Abstract
Targeting the tyrosine kinase activity of Bcr-Abl with STI571 is an attractive therapeutic strategy in chronic myelogenous leukemia (CML). A few CML cell lines and primary progenitors are, however, resistant to this compound. We investigated the mechanism of this resistance in clones of the murine BaF/3 cells transfected with BCR-ABL and in 4 human cell lines from which sensitive (s) and resistant (r) clones were generated by various methods. Although the resistant cells were able to survive in the presence of STI571, their proliferation was approximately 30% lower than that of their sensitive counterparts in the absence of the compound. The concentration of STI571 needed for a 50% reduction in viable cells after a 3-day exposure was on average 10 times higher in the resistant (2-3 μmol/L) than in the sensitive (0.2-0.25 μmol/L) clones. The mechanism of resistance to STI571 varied among the cell lines. Thus, in Baf/BCR-ABL-r, LAMA84-r, and AR230-r, there was up-regulation of the Bcr-Abl protein associated with amplification of the BCR-ABL gene. In K562-r, there was no Bcr-Abl overexpression, but the IC50 for the inhibition of Bcr-Abl autophosphorylation was increased in the resistant clones. Sequencing of the Abl kinase domain revealed no mutations. The multidrug resistance P-glycoprotein (Pgp) was overexpressed in LAMA84-r, indicating that at least 2 mechanisms of resistance operate in this cell line. KCL22-r showed neither Bcr-Abl up-regulation nor a higher threshold for tyrosine kinase inhibition by STI571. We conclude that BCR-ABL–positive cells can evade the inhibitory effect of STI571 by different mechanisms, such as Bcr-Abl overexpression, reduced intake mediated by Pgp, and, possibly, acquisition of compensatory mutations in genes other than BCR-ABL.
The t(9;22)(q34;q11) reciprocal chromosomal translocation occurs in nearly all patients with chronic myelogenous leukemia (CML) and in approximately 25% of adults and 5% of children with acute lymphoblastic leukemia (ALL).1 This translocation gives origin to a 22q−, or Philadelphia (Ph), chromosome that contains a BCR-ABL hybrid gene, the molecular hallmark of CML and of Ph-positive ALL.2BCR-ABLencodes an oncogenic fusion protein of 190, 210, or 230 kd, depending on the breakpoint on the BCR gene (reviewed in 1). The unifying feature of all these Bcr-Abl fusion proteins is their deregulated protein tyrosine kinase activity. The latter is responsible for the in vitro transformation effect and the in vivo leukemogenic property of Bcr-Abl.3,4 Targeting the tyrosine kinase activity of Bcr-Abl is, therefore, an attractive therapeutic strategy in CML or in BCR-ABL–positive ALL. Toward this aim, several inhibitors of tyrosine kinase were recently developed.5,6Among these molecules, STI571, previously known as CGP57148B, a 2-phenylaminopyrimidine derivative, is among the most promising and selective inhibitors of Bcr-Abl tyrosine kinase activity. It inhibits competitively the binding of adenosine triphosphate (ATP) to the kinase domain of Abl at micromolar concentrations. Most other serine/threonine and tyrosine kinases are unaffected, but there is a suppressive effect against the Kit and PDGF receptor kinase activities.7 8
We and others8,9 have reported that STI571 specifically abrogates CML granulocyte macrophage–colony-forming unit (GM-CFU) and erythroid burst-forming unit (BFU-e) colony formation over a 2-log dose range, with a maximum differential effect at 1 μmol/L. The inhibitor also suppresses proliferation of most Ph-positive cell lines.9,10 Nevertheless, a few BCR-ABL–positive GM-CFU colonies from peripheral blood or bone marrow survive in the presence of the compound, and 2 of 10 Ph-positive cell lines were found to be resistant to STI571.9 The reasons for this reduced sensitivity to STI571 in some cells is unknown, but it is an issue of considerable relevance as this compound enters clinical trials.11
In this study, we have generated resistant and sensitive sublines from different Ph-positive cell lines to investigate the mechanism of resistance to STI571. We show that the Bcr-Abl protein is significantly overexpressed in some, but not all, resistant cell lines. Similarly, the multidrug resistance P-glycoprotein (Pgp) is overexpressed in 1 of the resistant cell lines. Overall, our data suggest thatBCR-ABL–positive cells can evade the inhibitory effect of this tyrosine kinase inhibitor by various mechanisms.
Materials and methods
Cell lines
BaF/3 cells were grown in RPMI 1640 medium (Gibco, Paisley, UK) supplemented with penicillin, streptomycin, l-glutamine, and 10% fetal bovine serum (FBS), herein referred to as RF-10, with 10% WEHI-conditioned medium as a source of murine IL-3. The Baf/BCR-ABL line was obtained by standard electroporation transfection with the wild-type (wt) BCR-ABL–containing pGD210 expression vector12 (kindly provided by Dr George Daley, Whitehead Institute, Cambridge, MA), as described.13
Nine BCR-ABL–positive human cell lines were used in this study: K562, KYO1, LAMA84, EM2, EM3, BV173, AR230, KU812, and KCL22. They were all grown in RF-10. These cell lines were purchased from cell repository banks (American Tissue Culture Collection, Rockville, MD; European Collection of Cell Cultures, Winchester, UK; German Collection of Micro-organisms and Cell Cultures, Braunschweig, Germany) or were kindly donated by the originators.
Generation of resistant cell lines by cloning in methylcellulose
Exponentially growing cells from each cell line were plated at 0.5 × 103 to 1 × 104 cells/mL in Iscove methylcellulose medium (Methocult H4430; Stemcell Technologies, Vancouver, BC, Canada) supplemented with 10% fetal bovine serum. STI571 (kindly provided by Dr Elisabeth Buchdunger, Novartis, Basel, Switzerland) was added directly to the methylcellulose at different concentrations (0.5-10 μmol/L) from a 10-mmol/L stock solution in distilled water. Colonies were checked and selected for isolation at different days of the culture, depending on the cell line (day 5, 7, 10, or 12). Individual and well-separated colonies were plucked with a micropipette fitted with a sterile plugged tip, transferred to liquid culture, and expanded in RF-10 in the same concentration of STI571 used in semisolid culture.
Generation of resistant cell lines in liquid culture
Cell lines maintained in liquid culture, as described above, were gradually exposed to increasing concentrations of STI571 at a rate of 0.1-μmol/L increment every 10 days of culture. After approximately 3 months, sublines of cells growing in 1 μmol/L STI571 were maintained continuously in culture in this dose of the inhibitor. Parental, sensitive cell lines were maintained in parallel cultures without STI571 to be used as controls.
Cell proliferation assay
Cell proliferation assay was performed using MTS tetrazolium (Cell Titer96 Aqueous; Promega, Madison, WI), which measures numbers of viable cells. Between 2 × 103 and 2 × 104 cells were washed twice in RF-10 and plated in quadruplicate into microtiter-plate wells in 100 μL RF-10 plus various doses of STI571. Controls using the same concentrations of STI571 without cells were set up in parallel. Twenty microliters MTS was added to the wells at daily intervals. Two hours after MTS was added, the plates were read in a microplate autoreader (Dynex Technologies, Billingshurst, UK) at 490-nm wavelength. Results are expressed as the mean optical density of the 4-well set for each STI571 dose. All experiments were repeated at least 3 times.
Cell viability assay
Cells were plated at a density of 2 to 2.5 × 105cells/mL in RF-10 with or without the inhibitor and, in some experiments, with or without 5 μg/mL verapamil (Isoptine; Laboratoires Knoll, Levallois–Perret, France). Aliquots were taken out at 24-hour intervals for assessment of cell viability by trypan blue exclusion.
Apoptosis assay
For determination of apoptotic death, the level of caspase-3 activation was assessed by measurement of its capacity to cleave an Ac-Asp-Glu-Val-Asp-amino-4-methyl-coumarine (Ac-DEVD) substrate, as modified from a previously published method.14 Aliquots of 7.5 × 104 cells were cultured in triplicate in RF-10 into 96-well plates in the presence or absence of 1 μmol/L STI571. Triplicate wells with RF-10 only were used as background control. After 3 days the plate was centrifuged at 1500 rpm for 5 minutes, the culture supernatant was removed, and the cells were resuspended in 50 μL of a buffer containing 10 mmol/L HEPES, 5 mmol/L dithiothreitol, 2 mmol/L EDTA, 0.02% saponin, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL pepstatin A, 10 μg/mL leupeptin, and 72 mmol/L fluorogenic Ac-DEVD substrate (UBI Euromedex, Souffelweyerssheim, France). Development of the reaction was read on an automatic spectrofluorometer (Victor 2 Multilabel Counter; Wallac and Perkin Elmer, Akron, OH), using λexc = 380 nm and λem = 480 nm. After this reading, 200 μL of a 15 mg/mL propidium iodide solution was added to each well, and a new reading was taken at λexc = 360 nm and λem = 600 nm to evaluate the proliferation of cells. The caspase-3 activity was calculated for 105 cells after taking into account the degree of cell proliferation.
Western blot analysis
Protein lysates were prepared according to the method of Kabarowski et al.15 Protein concentrations were determined by the Bradford method (Dc Protein Assay; BIO-RAD, Hercules, CA). Approximately 250 μg protein was resolved on 7% SDS-PAGE gels, blotted onto polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA) by semi-dry electrophoretic transfer, probed with individual antibodies, and visualized by the ECL system (Amersham, Little Chalfont, UK). The following antibodies were used: PY-99 anti-phosphotyrosine (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Abl Ab-3 (Calbiochem–Novabiochem, Nottingham, UK), A-2066 anti-actin (Sigma Chemical, St. Louis, MO), and anti-Bcr (BCR 157; kind gift from R. Arlinghaus, MD Anderson Cancer Center, Houston, TX). Secondary antibodies were horseradish peroxidase-conjugated rabbit antimouse IgG and swine antirabbit IgG (DAKO, Glostrup, Denmark).
Northern blot analysis
RNA was extracted by the acid guanidinium thiocyanate method.16 Fifteen micrograms per lane were resolved on a 0.8% agarose gel and transferred onto nylon membranes (HYBOND; Amersham). The filters were prehybridized, hybridized, and washed according to the manufacturer's instructions. ABL andBCR cDNA probes were prepared by reverse transcription–polymerase chain reaction (RT-PCR) amplification with primers A2+, 5′ TTCAGCGGCCAGTAGCATCTGACTT, andA4e−, 5′) CTTCAAGGTCTTCACGGCCACCGT, for ABLand BCRB+, 5′ CCCCCGGAGTTTTGAGGATTGC, andBE2−, 5′ AGGTAGATCTCCTCGCTAGCCAGGATT, forBCR. The probes were labeled with the Megaprime system (Amersham). After posthybridization washes, the membranes were exposed to autoradiography film for 12 to 48 hours.
Southern blot analysis
Genomic DNA was isolated using the QIAmp Tissue Kit (QIAGEN, Crawley, UK) digested with appropriate restriction enzymes, electrophoresed on 0.8% agarose gels, and transferred to a nylon membrane (HYBOND; Amersham). Southern hybridizations were carried out according to protocols standardized by the manufacturers for this membrane. ABL and BCR cDNA probes prepared as described for the Northern blots were labeled with the Megaprime system (Amersham).
Sequence of the ABL kinase domain
The kinase domain of ABL was amplified by RT-PCR with primers NTPB+, 5′ AAGCGCAACAAGCCCACTGTCTAT, andNTPE−, 5′ CTTCGTCTGAGATACTGGATTCCT. PCR products were cloned into the pCR2.1 TA cloning vector (Invitrogen, Groningen, The Netherlands) and sequenced with M13 primers on an ABI prism 377 automated DNA sequencer (PE Applied Biosystems; Perkin Elmer, Cheshire, UK). Sequence analysis was performed using the GCG version 10 software (Griffith University, Brisbane, Australia).
Flow cytometric analysis
A phycoerythrin-conjugated monoclonal antibody (mAb) Fab' fragment of a murine anti-human Pgp (clone UIC2; Immunotech, Marseille, France) was used to determine the expression of the MDR-1 gene product on the different cell lines. HL-60 cells stably transfected withMDR-1 (a kind gift from Dr S. Devereux, University College Hospital, London, UK) was used as a positive control for Pgp expression. For detection of the Bcr-Abl protein, cells were fixed in 1% paraformaldehyde/phosphate-buffered saline for 10 minutes at room temperature, permeabilized with 0.3% saponin, and stained with an anti-Abl antibody (24-11; Santa Cruz Biotechnology) and then by fluorescein isothiocyanate-conjugated goat antimouse IgG (Becton Dickinson, San Jose, CA) as the secondary reagent. Appropriate isotypic controls were used in all experiments. Stained cells were analyzed on a FACScan with the aid of the Cell Quest software (Becton Dickinson).
Fluorescence in situ hybridization analysis
Interphase nuclei were hybridized with fluorescently labeled probes for ABL and BCR (Vysis, Downers Grove, IL) as previously described.9
Results
Generation of cell lines with differential sensitivity to STI571
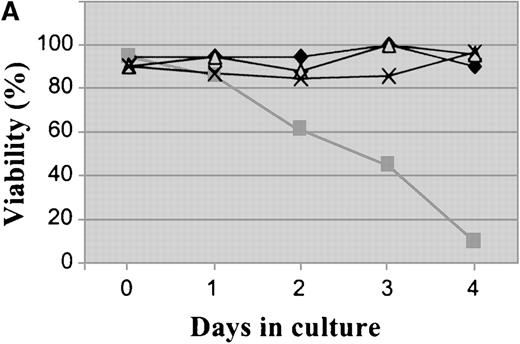
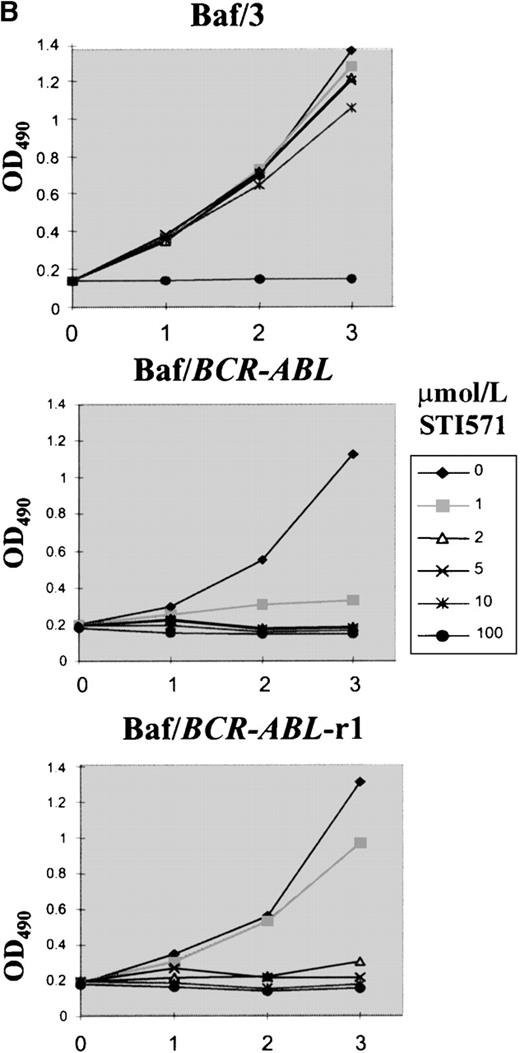
Plating of Baf/BCR-ABL cells in methylcellulose containing different doses of STI571 resulted in an 85% to 99% reduction in clonogenicity, as compared to that of cells seeded in the absence of STI571. Nine clones resistant to 1 to 4 μmol/L STI571 were selected after initial passaging into liquid culture and were expanded thereafter in RF-10 supplemented with STI571. Sensitive Baf/BCR-ABL clones were obtained from colonies growing in the absence of the inhibitor. Resistance was defined by the capacity to survive indefinitely in the continuous presence of a given concentration of STI571, as illustrated on Figure1A for 1 of the 4 Baf/BCR-ABL-r clones resistant to 1 μmol/L of the compound. Similar results were obtained for cells resistant to 2, 3, and 4 μmol/L (2, 2, and 1 clone, respectively). In this study we focused mainly on the BaF/BCR-ABL cells resistant to 1 μmol/L. Whereas proliferation of the parental Ba/F3 cells was totally unaffected by STI571 concentrations up to at least 10 μmol/L, growth of the Baf/BCR-ABL-s cell line was, as expected, profoundly inhibited by as little as 1 μmol/L of the compound (Figure 1B). Baf/BCR-ABL-r clones survived and proliferated at the specific dose used for cloning but remained sensitive to higher concentrations of STI571 (Figure 1B).
Growth characteristics of BCR-ABL–transformed Baf/3 cells resistant to STI571.
(A) Cell viability assessed by trypan blue exclusion of Baf/BCR-ABL cells (⧫) and Baf/BCR-ABL cloned in 1 μmol/L STI571 (Baf/BCR-ABL-r1) (▪), treated (▵) or not treated (X) with 1 μmol/L STI571. (B) Cell proliferation of Baf/3 cells, Baf/BCR-ABL-s, and Baf/BCR-ABL-r1 under the effect of various concentrations of STI571, as assessed by MTS uptake. Results are expressed as the mean OD490 of quadruplicate cultures, which is directly proportional to the number of viable cells.
Growth characteristics of BCR-ABL–transformed Baf/3 cells resistant to STI571.
(A) Cell viability assessed by trypan blue exclusion of Baf/BCR-ABL cells (⧫) and Baf/BCR-ABL cloned in 1 μmol/L STI571 (Baf/BCR-ABL-r1) (▪), treated (▵) or not treated (X) with 1 μmol/L STI571. (B) Cell proliferation of Baf/3 cells, Baf/BCR-ABL-s, and Baf/BCR-ABL-r1 under the effect of various concentrations of STI571, as assessed by MTS uptake. Results are expressed as the mean OD490 of quadruplicate cultures, which is directly proportional to the number of viable cells.
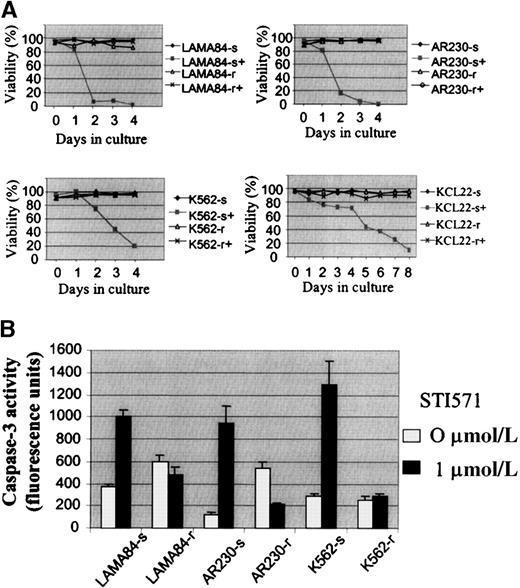
Several attempts to generate STI571-resistant clones from the human cell lines AR230, BV173, EM2, EM3, K562, KYO1, KU812, and LAMA84 by direct plating in STI571-treated methylcellulose failed. An alternative strategy was then devised by which each line was seeded into 0.1 μmol/L STI571 in liquid culture and exposed to 0.1-μmol/L increments in the concentration of the drug every 10 days. No cells from BV173, EM2, KYO1, or KU812 survived doses larger than 0.2 to 0.3 μmol/L. In contrast, subpopulations of cells from AR230, LAMA84, and K562 were able to survive and grow in the presence of 1 μmol/L STI571 after 3 months of the initiation of this culture system. The threshold for EM3 cells was lower, with 1 clone resistant to 0.5 μmol/L and 1 to 0.8 μmol/L STI571 being derived; this cell line was, therefore, not included in the remaining studies. KCL22, a line that was initially found to be resistant to STI571, was subcloned in methylcellulose, and 25 colonies were then expanded in liquid culture. Among these, 2 clones each of cells sensitive and resistant to 1 μmol/L STI571 were selected for further analysis. As for the murine Baf/BCR-ABL-r clones, resistance in the human lines was defined as the capacity to survive in the continuous presence of 1 μmol/L STI571 (Figure2A). Attempts to increase the dose of STI571 above this threshold were unsuccessful.
Growth characteristics of the human CML cell lines resistant to STI571.
(A) Cell viability assessed by trypan blue exclusion of LAMA84, AR230, K562, and KCL22 sensitive (s) and resistant (r) clones cultured in the presence (+) or absence of 1 μmol/L STI571. (B) Caspase 3 activity in 105 cells after 3 days in culture without (0 μmol/L) or with (1 μmol/L) STI571. Results represent the mean ± SD of triplicate cultures.
Growth characteristics of the human CML cell lines resistant to STI571.
(A) Cell viability assessed by trypan blue exclusion of LAMA84, AR230, K562, and KCL22 sensitive (s) and resistant (r) clones cultured in the presence (+) or absence of 1 μmol/L STI571. (B) Caspase 3 activity in 105 cells after 3 days in culture without (0 μmol/L) or with (1 μmol/L) STI571. Results represent the mean ± SD of triplicate cultures.
By means of a caspase-3 activation assay, we confirmed that theBCR-ABL–positive cell lines exposed to STI571 die by apoptosis, as previously reported.9 Figure 2B illustrates the reduction in apoptotic death in LAMA84-r, AR230-r, and K562-r, compared with their parental sensitive lines, when treated with 1 μmol/L of the inhibitor for 3 days.
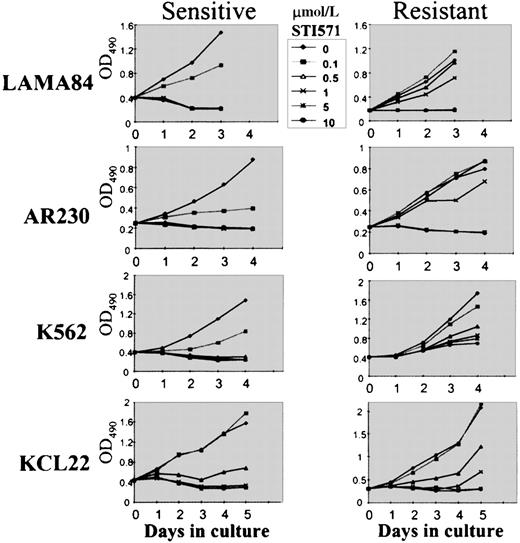
To compare the growth kinetics of the various cell lines, MTS cell proliferation assays were performed on cells exposed to 0.1, 0.5, 1, 5, and 10 μmol/L STI571 (Figure 3). Because we showed that the growth inhibition induced by STI571 in mostBCR-ABL–positive cell lines occurred within 48 to 72 hours,9 we evaluated at day 3 the dose of STI571 necessary to induce a 50% decrease in the uptake of MTS (IP50), as measured by the optical density index. Thus, the IP50 for LAMA84-s, AR230-s, and K562-s was, respectively, 0.2, 0.25, and 0.2 μmol/L, in contrast to 2, 3, and 3 μmol/L for their resistant counterparts. This indicated that the concentration of STI571 needed for a 50% reduction in the number of viable cells after 3-day exposure to the compound was on average 10 times higher in the resistant cells than in the sensitive cells (Figure 3). Nevertheless, although the resistant clones survived and proliferated at 1 μmol/L STI571, their rate of proliferation was approximately 30% lower than that of their sensitive counterparts in the absence of the compound, and, as found for Baf/BCR-ABL-r cells, they remained sensitive to the higher doses of STI571. For KCL22 cells, significant differences in growth inhibition between the sensitive and the resistant clones were observed only after 5 days of culture in the various concentrations of STI571 (Figure 3). This was because the sensitive KCL22 clones required more than 8 days of exposure to STI571 for a reduction to less than 10% viability, in contrast to the other sensitive lines in which this effect was achieved in 3 to 5 days of STI571-treated cultures (Figure 2A).
Cell proliferation of LAMA84, AR230, K562, and KCL22 sensitive (s) and resistant (r) clones under the effect of various concentrations of STI571, as assessed by MTS uptake.
Results are expressed as the mean OD490 of quadruplicate cultures, which is directly proportional to the number of viable cells.
Cell proliferation of LAMA84, AR230, K562, and KCL22 sensitive (s) and resistant (r) clones under the effect of various concentrations of STI571, as assessed by MTS uptake.
Results are expressed as the mean OD490 of quadruplicate cultures, which is directly proportional to the number of viable cells.
Some resistant cell lines overexpress Bcr-Abl
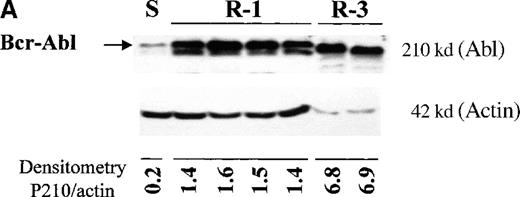
The level of Bcr-Abl protein in the different cell lines was studied by immunoblotting with an anti-Abl antibody. All Baf/BCR-ABL-r clones showed significantly high levels of Bcr-Abl, compared with the sensitive parental line, and the level of overexpression increased with the degree of resistance (Figure 4A). Thus, clones resistant to 1 μmol/L STI571 (lanes 2-5) expressed 5 to 9 times more Bcr-Abl than Baf/BCR-ABL-s (lane 1), whereas those resistant to 3 μmol/L STI571 showed a 34-fold overexpression of the oncoprotein. These values were calculated by taking into account the densitometry of the P210BCR-ABL band only, and they may, therefore, be an underestimation of the overall Bcr-Abl production because several reactive bands of multiple sizes, suggestive of Bcr-Abl degradation fragments, were also detected in the resistant cells (Figure 4B). The higher BCR-ABL expression in Baf/BCR-ABL-r cells was also observed at the mRNA level by Northern blot analysis (data not shown).
BCR-ABL expression in Baf/BCR-ABL clones.
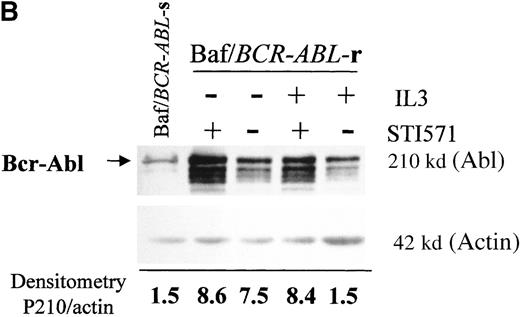
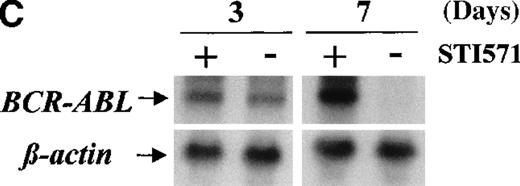
Western blots probed with anti-Abl (upper part of the filters) and anti-actin (lower part of the filters) antibodies. (A) Lane 1: Baf/BCR-ABL-s. Lanes 2-5: 4 Baf/BCR-ABL clones resistant to 1 μmol/L STI571. Lanes 6, 7: 2 Baf/BCR-ABLclones resistant to 3 μmol/L STI571. Note that one tenth the protein was loaded onto the latter 2 lanes to enable resolution of the 210-kd Bcr-Abl band without saturation of the image. Densitometric Bcr-Abl/actin ratio for each sample showed an average 7-fold Bcr-Abl overexpression in the clones resistant to 1 μmol/L STI571 and 34-fold in those resistant to 3 μmol/L in comparison with the level of expression in the sensitive parental line. (B) Modulation of Bcr-Abl expression in Baf/BCR-ABL cells grown for 3 days in the presence (+) or absence (−) of IL-3, STI571, or both. The P210/actin ratios indicate that the removal of STI571 leads to a weak reduction in the level of Bcr-Abl protein and that the effect is significantly enhanced by the addition of IL-3. These results were reproduced in 4 identical experiments. (C) BCR-ABL mRNA levels in Baf/BCR-ABL-r1 cells after 3- and 7-day withdrawal of STI571 from the culture.
BCR-ABL expression in Baf/BCR-ABL clones.
Western blots probed with anti-Abl (upper part of the filters) and anti-actin (lower part of the filters) antibodies. (A) Lane 1: Baf/BCR-ABL-s. Lanes 2-5: 4 Baf/BCR-ABL clones resistant to 1 μmol/L STI571. Lanes 6, 7: 2 Baf/BCR-ABLclones resistant to 3 μmol/L STI571. Note that one tenth the protein was loaded onto the latter 2 lanes to enable resolution of the 210-kd Bcr-Abl band without saturation of the image. Densitometric Bcr-Abl/actin ratio for each sample showed an average 7-fold Bcr-Abl overexpression in the clones resistant to 1 μmol/L STI571 and 34-fold in those resistant to 3 μmol/L in comparison with the level of expression in the sensitive parental line. (B) Modulation of Bcr-Abl expression in Baf/BCR-ABL cells grown for 3 days in the presence (+) or absence (−) of IL-3, STI571, or both. The P210/actin ratios indicate that the removal of STI571 leads to a weak reduction in the level of Bcr-Abl protein and that the effect is significantly enhanced by the addition of IL-3. These results were reproduced in 4 identical experiments. (C) BCR-ABL mRNA levels in Baf/BCR-ABL-r1 cells after 3- and 7-day withdrawal of STI571 from the culture.
We next examined whether IL3 or STI571 could modify the level of Bcr-Abl protein in the resistant clones (Figure 4B). Removal of STI571 from the culture for 3 days led to a slight reduction in the level of Bcr-Abl protein, an effect that was enhanced by the addition of IL-3 (see p210/actin ratios). A reduction in BCR-ABL expression was also observed at the transcriptional level, estimated as 1.3-fold after 3 days and 11-fold after 7 days of STI571 withdrawal (Figure 4C). These data indicate that Bcr-Abl expression can be modulated by the tyrosine kinase inhibitor itself in Baf/BCR-ABL-r cells and that the addition of IL-3, combined with STI571 withdrawal, completely abrogated the reactive Bcr-Abl overexpression of these clones.
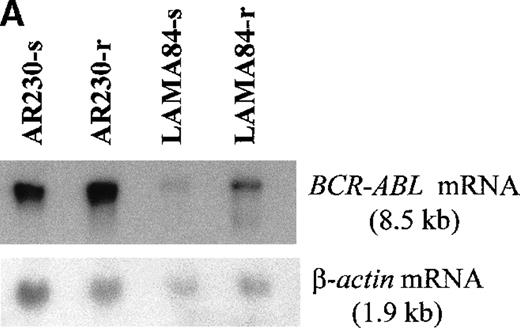
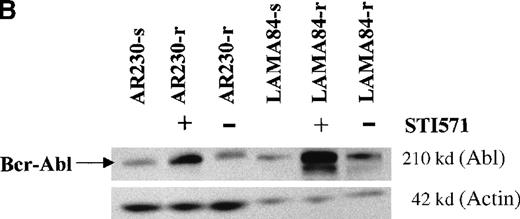
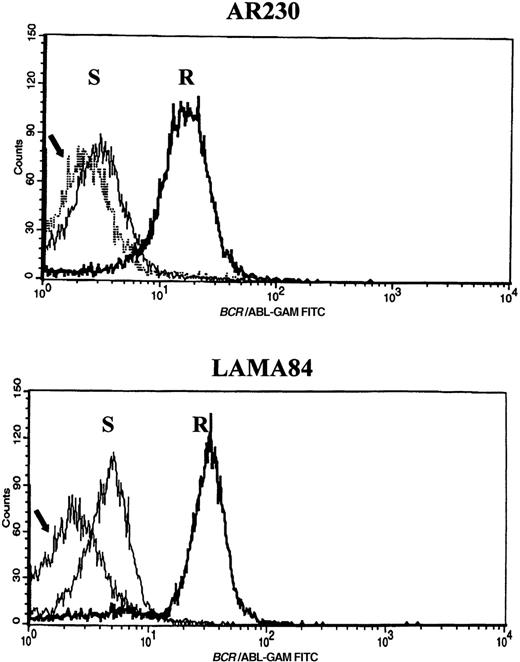
Screening of the human cell lines yielded similar findings in some cases. Northern blot hybridization with ABL and BCRcDNA probes revealed a 1.6- and a 5.5-fold increase in BCR-ABLmRNA expression in AR230-r and LAMA84-r, respectively, compared with their sensitive counterparts (Figure 5A). This overexpression was translated into protein overproduction as shown by a 6- and a 12-fold increase in Bcr-Abl levels in AR230-r and LAMA84-r, respectively, on immunoblot with an anti-Abl antibody (Figure5B). Because neither AR230 nor LAMA84 contains a normal ABL gene, we were able to confirm the overexpression of Bcr-Abl in their resistant clones by fluorocytometry of permeabilized cells stained with an anti-Abl mAb (Figure 6). After removal of STI571 from the culture, the degree of Bcr-Abl overexpression was significantly lower in both AR230-r and LAMA84-r; it was reduced to 1.5 and 5.6 times the levels found in AR230-s and LAMA84-s, respectively (Figure 5B). A similar 2.5-fold reduction in the BCR-ABL mRNA level was observed after LAMA84-r cells were withdrawn from STI571 for 7 days (data not shown).
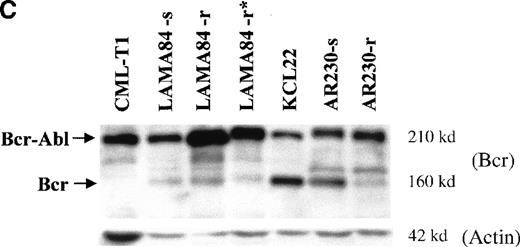
BCR-ABL expression in AR230 and LAMA84.
(A) Northern blot analysis of BCR-ABL (with an ABLprobe) and β-actin (loading control) mRNA in the sensitive and resistant cells. Densitometric analysis of the BCR-ABL/actin ratio showed a 1.6- and a 5.5-fold increase in AR230-r and LAMA84-r, respectively, compared with their sensitive parental lines. (B) Western blot probed with anti-Abl (upper part of the filter) and anti-actin (lower part of the filter) antibodies. Bcr-Abl overexpression in relation to the sensitive counterparts was estimated by densitometry of the Bcr-Abl/actin ratio as 6-and 12-fold in AR230-r and LAMA84-r, respectively; these values were reduced to 1.5- and 5.6-fold, respectively, 7 days after withdrawal of STI571 from the culture. (C) Western blot probed with anti-Bcr (upper part of the filter) and anti-actin (lower part of the filter) antibodies. CML-T1, which does not express normal Bcr protein, and KCL22, which does, were used as negative and positive controls, respectively. LAMA84-r overexpresses both Bcr (160 kd) and Bcr-Abl (210 kd) compared withA84-s. LAMA84-r* corresponds to the subline maintained in culture without STI571 for 1 week, in which reductions in Bcr-Abl and Bcr expression can be observed. AR230-r cells express a lower level of Bcr protein than AR230-s in spite of the Bcr-Abl overexpression.
BCR-ABL expression in AR230 and LAMA84.
(A) Northern blot analysis of BCR-ABL (with an ABLprobe) and β-actin (loading control) mRNA in the sensitive and resistant cells. Densitometric analysis of the BCR-ABL/actin ratio showed a 1.6- and a 5.5-fold increase in AR230-r and LAMA84-r, respectively, compared with their sensitive parental lines. (B) Western blot probed with anti-Abl (upper part of the filter) and anti-actin (lower part of the filter) antibodies. Bcr-Abl overexpression in relation to the sensitive counterparts was estimated by densitometry of the Bcr-Abl/actin ratio as 6-and 12-fold in AR230-r and LAMA84-r, respectively; these values were reduced to 1.5- and 5.6-fold, respectively, 7 days after withdrawal of STI571 from the culture. (C) Western blot probed with anti-Bcr (upper part of the filter) and anti-actin (lower part of the filter) antibodies. CML-T1, which does not express normal Bcr protein, and KCL22, which does, were used as negative and positive controls, respectively. LAMA84-r overexpresses both Bcr (160 kd) and Bcr-Abl (210 kd) compared withA84-s. LAMA84-r* corresponds to the subline maintained in culture without STI571 for 1 week, in which reductions in Bcr-Abl and Bcr expression can be observed. AR230-r cells express a lower level of Bcr protein than AR230-s in spite of the Bcr-Abl overexpression.
Flow cytometric histograms of AR230 and LAMA84 sensitive (S) and resistant (R) clones stained with an anti-Abl mAb.
The arrow indicates the profile of a BCR-ABL–negative cell line (HL60) stained with the same antibody. Relative fluorescence values are shown on the x axis.
Flow cytometric histograms of AR230 and LAMA84 sensitive (S) and resistant (R) clones stained with an anti-Abl mAb.
The arrow indicates the profile of a BCR-ABL–negative cell line (HL60) stained with the same antibody. Relative fluorescence values are shown on the x axis.
Because the BCR-ABL gene is under control of the BCRpromoter,17 18 we also analyzed the fusion protein expression using an anti-Bcr antibody to check the level of the normal Bcr protein. Figure 5C confirms that Bcr-Abl is overexpressed in LAMA84-r cells, but there is no significant change in the level of Bcr in this clone. In contrast, AR230-r cells express very small amounts of Bcr protein compared with the parental AR230-s.
In the other 2 cell lines, K562-r and KCL22-r, no up-regulation of BCR-ABL mRNA or protein expression was observed. The overall results show that resistance to STI571 is mediated by Bcr-Abl overexpression in some, but not all, cell lines and that this overexpression can be reversed at least partially by withdrawal of the tyrosine kinase inhibitor from the culture.
Some resistant cell lines have amplification of the BCR-ABL gene
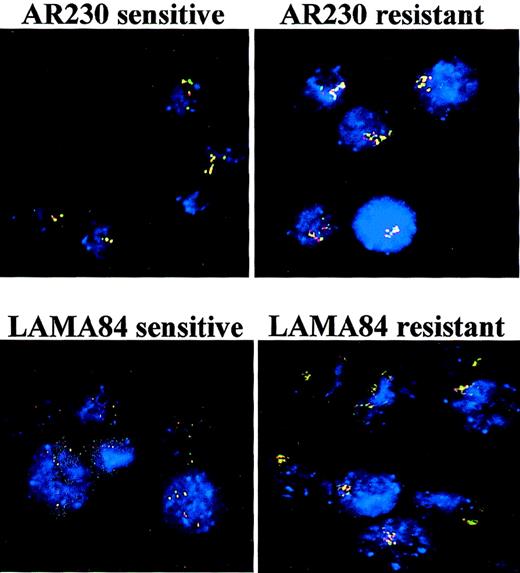
To investigate the cause of Bcr-Abl overexpression in some of the resistant cell lines, we performed fluorescence in situ hybridization (FISH) analysis of interphase nuclei with probes against theBCR and the ABL genes. Similar to what has been described for K562,19 the parental AR230 and LAMA84 cell lines had multiple copies of the BCR-ABL gene (Table1). However, resistant clones from both lines exhibited significant increases in the number of BCR-ABLcopies (Figure 7). For AR230-r there was an approximately 3-fold increase, whereas for LAMA84-r there was a nearly 6-fold amplification, with as many as 19 to 28 fusion signals discernible per cell (Table 1). In the resistant cell lines, the hybrid signals tended to cluster in individual regions of the nucleus (Figure7). No significant change in the number of fusion genes was observed in either resistant cell line when STI571 was withdrawn for 7 days from the culture media (Table 1).
Number of BCR-ABL fusion signals as detected by FISH
| Cell line . | Sensitive . | Resistant +STI571 . | Resistant −STI571 . |
|---|---|---|---|
| AR230 | 2.9 ± 0.6 | 9.6 ± 1.8 | 8.6 ± 1.5 |
| (2-4) | (7-12) | (7-12) | |
| n = 15 | n = 15 | n = 15 | |
| LAMA84 | 4.3 ± 0.7 | 23.8 ± 2.5 | 23.1 ± 2.9 |
| (3-5) | (19-28) | (18-29) | |
| n = 15 | n = 30 | n = 30 |
| Cell line . | Sensitive . | Resistant +STI571 . | Resistant −STI571 . |
|---|---|---|---|
| AR230 | 2.9 ± 0.6 | 9.6 ± 1.8 | 8.6 ± 1.5 |
| (2-4) | (7-12) | (7-12) | |
| n = 15 | n = 15 | n = 15 | |
| LAMA84 | 4.3 ± 0.7 | 23.8 ± 2.5 | 23.1 ± 2.9 |
| (3-5) | (19-28) | (18-29) | |
| n = 15 | n = 30 | n = 30 |
FISH analysis of AR230 and LAMA84 sensitive and resistant clones, with probes for the ABL (red signal) and theBCR (green signal) genes.
BCR-ABL is identified as a red–green or yellow fused signal.
FISH analysis of AR230 and LAMA84 sensitive and resistant clones, with probes for the ABL (red signal) and theBCR (green signal) genes.
BCR-ABL is identified as a red–green or yellow fused signal.
Attempts at FISH analysis in the Baf/BCR-ABL–derived cell lines using different sources of genomic BCR and ABLprobes were unsuccessful. Therefore, we investigated the possibility of gene amplification in these lines by Southern hybridization with cDNA probes spanning sequences present in the pGD210 provirus3used for the transduction of BCR-ABL into Ba/F3. On average, 5.7-fold stronger signals for BCR and ABL sequences were observed in Baf/BCR-ABL-r clones resistant to 1 μmol/L and 3 μmol/L than in Baf/BCR-ABL-s cells in duplicate experiments (data not shown).
Some resistant cell lines require higher STI571 concentrations for the inhibition of Bcr-Abl phosphorylation
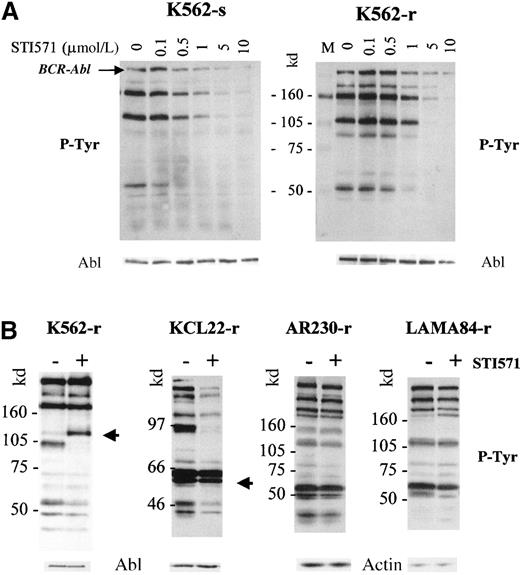
To investigate whether resistance to STI571 resulted from a lack or a reduction in inhibition of the Bcr-Abl kinase activity, we analyzed the effect of the compound on the phosphorylation pattern of the various cell lines. Sensitive and resistant clones were washed with RF-10, incubated in graded concentrations of STI571 for 2 hours, and processed for Western blots, which were probed with an anti-phosphotyrosine antibody (Figure 8). Phosphorylation of various proteins decreased in a dose-dependent manner in the sensitive and resistant clones. However, the doses of STI571 necessary to inhibit overall tyrosine phosphorylation were higher for K562-r (Figure 8A), LAMA84-r, and AR230-r (not shown) than for the respective parental clones. Densitometric quantification of the phosphorylated p210BCR-ABL band was used to calculate the IC50, that is, the dose of STI571 that inhibits 50% of the phosphorylation of Bcr-Abl itself (Table2). We found that the IC50 for the human CML cell lines was approximately 0.5 μmol/L but that a dose twice as high was required to achieve the same effect on the Ba/F3 murine cell line transfected with BCR-ABL. In the latter, as well as in KCL22, no difference was observed in the IC50 between the sensitive and the resistant clones. In contrast, LAMA84-r and AR230-r showed a 2.5-fold increase in the STI571 IC50, and this index was increased 4-fold in K562-r.
Phosphotyrosine immunoblots and control staining of the same blots (after strip-washes) with anti-Abl or anti-actin antibodies.
(A) K562-sensitive and -resistant clones after a 2-hour incubation with graded concentrations of STI751. (B) K562-, KCL22-, AR230-, and LAMA84-resistant sublines incubated for 8 days in the presence (+) or absence (−) of 1 μmol/L STI571. The arrows indicate protein bands of approximately 110 and 55 to 58 kd, which become or remain hyperphosphorylated in K562-r and KCL22-r, respectively.
Phosphotyrosine immunoblots and control staining of the same blots (after strip-washes) with anti-Abl or anti-actin antibodies.
(A) K562-sensitive and -resistant clones after a 2-hour incubation with graded concentrations of STI751. (B) K562-, KCL22-, AR230-, and LAMA84-resistant sublines incubated for 8 days in the presence (+) or absence (−) of 1 μmol/L STI571. The arrows indicate protein bands of approximately 110 and 55 to 58 kd, which become or remain hyperphosphorylated in K562-r and KCL22-r, respectively.
IC50 values for inhibition of Bcr-Abl phosphorylation by STI571
| Cell lines . | IC50 (μM STI571) . | |
|---|---|---|
| Sensitive sublines . | Resistant sublines . | |
| Baf/BCR-ABL | 1 | 1 |
| LAMA84 | 0.4 | 1 |
| AR230 | 0.4 | 1 |
| K562 | 0.5 | 2 |
| KCL22 | 0.4 | 0.4 |
| Cell lines . | IC50 (μM STI571) . | |
|---|---|---|
| Sensitive sublines . | Resistant sublines . | |
| Baf/BCR-ABL | 1 | 1 |
| LAMA84 | 0.4 | 1 |
| AR230 | 0.4 | 1 |
| K562 | 0.5 | 2 |
| KCL22 | 0.4 | 0.4 |
We next analyzed the tyrosine phosphorylation profile of proteins in the resistant clones after 8 days in culture with and without 1 μmol/L STI571. As expected, the phosphotyrosine content of the 3 resistant cell lines that required higher STI571 concentrations for effective Bcr-Abl inhibition remained largely unchanged (Figure8B). In K562-r however, an approximately 110-kd protein became hyperphosphorylated in the presence of 1 μmol/L STI571, whereas phosphorylation of a slightly smaller band at approximately 102 kd was exceptionally inhibited under these conditions. As a further confirmation of the IC50 measurements, the phosphotyrosine content of most proteins in KCL22-r was significantly reduced in the presence of the compound, but a group at approximately 55 to 58 kd remained strongly phosphorylated (Figure 8B). Thus, the possibility that other cellular kinases unresponsive to STI571 activate a part of the Bcr-Abl pathway cannot be excluded for K562-r and KCL22-r. Altogether, the results suggest that resistance to STI571 in AR230-r, LAMA84-r, and K562-r at least in part results from a reduced inhibition of Bcr-Abl kinase activity by concentrations of the compound effective in their sensitive parental lines.
Expression and function of the Pgp multidrug resistance protein
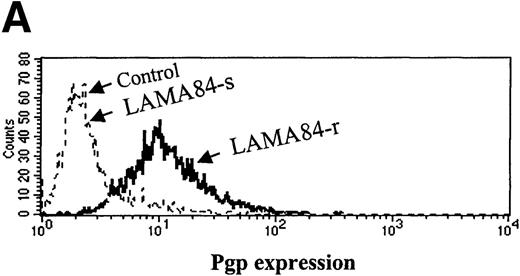
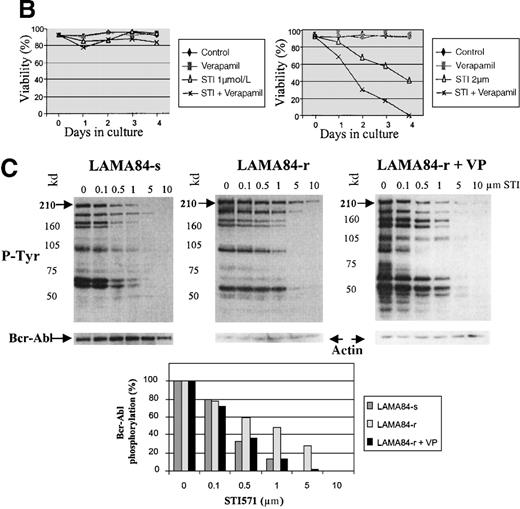
Inappropriate expression of the MDR-1 gene that codes for the Pgp glycoprotein has been frequently implicated in the mechanism of resistance to different drugs used in chemotherapy. Overexpression of Pgp can functionally modify the uptake of several drugs.20We studied the pattern of Pgp expression by flourocytometry in the 3 resistant clones that showed increased requirements for STI571 for Bcr-Abl kinase inhibition. AR230-r and K562-r showed negligible, baseline levels of Pgp that overlapped the profiles of their sensitive counterparts. In contrast, LAMA84-r exhibited a significant overexpression of Pgp: more than 50% of the resistant cells showed a strong reaction to the anti-Pgp antibody compared with only 2% of the LAMA84-s parental line (Figure 9A). The experiments that followed aimed at establishing the functional relevance of this overexpression. LAMA84-r cells were incubated with verapamil, a known inhibitor of Pgp, and were assessed for viability on exposure to different concentrations of STI571. Although no significant change in their usual resistance to 1 μmol/L STI571 was elicited, a clear enhancement of sensitivity to the higher 2 μmol/L dose of the compound was observed when verapamil was added to the culture (Figure9B). Because the STI571 IC50 for LAMA84-r is higher than that for LAMA84-s, we also established the IC50 for LAMA84-r after incubation with verapamil to investigate whether such treatment could modify and decrease the doses of STI571 necessary to inhibit Bcr-Abl tyrosine kinase (Figure 9C). In fact, the IC50 for LAMA84-r cells, which is 1 μmol/L (Table 2), was reversed to 0.4 μmol/L after the addition of verapamil, the same value found for LAMA84-s. No change in the IC50 for the latter was detected on treatment with verapamil. We conclude that LAMA84-r cells overexpress Pgp, a phenomenon that may impair the uptake of STI571 by this resistant subline.
Expression and function of the Pgp MDR-1 gene product in LAMA84.
(A) Flow cytometric analysis of Pgp expression in LAMA84-r and LAMA84-s clones. Note that the histogram for the latter overlaps that of cells stained with the isotypic control. (B) Effect of verapamil on the viability of LAMA84-r cells incubated with 1 μmol/L (left panel) or 2 μmol/L (right panel) STI571. (C) Effect of verapamil on the IC50 of LAMA84 cells. Upper panels show Western blots of LAMA84-s, LAMA84-r, and LAMA84-r treated with verapamil for 2 hours, probed with an anti-phosphotyrosine (pTyr) and with anti-Abl (Bcr-Abl band shown) or anti-actin antibodies. Lower panel illustrates the decrease in the IC50 for Bcr-Abl phosphorylation in LAMA84-r cells exposed to verapamil (VP), as calculated by densitometric analysis of the ratio pTyr-Bcr-Abl/Bcr-Abl or pTyr-Bcr-Abl/actin. We have ascertained that verapamil does not modify the IC50 in LAMA84-s cells (data not shown).
Expression and function of the Pgp MDR-1 gene product in LAMA84.
(A) Flow cytometric analysis of Pgp expression in LAMA84-r and LAMA84-s clones. Note that the histogram for the latter overlaps that of cells stained with the isotypic control. (B) Effect of verapamil on the viability of LAMA84-r cells incubated with 1 μmol/L (left panel) or 2 μmol/L (right panel) STI571. (C) Effect of verapamil on the IC50 of LAMA84 cells. Upper panels show Western blots of LAMA84-s, LAMA84-r, and LAMA84-r treated with verapamil for 2 hours, probed with an anti-phosphotyrosine (pTyr) and with anti-Abl (Bcr-Abl band shown) or anti-actin antibodies. Lower panel illustrates the decrease in the IC50 for Bcr-Abl phosphorylation in LAMA84-r cells exposed to verapamil (VP), as calculated by densitometric analysis of the ratio pTyr-Bcr-Abl/Bcr-Abl or pTyr-Bcr-Abl/actin. We have ascertained that verapamil does not modify the IC50 in LAMA84-s cells (data not shown).
Sequencing of the Abl kinase domain
For STI571-resistant cells that did not overexpressBCR-ABL but that did have higher IC50 values than their sensitive counterparts, such as K562-r, we asked whether resistance to the compound could be caused by a modification in the ATP binding site of the Abl kinase domain, thought to be the target of STI571. To address this question, we sequenced the entire kinase domain of K562-sensitive and -resistant cells and of AR230-sensitive and -resistant clones (as controls) and compared these to the published sequences.21 22 No mutation was found in an 856-bp fragment, including the Abl kinase domain, in any of the cell lines.
Discussion
STI571 can be regarded as the first member of a new family of drugs termed signal transduction inhibitors. It was designed based on the structure of the ATP binding site of the kinase domain, and it displays specificity at the submicromolar level for the Abl, PDGF, and Kit receptor kinases and selectively kills BCR-ABL–expressing cells.7,8,23 Nevertheless, we observed previously that rare Ph-positive cell lines are unaffected by concentrations of STI571 that suppress the proliferation of most CML cell lines and that a few primary BCR-ABL positive progenitors from peripheral blood or bone marrow are resistant to the compound.9 Relapse from tumor growth in mice injected with BCR-ABL–positive human cell lines and treated with STI571 has also been reported,24 suggesting the development of resistance in vivo. To study this phenomenon, we generated sublines with differential sensitivity to STI571 from BCR-ABL positives cell lines and investigated the possible mechanisms of their resistance to this compound.
The first remarkable observation from this exercise was the overall difficulty in generating resistant clones from the STI571-sensitive cell lines. This was particularly pronounced among the human Ph-positive lines. Although all 8 STI571-sensitive cell lines were clonogenic in methylcellulose, none was able to generate colonies in the presence of the compound. Even when subjected to a gradual exposure to STI571 in liquid culture, no cell survival was observed in 4 of the 8 lines at concentrations above 0.2 μmol/L. The inability to isolate resistant clones from some cell lines did not correlate with obvious cellular and molecular characteristics, such as blast crisis lineage, p53 status, FAS receptor expression, or structure (junction) of theBCR-ABL mRNA (data not shown). In the 4 cell lines from which clones of STI571-resistant cells were obtained, these represented rare survivors from massive cell killing at each step of dose increase, suggesting that they arose in response to selective pressure of the inhibitor. These results emphasize the specificity and high efficacy of this drug for the control of proliferation of BCR-ABL–positive cells and show that development of resistance among these cells is, at least in vitro, a rare phenomenon.
Nevertheless, occasional cells do escape the pro-apoptotic effect of STI571 compound9 and are able to establish a subline of cells that can grow continuously in the presence of pharmacologic doses24 of the compound. It is interesting to observe that although these resistant sublines are not killed by the tyrosine kinase inhibitor, their proliferation is slowed in comparison with the parental cultures (Figure 2C), suggesting that a delaying effect over the cell cycle is still elicited by the drug. This pattern of growth was also described in cell lines resistant to other drugs, such as cytosine arabinoside, colchicine, vinca alkaloids, epidophyllotoxins, anthracycline, and actinomycin D, with cross-resistance between them characterizing the multidrug resistance phenotype (reviewed in Arceci25).
The mechanisms by which a rare subpopulation of cells within susceptible BCR-ABL–positive lines becomes resistant to STI571 differ between the different lines. The most common, detected in theBCR-ABL–transfected murine Ba/F3 cells and in the human LAMA84 and AR230 CML cell lines, is overexpression of the Bcr-Abl protein. By increasing the amount of oncoprotein requiring inhibition of its kinase activity, the cell can survive concentrations of STI571, which then become suboptimal for competing out all the ATP binding. This is most clearly illustrated by the various Baf/BCR-ABL-r clones in which the degree of Bcr-Abl overexpression was directly correlated with the concentration of STI571 to which the individual clone was resistant (Figure 4). Similar behavior in the development of resistance to other chemotherapeutic agents has been described in cases of Pgp overproduction.26 27
The molecular basis for Bcr-Abl overexpression in all 3 resistant lines is, at least in part, a significant amplification of theBCR-ABL gene itself. The precedent for gene amplification as the underlying cause of drug resistance has been extensively documented for the MDR and the multidrug-resistance related protein genes.28,29 In our study, however, this finding was somewhat surprising in view of the fact that the degree of overexpression could be relatively rapidly modulated in the 3 cell lines by withdrawal of the inhibitor and by addition of IL-3 in the case of Baf/BCR-ABL-r. As expected this reduction in the level of Bcr-Abl overexpression was not accompanied by a similar decrease in the multiplicity of BCR-ABL genes. It thus appears that mechanisms responsible for controlling transcription and translation from the amplified genes are also in operation in each resistant line. If such a control were effected at the level of the BCR-ABLgene promoter, it might be expected that expression of the normalBCR mRNA and protein would be similarly increased in resistant clones. This was not the case for LAMA84-r cells, and, in fact, the opposite phenotype—that is, a decrease in normal Bcr expression—was observed in AR230-r. Although the molecular basis for this phenomenon is unknown, the reduced levels of Bcr protein could contribute to the resistance of AR230-r cells to STI571 because Bcr has been described as a negative regulator of Bcr-Abl.30
An alternative mechanism for the modulation of Bcr-Abl expression in the resistant lines would be a decrease in Bcr-Abl protein degradation through the inhibition of a cellular protease. This is, in fact, a frequent phenomenon in the regulation of apoptotic processes in which a family of inhibitors of apoptosis proteins can modulate cell death by the abrogation of caspase activity.31 However, an increase rather than a reduction in Bcr-Abl degradation products was detected in Baf/BCR-ABL-r cells (Figure 3B), suggesting that this would be an unlikely explanation for the reversible Bcr-Abl overexpression in this cell line.
In 2 of the resistant human cell lines, K562-r and KCL22-r, the levels of Bcr-Abl protein were comparable to those in their sensitive counterparts, indicating that other mechanisms underlie the resistance of these lines to STI571. The possibility that these cells had developed increased requirements for the compound for effective tyrosine kinase inhibition proved to be the case for K562-r, as well as for LAMA84-r and AR230-r, but not for KCL22-r, as shown by their respective STI IC50 for the inhibition of Bcr-Abl phosphorylation (Table 2). This biologic behavior has also been described in the development of resistance to other agents, such as anthracycline and vinca alkaloids in leukemic cells, and is usually attributed to a decrease in the cellular uptake of the drug.32,33 The most extensively studied mediator of this phenomenon is the Pgp protein encoded by the MDR-1 gene, which affects the uptake of a soluble compound by “pumping out” the drug through the plasma membrane.20 25 Measurement of Pgp expression in the 3 STI571-resistant cell lines with high IC50 showed that 1 of them, LAMA84-r, did indeed overexpress Pgp, in comparison with the LAMA84-sensitive line. Moreover, inhibition of Pgp with verapamil, a potent blocker of the pump, led to an improved uptake of STI571 in LAMA84-r, as shown by the reduction of its IC50. It is important to note, though, that the inhibition of Pgp also resulted in enhancement in the sensitivity of LAMA84-r to higher doses of STI571, but it was insufficient to overcome their resistance to the 1 μmol/L concentration in which they normally survive, probably because this cell line carries a second, independent mechanism of resistance (Bcr-Abl overexpression), which on its own is able to sustain the basic resistant phenotype.
The reasons for the increased requirement for STI571 in K562-r and AR230-r were not apparent. Mutations in the Bcr-Abl tyrosine kinase domain, which could partially prevent or hamper the binding of the compound to the ATP binding site, were not found in either cell line. It is possible, however, that other proteins such as the recently described multidrug-resistance related protein34 may be involved in reducing the STI571 uptake in these cells.
The resistance of KCL22 is the most intriguing of all because none of the obvious mechanisms investigated in this study were present in this line. It should be noted that the original, parental KCL22 is the only line among 12 CML cell lines tested in our laboratory9 (and unpublished observations) that is largely resistant to STI571. Even the few sensitive clones that could be isolated from this line showed some degree of resistance to the inhibitor, represented by a longer survival in 1 μmol/L STI571 than that exhibited by the other sensitive cell lines (Figure 2A). The fact that KCL22 can resist the apoptotic effect of STI571 without increasing the level of Bcr-Abl expression and still undergoing effective Bcr-Abl tyrosine kinase inhibition suggests that this cell line has evolved an alternative abnormality to circumvent the Bcr-Abl–dependent susceptibility to STI571. The nature of such abnormality cannot be inferred from this study, but it may be related to a group of 55- to 58-kd proteins that remain constitutively hyperphosphorylated when tyrosine phosphorylation of all Bcr-Abl–dependent proteins is well inhibited (Figure 8B). Investigations aiming at identifying this putative genetic event are in progress.
In conclusion, our data show that resistance to STI571 amongBCR-ABL–positive cells is a rare phenomenon and may develop through multiple mechanisms, such as Bcr-Abl overexpression, reduction in the uptake of the compound by Pgp overexpression, or possibly by excessive degradation. We cannot exclude that the acquisition of compensatory mutations in genes other than BCR-ABL also plays a part. It is possible that the same or similar mechanisms operate in a few primary CML progenitors that escape the in vitro antiproliferative effect of the compound.9 Thus amplification of theBCR-ABL gene, usually detected as the emergence of extra Ph-chromosomes, is a frequent event in blast crisis of CML,35 when the transformed leukemic clone becomes refractory to virtually all chemotherapeutic agents. Similarly, an increase in BCR-ABL mRNA expression has been described in 1 study as a marker of disease progression,36 though the magnitude of this overexpression has been questioned.37 It has also been reported that during the evolution of the disease to blast crisis, patients with CML may become resistant to treatment because of Pgp overexpression.38 Because STI571 is being tested in clinical trials for its therapeutic benefits in CML,11 it will be important to determine whether CML stem cells are able to develop in vivo these reactive strategies to survive the effects of this potent drug.
Acknowledgments
We thank Dr Elisabeth Buchdunger (Novartis, Basel, Switzerland) for providing STI571 and Dr R. Arlinghaus (MD Anderson Cancer Center, Houston, TX) for the anti-Bcr antibody. The HL60/MDR cell line was kindly provided by Dr S. Devereux (University College of London, London, UK).
Supported by grants from the Leukaemia Research Fund (UK), the Fondation contre la leucemie, Association pour la recherche contre cancer, and the Fondation de la recherche medicale (France), Dr. Ernst und Anita Bauer Stiftung (Nürnberg, Germany), and Dr. Mildred Scheel–Stiftung für Krebsforschung (Germany).
Reprints:Junia V. Melo, Department of Haematology, Imperial College School of Science, Technology and Medicine, Hammersmith Hospital, Ducane Rd, London W12 ONN, United Kingdom; e-mail: j.melo@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal