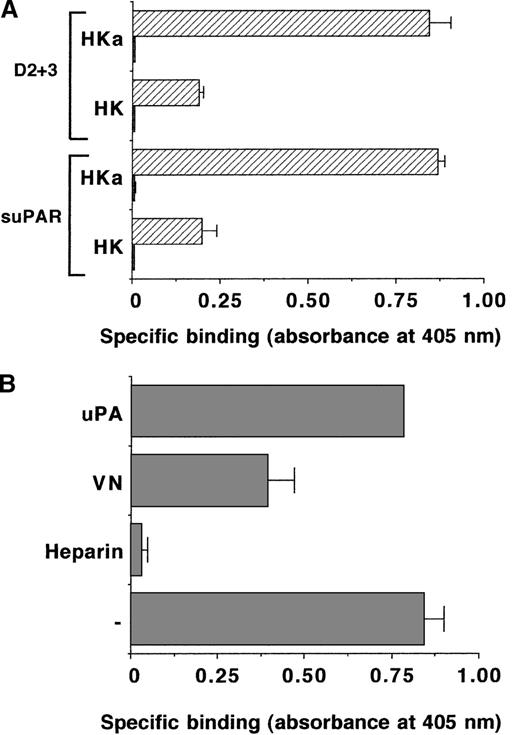
Binding of kininogen to uPAR
Subsequent experiments revealed that HKa and the isolated domain 5 inhibited the binding of 125I-VN to immobilized uPAR in a Zn2+-dependent manner, whereas HK, domain 3, or domain 6 were ineffective (Figure 5). In the absence of Zn2+, HKa and domain 5 presented only a weak inhibition of the uPAR-VN interaction (data not shown). This property of kininogen could be explained by the direct binding of HKa and the domain 5 to VN as described above. Because uPAR has been identified as a binding site on endothelial cells for HKa but not for HK,20 direct binding of HKa to suPAR was tested. As shown in Figure 6A, HKa but not HK bound specifically to immobilized suPAR in a Zn2+-dependent fashion. Moreover, HKa also interacted with the truncated 2-domain form of uPAR that lacks domain 1. The HKa-uPAR interaction was blocked by VN and also heparin, whereas uPA had no inhibitory effect (Figure 6B). The binding of HKa to uPAR could be attributed to domain 5, because the recombinant GST-D5 but not GST-D3 or GST-D6 inhibited the interaction (data not shown).
The effect of different kininogen forms on the binding of 125I-VN to immobilized uPAR.
The binding of 125I-VN to immobilized uPAR together with 20 nmol/L uPA and 50 μmol/L ZnCl2 was studied in the absence or presence of HK (open circles), HKa (filled circles), domain 3 (open squares), domain 5 (filled squares), or domain 6 (open triangles). Specific binding is expressed as percentage of control, which represents the binding of 125I-VN to uPAR in the absence of uPA. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
The effect of different kininogen forms on the binding of 125I-VN to immobilized uPAR.
The binding of 125I-VN to immobilized uPAR together with 20 nmol/L uPA and 50 μmol/L ZnCl2 was studied in the absence or presence of HK (open circles), HKa (filled circles), domain 3 (open squares), domain 5 (filled squares), or domain 6 (open triangles). Specific binding is expressed as percentage of control, which represents the binding of 125I-VN to uPAR in the absence of uPA. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of HK or HKa to immobilized uPAR.
(A) The binding of HK or HKa (each 2 μg/mL) to immobilized suPAR or the truncated 2-domain uPAR (D2 + 3) (each 5 μg/mL) was studied in the absence (filled bars) or presence (hatched bars) of 50 μmol/L ZnCl2. Specific binding is shown as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments. (B) The binding of 2 μg/mL HKa together with 50 μmol/L ZnCl2 to immobilized suPAR was studied in the absence (−) or presence of 10 μg/mL heparin, 20 μg/mL VN, or 50 nmol/L uPA. Specific binding is expressed as absorbance at 405 nm. Data represent mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Binding of HK or HKa to immobilized uPAR.
(A) The binding of HK or HKa (each 2 μg/mL) to immobilized suPAR or the truncated 2-domain uPAR (D2 + 3) (each 5 μg/mL) was studied in the absence (filled bars) or presence (hatched bars) of 50 μmol/L ZnCl2. Specific binding is shown as absorbance at 405 nm. Data are mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments. (B) The binding of 2 μg/mL HKa together with 50 μmol/L ZnCl2 to immobilized suPAR was studied in the absence (−) or presence of 10 μg/mL heparin, 20 μg/mL VN, or 50 nmol/L uPA. Specific binding is expressed as absorbance at 405 nm. Data represent mean ± SEM (n = 3) of a typical experiment, and similar results were obtained in 3 separate experiments.
Colocalization of VN and HK in sections of human atherosclerotic plaques
Consequences for the above-mentioned molecular relations in (patho)physiology depend on the availability of the different components at sites of tissue remodeling. As an example, the colocalization of VN with PAI-1 or uPAR could be demonstrated in diseased vessel sections (Lupu et al and Chavakis et al, unpublished observations), implying direct functional interactions. Double-labeling immunogold electron microscopy was performed in sections from human atherosclerotic coronary arteries obtained from cardiac surgery. VN was localized both bound to plasma membranes of cells, particularly smooth muscle cells, as well as associated with different fibrillar or amorphous structures of the extracellular matrix. HK was found in similar locations, and frequently VN and HK were localized in close proximity or direct apposition (Figure7). These data indicate that the above-described close molecular interaction could also take place in vivo.
Localization of vitronectin and kininogen in human advanced atherosclerotic lesions by double-labeling immunogold electron microscopy.
Vitronectin (10 nm gold particles, arrowheads) is detected both bound to plasma membranes of smooth muscle cells (SMC) (A and B) as well as in association with different fibrillar or amorphous extracellular matrix structures (ECM) (A, B, and C). Kininogen, labeled by 15 nm gold particles (arrows), was found in similar locations. Frequently, the 2 proteins were localized in close proximity, occasionally in direct apposition, indicating close molecular interaction (arrows and arrowheads); bars = 0.5 μm.
Localization of vitronectin and kininogen in human advanced atherosclerotic lesions by double-labeling immunogold electron microscopy.
Vitronectin (10 nm gold particles, arrowheads) is detected both bound to plasma membranes of smooth muscle cells (SMC) (A and B) as well as in association with different fibrillar or amorphous extracellular matrix structures (ECM) (A, B, and C). Kininogen, labeled by 15 nm gold particles (arrows), was found in similar locations. Frequently, the 2 proteins were localized in close proximity, occasionally in direct apposition, indicating close molecular interaction (arrows and arrowheads); bars = 0.5 μm.
Discussion
Apart from its role as precursor of bradykinin that contributes important vasodilator functions in vascular homeostasis, high molecular weight kininogen exhibits antiadhesive properties38,39,55especially in the 2-chain, kinin-free form (HKa). In the present study, we continued to define the underlying mechanisms of the antiadhesive function of HKa. The characterization of the binding interactions to different adhesion receptors as well as to VN-rich matrices revealed that HKa is responsible for the disruption of uPAR and αvβ3 integrin–dependent cellular contacts. HKa was shown to directly bind to VN and uPAR, to abrogate endothelial and leukocytic cell adhesion, and thereby could play a regulatory role in tissue remodeling of the vasculature. Binding of HKa to VN was blocked by recombinant uPAR and heparin as well as by active PAI-1 but not by cRGDfV, indicating that HKa does not interfere with the cell attachment sequence of VN but competes with PAI-1 or uPAR for binding to the amino terminal “somatomedin B” domain of VN, which is proximal to the integrin recognition motif.56 This hypothesis is also strengthened by the fact that the kininogen-VN interaction was blocked by a GST-fusion peptide consisting of the amino terminal portion of VN (amino acids 1-77). The antiadhesive function of HKa is particularly expressed in the His-Gly-Lys–rich domain 5, which contains the major cell binding domain and resembles the activity of PAI-1 as a prominent counter-adhesive factor in VN-dependent cell adhesion mediated by both integrins and uPAR.
Our observations further define the requirements for this function of HKa, conferred by its domain 5. HKa or domain 5 but not domains 3 or 6 inhibited the αvβ3 integrin–dependent adhesion of endothelial cells to VN but did not promote detachment of cells that had already adhered to VN. HK/HKa did not block endothelial cell adhesion to fibronectin and, in contrast to Asakura and coworkers,38 we could not demonstrate HKa-dependent blockade of adhesion of endothelial cells to FBG, because HKa did not directly bind to the αvβ3 integrin or this integrin ligand (data not shown). However, HKa preincubated onto FBG-coated surfaces prior to the adhesion step resulted in partial inhibition of cell adhesion. This was due to the Vroman effect53—namely, the displacement of FBG from the plate by HKa. This effect did not take place when the surface was coated with VN, which is in accordance with the known “resistance” of VN to the Vroman effect.54In addition, HKa or domain 5 completely abolished the uPAR-dependent adhesion to VN of differentiated U937 myelomonocytic cells as well as of uPAR-transfected BAF-3 cells. As a consequence and in contrast to the effect on integrin-mediated adhesion with endothelial cells, HKa promoted the detachment of adherent U937 cells, because HKa competed with both uPAR and the substrate VN. Thus, the inhibitory effect of HKa appears to be specific for VN-rich tissue matrices such as in provisional wounds and can be attributed to direct blockade of the substrate and of uPAR.
Instantaneously, HKa was antiadhesive when added together with the cells or when preincubated onto the VN substrate, whereas HK, which does not bind VN, was antiadhesive after a lag-phase only in the first case. These observations can be best explained by the fact that HK is converted to HKa by proteases in the cell cultures to become a potent antiadhesive factor, and data supporting this hypothesis were presented. Cell surface–dependent conversion of HK to HKa was recently described,57 implying that HK itself appears to be required for prekallikrein activation and thereby promotes its own conversion into HKa.
The consequences of the antiadhesive properties of kininogen are several. After tissue damage, leukocytes are recruited to the injured site, and neutrophil and monocyte adhesion to a provisional matrix is pivotal for this process. In the inflamed or injured tissue, the release of bradykinin from HK is enhanced, which also leads to a localized infiltration of additional inflammatory cells. Moreover, through inhibition of αv integrin–dependent adhesion, HKa could contribute to angiogenesis-regulating activities, because the αvβ3 integrin–VN interaction appears to be crucial during neovascularization in different tissues.58 Peptides derived from kininogen's domain 5 inhibit new vessel formation in the chicken chorioallantoic membrane,59 and work is in progress to define the role of HKa or fragments thereof in angiogenic processes in malignancies or in diabetic retinopathy.
The binding of HKa to both uPAR and VN on the cell surface may approximate kallikrein and prourokinase, thereby leading to potent plasmin formation. In addition, HKa binds directly to plasminogen,60 which could further amplify plasmin generation. Indeed, we observed colocalization of VN and kininogen at sites of tissue remodeling such as in the vasculature and, by immunogold electron microscopy, HK/VN-colocalization was demonstrated in human atherosclerotic coronary arteries. These data indicate that the described interactions between kininogen and VN may take place in vivo. Depending on its local concentration, however, HKa could also interfere with the assembly of the ternary uPA/uPAR/VN complex on the surface of vascular cells or the extracellular matrix, which augments plasmin generation.61 These interactions not only define HKa as an intrinsic regulator of the fibrinolytic system but, through its functions as antiadhesive component, HKa also may play a regulatory role in angiogenesis, atherogenesis, or cancer metastasis.
Prior to the findings that HKa binds to VN and uPAR, other kininogen binding proteins were identified that include cellular receptors such as Mac-1 on neutrophils33 or glycoprotein Ib on unactivated platelets62 as well as cell-associated thrombospondin on activated platelets.63 Moreover, cytokeratin-134 and gC1qR were identified as binding proteins for HK on endothelial cells,35,36 the latter also serving as binding protein for multimeric VN.64Interestingly, VN is found on the surface of several other cell types when cultivated in serum and can possibly account for an appreciable degree of binding sites for kininogen and other cellular ligands.20 In particular, specific binding of both VN and kininogen tp various bacterial strains could play a role in the infection process and the host response toward bacterial entry.65 66
Finally, recent studies with VN knockout mice67 and with kininogen-deficient rats68 indicated a similar phenotype in both cases that was more sensitive to thrombosis. Thus, the proposed antithrombotic properties of HK and its fragments could be mediated at least in part by interactions with VN, because loss of VN in mice is expected to influence the functional status of its ligands such as PAI-1 and kininogen as well. Because several ligands of VN exhibit opposing functional activities in hemostasis and cell adhesion, a detailed dissection of transgene animal models can lead to further information on the pathophysiologic role of this multifunctional adhesive protein.
Acknowledgments
The excellent technical assistance of U. Schubert and T. Schmidt is gratefully acknowledged. We appreciate the contribution of Dan Johnson and Dr Yen Lin in the preparation and characterization of the recombinant HK domains and of Thomas Renné for providing monoclonal and polyclonal antibodies to HK. We also acknowledge the generous gift of reagents from Drs H. Roger Lijnen, Gunilla Hoyer-Hansen, and Niels Behrendt.
This work is part of the MD/PhD thesis of T.C. at the Institute for Biochemistry, Medical Faculty, Justus-Liebig-University, Giessen, Germany. Supported in part by a grant (Pr 327/1-4) from the Deutsche Forschungsgemeinschaft (Bonn, Germany) to K.T.P. and by National Institutes of Health grants HL56914 and CA63938 to R.W.C.
Reprints:Klaus T. Preissner, Institut für Biochemie, Fachbereich Humanmedizin, Justus-Liebig-Universität, Friedrichstrasse 24, D-35392 Giessen, Germany; e-mail:klaus.t.preissner@biochemie.med.uni-giessen.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal