Abstract
Lymphoid and dendritic cells of donor origin can be detected in the recipient several years after a solid organ transplantation. This phenomenon is termed microchimerism and could play a role in the induction of tolerance. The fate of other hematopoietic cells transferred by liver transplantation, in particular of stem and progenitor cells, is unknown. For this reason, we studied peripheral blood and bone marrow samples of 12 patients who had received a liver transplant from an HLA-DR mismatched donor. Eight patients were long-term survivors between 2.8 and 10.1 years after allografting. CD34+ cells from bone marrow were highly enriched with the use of a 2-step method, and a nested polymerase chain reaction was applied to detect donor cells on the basis of allelic differences of the HLA-DRB1 gene. Rigorous controls with DRB1 specificities equal to the donor and host were included. In 5 of 8 long-term liver recipients, donor-specific CD34+ cells could be detected in bone marrow. Microchimerism in the CD34+ cell fraction did not correlate to the chimeric status in peripheral blood. In conclusion, our results demonstrate a frequent microchimerism among bone marrow–derived CD34+ cells after liver transplantation. The functional role of this phenomenon still needs to be defined.
The liver is a site of hematopoiesis in fetuses and, under certain pathologic conditions, in adults. Evidence for the presence of hematopoietic stem cells (HSCs) in the adult liver comes from murine transplantation studies.1,2 Taniguchi et al identified Sca1+ c-kit+ cells in the adult mouse liver, which were capable of reconstituting bone marrow of lethally irradiated recipient mice.1 In the same line, lethally irradiated rats could be reliably rescued by orthotopic liver transplantation.2 Recently, Crosbie et al detected and characterized CD34+ HSCs in the adult human liver by flow cytometry and tissue culture, showing multilineage hematopoietic colony formation.3 CD34 expression was found on 0.81% to 2.35% of isolated hepatic mononuclear cells by flow cytometry, and about 50% of these cells expressed CD38 as a marker for lineage commitment.3 These data suggest that the liver of the adult may still be an important homing site for lymphohematopoietic cells. The contribution of liver-derived HSCs to hematopoiesis is not known. Liver transplantation can serve as a model to study the fate of lymphohematopoietic cells derived from the liver.
Starzl et al demonstrated the persistence of donor cells in peripheral blood, lymph nodes, skin, and bone marrow even years after liver transplantation, a phenomenon that is called microchimerism.4-6 Donor leukocytes are readily detectable in the recipient's blood in the first days after transplantation but usually decrease thereafter to a level undetectable by flow cytometry.7 The transplantation of immunocompetent cells along with the liver may result in graft versus host disease (GvHD), an immunologic reaction against the recipient. Rare cases of GvHD after liver transplantation have been reported in the literature, and complete chimerism of peripheral blood in these patients has been observed.8-10
The immunologic and clinical relevance of microchimerism still remains a contentious issue. It has been postulated that microchimerism may play a role in the induction of tolerance and hence result in a reduction of rejection episodes and immunosuppressive therapy.11 12
We hypothesized that human hematopoietic stem and progenitor cells are transferred by liver transplantation and migrate to the recipient's bone marrow. Therefore, we studied bone marrow–derived CD34+ cells and peripheral blood of patients who had received a liver transplant 2.8 to 10.1 years ago to look for the presence of donor cells using a nested polymerase chain reaction (PCR) for major histocompatibility complex class II polymorphism.
Patients and methods
Patients
We enrolled 12 patients in our study after obtaining informed consent from each. Eight patients had received a liver transplant at least 2 years earlier. The median time after transplantation was 5.1 years, with a range from 2.8 to 10.1 years. We studied 4 patients before and 2 to 4 months following transplantation.
The patients' characteristics are detailed in Table1. Clinical data, such as the number of rejection episodes and immunosuppressive therapy at the time of the study, are listed in Table 2. The patients were treated in the Departments of Surgery and Internal Medicine of the University of Heidelberg.
Patient characteristics
| Patient . | Indication for OLT . | Years after OLT . | Sex . | Age at OLT . | HLA-DRB . | Donor . | HLA-DRB . | |
|---|---|---|---|---|---|---|---|---|
| Sex . | Age . | |||||||
| 1 | HCC; HBV-cirrhosis | 5.1 | M | 29 | 1;15 | M | 23 | 17;13 |
| 2 | HBV-cirrhosis | 4.8 | M | 31 | 11;— | M | 27 | 7;11 |
| 3 | Cirrhosis of unknown origin | 2.8 | F | 16 | 17;4 | M | 8 | 7;11 |
| 4 | Porphyria | 5.0 | F | 59 | 8;12 | F | 38 | 10;11 |
| 5 | PBC | 6.6 | F | 52 | 1;14 | F | 16 | 4;11 |
| 6 | Toxic liver failure of unknown origin | 4.9 | F | 24 | 1;13 | F | 32 | 17;11 |
| 7 | PBC | 6.8 | F | 61 | 17;14 | M | 31 | 15;14 |
| 8 | Alcoholic cirrhosis | 10.1 | M | 46 | 4;7 | M | 30 | 3;7 |
| 9 | HCC; HCV-cirrhosis; alcoholic cirrhosis | 0.3 | M | 45 | 15;17 | M | 40 | 11;— |
| 10 | HCC; alcoholic cirrhosis | 0.2 | M | 47 | 13;— | M | 18 | 7;13 |
| 11 | HCC; HCV-cirrhosis | 0.3 | M | 43 | 4;7 | M | 50 | 1;11 |
| 12 | HCC; alcoholic cirrhosis | 0.3 | M | 47 | 4;11 | M | 26 | 7;— |
| Patient . | Indication for OLT . | Years after OLT . | Sex . | Age at OLT . | HLA-DRB . | Donor . | HLA-DRB . | |
|---|---|---|---|---|---|---|---|---|
| Sex . | Age . | |||||||
| 1 | HCC; HBV-cirrhosis | 5.1 | M | 29 | 1;15 | M | 23 | 17;13 |
| 2 | HBV-cirrhosis | 4.8 | M | 31 | 11;— | M | 27 | 7;11 |
| 3 | Cirrhosis of unknown origin | 2.8 | F | 16 | 17;4 | M | 8 | 7;11 |
| 4 | Porphyria | 5.0 | F | 59 | 8;12 | F | 38 | 10;11 |
| 5 | PBC | 6.6 | F | 52 | 1;14 | F | 16 | 4;11 |
| 6 | Toxic liver failure of unknown origin | 4.9 | F | 24 | 1;13 | F | 32 | 17;11 |
| 7 | PBC | 6.8 | F | 61 | 17;14 | M | 31 | 15;14 |
| 8 | Alcoholic cirrhosis | 10.1 | M | 46 | 4;7 | M | 30 | 3;7 |
| 9 | HCC; HCV-cirrhosis; alcoholic cirrhosis | 0.3 | M | 45 | 15;17 | M | 40 | 11;— |
| 10 | HCC; alcoholic cirrhosis | 0.2 | M | 47 | 13;— | M | 18 | 7;13 |
| 11 | HCC; HCV-cirrhosis | 0.3 | M | 43 | 4;7 | M | 50 | 1;11 |
| 12 | HCC; alcoholic cirrhosis | 0.3 | M | 47 | 4;11 | M | 26 | 7;— |
OLT indicates orthotopic liver transplantation; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; PBC, primary biliary cirrhosis; HCV, hepatitis C virus; —, blank on the locus; M, male; and F, female.
Chimerism and clinical course
| Patient . | Donor-specific cells in . | Rejection episodes . | Current immunosuppressive therapy . | |
|---|---|---|---|---|
| CD34+ cells . | PB . | |||
| 1 | Pos. | Pos. | 0 | CycloA |
| 2 | Pos. | Pos. | 3 | FK506 |
| 3 | Pos. | Pos. | 2 | FK506 |
| 4 | Pos. | Neg. | 2 | CycloA |
| 5 | Pos. | Neg. | 5 | CycloA |
| 6 | Neg. | Pos. | 3 | CycloA + MP |
| 7 | Neg. | Pos. | 3 | FK506 + MP |
| 8 | Neg. | Neg. | 3 | CycloA + MP |
| 9 | Pos. | Pos. | 1 | FK506 + MP |
| 10 | Neg. | Neg. | 0 | FK506 + MP |
| 11 | Neg. | Neg. | 2 | CycloA + MP |
| 12 | not evaluable | not evaluable | 0 | CycloA + MP |
| Patient . | Donor-specific cells in . | Rejection episodes . | Current immunosuppressive therapy . | |
|---|---|---|---|---|
| CD34+ cells . | PB . | |||
| 1 | Pos. | Pos. | 0 | CycloA |
| 2 | Pos. | Pos. | 3 | FK506 |
| 3 | Pos. | Pos. | 2 | FK506 |
| 4 | Pos. | Neg. | 2 | CycloA |
| 5 | Pos. | Neg. | 5 | CycloA |
| 6 | Neg. | Pos. | 3 | CycloA + MP |
| 7 | Neg. | Pos. | 3 | FK506 + MP |
| 8 | Neg. | Neg. | 3 | CycloA + MP |
| 9 | Pos. | Pos. | 1 | FK506 + MP |
| 10 | Neg. | Neg. | 0 | FK506 + MP |
| 11 | Neg. | Neg. | 2 | CycloA + MP |
| 12 | not evaluable | not evaluable | 0 | CycloA + MP |
CycloA indicates cyclosporine A; FK506, tacrolimus; MP, methylprednisolone; PB, peripheral blood.
Cell preparation
Mononuclear cells (MNCs) from peripheral blood and bone marrow were separated by Ficoll-Hypaque density gradient centrifugation (density 1.077g/cm3) (Ficoll-Hypaque, Pharmacia, Uppsala, Sweden). The cells were washed twice with phosphate-buffered saline (PBS).
Aliquots from peripheral blood and bone marrow MNCs were incubated for 30 minutes at 4°C in the presence of an anti-CD45 fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody and an anti-CD34 (HPCA-2) phycoerythrin (PE)–conjugated monoclonal antibody (all from Becton Dickinson, Heidelberg, Germany). The cells were washed twice with PBS, and red blood cells were removed with the use of a fluorescence-activated cell sorting (FACS) lysis solution (Becton Dickinson). A total of 10 000 cells were acquired. Immunofluorescence analysis was performed with a 5-parameter FACScan (Becton Dickinson) equipped with an argon-ion laser tuned at 488 nm and 15 mW. Emission from FITC and PE was measured with the use of filters of 530 nm and 585 nm, respectively. The side scatter characteristics (SSCs)–versus–CD45-fluorescence dot plot was used to discriminate between the smallest hematopoietic cell population and erythrocytes or debris. After Ficoll-Hypaque density centrifugation, the fraction of CD45+ cells in peripheral blood and bone marrow was greater than 95%. The CD34+ cells were analyzed in a fluorescence-versus-SSC plot. Only cells with a lymphoid or lymphomonocytoid appearance were counted as CD34+ cells, and their proportion was calculated in relation to that of CD45+ cells. The percentage of false-positive events determined by isotype-specific control was less than 0.5% and was subtracted from the percentage of CD34+cells.13
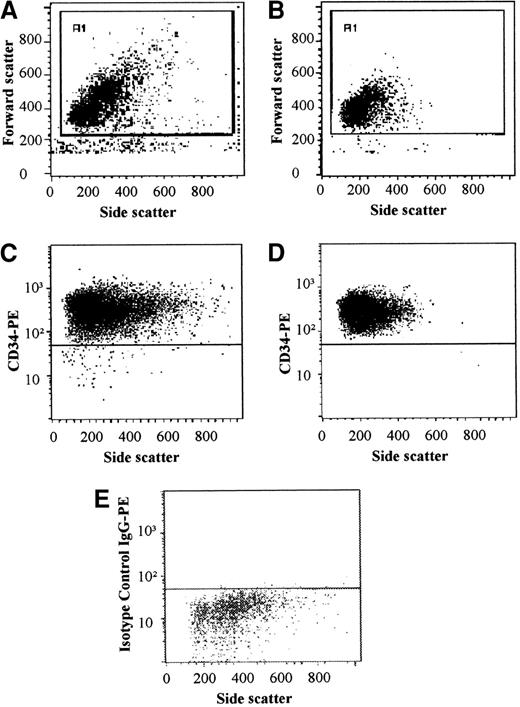
The CD34+ cell fraction from bone marrow samples was enriched with the use of a 2-step method as previously described.14 The first enrichment step consisted of an immunomagnetic separation (Minimacs, Miltenyi-Biotec GmbH, Bergisch Gladbach, Germany), according to the manufacturer's instructions. The majority of the enriched CD34+ cells were incubated for 30 minutes at 4°C in the presence of an anti-CD34 (HPCA-2) PE–conjugated monoclonal antibody (Becton Dickinson); 1 aliquot was incubated with an isotype-specific control. After the CD34+ cells were washed with PBS, their purity was analyzed in a fluorescence-versus-SSC plot (Figure1). The percentage of false-positive events determined by isotype-specific control was below 0.5% and was subtracted from the percentage of CD34+ cells. The cell fraction was further highly enriched by FACS with a FACSVantage (Becton Dickinson). Only cells with a lymphoblastoid appearance were sorted according to side and forward scatter characteristics. Final purity was analyzed by flow cytometry (Figure 1). The high purity of this procedure was demonstrated for patients with follicular lymphoma showing no detection of contaminating cells positive for the translocation t(14;18).14
Bone marrow–derived CD34+ cells were enriched with the use of a 2-step method.
Purity was analyzed after Minimacs (A, FSC/SSC; C, CD34/SSC; E, isotype control IgG/SSC), obtaining a mean purity of greater than 95%. Only cells with lymphoblastoid characteristics were sorted. Final purity after FACS with a FACSVantage was greater than 99.5% (B, FSC/SSC; D, CD34/SSC).
Bone marrow–derived CD34+ cells were enriched with the use of a 2-step method.
Purity was analyzed after Minimacs (A, FSC/SSC; C, CD34/SSC; E, isotype control IgG/SSC), obtaining a mean purity of greater than 95%. Only cells with lymphoblastoid characteristics were sorted. Final purity after FACS with a FACSVantage was greater than 99.5% (B, FSC/SSC; D, CD34/SSC).
DNA preparation and PCR amplification
Total DNA was extracted from enriched CD34+cells and from peripheral blood MNCs according to a standard protocol based on cell lysis, proteinase K digestion, and chloroform purification. The amount of DNA was measured by UV spectrophotometry. The entire second exon of the HLA-DRB1 gene was amplified with the use of commercially available primers to consensus regions (INNO-LiPA DRB key, Innogenetics, Zwijndrecht, Belgium).15 The primers were added to a PCR mix containing KCl, Tris HCl, MgCl2, gelatin, and deoxyribonucleoside trisphosphates (dNTPs). PCR was carried out in a final volume of 50 μL, with 0.3 units recombinant Taq polymerase (Perkin Elmer, Branchberg, NJ) and 0.1 μg template DNA.
The reactions were performed in a Perkin-Elmer 9600 thermocycler. The amplification profile was 5 minutes' denaturation at 95°C followed by 35 cycles of denaturation at 95°C for 30 seconds, primer annealing at 58°C for 20 seconds, and primer extension at 72°C for 30 seconds. At the end, an extension step of 10 minutes at 72°C was added.15 PCR products were separated by electrophoresis on a 2% agarose gel and visualized by ethidium bromide staining. After successful amplification, the PCR product was diluted 1:10 in H2O, and 1μL of the diluted PCR product was subjected to a second round of amplification with sequence specific primers (SSPs) from the Collaborative Transplant Study.16 The cycling conditions for this nested PCR assay were 30 cycles of denaturation at 94°C (20 seconds) and primer annealing/elongation at 69°C (50 seconds). Here also, 0.3 μL Taq polymerase was used per reaction. Samples were electrophoresed on an ethidium bromide–stained 2% agarose gel.
This procedure allowed the assignment of all broad HLA-DRB1 specificities as well as their serologic splits (HLA-DR 1-18). DNA samples from healthy blood donors with DRB1 specificities identical to those of the allograft donor and recipient were mixed and analyzed in model reactions in order to evaluate allele combinations that could result in either false-positive reactions or reduced amplification efficiency. In 4 patients, samples of the donor and recipient before transplantation were available to serve as rigorous baseline control. Each patient's posttransplantation DNA was submitted to the complete analysis repeatedly, and a chimeric state was defined if at least 2 out of 3 assays showed donor-specific positive amplification signals.
Results
Microchimerism in peripheral blood
We used a nested PCR with SSPs (SSP-PCR) for exon 2 of the HLA-DRB1 gene to detect donor cells in the allograft recipient (Figures2 and 3). We determined the sensitivity of this approach by spiking experiments to be capable of detecting 1 donor cell among 1000 to 10 000 recipient cells with high specificity (unpublished data, C. Horvath et al, August 1998). A chimeric status was defined when the results of at least 2 out of 3 PCR reactions were positive. In the peripheral blood, 5 out of 8 patients (63%) who had been followed for longer than 2 years after orthotopic liver transplantation were chimeras according to PCR. We prospectively studied 4 patients and obtained DNA samples before liver transplantation and 2 to 4 months following transplantation. We could not assess 1 allograft recipient because of cross-reactivities of his HLA-type with the donor's type. Of the other 3 patients, 1 patient clearly demonstrated donor-type HLA-DRB specificities in the PCR.
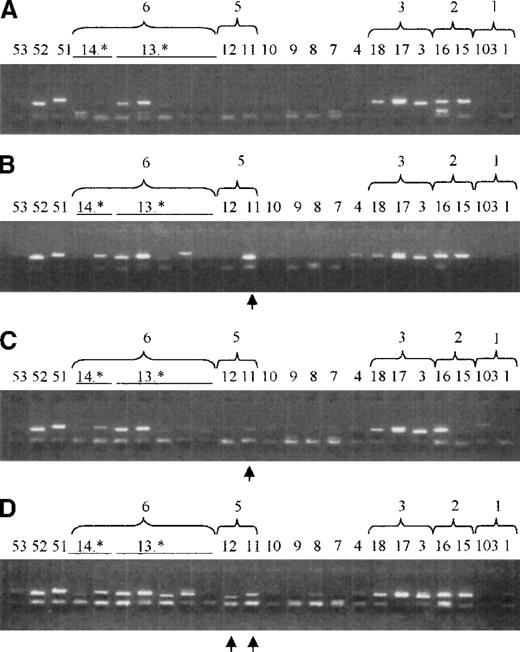
Microchimerism in peripheral blood and CD34+ cells in a patient (patient 1) 5.1 years after orthotopic liver transplantation as detected by nested SSP-PCR for the HLA-DRB gene.
Donor-specific bands are indicated by an arrow. 14.* and 13.* indicate different DRB subtypes. (A) Control DNA sample of a blood donor with the same HLA-DR type (DR1 and DR2(15) as patient 1 before orthotopic liver transplantation (patient 1's original DNA was no longer available). DR15 and DR16 are both serological splits of DR2, and they have a strong sequence homology. This causes a cross-reactivity in the nested PCR-SSP assay. DR51 is the product of the DRB5 gene, which is in linkage with DR15. (B) DNA mix containing the same sample as in panel A in 1:1 dilution with a DNA sample of a control, who had the same HLA type as patient 1's donor [(DR3(17)and DR6(13)]. The bands with primers 14* and 18 are donor-specific bands derived from sequence homology with DR13 and DR17, respectively. The band at DR52 is the PCR product of the DRB3 gene, which is in linkage with the donor's DR13. (C) DNA from peripheral blood from patient 1 obtained 5.1 years after orthotopic liver transplantation. Recipient-specific bands are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13.*, and 52. No other bands are observed. (D) DNA from bone marrow–derived CD34+ cells from patient 1. Bands specific for the recipient are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13*, and 52. Further bands (7, 8, 12, and 53) may be associated with previous blood transfusions.20
Microchimerism in peripheral blood and CD34+ cells in a patient (patient 1) 5.1 years after orthotopic liver transplantation as detected by nested SSP-PCR for the HLA-DRB gene.
Donor-specific bands are indicated by an arrow. 14.* and 13.* indicate different DRB subtypes. (A) Control DNA sample of a blood donor with the same HLA-DR type (DR1 and DR2(15) as patient 1 before orthotopic liver transplantation (patient 1's original DNA was no longer available). DR15 and DR16 are both serological splits of DR2, and they have a strong sequence homology. This causes a cross-reactivity in the nested PCR-SSP assay. DR51 is the product of the DRB5 gene, which is in linkage with DR15. (B) DNA mix containing the same sample as in panel A in 1:1 dilution with a DNA sample of a control, who had the same HLA type as patient 1's donor [(DR3(17)and DR6(13)]. The bands with primers 14* and 18 are donor-specific bands derived from sequence homology with DR13 and DR17, respectively. The band at DR52 is the PCR product of the DRB3 gene, which is in linkage with the donor's DR13. (C) DNA from peripheral blood from patient 1 obtained 5.1 years after orthotopic liver transplantation. Recipient-specific bands are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13.*, and 52. No other bands are observed. (D) DNA from bone marrow–derived CD34+ cells from patient 1. Bands specific for the recipient are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13*, and 52. Further bands (7, 8, 12, and 53) may be associated with previous blood transfusions.20
Microchimerism in peripheral blood and CD34+ cells in a patient (patient 9) surveyed before and 3 months after orthotopic liver transplantation as detected by nested SSP-PCR for the HLA-DRB gene.
Donor-specific bands are indicated by an arrow. 14.* and 13.* indicate different DRB subtypes. (A) DNA sample of patient 9 (DR215 and DR3) before transplantation, used to determine the patient's chimeric state before orthotopic liver transplantation. DR17 and DR18 are both serologic splits of DR3 and they have a strong sequence homology. This causes a cross-reactivity in the nested SSP-PCR assay. DR51 is the product of the DRB5 gene; DR52 is the product of the DRB3 gene. These genes are in linkage with DR2 and DR3, respectively. Unspecific additional bands are DR13.*. (B) DNA mix containing the same sample as in panel A in 1:1 dilution with an original DNA of patient 9's liver donor (DR511). DR11 and DR12 are both serologic splits of DR5, and they have a strong sequence homology. This may cause a cross-reactivity in the nested PCR-SSP assay. (C) DNA from peripheral blood from patient 9 at 3 months after orthotopic liver transplantation. The recipient-specific band is 11, whereas the band in 103 is not specific for this donor/recipient combination. (D) DNA from bone marrow–derived CD34+ cells. Bands specific for the recipient are 11 and 12. No further bands can be detected.
Microchimerism in peripheral blood and CD34+ cells in a patient (patient 9) surveyed before and 3 months after orthotopic liver transplantation as detected by nested SSP-PCR for the HLA-DRB gene.
Donor-specific bands are indicated by an arrow. 14.* and 13.* indicate different DRB subtypes. (A) DNA sample of patient 9 (DR215 and DR3) before transplantation, used to determine the patient's chimeric state before orthotopic liver transplantation. DR17 and DR18 are both serologic splits of DR3 and they have a strong sequence homology. This causes a cross-reactivity in the nested SSP-PCR assay. DR51 is the product of the DRB5 gene; DR52 is the product of the DRB3 gene. These genes are in linkage with DR2 and DR3, respectively. Unspecific additional bands are DR13.*. (B) DNA mix containing the same sample as in panel A in 1:1 dilution with an original DNA of patient 9's liver donor (DR511). DR11 and DR12 are both serologic splits of DR5, and they have a strong sequence homology. This may cause a cross-reactivity in the nested PCR-SSP assay. (C) DNA from peripheral blood from patient 9 at 3 months after orthotopic liver transplantation. The recipient-specific band is 11, whereas the band in 103 is not specific for this donor/recipient combination. (D) DNA from bone marrow–derived CD34+ cells. Bands specific for the recipient are 11 and 12. No further bands can be detected.
Microchimerism in bone marrow–derived CD34+ cells
CD34+ cells from bone marrow were enriched in a 2-step method consisting of an immunomagnetic procedure and FACS. In the 8 long-term allograft survivors, the median proportion of bone marrow–derived CD34+ cells before enrichment was 1.50% (range, 1.09% to 3.29%), whereas the median proportion of CD34+ cells in the bone marrow of 4 patients studied at 2 to 4 months after transplantation ranged between 0.57% and 1.19%. (median, 0.74%). After the first enrichment procedure, the use of the Minimacs, the mean purity of the CD34+ cells obtained was 95.06% ± 2.20% (Figure 1). The purity of the CD34+cells obtained after FACS was greater than 99.5% (median, 99.8%; range, 99.62% to 99.98%). In 5 of 8 (63%) long-term allograft recipients, donor-specific CD34+ cells could be detected in the bone marrow (Figure 2, Table 2). No correlation was found between chimerism in CD34+ cells and peripheral blood MNCs; 3 patients were positive in peripheral blood and HSCs, while 4 patients were positive in either HSCs or peripheral blood. Only 1 patient was negative in peripheral blood as well as in HSCs. In the prospectively studied patients, CD34+ cells were chimeric in 1 patient, who also demonstrated microchimerism in peripheral blood, whereas in 2 patients no cells of donor origin could be detected in blood or bone marrow–derived CD34+ cells.
Clinical course
We included 8 long-term survivors after liver transplantation in the study on the basis of a good graft function. Nevertheless, most patients had experienced 2 or more rejection episodes and required immunosuppressive therapy at the time of the study. Only 1 patient with microchimerism in peripheral blood and in the CD34+ cell fraction had not experienced any rejection episode. In the long-term allograft recipient without microchimerism in peripheral blood and CD34+ cells, cyclosporine had previously been reduced to half of the dose as part of an earlier study, but the patient developed a severe rejection episode and was reset to full therapeutic dose.17 The cohort of patients was too small to make any firm conclusions about microchimerism and clinical outcome.
Patients studied before and at short term after liver transplantation might be less biased for better graft function, and only 1 patient demonstrated microchimerism in peripheral blood and CD34+ cells.
Discussion
Cells of donor origin can be found in the recipient's peripheral blood, skin, lymph nodes, and bone marrow even years after liver transplantation. We could demonstrate microchimerism in the CD34+ cell population of the recipient's bone marrow and therefore conclude that hematopoietic stem and progenitor cells are transferred with the liver transplant. Vascular endothelial cells also express the CD34 antigen.18 These larger cells should have been eliminated from the analysis by the process of isolation and gating during FACS according to physical parameters. Nevertheless, we cannot exclude that donor cells of endothelial origin could contribute to microchimerism in the CD34+ cell fraction.
We applied a nested PCR for the polymorphic allele of HLA-DRB1 to detect cells of donor origin. Sensitivity and specificity depend on the donor/recipient HLA-DRB1 type combinations and the degree of mismatch between donor and recipient.19 We detected allogeneic chimerism in the CD34+ cells in 4 of 5 patients with 2 mismatches and only in 1 of 3 patients with 1 HLA-DRB mismatch. Using a similar nested PCR method, Spriewald et al reported microchimerism in 87.5% of patients with 2 HLA-DRB mismatches, in contrast to 50% in patients with 1 mismatch.19 Spiking experiments with third-party DNA with the same HLA-DRB1 specificities appear to be crucial to control for nonspecific amplifications. The use of pretransplant DNA as control may further reduce the risk of misinterpretation.
In peripheral blood, we found microchimerism in 5 of 8 patients (63%), which is in line with reports from other groups on the frequency of long-term microchimerism after solid organ transplantation. Similarly, we found cells of donor origin among the bone marrow–derived CD34+ cell fraction in 5 of 8 long-term liver recipients.
One can speculate that CD34+ cells egress from the liver and engraft in the bone marrow during the early phase after liver transplantation. The permanent homing implies that the HSCs have escaped from the host-versus-graft reaction. On the other hand, the presence of CD34+ cells in the recipient's bone marrow could be due to a continuous production of HSCs in the liver. The continuous production could sustain the permanent presence of donor-derived HSCs in the recipient's bone marrow, although these donor cells are still targets of the allograft reaction and are perpetually being eliminated.
Our results give additional evidence for the presence of HSCs in the adult human liver. Recently, Bodó et al described the development of donor-derived acute promyelocytic leukemia in the recipient of a liver transplant, indicating that donor myeloid cells had been transferred with the graft 2 years earlier.20 A recent study by Crosbie et al gave further evidence for the presence of HSCs in the adult human liver.3 This group isolated CD34+ hepatic MNCs, which expressed CD45, CD38, and HLA-DR and formed multilineage hematopoietic colonies after tissue culture. The localization of HSCs in the liver is not clear. Endothelial or parenchymal cells might deliver the microenvironmental support, and an association with endothelial cells might not be restricted to liver, but also occur in other parenchymal organs. It will be interesting to study microchimerism in HSCs after transplantation of solid organs other than liver.
Microchimerism in peripheral blood might be the consequence of proliferation and differentiation of HSCs engrafted in the recipient's bone marrow. Other long-lived donor cells as memory T cells might contribute to sustain microchimerism. This could explain our results in 2 patients in whom we could find microchimerism only in the peripheral blood, not in the CD34+ cell fraction. On the other hand, these results might reflect the limitations of the sensitivity of our method.
Apart from donor-specific bands, we could identify additional HLA-DRB1 specificities in 3 patients. Other mechanisms that lead to a state of microchimerism have been described. For this reason, it appears advantageous to use the polymorphic HLA-DRB region to discriminate between donor and recipient cells rather than simply the Y chromosome, which limits the analysis to the male-to-female transplant situation.4 Additional chimeric bands could be due to blood transfusions in the past.21 Bianchi et al showed that male CD34+ cells could be detected in women up to 27 years after delivery of a male child.22 Fetal lymphohematopoietic cells that cross the placenta and enter the maternal circulation could play a role in the induction of autoimmune diseases such as scleroderma.23 24
The clinical and immunologic relevance of microchimerism and in particular of HSCs in microchimerism is not defined. Starzl et al developed a model in which tolerance is the consequence of a balance between 2 immune systems.11 The presence of donor cells indicates tolerance, but it could be the effect and not the cause of tolerance. CD34+ cells themselves might function as veto cells and suppress alloreactive T cells.25 Khan et al demonstrated that allogeneic HSCs could differentiate into thymic dendritic cells, which might induce central tolerance to an organ transplant by negative selection.26 Our cohort of patients was too small for a correlation between the presence of microchimerism and clinical outcome. Nevertheless, most patients with or without microchimerism had experienced 2 or more rejection episodes and required immunosuppressive therapy at the time of the study.
The induction of tolerance may also depend on the quantity of chimeric cells. Minute quantities of donor cells as in microchimerism are not sufficient to induce a balance between 2 immune systems. Clinical trials are underway in several institutions to determine whether donor bone marrow cells can increase the proportion of chimeric cells and enhance organ allograft survival.27 28
Acknowledgments
M. Weis, M. Pförsich, and E. Sollich for excellent technical assistance and K. Hexel (Department of Tumor Immunology, German Cancer Research Center, Heidelberg) and L. Volk for excellent technical support with FACS-analysis and FACS-sorting.
Reprints:Stefan Hohaus, Istituto di Semeiotica Medica, Divisione di Ematologia, Università Cattolica S. Cuore, Largo A. Gemelli, 1 00168 Rome, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Microchimerism in peripheral blood and CD34+ cells in a patient (patient 1) 5.1 years after orthotopic liver transplantation as detected by nested SSP-PCR for the HLA-DRB gene. / Donor-specific bands are indicated by an arrow. 14.* and 13.* indicate different DRB subtypes. (A) Control DNA sample of a blood donor with the same HLA-DR type (DR1 and DR2(15) as patient 1 before orthotopic liver transplantation (patient 1's original DNA was no longer available). DR15 and DR16 are both serological splits of DR2, and they have a strong sequence homology. This causes a cross-reactivity in the nested PCR-SSP assay. DR51 is the product of the DRB5 gene, which is in linkage with DR15. (B) DNA mix containing the same sample as in panel A in 1:1 dilution with a DNA sample of a control, who had the same HLA type as patient 1's donor [(DR3(17)and DR6(13)]. The bands with primers 14* and 18 are donor-specific bands derived from sequence homology with DR13 and DR17, respectively. The band at DR52 is the PCR product of the DRB3 gene, which is in linkage with the donor's DR13. (C) DNA from peripheral blood from patient 1 obtained 5.1 years after orthotopic liver transplantation. Recipient-specific bands are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13.*, and 52. No other bands are observed. (D) DNA from bone marrow–derived CD34+ cells from patient 1. Bands specific for the recipient are 1.1, 15, (16), and 51, whereas donor-specific bands are 17, 13*, and 52. Further bands (7, 8, 12, and 53) may be associated with previous blood transfusions.20](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/2/10.1182_blood.v96.2.763/5/m_bloo01409002w.jpeg?Expires=1769084824&Signature=Wan7zYxQAmkQ9f2-96qT8WCZfcLKSjDAN4q7zV6AsrjNwBsMBmlL901PZarZGtucmCrte2LWOam~5Lk5xQEKzKk9wXlZEU0j1bRl~X61-oeR3GgQ2BVmhTPb6mhnnEhSVF4P~KIEmXopVppVKFznKasa7EIVs9tP9mO6xPxJHalkCiqkMA~N9nNG8cb05YYw4ws-3kDtnGoRTy2nLkJcgz8ln5PR4ATFAmmHDQVnrHystAOdhuBv7A2OUJUaRo-PCczi6~L-rlndtodrvCLUVHyy4N3FJ-Wa0LHRcufHDBnYp2M5rUkKXXDpNy9-U9t9et4~j2PIUO2wKnif7QP5Nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal