Abstract
The encounter with allogeneic major histocompatibility complex (MHC) molecules expressed on donor leukocytes during transfusion of blood products has been shown to impact the recipient's immune responses in a number of settings. To better understand the responses induced by the transfer of allogeneic cells, a murine model was used to characterize the recipient responses that control the fate of the allogeneic lymphoid cells. Recipient CD8+ cells could rapidly eliminate a large number of donor cells within 3 days after injection. When elimination responses were studied in the absence of CD8+ cells, it was found that alloantibody production was the secondary elimination mechanism. Optimal recipient CD8+ and B cell responses in this model required help from CD4+ cells that could be provided by 3 different pathways. Although recipient CD4+ cells could provide help when activated by direct recognition of allogeneic MHC class II molecules expressed on donor cells or by indirect recognition of processed alloantigen presented on recipient antigen-presenting cells (APCs), the most rapid recipient responses were generated by help provided by donor CD4+ cells. Purified donor CD4+ cells were also able to induce these rapid responses, indicating that activated donor CD4+cells expressing allogeneic MHC molecules were able to effectively stimulate responses by both recipient CD8+ and B cells.
Immune responses to allogeneic major histocompatibility complex (MHC) molecules are unique because of the high frequency of T cells that respond to these antigens. In vivo, the human immune system encounters alloantigen during pregnancy and after the transfusion of blood products or transplantation. The immune responses to alloantigen in these settings can have a number of immunologic consequences. For example, blood transfusion has been shown to result in the production of alloantibodies,1 increased incidence of bacterial infection,2 increased risk of tumor relapse especially for certain categories of tumors,2 transfusion associated graft-versus-host disease,3 prolonged allograft survival,4 antileukemic responses,5 and reversal of recurrent spontaneous abortion in some women.6These findings have raised questions about the mechanism by which a blood transfusion can affect all these responses. To define this mechanism, investigators have begun to study some of the immune responses such as the production of cytokines that are induced by transfusion. Although there may be cytokines already present in the stored blood product,7 several studies have suggested that blood transfusion preferentially produces Th2 cytokines such as IL-4 and IL-10.8-10 In the murine model, the transfusion of intact cells appeared to induce both Th1 and Th2 cytokines, whereas transfusion of apoptotic cells appeared to preferentially induce Th2 cytokines.11 This has led to the hypothesis that transfusion influences immune responses by preferentially producing Th2 cytokines.12
Posttransfusion responses also have been studied by measuring the fate of the donor leukocytes. It was found that 99.9% of the leukocytes are eliminated within 2 days after a blood transfusion, with the residual cells being eliminated by day 6.13 Similar elimination times were reported in the canine and murine model.13-15 To study the immune responses that result from the transfer of allogeneic cells, it was decided to characterize the mechanisms responsible for the elimination of allogeneic donor cells. A murine model was chosen for these studies because it is often difficult in human patients to distinguish the responses caused by transfusion from those responses caused by the underlying condition that requires the transfusion. An additional advantage of the murine model is the ability to carefully control donor/recipient combinations by using inbred as well as knockout (KO) or transgenic strains. Initial experiments confirmed that a large number of fully allogeneic donor splenocytes were eliminated within 3 days by naive murine recipients.16 This rapid elimination was found to be mediated by recipient CD8+cells predominantly with the use of the perforin pathway for lysis of the donor cells.16 This paper shows that alloantibody production is the secondary mechanism of elimination in the absence of CD8+ cells.
These findings raised the question of the role of CD4+cells in regulating the recipient CD8+ and B-cell responses in this model. CD4+ cells have been shown to be activated in response to alloantigens using 2 different pathways. The first pathway is a direct recognition of allogeneic MHC II antigens on donor cells. The second pathway is the indirect recognition of peptides of processed alloantigen presented by self-MHC II molecules on recipient antigen-presenting cells (APCs). Both of these pathways have been shown to provide help for the induction of CD8+ and B-cell responses after transplantation in vivo.17-19 Although help for recipient CD8+ and B-cell responses to allogeneic donor cells could be provided by recipient CD4+ cells that had been activated by either the direct or indirect pathway, the most efficient help for both recipient CD8+ and B-cell responses in this model system was found to be provided by activated donor CD4+ cells. The same results were found when purified donor CD4+ cells were used indicating that activated CD4+ cells are effective APCs when the antigen being recognized is the allogeneic MHC molecules expressed on their cell surface.
Materials and methods
Mice
The mouse strains that were used for these experiments are described in Table 1. All mice were obtained from Jackson Laboratory (Bar Harbor, ME), unless indicated otherwise.
Description of mouse strains used for these experiments
| Strain name (abbreviation) . | Strain name (full) . | H-2 . | Description of strain . |
|---|---|---|---|
| CD8 KO | C57BL/6-Cd8atm1Mak | b | Lacks CD8+ T cells |
| B-less | C57BL/6-Igh-6tm1Cgn | b | Lacks B cells |
| CD4 KO | C57BL/6J-Cd4tm1Knw | b | Lacks CD4+ cells |
| MHC II KO | C57BL/6TacfBR-[KO]Ab N5* | b | Lacks CD4+ cells and expression of MHC class II molecules |
| C57BL/6 | C57BL/6J | b | Inbred strain |
| DBA/2 | DBA/2J | d | Inbred strain |
| Strain name (abbreviation) . | Strain name (full) . | H-2 . | Description of strain . |
|---|---|---|---|
| CD8 KO | C57BL/6-Cd8atm1Mak | b | Lacks CD8+ T cells |
| B-less | C57BL/6-Igh-6tm1Cgn | b | Lacks B cells |
| CD4 KO | C57BL/6J-Cd4tm1Knw | b | Lacks CD4+ cells |
| MHC II KO | C57BL/6TacfBR-[KO]Ab N5* | b | Lacks CD4+ cells and expression of MHC class II molecules |
| C57BL/6 | C57BL/6J | b | Inbred strain |
| DBA/2 | DBA/2J | d | Inbred strain |
An abbreviated and full name are given.
These mice were obtained from Taconic, Germantown, NY.
Antibodies
The monoclonal antibodies that were used for these experiments included anti-Ly 2.2 (2.43, American Type Culture Collection [ATCC], Rockville, MD20), anti-CD8 (3.155, ATCC20) anti-CD4 (GK 1.5, ATCC).21 Directly labeled antibodies that were used for staining cells were obtained from Pharmingen, San Diego, CA (fluorescein isothiocyanate [FITC] anti-CD4, phycoerythrin anti-CD8) and from Serotec, Raleigh, NC (FITC anti-F4/80, a marker of macrophages).
Injection of donor cells and assay of recipient responses
To measure persistence of donor cells, splenocytes were labeled directly with FITC (Sigma Chemical Co, St Louis, MO) as described16 and these FITC-labeled donor cells were injected intravenously in the lateral tail vein of the indicated recipients. All recipients were injected with 1 spleen equivalent of donor cells, unless indicated otherwise. On the indicated day, the recipient mice were killed and the blood obtained by cardiac puncture using syringes containing 50 units heparin. The spleen and the inguinal, axillary, and mesenteric lymph nodes were also obtained from the recipients. The blood was spun for 10 minutes at ×700g and the plasma was collected and stored at −20°C for alloantibody assays. Single cell suspensions were prepared from the spleen and lymph nodes and a small aliquot of the cells were fixed in 0.5% paraformaldehyde and then analyzed for the presence of FITC-labeled donor cells using a FACS (FACScan, Becton-Dickinson). Each recipient mouse was analyzed individually, data from all similar mice in different experiments were combined and the mean and SD were determined. Statistical significance of the differences between groups was determined using the 2-sided Student t test.
For some experiments, mice were depleted of CD4+ cells by 2 intraperitoneal injections of 50 μL of anti-CD4 (GK1.5) ascites on days −3 and −2. Then on day 0, donor spleen cells were obtained from the anti-CD4–treated mice, labeled with FITC, and then injected intravenously into recipients that had also been injected with anti-CD4 using the same protocol. Detectable levels of anti-CD4 could still be found by day 7 in recipient mice that had been injected with this dose of anti-CD4 (data not shown).
In one set of experiments, recipient mice were injected with FITC-labeled purified CD4+ cells. The purified CD4+ cells were obtained by collecting donor spleen and lymph node cells, lysing the red blood cells with a red blood cell lysis solution (Gentra, Minneapolis, MN), then passing the cells over a T-cell enrichment column prepared as per manufacturer's instructions (R & D Systems, Minneapolis, MN) and collecting the nonadherent cells. The purified T cells were suspended at 40 × 106cells/mL, an equal volume of 3.155 culture supernatant was added and the cells incubated on ice for 1 hour and then washed twice in MLC medium.16 Then the cells were resuspended at 20 × 106 cells/mL in Low Tox rabbit complement (Cedarlane, Westbury, NY) diluted 1:20 in mixed lymphocyte culture (MLC) medium the cells were incubated for 5 minutes on ice and then 45 minutes at 37°C. After washing twice, the cells were stained for the number of CD4+ and F4/80+ cells. The purified CD4+ cells contained 84% to 88% CD4+cells and 0.2% to 2.8% F4/80+ cells. The starting populations contained 6.13% ± 3.05% F4/80+ cells.
Measurement of alloantibody production
The level of alloantibody was assessed by measuring the ability of plasma to stain thymocytes from the donor strain.11Thymocytes were prepared from the appropriate strain and reconstituted at 50 × 106 cells/mL in diluent (phosphate-buffered saline [PBS], containing 0.5% bovine serum albumin [BSA] and 0.1% sodium azide). Ten microliters of cells were incubated with 20 μL of plasma diluted 1:5 with diluent for 30 minutes on ice. After 2 washes with diluent, the thymocytes were incubated with FITC conjugates of goat antimouse IgM, IgG, IgG1, and IgG2a (Zymed, San Francisco, CA, 20 μL of a 1: 20 dilution) for 30 minutes on ice. Then the cells were washed twice and fixed with freshly prepared 0.5% paraformaldehyde. As controls, thymocytes were stained with plasma obtained from naive recipients. The samples were analyzed using a FACScan. The mean fluorescence channel number was obtained for each sample. The results are reported as the increase in mean channel number seen in the experimental sample compared with the control sample. The higher the increase in the mean channel number, the greater the concentration of alloantibodies in the plasma.
Results
Alloantibody production is the secondary donor cell elimination mechanism to recipient CD8+ cells
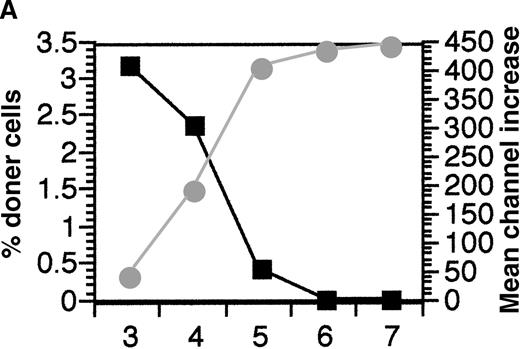
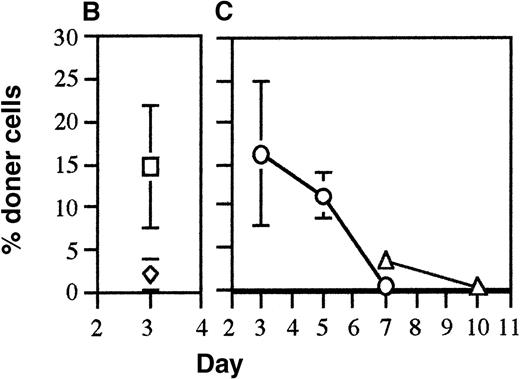
In previous experiments in which 1 spleen equivalent of fully allogeneic donor cells were injected per recipient, it was found that all donor cells were eliminated by 3 days after injection.16 This rapid elimination was shown to be mediated by recipient CD8+ cells using the perforin pathway as the major lytic pathway although the Fas/Fas ligand pathway and the tumor necrosis factor (TNF) pathway were also used to some degree in the absence of the perforin pathway. This rapid elimination pathway was driven by differences in the MHC and required both MHC class I and II differences to get effective elimination.16To examine whether the recipient CD8+ cells were the only effector mechanism responsible for the elimination of allogeneic donor splenocytes, the ability of CD8 KO mice to eliminate allogeneic donor splenocytes was measured. It was found that complete elimination of donor cells by CD8 KO mice was delayed from day 3 to day 6(Figure1A). These results indicated that an elimination mechanism other than recipient CD8+ cells was operating. Because preliminary experiments had shown that alloantibody-coated donor splenocytes were eliminated within 48 hours after injection (data not shown), the presence of IgG alloantibodies in the plasma of the same recipient CD8 KO mice was tested. The appearance of alloantibodies was found to be concordant with the elimination of the donor cells in these mice (Figure 1A). To confirm the role of alloantibodies in eliminating the donor cells, the elimination of allogeneic donor cells was tested in recipient mice lacking B cells (B-less). Higher percentages of donor cells were observed in these recipients because of the smaller number of recipient spleen cells per mouse. Despite the lack of B cells in the spleen, these B-less recipient mice were able to rapidly eliminate allogeneic donor splenocytes by day 3 (Figure 1B). This was not surprising as the B-less mice did not lack CD8+ cells. Thus, to test whether alloantibodies were involved in elimination of allogeneic cells, the B-less mice would have to be depleted of CD8+cells. The depletion of CD8+ cells was achieved by 1 or more injections of anti-CD8 and the ability of these CD8-depleted, B-less recipients to eliminate allogeneic cells was tested. The anti-CD8 that was used was allele-specific (anti-Ly 2.2) so that only recipient CD8+ cells would be bound by the antibody. The results indicated that there was prolonged persistence of the donor cells in these CD8-depleted recipient mice lacking B cells (compare Figure 1A with Figure 1C). Recipient CD8+ cells had started reappearing by day 7 after a single injection of anti-CD8 on day −1 (data not shown), thus the B-less mice were given injections of anti-CD8 on days −1 and 3 in a second experiment. The multiple injections of anti-CD8 resulted in even longer persistence of the donor cells (Figure 1C). One recipient B-less mouse that had been injected twice with anti-CD8 exhibited detectable donor antirecipient CTL (cytolytic T lymphocyte) on day 10 (data not shown), indicating that there was sufficient persistence of donor cells to induce graft-versus-host responses. These results confirmed the role of alloantibody production as the secondary elimination mechanism and also indicated that recipient CD4+ cells did not play a major role in directly eliminating donor cells.
Alloantibodies are responsible for elimination of donor cells in the absence of CD8 cells.
(A) To test if CD8+ cells were the only recipient mechanism responsible for elimination of allogeneic cells, FITC-labeled DBA/2 splenocytes were injected into CD8 KO mice and persistence was measured on days 3 to 7 (mean of 2 mice per day shown, ▪). In addition the presence of alloantibodies binding DBA/2 cells in the plasma of these mice was tested. (B) The persistence of one spleen equivalent of FITC C57BL/6 (◊) and DBA/2 splenocytes (□) when injected into B-less recipients was tested on day 3. The percentage of donor cells recovered was higher than normal because of the lack of B-cells in the spleen. (C) To test if B-less mice could still eliminate allogeneic cells in the absence of CD8+ cells, B-less recipients were injected with anti-CD8− and FITC DBA/2 splenocytes (1 spleen equivalent per recipient) were injected on day 0 and persistence measured in the recipient spleen cells on days 3, 5, and 7 (○). Because recipient CD8+ cells could be detected on day 7 (data not shown), the experiments were repeated, injecting anti-CD8 intraperitoneally on days −1 and 3 and measuring the persistence of donor FITC-labeled DBA/2 cells on days 7 and 10 (▵).
Alloantibodies are responsible for elimination of donor cells in the absence of CD8 cells.
(A) To test if CD8+ cells were the only recipient mechanism responsible for elimination of allogeneic cells, FITC-labeled DBA/2 splenocytes were injected into CD8 KO mice and persistence was measured on days 3 to 7 (mean of 2 mice per day shown, ▪). In addition the presence of alloantibodies binding DBA/2 cells in the plasma of these mice was tested. (B) The persistence of one spleen equivalent of FITC C57BL/6 (◊) and DBA/2 splenocytes (□) when injected into B-less recipients was tested on day 3. The percentage of donor cells recovered was higher than normal because of the lack of B-cells in the spleen. (C) To test if B-less mice could still eliminate allogeneic cells in the absence of CD8+ cells, B-less recipients were injected with anti-CD8− and FITC DBA/2 splenocytes (1 spleen equivalent per recipient) were injected on day 0 and persistence measured in the recipient spleen cells on days 3, 5, and 7 (○). Because recipient CD8+ cells could be detected on day 7 (data not shown), the experiments were repeated, injecting anti-CD8 intraperitoneally on days −1 and 3 and measuring the persistence of donor FITC-labeled DBA/2 cells on days 7 and 10 (▵).
The requirement for CD4+ cell help for optimal recipient CD8+ and B-cell responses
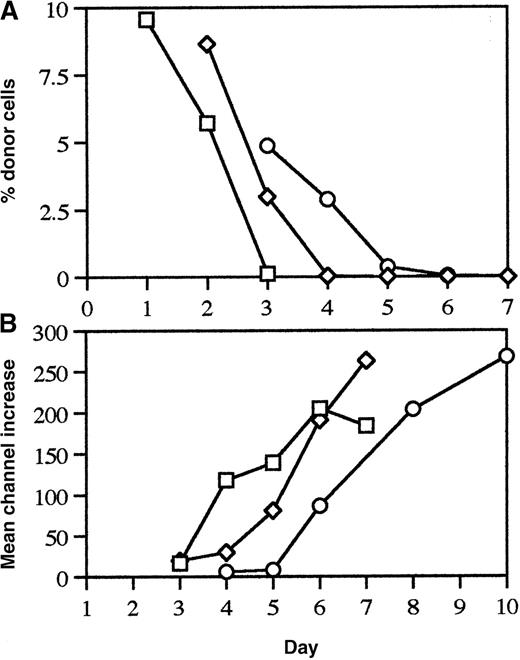
The studies completed to this point had shown that recipient CD8+ and B-cell responses were responsible for the elimination of allogeneic donor cells but had not tested whether these cells were able to mount these responses by themselves or required help. To test whether CD4+ cells played any role in facilitating these elimination responses, donor cells were obtained from mice depleted of CD4+ cells by administration of anti-CD4 and injected into recipient mice depleted of CD4+cells in a similar fashion. The results of these experiments indicated that the elimination of donor cells was delayed in a donor/recipient combination-dependent fashion in absence of functional CD4+cells (Figure 2).
Elimination of allogeneic donor cells in the absence of CD4+ cells.
To test if optimal recipient CD8+ and B-cell responses required help from CD4+ cells, C57BL/6 and DBA/2 mice were injected with anti-CD4 on days −3 and −2. Then on day 0 these mice were either used as source of donor cells or as recipients. Detectable levels of anti-CD4 could be maintained in the recipient mice for at least 10 days with this protocol. The CD4−donor splenocytes were labeled with FITC and then injected into the recipients which had also been injected with anti-CD4. Persistence of donor cells was measured in the spleen (shown) and lymph nodes on days 3, 5, 6, and 7 (mean of 3 mice per time point for DBA/2 into DBA/2 [□] and C57BL/6 into DBA/2 [◊] combinations and a mean of 2 mice per time point for the DBA/2 into C57BL/6 [○] combinations are shown).
Elimination of allogeneic donor cells in the absence of CD4+ cells.
To test if optimal recipient CD8+ and B-cell responses required help from CD4+ cells, C57BL/6 and DBA/2 mice were injected with anti-CD4 on days −3 and −2. Then on day 0 these mice were either used as source of donor cells or as recipients. Detectable levels of anti-CD4 could be maintained in the recipient mice for at least 10 days with this protocol. The CD4−donor splenocytes were labeled with FITC and then injected into the recipients which had also been injected with anti-CD4. Persistence of donor cells was measured in the spleen (shown) and lymph nodes on days 3, 5, 6, and 7 (mean of 3 mice per time point for DBA/2 into DBA/2 [□] and C57BL/6 into DBA/2 [◊] combinations and a mean of 2 mice per time point for the DBA/2 into C57BL/6 [○] combinations are shown).
As fresh immunocompetent splenocytes are being used as donor cells in this model system, it is possible that donor CD4+ cells, as well as recipient CD4+ cells, could be playing a role. As an initial test to determine the role of donor and/or recipient CD4+ cells in these elimination responses, the elimination of donor splenocytes on day 3 was assessed using CD4 KO and MHC II KO mice as the source of the donor splenocytes or as recipients. These results (Table 2) showed that the lack of CD4 cells in CD4 KO recipients did not change elimination by day 3 if the donor cells contained CD4+ cells, whereas there was significantly less elimination on day 3 if donor cells lacking CD4+ cells were used. These results indicated that the rapid elimination of allogeneic donor splenocytes by day 3 only required donor CD4+ cells. The lack of significant elimination on day 3 in MHC II KO recipients lacking CD4+cells indicates that, for the donor CD4+ cells to facilitate elimination, they needed to be activated by recognition of allogeneic MHC class II antigens expressed on recipient cells. The use of donor cells from MHC II KO mice that lacked CD4+ cells as well as expression of MHC II molecules resulted in no significant elimination on day 3. The decreased elimination of donor cells lacking expression of MHC II molecules and CD4+ cells compared with donor cells that only lacked CD4+ cells suggested that the recipient CD4+ cells could also provide help after being activated by recognition of allogeneic MHC class II antigens present on the donor cells.
The role of donor or recipient CD4+ cells on elimination responses
| Donor . | Recipient . | % donor cells (mean ± SD) . | No. of mice . | |
|---|---|---|---|---|
| Spleen . | Lymph nodes . | |||
| DBA/2 | C57BL/6 | 0.04 ± 0.03* | 0.10 ± 0.12* | 6 |
| DBA/2 | CD4 KO | 0.03 ± 0.01* | 0.03 ± 0.05* | 3 |
| DBA/2 | MHC II KO | 4.00 ± 0.58† | 3.30 ± 0.46*† | 3 |
| C57BL/6 | C57BL/6 | 5.82 ± 1.47† | 6.87 ± 1.70† | 6 |
| C57BL/6 | DBA/2 | 0.23 ± 0.09* | 0.39 ± 0.29* | 5 |
| CD4 KO | DBA/2 | 1.73 ± 0.70*† | 4.10 ± 0.86*† | 3 |
| MHC II KO | DBA/2 | 4.86 ± 1.23† | 7.39 ± 1.56† | 3 |
| DBA/2 | DBA/2 | 5.85 ± 1.69† | 6.74 ± 0.84† | 5 |
| Donor . | Recipient . | % donor cells (mean ± SD) . | No. of mice . | |
|---|---|---|---|---|
| Spleen . | Lymph nodes . | |||
| DBA/2 | C57BL/6 | 0.04 ± 0.03* | 0.10 ± 0.12* | 6 |
| DBA/2 | CD4 KO | 0.03 ± 0.01* | 0.03 ± 0.05* | 3 |
| DBA/2 | MHC II KO | 4.00 ± 0.58† | 3.30 ± 0.46*† | 3 |
| C57BL/6 | C57BL/6 | 5.82 ± 1.47† | 6.87 ± 1.70† | 6 |
| C57BL/6 | DBA/2 | 0.23 ± 0.09* | 0.39 ± 0.29* | 5 |
| CD4 KO | DBA/2 | 1.73 ± 0.70*† | 4.10 ± 0.86*† | 3 |
| MHC II KO | DBA/2 | 4.86 ± 1.23† | 7.39 ± 1.56† | 3 |
| DBA/2 | DBA/2 | 5.85 ± 1.69† | 6.74 ± 0.84† | 5 |
FITC-labeled splenocytes from the indicated donor mice were injected intravenously into the indicated recipient mice (1 spleen equivalent per recipient). On day 3, the spleen and lymph nodes were obtained from the recipient mice and the number of FITC-labeled donor cells determined by flow cytometric analysis. Results that are significantly lower (P < .05) than the syngeneic controls are indicated by an asterisk (*), whereas results that are significantly higher (P < .05) than the results obtained with the fully allogeneic control combination (C57BL/6 into DBA/2 or DBA/2 into C57BL/6) are indicated using an dagger (
).
Help from CD4+ cells for elimination responses can be provided using 3 different pathways
The results obtained so far indicated that, although donor CD4+ cells were required to achieve the most rapid recipient responses, elimination responses with slower kinetics could still be generated in the absence of donor CD4+ cells. To study the contribution of recipient CD4+ cells further and to test the role of the donor and/or recipient CD4+ cells in alloantibody production, time course experiments were conducted in which donor cells from normal C57BL/6, CD4 KO, and MHC II KO mice were injected into DBA/2 recipients and persistence of donor cells and alloantibody production was assessed on days 1 to 10. The use of normal C57BL/6 spleen cells as donor cells permits both donor and recipient CD4+ cells to be involved in these responses. The use of CD4 KO donor spleen cells eliminates the contribution of the donor CD4+ cells, but permits recipient CD4+ cells to provide help as a result of being activated either by direct recognition of donor cells expressing allogeneic MHC class II molecules or by indirect recognition of processed alloantigen presented by recipient APCs. The only pathway available for the provision of help when MHC II KO donor cells are used is by the activation of recipient CD4+ cells by indirect recognition of processed alloantigen presented by recipient APCs. The persistence of donor cells (Figure3) from CD4 KO (2.98 ± 1.98%, n = 7, P = .047) and MHC II KO (4.72 ± 1.46%, n = 3, P = .006) donors was significantly higher than the persistence of C57BL/6 donor cells (0.13 ± 0.09, n = 3) on day 3. These findings indicated that the presence of donor CD4+cells was necessary to achieve the most rapid elimination on day 3. On day 4, the persistence of MHC II KO donor cells (2.88 ± 1.48, n = 3, P = .01) was significantly higher than the persistence of CD4 KO donor cells (0.03 ± 0.02, n = 4). These results indicated that the presence of donor MHC II alloantigens in the absence of donor CD4+ cells slowed down the recipient elimination response by 1 day. The recognition of processed alloantigen alone resulted in elimination of donor cells by day 5, a small acceleration of recipient immune responses relative to the kinetics of response in the complete absence of donor and recipient CD4+ cells (compare results in Figure 3 with those found in Figure 2). In each of these donor/recipient combinations, alloantibody production could be detected the day after donor cells had been eliminated (Figure 3).
The effect of donor CD4+ cell and MHC class II expression on persistence of donor cells and alloantibody production.
DBA/2 recipient mice were injected intravenously with 1 spleen equivalent of FITC labeled splenocytes from C57BL/6 (□), CD4 KO (◊), MHC II KO (○) donor mice and then the spleen and plasma were obtained from the recipient mice on the indicated days. Persistence of donor cells in the spleen (panel A) and alloantibody levels in the plasma (panel B, mean of 2 to 7 mice per point) are shown.
The effect of donor CD4+ cell and MHC class II expression on persistence of donor cells and alloantibody production.
DBA/2 recipient mice were injected intravenously with 1 spleen equivalent of FITC labeled splenocytes from C57BL/6 (□), CD4 KO (◊), MHC II KO (○) donor mice and then the spleen and plasma were obtained from the recipient mice on the indicated days. Persistence of donor cells in the spleen (panel A) and alloantibody levels in the plasma (panel B, mean of 2 to 7 mice per point) are shown.
Purified donor CD4+ cells are able facilitate rapid elimination of themselves
The results obtained so far had indicated that donor CD4+ cells were necessary to obtain the most rapid elimination and alloantibody responses. To test whether the CD4+ cells were sufficient to induce these responses, CD4+ cells were purified, labeled with FITC, and injected into allogeneic or syngeneic recipients. Elimination of allogeneic purified CD4+ cells was obtained by day 3 (Table3) indicating that donor CD4+cells were sufficient to induce recipient responses. This showed that activated donor CD4+ cells were effective APCs when the antigen being presented was allogeneic MHC I molecules expressed on their cell surface.
A summary of experiments in which the indicated recipient mice were injected intravenously with FITC-labeled spleen cells or purified CD4+ cells from the indicated donor mice
| Donor cells . | Recipient . | % donor cells . | No. of mice . | |
|---|---|---|---|---|
| Spleen . | Lymph node . | |||
| DBA/2 CD4+ | C57BL/6 | 0.02 ± 0.02 | 0.04 ± 0.05 | 4 |
| C57BL/6 CD4+ | DBA/2 | 0.03 ± 0.03 | 0.08 ± 0.05 | 3 |
| C57BL/6 CD4+ | C57BL/6 | 1.35 ± 0.26 | 2.63 ± 0.35 | 2 |
| C57BL/6 | C57BL/6 | 1.49 ± 0.25 | 2.28 ± 0.13 | 3 |
| Donor cells . | Recipient . | % donor cells . | No. of mice . | |
|---|---|---|---|---|
| Spleen . | Lymph node . | |||
| DBA/2 CD4+ | C57BL/6 | 0.02 ± 0.02 | 0.04 ± 0.05 | 4 |
| C57BL/6 CD4+ | DBA/2 | 0.03 ± 0.03 | 0.08 ± 0.05 | 3 |
| C57BL/6 CD4+ | C57BL/6 | 1.35 ± 0.26 | 2.63 ± 0.35 | 2 |
| C57BL/6 | C57BL/6 | 1.49 ± 0.25 | 2.28 ± 0.13 | 3 |
The purified CD4+ cells contained 84% to 88% CD4+ cells and 13 to 25 × 106 cells were injected per recipient. Elimination of allogeneic donor CD4+ cells was significantly greater (P < .05) than elimination of syngeneic donor cells.
Discussion
These studies demonstrate that activated donor CD4+ cells expressing allogeneic MHC class I molecules on their cell surface are able to directly induce the activation of both recipient CD8+ and B cells in response to these alloantigens. This activation pathway induces the most rapid in vivo responses by the recipient CD8+ cells and B cells. When donor cells enter the spleen, they are emptied into the red pulp that contains a high density of MHC II–expressing cells, including macrophages and dendritic cells. This environment should provide ample opportunity for the alloreactive donor CD4+ cells to encounter recipient MHC II molecules and become activated. This high probability of alloreactive donor CD4+ cell activation, combined with the ability of the activated CD4+ cells to effectively present alloantigen, could provide an explanation for the rapidity of the recipient cell response.
Previous studies have examined the ability of T cells to act as antigen-presenting cells.22 In particular, the ability of activated human CD4+ T cells to act as antigen-presenting cells has been examined because these cells, in contrast to murine CD4+ cells, up-regulate expression of MHC class II molecules on activation. B-cell lymphoblastoid cells lines were compared with activated T-cell clones for their ability to induce cytolytic effector cells from human responder T cells.23The results of this comparison showed that the use of activated T cells as antigen-presenting cells preferentially induced the generation of CD8+ and CD4+ cytolytic effector cells. In contrast, the use of B-cell lines as APCs preferentially induced CD4+ cells to become cytokine-secreting cells. This finding suggests that, similar to our findings, activated T cells provide signals that foster the development of cytolytic cells.23 Although the presence of CD40L on activated CD4+ cells should provide appropriate costimulation for B cells, the additional costimulatory signal on activated donor CD4+ cells required for activation of recipient CD8+ cells needs to be defined and studies are underway to define this signal.
The persistence of donor lymphoid cells plays an important role in the immunologic consequences of the transfusion of blood products, and it has been proposed that persistence of donor cells may facilitate the establishment of tolerance.24 Characterization of the recipient responses that cause elimination of the donor cells could allow for the development of protocols that permit the prolonged persistence of donor cells. Previous studies had shown that recipient CD8+ cells were responsible for the rapid elimination of fully allogeneic cells.16 The studies described here show that alloantibody production is the secondary elimination mechanism in the absence of CD8+ cells. Although activated donor CD4+ cells are able to rapidly induce recipient CD8+ and B-cell responses, these studies also showed that, in the absence of donor CD4+ cells, it was still possible to generate recipient CD8+ and B-cell responses but with slower kinetics. The recipient CD4+cells were shown to be important for these responses and could be activated directly by recognition of allogeneic MHC II molecules on donor cells. Alternatively, if the allogeneic donor cells lacked expression of MHC II molecules the recipient CD4+cells could be activated by an indirect presentation route in which recipient APCs would process and present the alloantigen.25These results would indicate that blocking both donor and recipient CD4+ cell function would be necessary to obtain prolonged persistence of allogeneic donor cells.
Because the role of donor CD4+ cells to act as alloantigen-presenting cells in vivo has not been previously appreciated, it will be important to reassess the role of donor CD4+ cells in the regulation of the transfusion-induced immunomodulation. Attempts to inhibit the immune responses induced by transfusion have involved developing filters to remove leukocytes or to treating the blood product with ultraviolet light or gamma irradiation to impair the immune responses. A recent study found that ultraviolet light treatment and leukoreduction had equivalent effects in reducing the induction of alloantibodies after platelet transfusion.1 However, these treatments only reduced the incidence of alloantibodies from 45% in the control group to about 20% in the treated groups in the total cohort of patients. If the analysis was limited to previously pregnant women the incidence of alloantibodies was reduced from 62% in the control group to about 33% in the groups receiving treated platelets. It will be important to determine the impact that the presence and function of any residual alloreactive CD4+ cells would have on the induction of recipient immune responses. Studies examining the role of limiting numbers of donor CD4+ cells on recipient immune responses in the murine model have been initiated in the laboratory. The transfer of allogeneic lymphocytes is also used for therapeutic purposes.5 6 A better understanding of the elimination responses may allow for the development of protocols that permit increased persistence of the donor cells that could lead to better therapeutic effects or permit responses using fewer donor lymphocytes. Thus, the ability of CD4+ cells to act as alloantigen-presenting cells could have both detrimental and beneficial effects.
Acknowledgment
I would like to thank Jacqui Poore for her excellent technical assistance.
Supported by funding from the National Blood Foundation, Rhode Island Hospital, and NHLBI grant HL59241.
Reprints:Loren D. Fast, Division of Clinical Hematology, Rhode Island Hospital, 593 Eddy St, Providence, RI 02903; e-mail:loren_fast@brown.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Elimination of allogeneic donor cells in the absence of CD4+ cells. / To test if optimal recipient CD8+ and B-cell responses required help from CD4+ cells, C57BL/6 and DBA/2 mice were injected with anti-CD4 on days −3 and −2. Then on day 0 these mice were either used as source of donor cells or as recipients. Detectable levels of anti-CD4 could be maintained in the recipient mice for at least 10 days with this protocol. The CD4−donor splenocytes were labeled with FITC and then injected into the recipients which had also been injected with anti-CD4. Persistence of donor cells was measured in the spleen (shown) and lymph nodes on days 3, 5, 6, and 7 (mean of 3 mice per time point for DBA/2 into DBA/2 [□] and C57BL/6 into DBA/2 [◊] combinations and a mean of 2 mice per time point for the DBA/2 into C57BL/6 [○] combinations are shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.1144/5/m_bloo01546002x.jpeg?Expires=1766379085&Signature=doNnOka5nSKMvMR0DM8bFBBibu4oJyB14eDQAouAp5QBJw6pKMh-SRviDWHGxY~7zYTO0HYInJ71YTAfdskagNL8o4AcS6D~4FjedWMqJkEbRVGII9h3ZMN8ap045jDbxMm-N3HY6q4utTPRTnzy2NItRqTUSpWjZaCuLjvxKf4EW~WVWQrnsmP4Mfe6X4O2uRmTmoKn9KC06Fl-VBHUNl89UjEN7t2xZg6rAbj6ktw6FLNGiBaRvTbU6fWYmc8TlkXm25spI-uoSuWACDEz11SQL6BeifX6dv45p8nONLuX7Az3vdhy~U5oUiKDre5rKodVMMd9pULBxjmKg0gEMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal