Abstract
Vascular endothelial growth factor (VEGF) plays a major role in tumor angiogenesis. VEGF-C, however, is thought to stimulate the growth of lymphatic vessels because an expression of its specific receptor, VEGF receptor-3 (VEGFR-3), was demonstrated to be restricted to lymphatic vessels. Here we demonstrate that the inactivation of VEGFR-3 by a novel blocking monoclonal antibody (mAb) suppresses tumor growth by inhibiting the neo-angiogenesis of tumor-bearing tissues. Although VEGFR-3 is not expressed in adult blood vessels, it is induced in vascular endothelial cells of the tumor-bearing tissues. Hence, VEGFR-3 is another receptor tyrosine kinase involved in tumor-induced angiogenesis. Micro-hemorrhage in the tumor-bearing tissue was the most conspicuous histologic finding specific to AFL4 mAb-treated mice. Scanning microscopy demonstrated disruptions of the endothelial lining of the postcapillary venule, probably the cause of micro-hemorrhage and the subsequent collapse of the proximal vessels. These findings suggest the involvement of VEGFR-3 in maintaining the integrity of the endothelial lining during angiogenesis. Moreover, our results suggest that the VEGF-C/VEGFR-3 pathway may serve another candidate target for cancer therapy.
Angiogenesis is essential for tumor progression because it allows oxygenation and nutrient perfusion of the tumor. In the absence of neovascularization, a solid tumor cannot form a large mass.1-3 Angiogenesis is a complex multistep process by which blood vessels are formed from the preexisting vasculature. Previous studies of null mutant mice have demonstrated that development of the embryonic vascular system requires the coordinated expression of various receptor tyrosine kinases (RTKs) and their ligands.4 Among these ligands, vascular endothelial growth factor (VEGF) has been shown to play a major role throughout angiogenesis. VEGF action is mediated by 2 RTKs, VEGFR-1 (FLT-1) and VEGFR-2 (FLK-1/KDR), expressed primarily by endothelial cells (ECs). VEGF regulates multiple EC activities such as proliferation, migration, tube formation, and permeability.5-8 Some molecules, such as angiopoietins (Ang1 and Ang2)/Tie2, are implicated in the later stages of vascular development—ie, during vascular remodeling and maturation.9-11 Based on studies using reagents that neutralize each ligand, it has been suggested that those RTKs involved in the embryonic process are also involved in tumor angiogenesis.12-16
Recently, VEGFR-3 was identified as an endothelial-specific RTK related to VEGF receptors.17 VEGFR-3 is induced in all endothelial cells during early embryogenesis, though its expression eventually disappears from the vascular ECs of adult tissues.18 In contrast to its transient expression in vascular ECs, VEGFR-3 is expressed constitutively by the adult lymphatic endothelium.19 VEGF-C, a new member of the platelet-derived growth factor (PDGF)/VEGF family, was first identified as a ligand for VEGFR-3.20 Among several distinct forms of VEGF-C generated by stepwise proteolysis, the maturely processed VEGF-C could also activate VEGFR-2.21 Moreover, another ligand, named VEGF-D22 for VEGFR-3, has been identified recently, illustrating the complex ligand–receptor relationship, which poses a problem for understanding the role of VEGFR-3 during vasculogenesis.
Transgenic overexpression of VEGF-C in the skin has been shown to induce hyperplasia of the lymphatic vasculature (lymph-angiogenesis), leaving the vascular structure unaffected. This observation implies a lymphatic-specific role for VEGF-C/VEGFR-3.23,24 Another report using the cornea assay,25 however, demonstrated that VEGF-C could induce angiogenesis of adult tissues. Mice bearing a null mutation of the VEGFR-3 gene display defects in vascular remodeling, indicating a role for VEGFR-3 in angiogenesis.26 Nonetheless, because VEGFR-3 is expressed by vascular ECs in the embryo but not the adult, the role of VEGFR-3 in adult mice is yet to be determined. We generated an antagonistic monoclonal antibody (mAb) against VEGFR-3 to elucidate the role of VEGFR-3 in tumor angiogenesis. We show that VEGFR-3 is indeed involved in tumor angiogenesis and is essential for maintaining the integrity of the endothelial sheet.
Materials and methods
Mice
Six- to 8-week-old nude (nu/nu) mice were purchased from SLC (Shizuoka, Japan). VEGFR-3 null mutant embryos26 were maintained and mated in our animal facility.
Cell culture
The C6 rat glioblastoma cell line (a gift from Dr H. Kataoka, Kyoto University, Kyoto, Japan), the F-2 murine endothelial cell line27 (a gift from Dr K-I Toda, Kyoto University, Kyoto, Japan), and the 293 human embryonic kidney cell line (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's modified Eagle's medium (DMEM; GIBCO/BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) (HyClone Laboratories, Logan, UT). PC-3 human prostate adenocarcinoma cell line (a gift from Dr N. Itoh, Kyoto University, Kyoto, Japan) were maintained in RPMI 1640 medium (GIBCO/BRL) containing 10% FCS.
Gene transfection
For the preparation of Fc chimeric proteins, cells from the 293 cell line were plated on a 100-mm tissue culture dish to reach 70% confluence the next day and were transfected with 15 μg pCDM8-VEGFR-3-Fc (described below), pCDM8-VEGFR-1-Fc,28 or pCDM8-VEGFR-2-Fc29 plasmid DNA mixed with 25 μg Trans-IT (Mirus, Madison, WI) according to the manufacturer's instructions. Transfected cells were grown in DMEM/F12 medium, and culture supernatants were harvested 5 days after transfection. Fusion protein was purified using protein G–Sepharose columns (Pharmacia Biotech, Uppsala, Sweden).
Plasmid vector containing murine VEGFR-3 cDNA (P2B1S neo/mFLT417) was kindly provided by Dr W. I. Wood (Genentech, Cambridge, MA). cDNA containing full-length VEGFR-3 withNot I and Sal I restriction sites at the 5′ and 3′ ends, respectively, was obtained from P2R1S neo/mFLT4 and subcloned into the expression vector pCDNA3.
Generation of anti-VEGFR-3 monoclonal antibody
A 2.3-kb fragment of murine VEGFR-3 cDNA (positions 45-2354 in the GeneBank L07296), encoding the extracellular domain of VEGFR-3, was subcloned into the expression vector pCDM8-hIgG.29 Rat monoclonal antibodies against VEGFR-3-Fc protein were produced using standard methods as described.29 In brief, an 8-week-old Wistar rat was first immunized subcutaneously with 500 μg VEGFR-3-Fc protein in complete Freund's adjuvant (Difco, Detroit, MI) and then was administered 3 intraperitoneal shots of 250 μg VEGFR-3-Fc protein in Freund's incomplete adjuvant (Difco) in alternating weeks and finally was given an intravenous boost of 100 μg VEGFR-3-Fc protein. Three days after the boost, the spleen cells were harvested and fused with the murine myeloma X63Ag8. Undiluted supernatants from hybridoma were screened by enzyme-linked immunosorbent assay (ELISA) plates coated with 50 ng/mL VEGFR-3-Fc; VEGFR-2-Fc29 and VEGFR-1-Fc were used as controls.28 Positive hybridomas were cloned by the limiting dilution technique and were subcloned twice.
VEGF-C/VEGFR-3 binding inhibition assay by ELISA
The N-terminal signal sequence of mouse stem cell factor (MMU44725; 198-279) and the 5 repeated myc-tag sequences were inserted to a 5′ end of the cDNA fragment corresponding to the mature VEGF-C (U43142; 705-1052),21 which was generated by reverse transcription–polymerase chain reaction amplification of mRNA prepared from PC-3 cells (5-myc-VEGF-C). For the binding inhibition study, ELISA plates coated with 50 ng/mL VEGFR-3-Fc protein were first incubated with various dilutions of mAbs and then with the conditioned medium (CM) of 293 cells transfected by the 5-myc-VEGF-C gene. Binding with 5-myc-VEGF-C was detected by the anti-myc tag antibody (9E10) (Santa Cruz Biotechnology, Santa Cruz, CA) and then by horseradish peroxidase (HRP)-conjugated antimouse IgG (Zymed, San Francisco, CA). Plate-bound enzymic activity was detected by using 3′,3′,5′,5′-tetramethylbenzidine (Chemo-Sero Therapeutic Research Institute, Kumamoto, Japan), and absorbance of each well was measured using the ELISA reader.
Tumor transplantation
Six- to 8-week-old nude (nu/nu) mice (SLC, Shizuoka, Japan) underwent subcutaneous transplantation of 2 × 106 C6 rat glioblastoma cells or PC-3 prostate cancer cells in 0.1 mL phosphate-buffered saline (PBS) on the right flank. Subcutaneous injections of mAbs were given on the left flank of mice. Tumor size was measured in 2 dimensions, and the volume was calculated using the formula, width2 × length/2. After 14 days, all mice were humanely killed and autopsied.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde in PBS overnight, embedded in paraffin and sectioned at 5 to 7 μm. The sectioned specimens were incubated first in bleaching solution (methanol, 0.2% NaN3; 0.6% H2O2) for 30 minutes at room temperature to block endogenous peroxidase. After rehydration, the sections were blocked by incubation with 1% bovine serum albumin in PBS–0.1% Tween 20 (PBS-T) for 20 minutes at room temperature and then incubated overnight with respective primary antibodies: rat anti-VEGFR-3 mAb, AFL4 (20 μg/mL); rat anti-VEGFR-2 mAb, AVAS1229 (10 μg/mL); and rat mAb for murine PECAM-1 (5 μg/mL, Mec13.3; PharMingen, San Diego, CA). After they were washed 3 times in PBS-T for 10 minutes each at room temperature, the sections were incubated with 1 μg/mL HRP-conjugated anti-rat IgG(H+L) (Biosource, Camarillo, CA) for 1 hour at room temperature. After washing with 3 exchanges of PBS-T, the enzymatic reaction with enhanced DAB substrate kit (TSA–Indirect; NEN Life Science Products, Boston, MA) was allowed to proceed until the desired color intensity was reached. For immunohistochemical analysis of AFL4-treated mice, tissues were incubated with a biotinylated anti-PECAM-1 antibody (1/100) as a primary antibody, and HRP-conjugated streptavidin (Zymed) (1/1000) was used as a developing reagent.
The densities of PECAM-1+ and VEGFR-3+ vessels were calculated according to the method described by Gasparini and Harris.30 A minimum of 5 fields (×200) was counted per slide.
Whole-mount immunostaining was performed according to the protocol previously described.31 In some experiments, stained whole-mount specimens were embedded in polyester wax (BDH, Poole, UK) and sectioned.
Scanning electron microscopy (SEM)
Tumor masses with surrounding tissues were dissected carefully and fixed with 3% glutaraldehyde in 0.1 mol/L phosphate buffer (pH 7.4). The method of SEM observation was as previously reported.32In brief, the specimens were postfixed with OsO4 and were hydrolyzed with 8N HCl for 25-60 minutes at 60°C. After a brief rinse, the specimens were dehydrated through a graded series of ethanol, immersed in isoamyl acetate, critical-point dried, coated with platinum, and observed with an SEM (Hitachi S-800, Tokyo, Japan).
Western blot analysis
Western blot analysis was performed as described.29 The filter was probed with 2 μg/mL anti-VEGFR-3 mAb or a control mAb, followed by treatment with HRP-coupled goat anti-rat IgG, and visualized using the enhanced chemiluminescence reagent (NEN Life Science Products).
Phosphorylation assay
F2 cells were grown to subconfluence in DMEM supplemented with 10% FCS. The medium was removed and replaced with fresh DMEM containing 10% FCS with or without antibodies (AFL4, rat IgG fraction 50 μg/mL) for 15 minutes; this was followed by stimulation with one-fifth diluted VEGF-C CM. Fifteen minutes after VEGF-C stimulation, the cells were lysed in lysis buffer (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% NP-40, 0.2 mmol/L PMSF, 0.5 mmol/L sodium vanadate, 2 mmol/L sodium fluoride). VEGFR-3 was immunoprecipitated from cell lysates by protein G–Sepharose after incubation with the anti-VEGFR-3 mAb. Proteins were resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis under nonreducing conditions and were probed with HRP-conjugated anti-phosphotyrosine mAb (PY20; Transduction Laboratories, Lexington, KY). Filters were reprobed with anti-VEGFR-3 mAb to measure the amount of protein loaded on each lane.
Results
Production of an antagonistic anti-mouse VEGFR-3 mAb
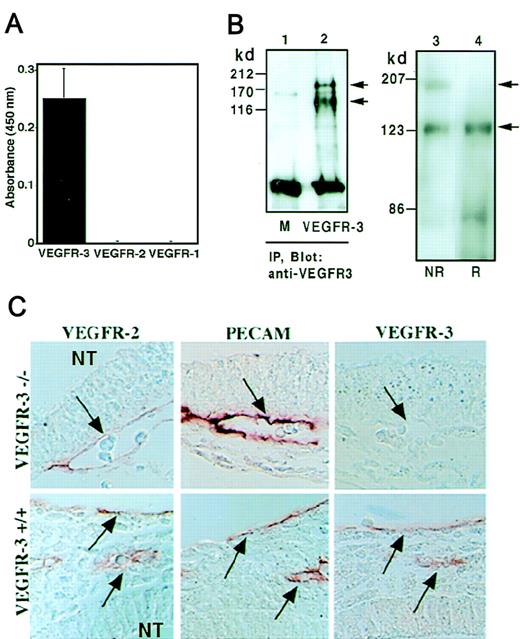
After screening more than 500 clones, 1 clone, AFL4 (IgG2ak), was isolated as reacting specifically to VEGFR-3-Fc but not to VEGFR-1-Fc or VEGFR-2-Fc (Figure 1A). Two polypeptides were precipitated and immunodetected with AFL4 in cell lysates of VEGFR-3 gene-transfected 293 cells but not from mock-transfected cells (Figure 1B; lanes 1, 2). Through immunoblot analysis of total extracts of the F-2 endothelial cell line,27 2 bands of 195- and 125-kd were detected with AFL4 (Figure 1B; lane 3) under nonreducing condition, whereas only the 125-kd band was observed under reducing conditions (Figure 1B; lane 4). These results are consistent with the previous observation that a 175-kd precursor of VEGFR-3 matures to a 195-kd form, which is then proteolytically cleaved into the 125-kd and 75-kd fragments,33 each linked by disulfide bonds.
Specificity of AFL4. (A) AFL-4 binding to VEGFR-1-Fc, VEGFR-2-Fc, or VEGFR-3-Fc was analyzed by ELISA. The absorbance at 450 nm is depicted. Each bar represents the mean ± SEM of triplicate assays. (B) Cell lysates from the 293 cell line transiently transfected with murine VEGFR-3 DNA (lane 2) or mock transfected (lane 1, M) were precipitated with AFL4 and immunoblotted with the same antibody. (IP, immunoprecipitation.) Two bands of 195- and 125-kd proteins were detected in lane 2 under reducing conditions. Through immunoblotting of the cell lysates from the murine EC line F2 with anti-VEGFR-3 mAb, 2 bands were detected in nonreducing conditions (NR), whereas only a 125-kd band was seen under reducing conditions (R) (lanes 3, 4). Arrows denote the positions of the unprocessed 195-kd form and the proteolytically processed 125-kd form of VEGFR-3. (C) Specificity of AFL4 in tissue sections. Sagittal sections were prepared from embryonic day 9.5 VEGFR-3−/− or VEGFR-3+/+ embryos and stained with AFL4, anti-PECAM-1 mAb, and anti-VEGFR-2 mAb. Arrows indicate blood vessels in the mesenchymal region around the neural tube (NT). Note that all PECAM-1+cells are also VEGFR-2+ and VEGFR-3+ at this stage of embryonic development.
Specificity of AFL4. (A) AFL-4 binding to VEGFR-1-Fc, VEGFR-2-Fc, or VEGFR-3-Fc was analyzed by ELISA. The absorbance at 450 nm is depicted. Each bar represents the mean ± SEM of triplicate assays. (B) Cell lysates from the 293 cell line transiently transfected with murine VEGFR-3 DNA (lane 2) or mock transfected (lane 1, M) were precipitated with AFL4 and immunoblotted with the same antibody. (IP, immunoprecipitation.) Two bands of 195- and 125-kd proteins were detected in lane 2 under reducing conditions. Through immunoblotting of the cell lysates from the murine EC line F2 with anti-VEGFR-3 mAb, 2 bands were detected in nonreducing conditions (NR), whereas only a 125-kd band was seen under reducing conditions (R) (lanes 3, 4). Arrows denote the positions of the unprocessed 195-kd form and the proteolytically processed 125-kd form of VEGFR-3. (C) Specificity of AFL4 in tissue sections. Sagittal sections were prepared from embryonic day 9.5 VEGFR-3−/− or VEGFR-3+/+ embryos and stained with AFL4, anti-PECAM-1 mAb, and anti-VEGFR-2 mAb. Arrows indicate blood vessels in the mesenchymal region around the neural tube (NT). Note that all PECAM-1+cells are also VEGFR-2+ and VEGFR-3+ at this stage of embryonic development.
To further evaluate the specificity of AFL4, serial transverse sections were prepared from E9.5 VEGFR-3−/− or VEGFR-3+/+ embryos and immunostained by AFL4. The AFL4 immunostaining was seen in the vascular endothelial lining of the wild-type embryo but not in that of the VEGFR-3−/−embryo, whereas the expression of VEGFR-2 and PECAM-1 was detected in both groups (Figure 1C). From these results, we concluded that AFL4 is specific to VEGFR-3 and can be used for various purposes, including immunoprecipitation, immunoblotting, and immunostaining of fixed tissues.
We next investigated whether AFL4 blocks the function of VEGFR-3. AFL4 could block the binding of myc-tagged VEGF-C to ELISA plates coated with VEGFR-3-Fc, whereas anti-VEGFR-2 mAb (AVAS12)29 could not, indicating that AFL4 recognizes the ligand-binding site of VEGFR-3 (Figure 2A). Although these analyses did not permit a precise determination of the binding affinity of AFL4 for VEGFR-3, the IC50 for AFL4 inhibition of VEGF-C binding to VEGFR-3 was estimated to be 0.5 μg/mL. We also examined whether the blocking of ligand binding leads to the suppression of receptor signaling. We stimulated F2 cells by VEGF-C in the presence of AFL4 or AVAS12. Tyrosine phosphorylation of immunoprecipitated VEGFR-3 was measured by anti-phosphotyrosine mAb. Compared with the control, AFL-4 treatment resulted in 6- and 3-fold reduction of VEGF-C–induced tyrosine phosphorylation at 125-kd and 195-kd bands, respectively (Figure 2B).
AFL4 blocks VEGFR-3 function by inhibiting VEGF-C binding.
(A) Inhibition of binding of VEGFR-3 to VEGF-C by AFL4. Culture supernatant of 293 cells transfected with 5 myc-tagged VEGF-C DNA was incubated with various doses of AFL4 (•) or anti-VEGFR-2 mAb (○) and added to microtiter plates coated with mVEGFR-3-Fc. Binding was quantified by using the anti-myc mAb as a primary antibody, and absorbance at 450 nm was determined. Data indicate the background-corrected mean ± SEM from triplicate wells. (B) Tyrosine phosphorylation of VEGFR-3 in F2 cells was induced by VEGF-C CM in the presence of control IgG (lanes 1, 3) or AFL4 (lanes 2, 4). Total cell lysates were immunoprecipitated with anti-VEGFR-3 mAb and subjected to serial immunoblotting with anti-phosphotyrosine antibody (upper) and anti-VEGFR-3 mAb (lower). Arrows and arrowheads denote the positions of 195-kd and 125-kd forms of VEGFR-3. Relative density of bands against 125-kd bands of the control lanes (lane 1, upper and lower panels) were 195-kd/125-kd 3.7/1 (lane 1, upper), 0.9/0.2 (lane 2, upper), 0.78/1 (lane 1, lower), and 0.6/1.1 (lane 2, lower). Reduction ratios after correction by the amount of protein were 0.32 and 0.18 for 195-kd and 125-kd bands, respectively.
AFL4 blocks VEGFR-3 function by inhibiting VEGF-C binding.
(A) Inhibition of binding of VEGFR-3 to VEGF-C by AFL4. Culture supernatant of 293 cells transfected with 5 myc-tagged VEGF-C DNA was incubated with various doses of AFL4 (•) or anti-VEGFR-2 mAb (○) and added to microtiter plates coated with mVEGFR-3-Fc. Binding was quantified by using the anti-myc mAb as a primary antibody, and absorbance at 450 nm was determined. Data indicate the background-corrected mean ± SEM from triplicate wells. (B) Tyrosine phosphorylation of VEGFR-3 in F2 cells was induced by VEGF-C CM in the presence of control IgG (lanes 1, 3) or AFL4 (lanes 2, 4). Total cell lysates were immunoprecipitated with anti-VEGFR-3 mAb and subjected to serial immunoblotting with anti-phosphotyrosine antibody (upper) and anti-VEGFR-3 mAb (lower). Arrows and arrowheads denote the positions of 195-kd and 125-kd forms of VEGFR-3. Relative density of bands against 125-kd bands of the control lanes (lane 1, upper and lower panels) were 195-kd/125-kd 3.7/1 (lane 1, upper), 0.9/0.2 (lane 2, upper), 0.78/1 (lane 1, lower), and 0.6/1.1 (lane 2, lower). Reduction ratios after correction by the amount of protein were 0.32 and 0.18 for 195-kd and 125-kd bands, respectively.
Lymphatic specific expression of VEGFR-3 protein in adult mouse
Although a previous study18 and Figure 2 demonstrated VEGFR-3 expression in the vascular EC of early embryos, AFL4 recognized only lymphatic vessels in later life. Figure3 showed the whole-mount immunostaining of the mesentery of E17 embryos, in which lymphatic vessels easily could be distinguished morphologically from vein and artery running in parallel (Figure 3A-C). In cross-sections, staining could be found only in the endothelial cells of the lymphatic vessels that did not contain blood cells in the lumen (Figure 3D).
Lymphatic-specific expression of VEGFR-3 in embryonic day 17 (E17) embryos and adult dermis.
(A) Whole-mount staining of the mesentery of E17 embryos by AFL4. A fraction of vascular system is stained. (B) Higher magnification of the marked area in panel A demonstrates that lymphatic vessels with typical sac-like structure are stained (arrows). (C) PECAM-1 staining of the same region shows that blood vessels (arrow) and lymphatic vessels (arrowhead) are stained. (D) A section of the E17 mesentery illustrating stained endothelial vessel (arrow) and an unstained blood vessel (arrowhead) (×200). Note that all stained vessels do not contain hematopoietic cells. (E) A section of an adult skin illustrates VEGFR-3+ (arrows) and VEGFR-3− vessels (×100). Higher magnification (inset, ×200) reveals that VEGFR-3+ vessels (arrows) do not contain hematopoietic cells, whereas unstained blood vessels do (arrowheads).
Lymphatic-specific expression of VEGFR-3 in embryonic day 17 (E17) embryos and adult dermis.
(A) Whole-mount staining of the mesentery of E17 embryos by AFL4. A fraction of vascular system is stained. (B) Higher magnification of the marked area in panel A demonstrates that lymphatic vessels with typical sac-like structure are stained (arrows). (C) PECAM-1 staining of the same region shows that blood vessels (arrow) and lymphatic vessels (arrowhead) are stained. (D) A section of the E17 mesentery illustrating stained endothelial vessel (arrow) and an unstained blood vessel (arrowhead) (×200). Note that all stained vessels do not contain hematopoietic cells. (E) A section of an adult skin illustrates VEGFR-3+ (arrows) and VEGFR-3− vessels (×100). Higher magnification (inset, ×200) reveals that VEGFR-3+ vessels (arrows) do not contain hematopoietic cells, whereas unstained blood vessels do (arrowheads).
We next examined VEGFR-3 expression in adult cutaneous tissues. Cells lining the luminal wall of a vascular structure (indicated by arrows) were immunostained by AFL4 (Figure 3E). In contrast to the lack of reactivity to blood vessels containing hematopoietic cells (indicated by arrowheads), all AFL4-reactive vessels did not contain blood cells, suggesting lymphatic specific expression of VEGFR-3. This staining pattern corroborates well with previous in situ analysis showing that VEGFR-3 is specific to ECs lining the lymphatic vessels.18
To determine the role of VEGFR-3 in the maintenance of the adult lymphatic system, 1 mg AFL4 was injected subcutaneously into 8-week-old mice on alternating days for up to 3 weeks. During this 3-week period of continuous AFL4-injection, we could not detect any gross abnormality in the treated mice compared with the control mice, which were treated with non-antagonistic anti-VEGFR-2 mAb or PBS (data not shown). Thus, VEGFR-3 appeared not to be essential for the maintenance of the adult lymphatic system.
AFL4 suppresses the growth of xenogenic tumors in nude mice
The antagonistic anti-VEGFR-3 mAb enabled us to examine the involvement of VEGFR-3 in tumor-induced neo-angiogenesis. For this purpose, we first used the C6 glioblastoma cell line, which grows aggressively in the nude mouse.33 C6 cell line has been shown to secrete VEGF34 and VEGF-C.35
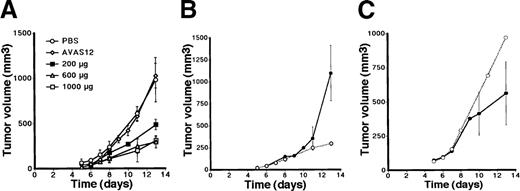
To determine the effect of AFL4 treatment on the C6 growth, 200, 600, or 1000 μg purified AFL4 was injected on alternating days for 12 days into mice grafted with 2 × 106 C6 tumor cells. As controls, 600 μg AVAS12, nonantagonistic anti-VEGFR-2 mAb was injected in the same manner. The size of tumor was measured from day 5 to day 14 after the tumor transplantation. As shown in Figure4A and Table 1, AFL4 treatment inhibited tumor growth at all doses. Because 200 μg showed less effect than other doses, we decided to use 600 μg for subsequent experiments.
Anti-VEGFR-3 suppresses the growth of C6 tumor cells implanted subcutaneously in nude mice.
(A) Protocol 1: days 0 to 12. Alternate-day treatment with PBS (n = 8), anti-VEGFR-2 (600 μg/dose; n = 4), or anti-VEGFR-3 (200 μg/dose, 600 μg/dose, 1000 μg/dose; n = 4 each). Tumor size at day 14 was summarized in Table 1. (B) Protocol 2: days 0 to 6. Injection of anti-VEGFR-3 treatment (600 μg/dose, closed circle; n = 4), compared with continuous injection (days 0-12) (open circle; n = 4). (C) Protocol 3: days 7 to 13. Anti-VEGFR-3 treatment (600 μg/dose, closed circle; n = 4) and PBS treatment (open circle; n = 4). The growth curves of tumors in PBS-treated mice were used as reference points for each figure.
Anti-VEGFR-3 suppresses the growth of C6 tumor cells implanted subcutaneously in nude mice.
(A) Protocol 1: days 0 to 12. Alternate-day treatment with PBS (n = 8), anti-VEGFR-2 (600 μg/dose; n = 4), or anti-VEGFR-3 (200 μg/dose, 600 μg/dose, 1000 μg/dose; n = 4 each). Tumor size at day 14 was summarized in Table 1. (B) Protocol 2: days 0 to 6. Injection of anti-VEGFR-3 treatment (600 μg/dose, closed circle; n = 4), compared with continuous injection (days 0-12) (open circle; n = 4). (C) Protocol 3: days 7 to 13. Anti-VEGFR-3 treatment (600 μg/dose, closed circle; n = 4) and PBS treatment (open circle; n = 4). The growth curves of tumors in PBS-treated mice were used as reference points for each figure.
Suppression of tumor growth by AFL4 treatment
| Antibodies . | μg/dose . | Tumor volume (mm3)‡ . |
|---|---|---|
| C6 tumor* | ||
| PBS | — | 982 ± 246 |
| Avas12† | 600 | 1027 ± 137 |
| AFL4 | 200 | 488 ± 58.51-153 |
| 600 | 296 ± 67.01-153 | |
| 1000 | 312 ± 38.41-153 | |
| PC-3 tumor | ||
| Avas12† | 600 | 291 ± 90.1 |
| AFL4 | 600 | Not detectable |
| Antibodies . | μg/dose . | Tumor volume (mm3)‡ . |
|---|---|---|
| C6 tumor* | ||
| PBS | — | 982 ± 246 |
| Avas12† | 600 | 1027 ± 137 |
| AFL4 | 200 | 488 ± 58.51-153 |
| 600 | 296 ± 67.01-153 | |
| 1000 | 312 ± 38.41-153 | |
| PC-3 tumor | ||
| Avas12† | 600 | 291 ± 90.1 |
| AFL4 | 600 | Not detectable |
To determine the timing of VEGFR-3 involvement in tumor progression, 3 protocols for antibody injection were tested: (1) day 0 to day 12, (2) day 0 to day 6, and (3) day 7 to day 13. The continuous injection of AFL4 suppressed tumor growth by approximately 75%. Because the discontinuation of treatment on day 7 (protocol 2) resulted in the prompt recovery of tumor growth, it is likely that VEGFR-3 is continuously required for tumor growth (Figure 4B). We also attempted to determine whether growth was suppressed if AFL4 injection was commenced from day 7 (protocol 3, Figure 4C). Although we did observe a reduction of tumor size, the effect of this protocol was not statistically significant (P = .06).
To examine whether AFL4 treatment suppresses the growth of other tumor types, a human prostatic cancer cell line, PC-3 was used. PC-3 cells were reported to secrete VEGF-C.20 PC-3 cells grew more slowly than C6 cells in the subcutaneous region of the nude mice. At day14, PC-3 tumors reached an average size of 291 ± 90.1 mm3 in control mice (Table 1), whereas we could not detect PC-3 tumor mass at day 14 in AFL4-treated mice (Table 1).
Induction of VEGFR-3 expression during tumor-induced angiogenesis
Such a dramatic suppression of tumor growth by AFL4 treatment implicates the role of VEGFR-3 in angiogenesis rather than in the formation of lymphatic vessels, though VEGFR-3 is not expressed in the blood vessels of normal tissues. Thus, we hypothesized that VEGFR-3 expression might be induced by tumor transplantation in the surrounding tissue. To test this possibility, we investigated VEGFR-3 expression in the tumor-bearing tissues. Sections of tumor and surrounding tissues were immunostained with AFL4 or anti-PECAM-1 mAb. VEGFR-3 expression was detected in intratumoral vessels (indicated by arrows) (Figure5A). Unlike normal tissues (Figure 3E), VEGFR-3+ vessels in this section contained blood cells, indicating that VEGFR-3 expression was induced in the tumor blood vessels. A similar staining pattern was seen for PECAM-1 staining of serial sections, demonstrating EC-specific expression of VEGFR-3 (Figure 5B). It should be noted, however, that not all EC in the tumor vessels expressed VEGFR-3. Intratumoral VEGFR-3+ vessel density, including lymphatics, was 30 ± 1.2 per high-power field, whereas that of normal skin tissue was 7.4 ± 0.3 per high-power field. Because the intratumoral PECAM-1+ vessel density was 92 ± 2.8, approximately 30% of intratumoral vessels become activated to express VEGFR-3+, and because PECAM-1+ vessel density in the normal skin was 38 ± 1.3, the intratumor region was indeed rich in blood vessels. VEGFR-3 staining was also induced in vessels surrounding the tumor (Figure 5C).
Induction of VEGFR-3 expression during tumor-induced angiogenesis.
Immunostaining for VEGFR-3 (A) and PECAM-1 (B) in adjacent sections of C6 subcutaneous tumors (day 7) in nude mice (×200). Intratumoral VEGFR-3+ (A, arrows) containing blood cells are also positive for PECAM-1 expression (B, arrows). Note that hemorrhages are not conspicuous at this stage. (C) Immunostaining for VEGFR-3 in a section of C6 tumors with surrounding tissues (×100). Note the presence of VEGFR-3+ (black arrows) and VEGFR-3−(white arrows) vessels, both containing hematopoietic cells. Arrowheads indicate VEGFR-3+ vessels that do not contain hematopoietic cells. Asterisk indicates the edge of a tumor.
Induction of VEGFR-3 expression during tumor-induced angiogenesis.
Immunostaining for VEGFR-3 (A) and PECAM-1 (B) in adjacent sections of C6 subcutaneous tumors (day 7) in nude mice (×200). Intratumoral VEGFR-3+ (A, arrows) containing blood cells are also positive for PECAM-1 expression (B, arrows). Note that hemorrhages are not conspicuous at this stage. (C) Immunostaining for VEGFR-3 in a section of C6 tumors with surrounding tissues (×100). Note the presence of VEGFR-3+ (black arrows) and VEGFR-3−(white arrows) vessels, both containing hematopoietic cells. Arrowheads indicate VEGFR-3+ vessels that do not contain hematopoietic cells. Asterisk indicates the edge of a tumor.
Histologic basis for tumor suppression in AFL4-treated mice
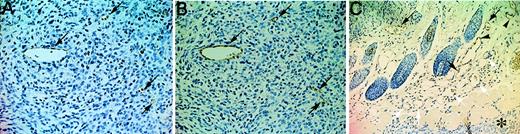
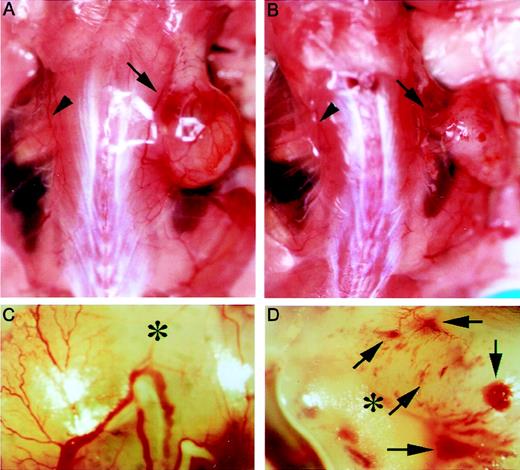
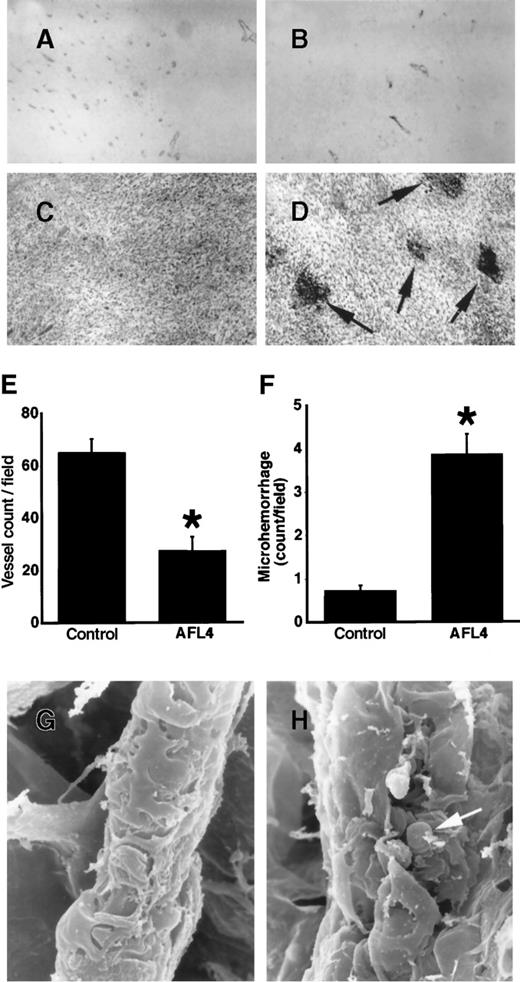
To gain insight into the VEGFR-3–dependent cellular processes during tumor-induced angiogenesis, we compared the vascular system surrounding tumors of AFL4-treated and control mice. In the control mouse, the size of the vascular trunk supplying branches to tumors was larger than the corresponding trunk in the tumor-free side of skin, suggesting an increase of overall blood flow in the tumor-bearing side (Figure 6A). As expected from the reduced tumor size in AFL4-treated mice, the vascular trunk governing tumor blood supply was smaller than that of the control mice (Figure 6B). In control mice, several branches of similar size stemmed from this trunk, which further divided into smaller branches (Figure 6A,C). In contrast, though the primary branches were detectable in the AFL4-treated mice, their sizes were variable, and they did not develop the fan-like architecture found in the control tumor (Figure 6B,D). Secondary and tertiary branches appeared to be very thin. Many micro-hemorrhages were found along the small branches (Figure 6B,D). Although massivebleeding was frequently found in the necrotic regions of tumors in the control mice, micro-hemorrhages were rare. This macroscopic observation was confirmed by microscopic analysis. Control tumor sections stained with hematoxylin–eosin showed enlarged vessels surrounding tumors (data not shown), whereas overall vascularity around the tumors was lower in the AFL4-treated group (data not shown). Moreover, the number of PECAM-1+ EC within the tumor mass was reduced to 40% in the control group (Figure 7A,B,E). Conversely, 4 times more micro-hemorrhagic regions were detected in the AFL4-treated group (Figure 7C,D,F).
AFL4 treatment inhibits tumor angiogenesis.
Tumor-bearing regions were photographed on day 14 after tumor transplantation. Gross appearance of representative vascularization of control (A) and AFL4-treated (B) mice. Arrows and arrowheads indicate the vascular trunks governing tumor blood supply and those of the tumor-free side, respectively. Note that the size of the trunk is larger on the tumor-bearing side than on the other side. Such a dilatation is not clearly seen in the AFL4-treated mouse. (C, D) Higher magnification than that in A and B, respectively. Secondary and tertiary branches are poorly developed in the AFL4-treated mouse. Note the many micro-hemorrhages in this AFL4-treated tumor (arrows). Asterisks indicate tumors.
AFL4 treatment inhibits tumor angiogenesis.
Tumor-bearing regions were photographed on day 14 after tumor transplantation. Gross appearance of representative vascularization of control (A) and AFL4-treated (B) mice. Arrows and arrowheads indicate the vascular trunks governing tumor blood supply and those of the tumor-free side, respectively. Note that the size of the trunk is larger on the tumor-bearing side than on the other side. Such a dilatation is not clearly seen in the AFL4-treated mouse. (C, D) Higher magnification than that in A and B, respectively. Secondary and tertiary branches are poorly developed in the AFL4-treated mouse. Note the many micro-hemorrhages in this AFL4-treated tumor (arrows). Asterisks indicate tumors.
Histology of intratumor vasculatures.
(A, B) Tumor vasculatures are visualized by anti-PECAM-1 immunostaining. Micrographs are of representative sections prepared from the control (A) and AFL4-treated mice (B) (×200). (C, D) Representative H&E-stained tumor sections from controls (C) and anti-VEGFR-3–treated mice (D) (×200). In anti-VEGFR-3–treated tumors, many micro-hemorrhages are observed (arrows). (E) Vessel counts per field were determined from at least 5 different vision fields of sections from control (n = 4; 65 ± 5.2/high-power field) and AFL4-treated mice (n = 4; 27.5 ± 5.3/high-power field) (×200). Data are plotted as mean ± SEM. *P < .01. (F) The number of micro-hemorrhages was scored in high-power fields (×200) of H&E-stained tumor sections (at least 5 different vision fields each of 4 tumors). Control, 0.73 ± 0.13/high-power field; AFL4, 3.9 ± 0.46/high-power field. Data are plotted as mean ± SEM. *P < .01. Statistical differences between groups were computed using the Student t test. (G) Representative SEM of a postcapillary venule in control tumors (×3800). (H) SEM of a postcapillary venule in anti-VEGFR-3–treated tumors reveals disruption of the endothelial sheet, exposing red blood cells (arrow) (×2200).
Histology of intratumor vasculatures.
(A, B) Tumor vasculatures are visualized by anti-PECAM-1 immunostaining. Micrographs are of representative sections prepared from the control (A) and AFL4-treated mice (B) (×200). (C, D) Representative H&E-stained tumor sections from controls (C) and anti-VEGFR-3–treated mice (D) (×200). In anti-VEGFR-3–treated tumors, many micro-hemorrhages are observed (arrows). (E) Vessel counts per field were determined from at least 5 different vision fields of sections from control (n = 4; 65 ± 5.2/high-power field) and AFL4-treated mice (n = 4; 27.5 ± 5.3/high-power field) (×200). Data are plotted as mean ± SEM. *P < .01. (F) The number of micro-hemorrhages was scored in high-power fields (×200) of H&E-stained tumor sections (at least 5 different vision fields each of 4 tumors). Control, 0.73 ± 0.13/high-power field; AFL4, 3.9 ± 0.46/high-power field. Data are plotted as mean ± SEM. *P < .01. Statistical differences between groups were computed using the Student t test. (G) Representative SEM of a postcapillary venule in control tumors (×3800). (H) SEM of a postcapillary venule in anti-VEGFR-3–treated tumors reveals disruption of the endothelial sheet, exposing red blood cells (arrow) (×2200).
To determine the morphologic basis of the micro-hemorrhage in the AFL4-treated tissues, the vessels connecting to the tumor were analyzed systemically by SEM. We could not detect any abnormalities in the morphology of the proximal vessels of AFL4-treated mice, suggesting that these vessels developed normally (data not shown). However, at the level of postcapillary venules, disruption of the endothelial lining was observed frequently in AFL4-treated mice (Figure 7G,H). Erythrocytes could be seen through the cleft. The formation of such clefts was barely detectable in the postcapillary venule surrounding the tumors in control mice.
Discussion
In this study we established an antagonistic mAb to VEGFR-3 (AFL4) and used AFL4 to evaluate the role of this RTK in tumor angiogenesis. Although VEGFR-3 is not expressed in the vascular EC of adult mice, our result showed that VEGFR-3 expression is induced in the vascular EC upon the implantation of tumor cells. Furthermore, the growth of C6 glioma cells and PC-3 prostate carcinoma cells was suppressed by the injection of AFL4, presumably because of the inhibition of the establishment of the vascular architecture in tumor-bearing tissues. Taking the previous study on VEGFR-3−/−embryos26 into account, besides embryonic angiogenesis, VEGFR-3 is involved in the neo-angiogenesis of adult tissue.
Sustained AFL4 treatment resulted in no gross anomalies in normal mice. Thus, during the 3 weeks of mAb injection, the architecture of the lymphatics and the vascular system remained unaffected in the absence of VEGFR-3 function; however, the effect over a longer period of time remains to be investigated. Recently, it has been demonstrated that the prolonged suppression of VEGF activity in the adult mouse has no effect on the maintenance of the vascular system, though it suppresses angiogenesis in the newborn mouse.36 The fully established lymphatic and vascular systems are basically resistant to treatment with various reagents that suppress neo-angiogenesis. Because of the neo-angiogenesis–specific effect of these reagents, this approach has been expected to be chosen for cancer therapy. Our current results demonstrate clearly that VEGFR-3 is a potentially useful molecule for targeting in future cancer therapy.
Compared with the phenotype of VEGFR-2−/− mice, in which formation of the primitive vascular plexus is impaired,37 it has been indicated that VEGFR-3 is involved at a later stage of vascular development, particularly in the remodeling of the primitive plexus to a higher-order architecture.26 The absence of secondary and tertiary branches in AFL4-treated mice suggests a VEGFR-3 role in the remodeling of tumor-induced neo-angiogenesis. Angiopoietins/Tie-2 has also been implicated in the remodeling process of embryonic and tumor-induced angiogenesis. It is likely that the molecular requirements for vascular development in the embryo and for tumor-induced neo-angiogenesis are essentially the same and involve an ordered expression of multiple tyrosine kinase receptors.
Which process of angiogenesis is affected by the inhibition of VEGFR-3? Although the role of VEGFR-3 in the remodeling of vascular formation has been implicated, these reports did not specify the process beyond the word remodeling.26 This may be, in part, because of an inherent difficulty in studying embryonic angiogenesis in which angiogenesis proceeds asynchronously according to region-specific timetables. In other words, various intermediate steps of angiogenesis are mixed within an embryo. In contrast, tumor-induced angiogenesis is a synchronous process that can be induced in a relatively homogeneous microenvironment. Moreover, the progression of angiogenesis during tumor growth has been described in detail. With these considerations in mind, we attempted to obtain insight into the histologic basis of the phenotype induced by AFL4-injection. Although AFL4 treatment appeared to be identical to other anti-angiogenic reagents in that it inhibited the supply of vascular branches to the tumor, we demonstrated that micro-hemorrhage, presumably because of the disruption of the endothelial lining at the postcapillary venule level, is a characteristic feature of AFL4-treated tissues. It is difficult to rule out the possibility that this effect is caused by the cytotoxic reaction of AFL4 to VEGFR-3+ EC, but we prefer to think that this disruption is derived from the blockage of VEGFR-3 function. This histologic sign has not been described in previous experiments in which other RTKs are blocked. Because micro-hemorrhages are too conspicuous to be overlooked, frequent micro-hemorrhages may be specific to VEGFR-3 inhibition.
How VEGFR-3-block caused the disruption of endothelial structure is difficult to specify. According to previous studies, sprouting of EC occurs only at the levels of capillaries, postcapillary venules, and precapillary arterioles, where no smooth muscle is present.38 By the repeated sprouting, splitting, and anastomosis of blood vessels at this level, the overall peripheral vascular bed in the tumor-bearing tissues increases. This increase in the vascular bed contributes to the reduction of regional vascular resistance, thereby resulting in the increased blood supply. The change of blood supply induces restructuring of the more proximal vessels connecting to tumor, as observed in the current study. Because the angiopoietins/Tie2 signal was shown to regulate interactions between ECs and smooth muscle cells,9-11 it is conceivable that this signal is required for vascular remodeling in which the distal blood vessel is restructured to the more proximal form associated with smooth muscles. In the Tie2-block experiment, however, micro-hemorrhages in tumor-bearing tissues has not been indicated. Hence, failure in interaction between ECs and smooth muscle cells may not lead to disruption of the endothelial structure.
The frequency of micro-hemorrhages found in AFL4 treated-mice suggests that AFL4 treatment inhibits maintenance of the integrity of the endothelial sheet during angiogenesis. Although further cell biology studies are required for an understanding of the underlying mechanisms, the presence of mural cells at the level of precapillary arterioles and postcapillary venules of AFL4-treated mice suggests that it may not result from inhibition of the interaction between EC and smooth muscle cells. It has been suggested that vascular permeability is increased at the site of tumor-induced angiogenesis. Thus, it is likely that the endothelial lining is agitated during neo-angiogenesis. Indeed, sprouting and pruning would imperil the integrity of the endothelial lining. Yet, micro-hemorrhage is not a frequent outcome of neo-angiogenesis, indicating that regulatory mechanisms maintain the integrity of the endothelial sheet even during dynamic restructuring of the vascular system. Therefore, we speculate that in the process of sprouting and pruning, during which integrity of the EC layer is disturbed, additional signals such as VEGFR-3 may be required for quick restoration of the EC sheet that otherwise leads to the formation of irreparable clefts. If such clefts causing micro-hemorrhage are generated during neo-angiogenesis, the rheologic resistance of the vascular system should increase, thereby resulting in the collapse of more proximal vessels as observed in the AFL4-treated mouse.
Acknowledgments
We thank Dr W. I. Wood (Genentech) for VEGFR-3 cDNA, Dr K. I. Toda for the F-2 cell line, and Dr N. Itoh for the PC-3 cell line. We also thank Drs H. Kataoka, M. Hirashima, and H. Yoshida for their helpful advice, and we thank Dr S. Fraser for critical reading of the manuscript.
Supported by grants from the Japanese Ministry of Education, Science and Culture (07CE2005, 07457085, and 06277102), from the Japanese Ministry of Health and Welfare, and from the Japan Society for the Promotion of Science Research.
Reprints:Hajime Kubo, Department ofMolecular Genetics, Graduate School of Medicine, Kyoto University, Shogoin Kawaharacho 53, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: kuboflt@kuhp.kyoto-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal