Abstract
In this report we describe the molecular defect underlying partial and severe quantitative von Willebrand factor (VWF) deficiencies in 3 families previously diagnosed with types 1 and 3 Von Willebrand-disease. Analysis of the VWF gene in affected family members revealed a novel C to T transition at nucleotide 1067 of the VWF complemetary DNA (cDNA), predicting substitution of arginine by tryptophan at amino acid position 273 (R273W) of pre–pro-VWF. Two patients, homozygous for the R273W mutation, had a partial VWF deficiency (VWF:Ag levels of 0.06 IU/mL and 0.09 IU/mL) and lacked high-molecular weight VWF multimers in plasma. A third patient, also homozygous for the R273W mutation, had a severe VWF deficiency (VWF:Ag level of less than 0.01 IU/mL) and undetectable VWF multimers in plasma. Recombinant VWF having the R273W mutation was expressed in COS-7 cells. Pulse-chase experiments showed that secretion of rVWFR273W was severely impaired compared with wild-type rVWF. However, the mutation did not affect the ability of VWF to form dimers in the endoplasmic reticulum (ER). Multimer analysis showed that rVWFR273W failed to form high-molecular-weight multimers present in wild-type rVWF. We concluded that the R273W mutation is responsible for the quantitative VWF deficiencies and aberrant multimer patterns observed in the affected family members. To identify factors that may function in the intracellular retention of rVWFR273W, we investigated the interactions of VWF expressed in COS-7 cells with molecular chaperones of the ER. The R273W mutation did not affect the ability of VWF to bind to BiP, Grp94, ERp72, calnexin, and calreticulin in COS-7 cells.
Von Willebrand factor (VWF) is a multimeric plasma glycoprotein with 2 essential roles in hemostasis. It acts as a carrier for factor VIII and mediates platelet adhesion to the subendothelium at sites of vascular damage.1 Mutations in the VWF gene, resulting in quantitative deficiencies or qualitative abnormalities of VWF, cause von Willebrand disease (VWD), which is the most common inherited bleeding disorder in humans. Current classification of VWD recognizes 3 types.2 Type 1 VWD is characterized by partial quantitative deficiency of VWF. It is generally inherited in an autosomal dominant manner and accounts for up to 70% of the cases. Type 3 VWD is characterized by a complete deficiency of VWF and is generally inherited in an autosomal recessive manner.1,2Type 2 VWD refers to qualitative deficiency of VWF and is subdivided into types 2A, 2B, 2M, and 2N.2 Types 2A and 2M variants show decreased platelet binding. In type 2A VWD, but not type 2M VWD, this is associated with an absence of high-molecular-weight multimers. Type 2B variants have an increased affinity for platelet glycoprotein Ib. Type 2N VWD refers to variants having a decreased affinity for factor VIII.2
The 178-kilobase (kb) gene encoding VWF has been localized to chromosome 12 and contains 52 exons. The 8.7-kb VWF messenger RNA (mRNA) encodes a pre-proprotein containing 2813 amino acids, including a 22-residue signal peptide, a 741-residue propeptide and a 2050-residue mature subunit.3,4 Pro-VWF is organized into repeats of 4 homologous domains. The domains from N to C terminus are D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK.5 Multimer assembly begins in the endoplasmic reticulum (ER) with the formation of pro-VWF dimers linked by interchain disulphide bonds between C-terminal cysteine residues.6,7 Multimerization continues in the Golgi apparatus8,9 through interchain disulphide bond formation between cysteine residues within the N-terminal of mature propeptide cleaved VWF. The propeptide, consisting of domains D1 and D2, plays an essential role in the assembly of VWF multimers. VWF lacking a propeptide has been shown to undergo dimerization, passage from the ER to the Golgi apparatus, and secretion as dimers.10 The D1 and D2 domains each contain Cys-X-X-Cys sequences that resemble the active sites of the protein disulphide isomerase (PDI) family of enzymes that catalyze the formation of disulphide bonds during synthesis of secretory proteins in the ER.11 Although PDI activity has not been shown for the VWF propeptide, mutation of a cysteine residue to glycine in either of the 2 Cys-X-X-Cys sequences abolishes multimerization and results in the secretion of dimers.12
There have been several mutations reported in the VWF gene sequence encoding the propeptide that result in VWD.13-21 The majority of these are nonsense and frameshift mutations causing type 3 VWD. Other propeptide mutations, including 3 missense mutations (N528S,17 G550R,20 and C623W 18), 2 in-frame insertions (ins625Gly18 and ins405AsnPro19), and 1 in-frame deletion (delR437-D44221), were associated with type 2A VWD. Only 1 missense mutation in the propeptide (W377C) has been reported to cause type 3 VWD.14This current report describes the molecular defect underlying VWD in 3 different families from Turkey previously diagnosed with types 1 and 3 VWD. Analysis of the VWF gene sequence in DNA from all 3 propositi and affected family members detected a novel mutation, C1067T, predicting substitution of arginine by tryptophan at amino acid 273 in the propeptide of pre–pro-VWF. When expressed in COS-7 cells, recombinant VWF (rVWF), having the R273W mutation (rVWFR273W), exhibited severely impaired secretion, failed to form high-molecular-weight multimers, but showed no difference in its ability to form disulphide-linked dimers when compared with wild-type rVWF.
To identify candidate proteins that may retain rVWFR273W intracellularly, we have investigated the interaction of rVWFR273W with molecular chaperones in COS-7 cells. We showed that, as with earlier reported VWF variants, rVWFR273W interacted with the molecular chaperones BiP, Grp94, and ERp72.22-24 We also showed for the first time that wild-type rVWF, as well as rVWFR273W, interacted with the molecular chaperones calnexin and calreticulin during biosynthesis.
Materials and methods
Plasmid pSVHVWF1 containing the full-length human VWF complementary DNA (cDNA) was kindly supplied by Dr Aida Inbal (Tel Aviv, Israel). A rabbit polyclonal antibody to canine calnexin was kindly supplied by Professor Neil Bulleid (Manchester, UK).25 Rabbit polyclonal antisera to canine calnexin (SPA-860), human calreticulin (SPA-600), mouse ERp72 (SPA-720), and rat monoclonal antibody to chicken Grp94 were purchased from StressGen (York, UK). A goat polyclonal antibody to human BiP (N-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The following polyclonal antibodies were purchased from Dako (Glostrup, Denmark): rabbit antihuman VWF, alkaline phosphatase–conjugated swine antirabbit IgG, horseradish peroxidase (HRP)–conjugated rabbit antigoat IgG, and HRP-conjugated rabbit antihuman VWF. The sources of all other reagents used have been described previously by Allen and coworkers.26
Laboratory assays and multimer analysis
Peripheral venous blood was collected from the propositi, their parents, and siblings in Turkey after informed consent. Bleeding times were determined using a Simplate-II device (General Diagnostics, Morris Plains, NJ). Blood samples and plasma were shipped to Aarhus, Denmark, where the laboratory assays for factor VIII coagulant activity (FVIII:C), FVIII antigen (FVIII:Ag), VWF antigen (VWF:Ag), VWF ristocetin cofactor (VWF:RiCoF), and colorimetric multimer analysis were performed as described previously.26-29 Blood samples and plasma were then shipped to Sheffield, UK, where the chemiluminescent multimer analysis,26 mutation detection, and recombinant expression experiments were performed.
Mutation detection
DNA fragments corresponding to the 2.2-kb promoter (nucleotides 1-2181),3 exons 1 to 52, and exon/intron boundaries of the VWF gene were amplified from genomic DNA by polymerase chain reaction (PCR), as described previously.26 Apart from the promoter and exons 9, 10, 15, 26, and 28 that were amplified using the oligonucleotide primers described by Allen and coworkers,26the fragments were amplified using the oligonucleotide primers designed by Zhang and coworkers.30,31 The variable number tandem repeats, VNTR-132,33 and VNTR-2,34 in intron 40 of the VWF gene and the single nucleotide polymorphisms (SNPs) −1185A/G and −1051G/A of the VWF gene promoter were analyzed as described previously.26,35 After amplification of exons 36 to 52, chemical cleavage mismatch analysis (CCMA) was used to screen fragments for the presence of mutations,36 while conformation sensitive gel electrophoresis (CSGE) was used to analyze exons 1 to 35 and the promoter, as described previously.26 37 Mutated DNA fragments were purified from 1% agarose gels, using a QIAEX kit (Qiagen, Crawley, UK) and the manufacturer's protocol, for sequence analysis to identify the nucleotide change. Manual sequencing was performed using a Thermosequenase cycle sequencing kit (Amersham International, Bucks, UK) as directed by the manufacturer. Automated sequencing was performed using an Applied Biosystems (Foster City, CA) DNA sequencer (model 373).
Determination of ABO genotypes
ABO blood group genotypes of family members were determined by restriction enzyme analysis of amplified DNA fragments corresponding to exons 6 and 7 of the ABO glycosyltransferase gene.38,39PCRs were identical in composition to those described previously.26 The sequences of the forward- and reverse-oriented primers used to amplify exon 6 were 5′-TGGCACCCTGCCAGCTCCAT and 5′-TCACTCGCCACTGCCTGGGT, respectively, whereas the sequences of those used to amplify exon 7 were 5′-TACTATGTCTTCACCGACCA (forward) and 5′-TAGAAATCGCCCTCGTCCTT (reverse). PCRs were subjected to the same amplification conditions as indicated previously26using an annealing temperature of 57°C. The deletion of a single cytosine at nucleotide position 258 of the cDNA that distinguishes the O allele from the A and B alleles was detected by digestion of amplified exon 6 with Asp718. Digestion of amplified exon 7 with AluI was used to detect the G to A transition at nucleotide 700 that distinguishes the B allele from the A and O alleles.
Plasmid construction
Plasmid pSVHVWF1 contains full-length wild-type human VWF cDNA cloned into the expression vector pSV7D.40 Plasmid pSVVWFR273W contained a C to T transition at nucleotide 1067 of the VWF cDNA and was generated by mutagenesis of a shuttle vector pKpnI that contains a 4.5-kb fragment (nucleotides 401-4851) of the VWF cDNA obtained by KpnI digestion of pSVHVWF1 cloned into pGEM3Z. Mutagenesis was performed using the GeneEditor system (Promega, Madison, WI) and the mutagenic oligonucleotide: 5′GAGTACGCCTGGACCTGTGC3′ (nucleotides 1058-1077 in the VWF cDNA). Clones containing the appropriate mutation were confirmed by sequence analysis. The mutated fragment was subcloned into the KpnI sites of pSVHVWF1 to obtain pSVVWFR273W. The presence of the appropriate mutation in pSVVWFR273W was confirmed again by sequence analysis.
Transient transfection of COS-7 cells with wild-type pSVHVWF1 and pSVVWFR273W
COS-7 cells, grown to 60% confluence in 10-cm dishes, were transfected using DEAE-dextran (0.4 mg/mL) and the appropriate plasmid DNA (15 μg per dish) as described previously.26
Steady-state and pulse-chase analysis of von Willebrand factor secretion
Steady-state and pulse-chase analysis of VWF processing and secretion were performed as described previously.26
Metabolic labeling, cross-linking, and coimmunoprecipitation
To coimmunoprecipitate metabolically labeled VWF with antibodies to calnexin and calreticulin, 48 hours after transfection, COS-7 cells were washed twice with cysteine- and methionine-free DMEM and incubated with the same medium for 1 hour. The cells were then incubated for 90 minutes with 100 μCi of Promix [35S]-cysteine and [35S]-methionine per 10-cm dish in 5 mL of cysteine- and methionine-free DMEM. The medium was removed and the cells washed twice with ice-cold phosphate-buffered saline (PBS) before lysis with 0.75 mL of CHAPS (3-[(3-Cholamidopropyl)dimethyl-ammonia]-1-propanesulphonate) lysis buffer (50 mmol/L HEPES, pH 7.4, containing 2% CHAPS, 200 mmol/L NaCl, 10 μg/mL soybean trypsin inhibitor [SBTI], 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], and 0.02% sodium azide) on ice for 1 hour. Cell lysates were adjusted to 1 mL with lysis buffer then centrifuged for 15 minutes at 12 000g to pellet nuclei and cell debris. Cell lysates were precleared by incubation with 0.5% protein-A sepharose at 4°C for 1 hour, then incubated overnight at 4°C with 0.5% protein-A sepharose and the appropriate antibody. The sepharose beads were pelleted, washed 4 times with CHAPS lysis buffer, then prepared for either SDS-PAGE as described below or sequentially immunoprecipitated with anti-VWF as follows. The washed protein-A sepharose pellets were resuspended in 200 μL of 50 mmol/L Tris/HCl, pH 8.0, containing 1% wt/vol SDS, and boiled for 5 minutes. The samples were cooled and then diluted to 4 mL with lysis buffer to dilute out the SDS 20-fold and immunoprecipitated with rabbit anti-VWF polyclonal antibody, as described above.
To coimmunoprecipitate BiP with antibodies to VWF, COS-7 cells were washed 72 hours after transfection with ice-cold PBS, then lysed with 0.75 mL of CHAPS lysis buffer on ice for 1 hour. To preserve BiP/ VWF complexes for coimmunoprecipitation of BiP with antibodies to VWF, lysates were prepared in the presence of 1 mU/mL apyrase. The lysates were then immunoprecipitated with polyclonal rabbit antihuman VWF antibody, as described for calnexin and calreticulin immunoprecipitates previously.
To coimmunoprecipitate Grp94 and ERp72 with antibodies to VWF, COS-7 cells were treated before lysis with the membrane-permeable, thiol-cleavable, cross-linking agent (Dithiobis[succinimidylpropionate]) (DSP) as follows. COS-7 cells were washed 72 hours after transfection with ice-cold PBS, then incubated for 30 minutes on ice with PBS containing 100 μg/mL DSP. The cells were washed with PBS containing 10 mmol/L glycine, then incubated on ice for 10 minutes in the same buffer to quench any unreacted cross-linker. The cells were washed once with ice-cold PBS, then lysed with 0.75 mL of CHAPS lysis buffer on ice for 1 hour. The lysates were then immunoprecipitated with polyclonal rabbit antihuman VWF antibody, as described previously. All antibodies used for immunoprecipitation were used at a working dilution of 1:200.
Isolation of microsomes from COS-7 cells
Microsomes were prepared from COS-7 cells using a modification of the method of Austen and coworkers.41 COS-7 cells were transfected with pSVHVWF1, pSVVWFR273W, or mock transfected without expression plasmid, as described previously.26 The cells were washed twice with PBS 72 hours after transfection, then trypsinized with 2 mL 1 × trypsin/EDTA solution (Life Technologies, Glasgow, UK). The cells from 5 dishes for each transfection were pooled, then centrifuged at 1600 rpm for 3 minutes. The cells were washed with 10 mL ice-cold buffer A (50 mmol/L Tris, pH 7.4, containing 0.25 mol/L sucrose, 25 mmol/L KCl, 0.5 mmol/L MgCl2, 0.1 mmol/L PMSF, and 100 μg/mL SBTI), pelleted, washed again in buffer A, then resuspended in 2 mL of buffer A. The cells were homogenized on ice using 60 strokes of a Dounce homogenizer. The homogenates were centrifuged at 1000 rpm for 5 minutes to yield a postnuclear supernatant that was centrifuged at 150 000g (60 000 rpm in a TL100.2 Beckman bench top ultracentrifuge rotor [Beckman, Fullerton, CA]) for 10 minutes at 4°C. The microsomes were resuspended in 50 μL of 0.25 mol/L sucrose, containing 0.3 mmol/L PMSF.
Sodium dodecylsulfate-polyacrylamide gel electrophoresis and immunoblotting
Immunoprecipitated sepharose beads were resuspended in SDS-PAGE sample buffer (0.25 mol/L Tris-HCl, pH 6.8, containing 2% wt/vol SDS, 20% vol/vol glycerol, 50 mmol/L DTT, and 0.004% wt/vol bromophenol blue). Electrophoresis and autoradiography were performed as described previously.26 For BiP, Grp94, and ERp72 detection by immunoblotting, proteins were electrophoretically transferred from SDS-polyacrylamide gels onto nitrocellulose membranes. These were probed with either the goat anti-BiP polyclonal antibody N-20, rat anti-Grp94 monoclonal antibody, or rabbit anti-ERp72 polyclonal antibody, followed by polyclonal HRP conjugated rabbit antigoat, rabbit antirat, or swine antirabbit immunoglobulins, respectively, to facilitate antigen detection by enhanced chemiluminesence. For quantitation of VWF in calnexin and calreticulin immunoprecipitates, autoradiographs were subjected to densitometry by phosphorimage analysis using a Biorad GS250 molecular imager (Hertfordshire, UK).
Results
Patient histories and phenotypic results
The propositi from families A (AII:1, Figure1[iA]), B (BII:3, Figure 1[iB]), and C (CII:6, Figure 1[iC]) were Turkish boys with histories of severe bleeding problems that included epistaxsis, easy bruising, bleeding from gums, tonsils, and superficial cuts. The parents of all 3 propositi were first cousins. None of the parents of the 3 propositi (AI:1, AI:2, BI:1, BI:2, CI:1, and CI:2, Figure 1) had any bleeding symptoms. The phenotypic data for all 3 families are summarized in Table 1. The propositus AII:1 had a prolonged bleeding time, reduced VWF:Ag (0.06 IU/mL), FVIII:Ag (0.18 IU/mL), FVIII:C (0.20 IU/mL), and VWF:RiCoF (0.06 IU/mL) levels. The propositus BII:3 had a prolonged bleeding time, reduced VWF:Ag (0.09 IU/mL), FVIII:Ag (0.33 IU/mL), FVIII:C (0.21 IU/mL), and VWF:RiCoF (0.04 IU/mL) levels. The propositus CII:6 had a prolonged bleeding time, reduced VWF:Ag (less than 0.01 IU/mL), FVIII:Ag (0.06 IU/mL), FVIII:C (0.09 IU/mL), and VWF:RiCoF (less than 0.01 IU/mL) levels. The propositus AII:1 was homozygous for the ABO blood group O allele, whereas the propositi BII:3 and CII:6 were homozygous for the A allele (Table 1).
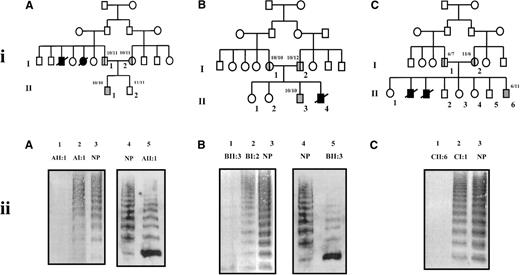
Pedigrees of and multimer analysis of VWF in plasma from families.
(i) Pedigrees of families A (A), B (B), and C (C). Squares denote males, circles denote females. Slashed symbols represent deceased family members. Inheritance of the intron 40 VNTR-1 is shown as number of ATCT repeat units.32 33 (ii) Multimer analysis of VWF in plasma from members of families A, B, and C. Plasma samples were electrophoresed on 2% SDS-agarose gels. In A-C, lanes 1-3 contain equal volumes of plasma (2 μL). In A and B, lanes 4 and 5 contain the appropriate volume of plasma containing 0.5 mU VWF as determined by ELISA. VWF was transferred onto nitrocellulose by electroblotting. In A-C, lanes 1-3 were developed using polyclonal rabbit antihuman VWF antibody and a secondary alkaline phosphatase-conjugated swine antirabbit IgG polyclonal antibody and colorimetric staining. In A and B, lanes 4 and 5 were developed using rabbit antihuman VWF polyclonal antibody and a secondary horseradish peroxidase-conjugated swine antirabbit IgG polyclonal antibody and enhanced chemiluminescence. NP denotes plasma pooled from 20 healthy individuals.
Pedigrees of and multimer analysis of VWF in plasma from families.
(i) Pedigrees of families A (A), B (B), and C (C). Squares denote males, circles denote females. Slashed symbols represent deceased family members. Inheritance of the intron 40 VNTR-1 is shown as number of ATCT repeat units.32 33 (ii) Multimer analysis of VWF in plasma from members of families A, B, and C. Plasma samples were electrophoresed on 2% SDS-agarose gels. In A-C, lanes 1-3 contain equal volumes of plasma (2 μL). In A and B, lanes 4 and 5 contain the appropriate volume of plasma containing 0.5 mU VWF as determined by ELISA. VWF was transferred onto nitrocellulose by electroblotting. In A-C, lanes 1-3 were developed using polyclonal rabbit antihuman VWF antibody and a secondary alkaline phosphatase-conjugated swine antirabbit IgG polyclonal antibody and colorimetric staining. In A and B, lanes 4 and 5 were developed using rabbit antihuman VWF polyclonal antibody and a secondary horseradish peroxidase-conjugated swine antirabbit IgG polyclonal antibody and enhanced chemiluminescence. NP denotes plasma pooled from 20 healthy individuals.
Phenotypic data for families A, B, and C
| Family member | AI:1 | AI:2 | AII:1 | BI:1 | BI:2 | BII:3 | CI:1 | CI:2 | CII:6 | Normal range |
| Bleeding time (min) | 5 | 4 | 15 | 6 | 4 | 15 | 2.5 | nd | >20 | <8 |
| VWF:Ag (IU/mL) | 0.43 | 0.45 | 0.06 | 0.54 | 0.51 | 0.09 | 0.47 | 0.79 | <0.01 | 0.46-1.46 |
| FVIII:Ag (IU/mL) | 0.69 | 0.71 | 0.18 | 1.03 | 0.70 | 0.33 | 0.91 | 1.66 | 0.06 | 1.0 (mean value) |
| FVIII:C (IU/mL) | 0.60 | 0.33 | 0.20 | 0.98 | 0.58 | 0.21 | 0.70 | nd | 0.09 | 0.52-1.40 |
| VWF:RiCof (IU/mL) | 0.36 | 0.25 | 0.06 | 0.42 | 0.45 | 0.04 | 0.60 | nd | <0.01 | 0.5-1.72 |
| ABO blood group | O/O | O/O | O/O | A/O | A/O | A/A | A/A | A/A | A/A |
| Family member | AI:1 | AI:2 | AII:1 | BI:1 | BI:2 | BII:3 | CI:1 | CI:2 | CII:6 | Normal range |
| Bleeding time (min) | 5 | 4 | 15 | 6 | 4 | 15 | 2.5 | nd | >20 | <8 |
| VWF:Ag (IU/mL) | 0.43 | 0.45 | 0.06 | 0.54 | 0.51 | 0.09 | 0.47 | 0.79 | <0.01 | 0.46-1.46 |
| FVIII:Ag (IU/mL) | 0.69 | 0.71 | 0.18 | 1.03 | 0.70 | 0.33 | 0.91 | 1.66 | 0.06 | 1.0 (mean value) |
| FVIII:C (IU/mL) | 0.60 | 0.33 | 0.20 | 0.98 | 0.58 | 0.21 | 0.70 | nd | 0.09 | 0.52-1.40 |
| VWF:RiCof (IU/mL) | 0.36 | 0.25 | 0.06 | 0.42 | 0.45 | 0.04 | 0.60 | nd | <0.01 | 0.5-1.72 |
| ABO blood group | O/O | O/O | O/O | A/O | A/O | A/A | A/A | A/A | A/A |
Multimer analysis of plasma von Willebrand factor
Multimer analysis of equal volumes (2 μL) of plasma from the members of families A (Figure 1, iiA, lanes 1-3), B (Figure 1, iiB, lanes 1-3), and C (Figure 1, iiC, lanes 1-3) failed to detect multimers in plasma from all 3 propositi (AII:1, Figure 1, iiA, lane 1; BII:3, Figure 1, iiB, lane 1; CII:6, Figure 1, iiC, lane 1). All sizes of VWF multimers were detected in plasma from other family members (Figure 1). To determine whether failure to detect multimers in plasma from the propositi was due to the low concentration of VWF in the plasma or to the absence of VWF multimers, appropriate volumes of plasma containing identical amounts of VWF (0.5 mU) were analyzed (Figure 1, iiA, iiB, lanes 4 and 5) and compared with normal plasma. There was a reduction in intensity of high-molecular-weight multimers in plasma from the propositi of families A and B; in both cases, there was a marked increase in the intensity of the protomer band compared with that in normal plasma. Detection of VWF multimers in plasma from the propositus of family C (CII:6) was not possible because of the insufficient amounts of VWF present in the limited volume of plasma available.
Candidate mutation Arg273Trp
CSGE and CCMA were used to analyze all exons, exon/intron boundaries, and the promoter of the VWF gene in DNA from all 3 propositi. In all 3 cases, CSGE analysis detected a change in exon 7 that, on sequencing, was shown to result from a C to T transition at position 1067 of the VWF cDNA, predicting the substitution of arginine by tryptophan at amino acid 273 in pre–pro-VWF. All 3 propositi were homozygous for the C1067T transition, whereas the parents were heterozygous for the same defect. In the case of families A and B, the mutation was inherited with the 10 repeat allele of the intron 40 VNTR-1, whereas in family C, the propositus inherited the mutation with the paternal 6 repeat allele and the maternal 11 repeat allele of the intron 40 VNTR-1 and with the paternal 156-bp allele and the maternal 164-bp allele of the intron 40 VNTR-2. The occurrence of this nucleotide change on at least 3 separate occasions led us to examine whether it is a polymorphic change. DNA corresponding to exon 7 of the VWF gene was therefore amplified from genomic DNA samples from 35 Turkish individuals and 35 normal white individuals and screened for the C1067T transition. None of the 140 alleles screened carried this change, providing support for the C1067T transition being a mutation and not a rare polymorphism (results not shown).
Von Willebrand factor promoter polymorphisms
Different haplotypes of the single nucleotide polymorphisms, −1234C/T, −1185A/G, and −1051G/A of the VWF promoter, have been shown to be associated with plasma VWF levels.35To investigate a possible influence of these SNPs on VWF levels in plasma from members of families A, B, and C, we determined the promoter genotypes at nucleotides −1185 and −1051 for the propositi and their parents (Table 2). The propositi from all 3 families had the same genotype −1185GG/−1051AA.
VWF −1185A/G and −1051G/A promoter genotypes in members of families A, B, and C
| Family member . | Genotype . | |
|---|---|---|
| −1185 . | −1051 . | |
| AI:1 | G/G | A/A |
| AI:2 | A/G | G/A |
| AII:1* | G/G | A/A |
| AII:2 | A/G | G/A |
| BI:2 | G/G | A/A |
| BII:3* | G/G | A/A |
| CI:1 | A/G | G/A |
| CI:2 | G/G | A/A |
| CII:6* | G/G | A/A |
| Family member . | Genotype . | |
|---|---|---|
| −1185 . | −1051 . | |
| AI:1 | G/G | A/A |
| AI:2 | A/G | G/A |
| AII:1* | G/G | A/A |
| AII:2 | A/G | G/A |
| BI:2 | G/G | A/A |
| BII:3* | G/G | A/A |
| CI:1 | A/G | G/A |
| CI:2 | G/G | A/A |
| CII:6* | G/G | A/A |
Asterisk denotes the propositi from each family.
Steady-state analysis of rVWFR273W secretion
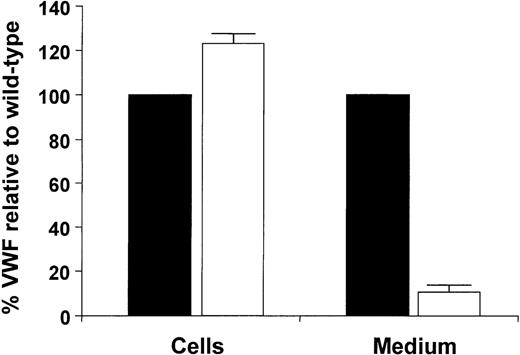
To investigate the synthesis and secretion of VWF having the R273W amino acid substitution, the expression vectors pSVHVWF1 and pSVVWFR273W were used to transfect COS-7 cells. Intracellular and secreted rVWFR273W levels were quantified by enzyme-linked immunosorbent assay (ELISA) and compared with those of wild-type rVWF (Figure 2).26 27 Secretion of rVWFR273W was decreased to 10% ± 4 (n = 4) and intracellular levels of rVWFR273W were increased to 122% ± 3 (n = 4) relative to the wild-type rVWF.
Steady-state analysis of rVWFR273W expressed in COS-7 cells.
The cells were transfected with the appropriate plasmid DNA, then placed in serum-free medium 24 hours after transfection. Cell lysates and medium supernatants were harvested after an additional 48 hours and the quantities of VWF in each fraction were measured by ELISA. Each bar represents the average values with Sds of 4 transfections and are expressed as percentages relative to wild-type VWF in 1 mL of lysate (26 mU ± 1 mU, n = 4) or 5 mL medium (69 mU ± 10 mU, n = 4). Wild-type is indicated by ▪, Arg273Trp is indicated by □.
Steady-state analysis of rVWFR273W expressed in COS-7 cells.
The cells were transfected with the appropriate plasmid DNA, then placed in serum-free medium 24 hours after transfection. Cell lysates and medium supernatants were harvested after an additional 48 hours and the quantities of VWF in each fraction were measured by ELISA. Each bar represents the average values with Sds of 4 transfections and are expressed as percentages relative to wild-type VWF in 1 mL of lysate (26 mU ± 1 mU, n = 4) or 5 mL medium (69 mU ± 10 mU, n = 4). Wild-type is indicated by ▪, Arg273Trp is indicated by □.
Pulse-chase analysis of rVWFR273W secretion
To further investigate the reduced secretion of rVWFR273W from COS-7 cells, a pulse-chase approach was adopted. Transfected COS-7 cells were pulse-labeled for 20 minutes, then chased for various times up to 96 hours in unlabeled growth medium. The labeled VWF in cell lysates and medium samples was immunoprecipitated and analyzed by SDS-PAGE and autoradiography (Figure3). Immediately after the pulse (0 hour of chase, Figure 3), similar amounts of wild-type rVWF and rVWFR273W were immunoprecipitatedfrom the cell lysates, indicating that an equivalent amount of rVWFR273W was synthesized compared with wild-type rVWF. In the case of wild-type rVWF, there was a decrease in the amount of VWF immunoprecipitated from the cells over the 96-hour time course and a concomitant increase in the amount of VWF immunoprecipitated from the medium until after 96 hours, when most of the wild-type rVWF had been chased out of the cells into the medium. In the case of rVWFR273W, similar to that with wild-type rVWF, there was a decrease in the amount of VWF immunoprecipitated from the cells over the 96-hour time course until no detectable rVWFR273W remained. However, over the 96-hour time course, rVWFR273W was only weakly detected in immunoprecipitates from the medium compared with wild-type rVWF, suggesting impaired secretion from the COS-7 cells.
Pulse-chase analysis of rVWFR273W secretion from COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 20 minutes with [35S]-methionine and [35S]-cysteine, then chased for various times in the presence of nonradioactive methionine and cysteine. Radiolabeled cell lysates and medium samples were immunoprecipitated with a rabbit polyclonal antiserum to VWF. Immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. Upper bar denotes the position of pro-VWF, lower bar denotes the position of mature propeptide cleaved VWF.
Pulse-chase analysis of rVWFR273W secretion from COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 20 minutes with [35S]-methionine and [35S]-cysteine, then chased for various times in the presence of nonradioactive methionine and cysteine. Radiolabeled cell lysates and medium samples were immunoprecipitated with a rabbit polyclonal antiserum to VWF. Immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. Upper bar denotes the position of pro-VWF, lower bar denotes the position of mature propeptide cleaved VWF.
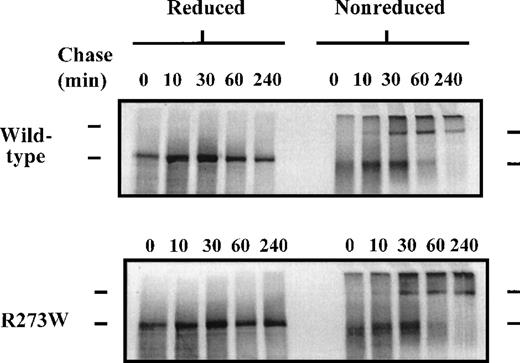
Pulse-chase analysis of rVWFR273W dimer formation
To determine the effect of the R273W mutation on the ability of rVWFR273W to form disulphide-linked dimers in the ER, transfected COS-7 cells were pulse-labeled for 20 minutes, then chased for various times up to 240 minutes in unlabeled growth medium. The labeled VWF in the cell lysates was immunoprecipitated and analyzed by reducing and nonreducing SDS-PAGE (Figure 4). Immediately after the pulse (Figure 4, 0-minute chase), both rVWF and rVWFR273W, when separated under reducing conditions, migrated as a single band of approximately 350 kd, corresponding to monomeric pro-VWF. Identical samples separated under nonreducing conditions migrated as a single diffuse band of approximately 350 kd, indicating that, immediately after the 20 minute pulse, both rVWF and rVWFR273W existed predominantly as pro-VWF monomers. The slight increase in mobility and the diffuse banding pattern of the VWF monomers when separated under nonreducing conditions, compared with identical samples separated under reducing conditions, indicated that intrachain disulphide bonds were present in the monomers immediately after the pulse. After 10 minutes of chase, a faint band of approximately 700 kd was detected in both rVWF and rVWFR273W immunoprecipitates separated under nonreducing conditions but not reducing conditions, indicating VWF dimers had begun to form 10 minutes after the pulse. There was no difference between the rVWF and rVWFR273W immunoprecipitates in either the increase in intensity of the dimer band or the decrease in intensity of the monomer band up to 240 minutes under nonreducing conditions. It was therefore concluded that the R273W mutation had no significant effect on the ability of VWF to form disulphide-linked dimers.
Pulse-chase analysis of dimerization of rVWFR273W in COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA and pulse-chased as in Figure 3. Radiolabeled cell lysates were immunoprecipitated with a rabbit polyclonal antiserum to VWF. Immunoprecipitates were divided into 2 aliquots, then separated by electrophoresis on 6% reducing and nonreducing SDS-polyacrylamide gels. Upper bar denotes the position of pro-VWF dimers, lower bar denotes the position of pro-VWF monomers.
Pulse-chase analysis of dimerization of rVWFR273W in COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA and pulse-chased as in Figure 3. Radiolabeled cell lysates were immunoprecipitated with a rabbit polyclonal antiserum to VWF. Immunoprecipitates were divided into 2 aliquots, then separated by electrophoresis on 6% reducing and nonreducing SDS-polyacrylamide gels. Upper bar denotes the position of pro-VWF dimers, lower bar denotes the position of pro-VWF monomers.
Multimer analysis of rVWFR273W
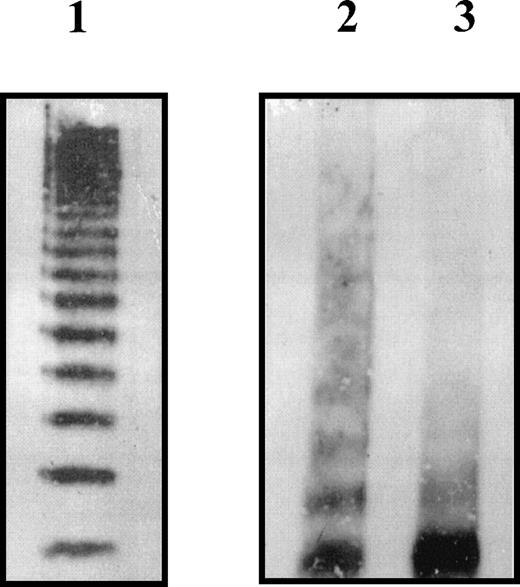
We examined the effects of the R273W amino acid substitution on VWF multimer structure by SDS-agarose electrophoresis of VWF secreted by COS-7 cells (Figure 5). Wild-type rVWF exhibited a full range of multimers when the appropriate volume of COS-7 growth medium containing 1 mU rVWF was analyzed (Figure 5, lane 1). Because of the inefficient secretion of rVWFR273W, it was not possible to analyze directly the appropriate volume of COS-7 growth medium containing 1 mU rVWFR273W. Instead, appropriate volumes of growth medium containing equivalent amounts of wild-type rVWF and rVWFR273W (10 mU) were immunoprecipitated with rabbit antihuman VWF polyclonal antibody before analysis by SDS-agarose electrophoresis (Figure 5, lanes 2 and 3). The multimer pattern of immunoprecipitated wild-type rVWF (Figure 5, lane 2) had a more smeared appearance when compared with wild-type rVWF electrophoresed without prior immunoprecipitation (Figure 5, lane 1). However, rVWFR273W (Figure 5, lane 3) showed a marked increase in the proportion of low-molecular-weight multimers compared with wild-type rVWF (Figure 5, lane 2).
Multimer analysis of rVWFR273W secreted by COS-7 cells.
The cells were transfected with the appropriate plasmid DNA then placed in serum-free medium 24 hours after transfection. Medium supernatants were harvested after an additional 48 hours. An appropriate volume of medium containing 1 mU of rVWF wild-type as determined by ELISA was analyzed on a nonreducing 2% SDS-agarose gel (lane 1). Ten milliunits of each rVWF as determined by ELISA was immunoprecipitated with rabbit antihuman VWF polyclonal antibody, then analyzed on a nonreducing 2% SDS-agarose gel (lane 2 wild-type rVWF, lane 3 rVWFR273W). Multimers were visualized using polyclonal rabbit antihuman VWF antibody and a secondary HRP-conjugated swine antirabbit IgG polyclonal antibody and enhanced chemiluminescence.
Multimer analysis of rVWFR273W secreted by COS-7 cells.
The cells were transfected with the appropriate plasmid DNA then placed in serum-free medium 24 hours after transfection. Medium supernatants were harvested after an additional 48 hours. An appropriate volume of medium containing 1 mU of rVWF wild-type as determined by ELISA was analyzed on a nonreducing 2% SDS-agarose gel (lane 1). Ten milliunits of each rVWF as determined by ELISA was immunoprecipitated with rabbit antihuman VWF polyclonal antibody, then analyzed on a nonreducing 2% SDS-agarose gel (lane 2 wild-type rVWF, lane 3 rVWFR273W). Multimers were visualized using polyclonal rabbit antihuman VWF antibody and a secondary HRP-conjugated swine antirabbit IgG polyclonal antibody and enhanced chemiluminescence.
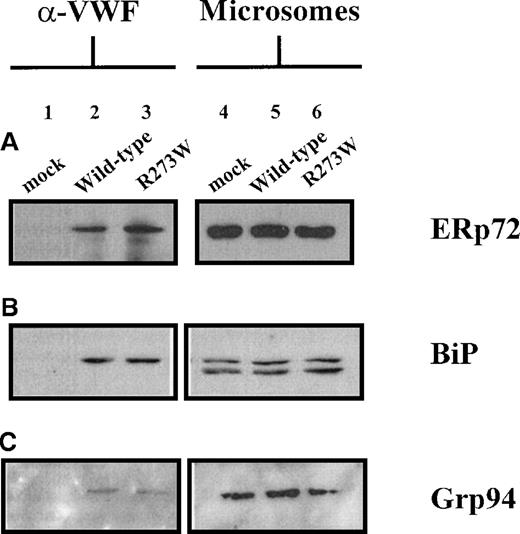
Interaction of rVWFR273W with BiP, Grp94, and ERp72
Several studies have shown that, during synthesis, VWF interacts transiently with the ER proteins BiP (Grp78), Grp94, and ERp7222-24 that assist folding and assembly of secretory proteins and retain incompletely folded proteins in the ER. To determine whether rVWFR273W interacts with BiP, Grp94, and ERp72, lysates prepared from COS-7 cells transfected with pSVHVWF1, or pSVVWFR273W or mock transfected, were immunoprecipitated with an anti-VWF polyclonal antibody. The resulting immunoprecipitates were subjected to reducing SDS-PAGE, then immunoblotted with antibodies raised to BiP, Grp94, or ERp72 (Figure 6). To provide positive detection of BiP, Grp94, and ERp72 by immunoblotting, ER-derived microsomes were prepared from duplicate COS-7 transfectants. Microsomes prepared from mock-transfected COS-7 cells (Figure 6A-C, lane 4), COS-7 cells transfected with wild-type pSVHVWF1 (Figure 6A-C, lane 5), or pSVVWFR273W (Figure 6A-C, lane 6) contained species of 72 kd (Figure 6A, lanes 4-6), 78 kd (Figure 6B, lanes 4-6), and 100 kd (Figure 6C, lanes 4-6), corresponding to ERp72, BiP, and Grp94, respectively. The immunoblot for BiP detection in microsomes also showed a faster migrating 70 kd species (Figure 6B, lanes 4-6). This represented contaminating cytosolic heat shock cognate protein (Hsc70), which cross-reacts with the antibody because the peptide immunogen used to raise the antibody (KEDVGTTVVGIDLGTTYSCVG) contains 13 residues (shown in bold) that are 100% conserved across the mammalian Grp78/Hsp70 family. VWF immunoprecipitates from wild-type pSVHVWF1 (Figure 6A-C, lane 2) and pSVVWFR273W (Figure 6A-C, lane 3) transfected COS-7 cells contained species corresponding to ERp72, BiP, and Grp94. In the case of Grp94, the bands were barely visible above the background on repeated occasions (Figure 6C, lanes 2,3). rVWFR273W and wild-type rVWF coimmunoprecipitated similar amounts of each chaperone, demonstrating that there was no detectable difference in the amount of each chaperone protein interacting with rVWFR273W and wild-type rVWF. The absence of the 70 kd species in VWF immunoprecipitates immunoblotted for BiP reflects the expected lack of interaction between ER resident VWF and the cytosolic chaperone Hsc70 (Figure 6B, lanes 2,3). The absence of any species in VWF immunoprecipitates from mock-transfected COS-7 cells (Figure 6A-C, lane 1) demonstrated that ERp72, BiP, and Grp94 precipitated with VWF antibodies was not due to nonspecific precipitation but represented bona fide interactions of rVWFR273W and wild-type rVWF with ERp72, BiP, and Grp94.
Detection of ERp72, BiP, and Grp94 in anti-VWF immunoprecipitates of rVWFR273W from COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA. Seventy-two hours after transfection, lysates were prepared from the cells and immunoprecipitated with polyclonal rabbit antihuman antibody as detailed in the “Materials and methods” section. Immunoprecipitates from mock (lane 1), pSVHVWF1 (lane 2), and pSVVWFR273W (lane 3) transfected cells were separated by electrophoresis on 10% reducing SDS-polyacrylamide gels. Microsomes (2 μL per lane) purified from mock (lane 4), pSVHVWF1 (lane 5), and pSVVWFR273W (lane 6) transfected cells were included to provide positive detection of ERp72, BiP, and Grp94. ERp72 (A), BiP (B), and Grp94 (C) were detected by immunoblotting as detailed in the “Materials and methods” section. ERp72 (A) and Grp94 (C) immunoblots were exposed to film for 5 minutes, whereas BiP (B) immunoblots were exposed for 2 minutes.
Detection of ERp72, BiP, and Grp94 in anti-VWF immunoprecipitates of rVWFR273W from COS-7 cells.
COS-7 cells were transfected with the appropriate plasmid DNA. Seventy-two hours after transfection, lysates were prepared from the cells and immunoprecipitated with polyclonal rabbit antihuman antibody as detailed in the “Materials and methods” section. Immunoprecipitates from mock (lane 1), pSVHVWF1 (lane 2), and pSVVWFR273W (lane 3) transfected cells were separated by electrophoresis on 10% reducing SDS-polyacrylamide gels. Microsomes (2 μL per lane) purified from mock (lane 4), pSVHVWF1 (lane 5), and pSVVWFR273W (lane 6) transfected cells were included to provide positive detection of ERp72, BiP, and Grp94. ERp72 (A), BiP (B), and Grp94 (C) were detected by immunoblotting as detailed in the “Materials and methods” section. ERp72 (A) and Grp94 (C) immunoblots were exposed to film for 5 minutes, whereas BiP (B) immunoblots were exposed for 2 minutes.
Interaction of wild-type rVWF and rVWFR273W with calnexin and calreticulin
Previous studies have demonstrated that the ER membrane protein calnexin and its soluble luminal homologue calreticulin serve to assist the folding of newly synthesized glycoproteins and retain misfolded mutant glycoproteins.42-44 To investigate the possibility that calnexin and calreticulin play a role in VWF maturation and participate in the retention of rVWFR273W, we performed coimmunoprecipitation studies to determine whether wild-type rVWF and rVWFR273W interact with calnexin and calreticulin. COS-7 cells transfected with wild-type pSVHVWF1 or pSVVWFR273W were radiolabeled. Lysates prepared from these transfectants were initially immunoprecipitated with antibodies raised to calnexin (Figure7, lanes 1-4), calreticulin (Figure 7, lanes 7-10), VWF (Figure 7, lanes 11,12), and a preimmune serum prepared from nonimmunized rabbits (Figure 7, lanes 5,6). The calnexin (Figure 7, lanes 1,2) and calreticulin (Figure 7, lanes 7, 8) immunoprecipitates contained many radiolabeled protein bands corresponding to a pool of newly synthesized interacting glycoproteins and included a species that migrated with an identical mobility to pro-VWF (Figure 7, lanes 11,12). To determine whether pro-VWF was present in the calnexin (Figure 7, lanes 1,2) and calreticulin (Figure7, lanes 7,8) immunoprecipitates, identical calnexin and calreticulin immunoprecipitates were reimmunoprecipitated with an antibody to VWF (Figure 7, lanes 3,4,9,10). The secondary VWF immunoprecipitates from primary calnexin immunoprecipitates showed faint bands corresponding to wild-type pro-VWF (Figure 7, lane 3) and R273W pro-VWF (Figure 7, lane 4), whereas those from primary calreticulin immunoprecipitates showed strong bands corresponding to wild-type pro-VWF (Figure 7, lane 9) and R273W pro-VWF (Figure 7, lane 10). Densitometry of the pro-VWF bands in the VWF immunoprecipitates revealed that 2% of the total radiolabeled wild-type pro-VWF (Figure 7, lane 3) and 2% of the total radiolabeled R273W pro-VWF (Figure 7, lane 4) were coimmunoprecipitated with antibodies to calnexin, whereas 25% of the total radiolabeled wild-type pro-VWF (Figure 7, lane 9) and 25% of the total radiolabeled R273W pro-VWF (Figure 7, lane 10) were coimmunoprecipitated with antibodies to calreticulin. The absence of pro-VWF in the immunoprecipitates obtained using preimmune serum (Figure 7, lanes 5,6) demonstrated that the VWF did not coimmunoprecipitate with antibodies to calnexin and calreticulin because of nonspecific interaction of VWF with the serum, but that it represented bona fide interactions of calnexin and calreticulin with VWF.
Coimmunoprecipitation of wild-type rVWF and rVWFR273W from COS-7 cells with anticalnexin and anticalreticulin.
COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 90 minutes with [35S]-methionine and [35S]-cysteine. Radiolabeled cell lysates were immunoprecipitated with rabbit polyclonal antiserum to calnexin (N) (lanes 1, 2, 3, and 4), rabbit serum prepared from nonimmunized rabbits (P) (lanes 5 and 6), rabbit polyclonal antiserum to calreticulin (R) (lanes 7, 8, 9, and 10), and rabbit polyclonal antiserum to VWF (V) (lanes 11 and 12). Wild-type (odd-numbered lanes) and R273W (even-numbered lanes) immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. For calnexin (lanes 1, 2, 3, and 4) and calreticulin (lanes 7, 8, 9, and 10) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from two 10-cm dishes of COS-7 cells. For preimmune (lanes 5 and 6) and VWF (lanes 11 and 12) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from half a 10-cm dish of COS-7 cells. Bars denote positions of Pro-VWF, calnexin (CNX), and calreticulin (CRT).
Coimmunoprecipitation of wild-type rVWF and rVWFR273W from COS-7 cells with anticalnexin and anticalreticulin.
COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 90 minutes with [35S]-methionine and [35S]-cysteine. Radiolabeled cell lysates were immunoprecipitated with rabbit polyclonal antiserum to calnexin (N) (lanes 1, 2, 3, and 4), rabbit serum prepared from nonimmunized rabbits (P) (lanes 5 and 6), rabbit polyclonal antiserum to calreticulin (R) (lanes 7, 8, 9, and 10), and rabbit polyclonal antiserum to VWF (V) (lanes 11 and 12). Wild-type (odd-numbered lanes) and R273W (even-numbered lanes) immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. For calnexin (lanes 1, 2, 3, and 4) and calreticulin (lanes 7, 8, 9, and 10) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from two 10-cm dishes of COS-7 cells. For preimmune (lanes 5 and 6) and VWF (lanes 11 and 12) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from half a 10-cm dish of COS-7 cells. Bars denote positions of Pro-VWF, calnexin (CNX), and calreticulin (CRT).
Discussion
We have studied 3 Turkish families previously diagnosed with types 1 and 3 VWD. Analysis of the VWF gene in DNA from affected members of all 3 families identified a C to T transition in exon 7 at nucleotide 1067 of the VWF cDNA that predicted substitution of arginine by tryptophan at amino acid position 273 (R273W) of pre–pro-VWF. In the case of the propositi in families A and B, previously diagnosed with type 1 VWD, the defect was inherited on the 10 repeat allele of the intron 40 VNTR-1 from both parents, suggesting that the propositi from families A and B may have inherited 2 copies of the same VWF allele and that the families may have been related to a common ancestor. In contrast, the propositus from family C, previously diagnosed with type 3 VWD, inherited the defect on 2 different parental alleles, which in turn were different from those on which the defect was inherited in families A and B. The repeat occurrence of this mutation on at least 3 different alleles in these families may reflect its location in a CpG dinucleotide, which has a 12-fold higher mutation frequency than other sequences.45
We reasoned that the differences in VWF levels in plasma from the propositus of family C, compared with those of the propositi of families A and B, may be linked to another factor. The SNPs of the VWF gene promoter, −1234C/T, −1185A/G, and −1051G/A, are known to influence plasma VWF levels, at least in individuals with blood group O.35 Highest plasma VWF levels are associated with the CC/AA/GG genotype, intermediate levels are associated with the CT/AG/GA genotype, and lowest levels are associated with the TT/GG/AA genotype. This prompted us to analyze DNA encoding the VWF gene promoter from members of all 3 families to determine the promoter genotypes of the families. However, as all 3 propositi had identical genotypes, we could not account for the differences in plasma VWF antigen levels on the basis of the promoter SNPs determined here. Previous studies have demonstrated a relationship between ABO blood group and plasma VWF antigen levels with blood group O associated with the lowest plasma VWF antigen levels, blood group AB associated with the highest levels, and blood groups A and B associated with intermediate levels.46 To investigate the possibility that ABO blood group was responsible for the differences in VWF levels in plasma associated with the R273W mutation, we determined the ABO genotypes of the propositi and their parents. However, as both propositi BII:3 and CII:6 were homozygous for the A allele and propositus AII:1 was homozygous for the O allele, we could not account for the differences in plasma VWF antigen levels on the basis of ABO blood group alone. Analysis of all 52 exons, exon/intron boundaries and 5′ and 3′ noncoding regions only detected 1 mutation in all 3 families. The reason for the more severe phenotype in family C is therefore unknown. It could reflect differences in nonanalyzed regions of the VWF gene. Although we cannot rule out polymorphic differences in the exons, we did not note any further differences in the coding regions with the screening methodologies used here. Finally, phenotypic differences between the propositi may have been due to the presence of as yet unidentified plasma VWF level modifying loci outside of the VWF gene.
To determine whether the R273W substitution could be responsible for the quantitative plasma VWF deficiencies in these families, mutated VWF molecules were expressed in COS-7 cells and analyzed for their ability to be secreted compared with normal VWF. Both steady-state and pulse-chase analysis of rVWFR273W secretion showed a relatively small amount of rVWFR273W present in the growth medium of COS-7 cells in comparison to wild-type rVWF. It is likely that the lack of rVWFR273W in the medium is a consequence of a block in intracellular transport leading to impaired secretion. Reduced rVWF secretion has been demonstrated previously for a number of variants associated with quantitative deficiencies of VWF.24,26 Pulse-chase analysis revealed that the R273W substitution had no effect on the ability of VWF to form disulphide-linked dimers. In contrast, the R273W substitution had a dramatic effect on the ability of VWF to form disulphide-linked multimers. Previous studies have shown the importance of the propeptide in the formation of interchain disulphide bonds between N-terminal regions of the mature VWF polypeptide. Mutations in the propeptide while impairing multimerization resulted in the secretion of dimers.10,12 Here we have demonstrated that the role of the propeptide in multimerization is impaired as a consequence of the R273W substitution. Similarly, there are several reported VWD-associated missense mutations in the mature VWF subunit that result in impaired multimerization.24 26
An increasing number of studies have shown that a subset of inherited human diseases associated with quantitative protein deficiencies result from misfolding of membrane and secretory proteins.42-44,47-49 In such cases, misfolding of mutated protein is often followed by binding to molecular chaperones of the ER. As well as their role in retention of normal immature proteins in the ER, molecular chaperones have been shown to be involved in the retrograde transport of misfolded mutant proteins from the ER into the cytosol where they are destroyed by the proteasome complex.50 Transient interactions of wild-type VWF with the ER chaperones BiP, Grp94, and ERp72 have been shown previously. However, the role of these proteins in the normal folding and assembly of VWF in the ER is not understood.22-24 Demonstration of the interaction of BiP with type 2A VWD, causing mutants that undergo impaired intracellular transport, has suggested a possible role for BiP in the quality control of VWF secretion.24 Here, too, we demonstrated the interaction of wild-type and mutated VWF with BiP, Grp94, and ERp72. However, there was no significant difference between wild-type and mutated VWF in their steady-state levels of binding to all 3 molecular chaperones. As misfolding of VWF, followed by intracellular retention, is the most likely mechanism of impaired secretion of rVWFR273W, we wanted to identify novel factors that may facilitate intracellular retention. The extensive N-linked glycosylation of each VWF subunit and previous studies42-44highlighting the importance of the glycosylation-specific molecular chaperones calnexin and calreticulin in secretory protein quality control made these proteins obvious candidates for investigation. Both wild-type rVWF and rVWFR273W were shown to interact with calnexin and calreticulin. Significantly large proportions (25%) of total radiolabeled wild-type rVWF and rVWFR273W were coprecipitated with calreticulin, whereas small amounts (2%) of total radiolabeled wild-type rVWF and rVWFR273W were coprecipitated with calnexin. There was no significant difference between wild-type rVWF and rVWFR273W in the amount of VWF that coprecipitated with both calnexin and calreticulin, indicating that the mutation did not affect the extent of binding to either chaperone in COS-7 cells. Given the high level of rVWF expression in transiently transfected COS-7 cells compared with endogenous VWF expression by endothelial cells and megakaryocytes in vivo, the experimental system described here may not reveal subtle differences in the extent of binding to a chaperone protein between wild-type and variant rVWF.
In conclusion, we have identified a missense mutation (R273W) in VWF that results in a quantitative deficiency of plasma VWF and defective multimerization. Recombinant expression studies showed that the mutation caused impaired secretion of VWF, confirming that it was responsible for the quantitative VWF deficiency in patient plasma. The failure of the mutant protein to form a full range of high-molecular-weight multimers confirmed that the mutation was sufficient to cause the aberrant multimer pattern in patient plasma. The mutated protein was shown to interact with 5 chaperone proteins of the ER quality control apparatus. Two of these interactions, with calnexin and calreticulin, were novel. In particular, a significantly large proportion of normal and mutant VWF exhibited interaction with calreticulin. Future studies are therefore required to determine the role of these molecular chaperones during the synthesis of normal VWF and in quality control mechanisms operating during the synthesis of disease-associated VWF variants.
Acknowledgments
We thank Dr Aida Inbal (Tel Aviv, Israel) for providing pSVHVWF1, Professor Neil Bulleid (Manchester, UK) for providing anticalnexin polyclonal antibody, Hazel Holden for oligonucleotide synthesis and automated sequencing, and Dr Mike Makris and Dr Eddie Hampton for useful discussion of this work.
Supported by a project grant from the British Heart Foundation (grant number PG97016).
Reprints:Simon Allen, Division of Molecular and Genetic Medicine, Royal Hallamshire Hospital, Glossop Rd, Sheffield S10 2JF, UK; e-mail: simon.allen@sheffield.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 3. Pulse-chase analysis of rVWFR273W secretion from COS-7 cells. / COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 20 minutes with [35S]-methionine and [35S]-cysteine, then chased for various times in the presence of nonradioactive methionine and cysteine. Radiolabeled cell lysates and medium samples were immunoprecipitated with a rabbit polyclonal antiserum to VWF. Immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. Upper bar denotes the position of pro-VWF, lower bar denotes the position of mature propeptide cleaved VWF.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.560/5/m_bloo01401003w.jpeg?Expires=1768252657&Signature=OWbrAY2PIk0IfbBz4yVyMjLPtoYMVoamLN8wx6i4vo10ZaUzVyjCMKunApx27r6EfvenRTCGAbs~Gy7FjbLJCJMJenZS75fJXM-chfEfoOjixv0-t9p7xSOn8DTgJ8XCvyNA5cX8Ep9mUELWfO8r9xgPoYwM1-H8OErfeTQMZD4ALpCEmi1sHLQ~0UPnzdgcbv4-p4ClW3p6sJoWkXW1h7iubxot~6nIRpUQS5sSuEbMkUdkRG9cWT75x-e~0qN0GaSByo3ArHFpIY-XodBVC-IUV56OMfYihFlUeSuy00Eg3orHZm8SEylMq7BByWu4aXcToOe9ATZXL28JfVQksw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Coimmunoprecipitation of wild-type rVWF and rVWFR273W from COS-7 cells with anticalnexin and anticalreticulin. / COS-7 cells were transfected with the appropriate plasmid DNA. Forty-eight hours after transfection, the cells were radiolabeled for 90 minutes with [35S]-methionine and [35S]-cysteine. Radiolabeled cell lysates were immunoprecipitated with rabbit polyclonal antiserum to calnexin (N) (lanes 1, 2, 3, and 4), rabbit serum prepared from nonimmunized rabbits (P) (lanes 5 and 6), rabbit polyclonal antiserum to calreticulin (R) (lanes 7, 8, 9, and 10), and rabbit polyclonal antiserum to VWF (V) (lanes 11 and 12). Wild-type (odd-numbered lanes) and R273W (even-numbered lanes) immunoprecipitates were separated by electrophoresis on 6% reducing SDS-polyacrylamide gels. For calnexin (lanes 1, 2, 3, and 4) and calreticulin (lanes 7, 8, 9, and 10) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from two 10-cm dishes of COS-7 cells. For preimmune (lanes 5 and 6) and VWF (lanes 11 and 12) immunoprecipitates, each lane contained the amount of protein immunoprecipitated from half a 10-cm dish of COS-7 cells. Bars denote positions of Pro-VWF, calnexin (CNX), and calreticulin (CRT).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.560/5/m_bloo01401007w.jpeg?Expires=1768252657&Signature=PUSpFcbNNLshpqTZyDTMPWphq6EbjUF~xOvNoxzFWU4TpurkZDPx5is3Da3-wwcTd6yjyPJcXHwcJZ8ALkQr4TKUZqt-FJDV3URULP67EX02ZYDsgEfry1EmdYMWXTNzaQdGnKeetMI0dK-47URlw12Wdjr9I~H~MuEbSDto-QUy~WtmvfcPNMD2IBHL~~nag~u2N-bkcyjLrnl12oHlI6OHRLTRS5uKYsmhwuhwchJxx8ca-0S9rg9tGIp9T92BMDT~OAB6PiUaGbuoxsiQ9ZQMxTLmm3jV2rkS1HNZOzMJPHzzwlkgG~aGlKkexkRUiMDCg1yOc3J5L77G7TbsRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal