Abstract
The diagnosis of Fanconi anemia (FA) is based on the association of congenital malformations, bone marrow failure syndrome, and hypersensitivity to chromosomal breaks induced by cross-linking agents. In the absence of typical features, the diagnosis is not easy to establish because there is no simple and cost-effective test; thus, investigators must rely on specialized analyses of chromosomal breaks. Because we observed elevated serum alpha-fetoprotein (sAFP) levels in FA patients, we investigated this parameter as a possible diagnostic tool. Serum AFP levels from 61 FA patients and 27 controls with acquired aplastic anemia or other inherited bone marrow failure syndromes were analyzed using a fluoroimmunoassay based on the TRACE technology. Serum AFP levels were significantly more elevated (P < .0001) in FA than in non-FA aplastic patients. In the detection of FA patients among patients with bone marrow failure syndromes, this assay had a sensitivity of 93% and a specificity of 100%. This elevation was not explained by liver abnormalities. Levels of sAFP were unchanged during at least 4 years of follow-up, and allogeneic bone marrow transplantation did not modify sAFP levels. Three of 4 FA patients with mosaicism as well as 5 of 6 FA patients with myelodysplastic syndrome were detected by this test. Heterozygous parents of FA patients had normal sAFP levels. Measurement of sAFP levels with this automated, cost-effective, and reproducible fluoroimmunoassay could be proposed for the preliminary diagnosis of FA whenever this disorder is suspected.
Fanconi anemia (FA) is an autosomal recessive disorder characterized initially by progressive pancytopenia with diverse congenital abnormalities, and later by increased predisposition to malignancy.1,2 Spontaneous chromosomal breaks enhanced by the adjunction of bifunctional alkylating agents such as mitomycin C, a delay in the G2 phase of the cell cycle, and predisposition to apoptosis are the main features shared by this genetically heterogeneous disorder.2-4 At least 8 genetic complementation groups (A-H) have been described.5 The FANCA, FANCC, and FANCG genes have been cloned and localized to chromosomes 16q24.3, 9q22.3, and 9p13, respectively.6-8 The normal functions of the corresponding proteins are largely unknown and the relationship between this dysfunction and the development of aplastic anemia or malignant transformation is thus far unclear.
Although the diagnosis is easily suspected in a child who presents a typical constitutional phenotype with pancytopenia, in approximately 30% of the cases the diagnosis is not made or is delayed because of the absence of associated skin, skeletal, or urogenital malformations or suggestive familial history.9 Currently, FA diagnosis is based on cytogenetic analysis showing increased chromosomal breaks after incubation with DNA cross-linking agents.10,11 A flow cytometric method based on the accumulation of FA cells in the G2/M phase compartment of the cell cycle after incubation with alkylating agents has also been proposed for the diagnosis of FA.3These tests require a high degree of expertise and can result in false-negative results in cases of myelodysplastic syndrome3 or because of mosaicism.12 Mosaicism is characterized by an inconclusive cytogenetic analysis performed on peripheral blood lymphocytes, a low or absent increase in the number of chromosome breaks, a mitomycin C–resistant lymphoblastoid cell line, and a normal cell cycle study. It has been demonstrated recently that mosaicism could be explained by the functional correction of a pathogenic mutation by an acquired sequence modification incis, resulting in a functionally normal protein.13FA diagnosis in patients with mosaicism is based on the analysis of chromosome breakage in fibroblasts or other nonhematopoietic tissues, or on mutation analyses.
In our center, we followed a large number of patients with FA, and we studied patients before and after allogeneic bone marrow transplantation (BMT). Serum alpha-fetoprotein (sAFP) levels were routinely measured in FA patients to detect liver adenomas,14 which are a potential complication occurring in patients treated with androgens. We found that the vast majority of FA patients had stable elevated sAFP levels over time, independent of any liver complication and also independent of any specific treatment such as androgens. We showed that this assay was simple, fast, sensitive, and specific for FA, characteristics that could recommend it as a reliable and simple detection test for FA.
Patients and methods
Patients
Serum AFP measurements were performed in 61 patients diagnosed with FA by cytogenetic analysis. There were 31 males and 30 females, with a median age of 13 years (range, 1-53 years). There were 5 pairs of siblings, including 2 sets of monozygotic twins. At the time of blood sample collection, 30 patients had received no treatment and 23 had negative serologies for hepatitis B and C viruses; normal liver function as estimated by transaminases, bilirubinemia, and alkaline phosphatase; and no history of liver adenoma. Of the 31 other patients, 5 had elevated transaminases, 1 had elevated alkaline phosphatase, and 3 had mixed cytolytic and cholestatic hepatitis. Five patients had a history of hepatic adenoma and 1 had hepatocellular carcinoma. Furthermore, 7 had positive serology for hepatitis B or C virus and 19 were undergoing treatment with androgens. Serum AFP measurements from 17 patients who had received an allogeneic BMT were analyzed, with pre- and posttransplant sAFP values available in 6 cases and posttransplant values alone in 11 cases.
Six patients had myelodysplastic marrow abnormalities, 3 of them having no hepatic disorder and having received no treatment. Twelve patients had a bone marrow clonal cytogenetic abnormality, 4 with marrow myelodysplastic changes.
Among the 61 FA patients, 4 patients were defined as having mosaicism on the basis of subnormal blood counts with normal cell cycle analysis and a low number of chromosome breaks or mitomycin C–resistant cell lines. The diagnosis of FA was confirmed by a positive family history and/or malformations, or by the existence of typical chromosome breaks on a previous test or in a nonhematologic tissue.
A negative control group consisted of 27 patients (9 males and 18 females) with acquired aplastic anemia (n = 19), paroxysmal nocturnal hemoglobinuria (n = 2), dyskeratosis congenita (n = 3), Diamond-Blackfan anemia (n = 1), Shwachman-Diamond syndrome (n = 1), or Glanzmann disease (n = 1). The median age was 26 years (range, 5-56 years). Nine of these patients had positive serology for hepatitis C and 11 had received an allogeneic BMT. A second negative control group was composed of 53 healthy persons (28 males and 25 females) with a median age of 12 years (range, 3-36 years). Finally, 7 controls were healthy heterozygous parents of FA patients.
Because this research involved routine tests, informed consent was obtained from patients and controls as required by French law.
Determination of AFP and carcinoembryonic antigen (CEA) levels in serum
An aliquot of serum was cryopreserved at −20°C for tumor marker analysis. Two different tumor markers were analyzed in serum: sAFP and sCEA. The method used was a fluoroimmunoassay performed on the Kryptor apparatus (Cis-Bio International, Gif-sur-Yvette, France). Briefly, this homogeneous-phase assay with monoclonal antibodies labeled with rare-earth cryptates15 is derived from the Time-Resolved Amplified Cryptate Emission (TRACE) technology and is based on the energy transfer from a donor (europium cryptate)-labeled antibody to an acceptor (fluorophore)-labeled antibody. This energy transfer is possible only when donor and acceptor are close enough; that is, when both antibodies are linked to the same antigen. The normal cutoff threshold (defined by the maximal value obtained for 95% of normal persons) was 8 kIU/L (IU determined by the first international standard WHO 72/225) for sAFP (n = 69) and 3.5 μg/L for sCEA (n = 79).
Statistical analysis
Comparison of sAFP distributions in patients and controls used the nonparametric Wilcoxon test. The accuracy of sAFP measurements for the diagnosis of FA was assessed through the use of sensitivity (true positive) and specificity (true negative), which are interpretable regardless of the prevalence of the disease in the tested population and can be estimated using the case-control study design. This assumes a binary (negative/positive) diagnostic test, so we first had to dichotomize the sAFP measurements according to a threshold value, with a positive test when sAFP was above the threshold and a negative test otherwise. Receiver operating characteristic (ROC) curves, which plot the true-positive rate versus the false-positive rate associated with increasing threshold values, were computed.16 Finally, to provide information about the ability of this diagnostic test to detect or rule out the disease, likelihood ratios were computed; these allow evaluation of the predictive value of a test without requiring knowledge of disease prevalence in the population. Analysis was performed with the SAS (SAS Institute, Cary, NC) software package.
Results
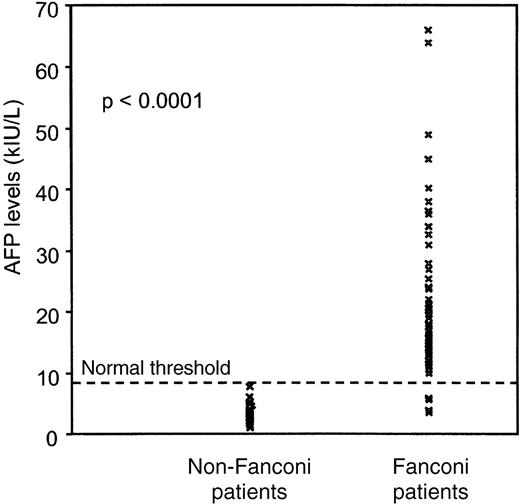
Whereas in the general healthy population, sAFP was less than 8 kIU/L, 57 of the 61 FA patients had elevated sAFP levels (median, 16 kIU/L; range, 3.5-66 kIU/L) (Figure 1). There was a slight difference between males (median, 19 kIU/L; range, 9-66 kIU/L) and females (median, 15 kIU/L; range, 3.5-36.5 kIU/L) (P = .06). After logarithmic transformation of the sAFP values, no significant correlation between age of the FA patients and sAFP levels was observed (P = .89). With a median value of 2.8 kIU/L (range, 1-7.5 kIU/L), the control group, including 27 patients with acquired aplastic anemia or other inherited non-FA bone marrow failure syndromes, had significantly lower sAFP levels (P < .0001) (Figure 1). A second control group, composed of 53 healthy persons (28 males and 25 females) age-matched with FA patients (median age, 12 years), had median sAFP values of 2.1 kIU/L (range, 0.7-5.8 kIU/L) (not shown).
Serum AFP values measured by fluoroimmunoassay in 61 Fanconi patients and 27 non-Fanconi aplastic patients.
Serum AFP values measured by fluoroimmunoassay in 61 Fanconi patients and 27 non-Fanconi aplastic patients.
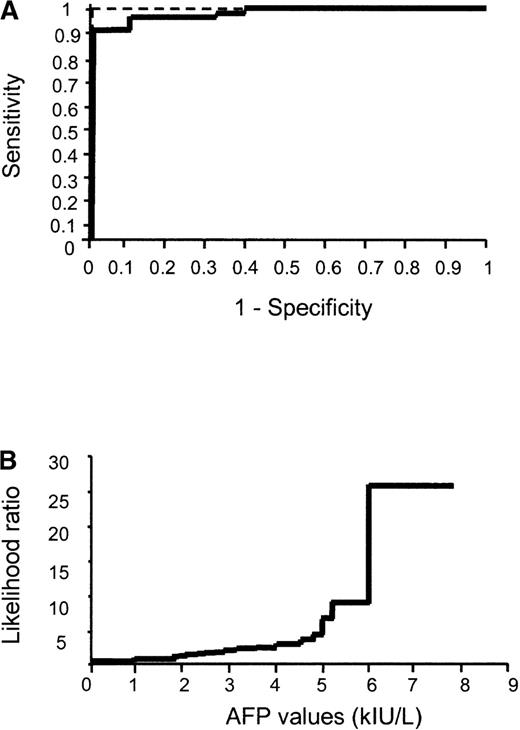
Using the ROC curve approach, we found that the threshold value of 8 kIU/L allowed the distinction of FA (sAFP ≥ 8 kIU/L) from other acquired or inherited bone marrow failure syndromes (sAFP < 8 kIU/L) with a sensitivity of 93%, a specificity of 100%, and a significant likelihood ratio of 26 (Figure 2).
ROC curve analysis of sAFP levels.
(A) ROC curve analysis performed by plotting sensitivity versus (1 − specificity) for each threshold value. Punctuated line represents ROC curve of a perfect test. (B) Evolution of the likelihood ratio for AFP measurement.
ROC curve analysis of sAFP levels.
(A) ROC curve analysis performed by plotting sensitivity versus (1 − specificity) for each threshold value. Punctuated line represents ROC curve of a perfect test. (B) Evolution of the likelihood ratio for AFP measurement.
With this cutoff value, 4 female patients among the FA group had normal sAFP values. Three were children with typical features of FA and positive cytogenetic and cell cycle studies. Two of them were monozygotic twins. The fourth patient was an adult with an unusual presentation. She was diagnosed at 40 years of age with a positive family history; her younger brother died of FA at 12 years of age and she had café au lait spots. Her blood counts were almost normal apart from an isolated macrocytosis. However, her bone marrow examination showed signs of myelodysplastic syndrome without clonal cytogenetic abnormalities. This patient was considered to have mosaicism because there were no spontaneous or caryolysin-induced chromosome breaks on cytogenetic analysis and her lymphoblastoid cell line was mitomycin C resistant. This observation demonstrates the risk of misdiagnosing FA. In this case, FA was suspected because of a squamous cell carcinoma of the uterine cervix and a family history of FA. The diagnosis was strengthened by a cell cycle study showing a slight accumulation of cells in G2/M phase after incubation with alkylating agents. Results in the FA group were not dependent on concurrent hepatic diseases or treatments (corticosteroids, androgens, or BMT) because all but 2 of the 23 untreated FA patients with normal hepatic function, negative hepatitis B and C serologies, and no detectable liver adenoma still had elevated sAFP levels (FA patients: median, 15.3 kIU/L; range, 3.8-66 kIU/L; versus the other 38 patients: median, 19 kIU/L; range, 3.5-49 kIU/L; P = .30).
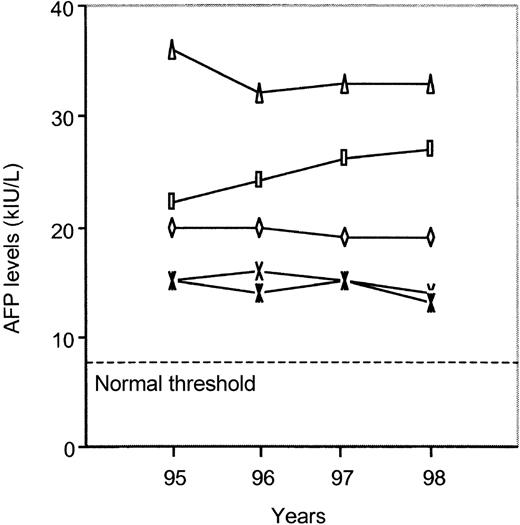
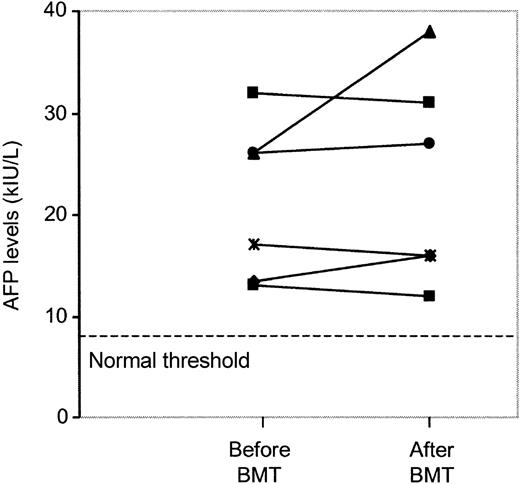
Patients with hepatocellular carcinoma have sAFP levels that increase rapidly over time. The FA patients we studied were stable for at least 4 years, without appearance of malignancy (Figure3). Furthermore, in 6 FA patients who received BMT, pre- and posttransplant sAFP levels were available. In all cases, the BMT procedure and the appearance of a normal allogeneic hematopoiesis did not modify sAFP levels (Figure4). Serum AFP levels remained stable after BMT in all but 1 patient, in whom a slight increase from 26 to 38 kIU/L was observed, without occurrence of liver adenoma or hepatocellular carcinoma. All but 1 of the 6 FA patients with myelodysplastic syndrome had elevated sAFP levels (5.9, 9, 12, 12, 26, and 26.5 kIU/L). Moreover, all FA patients with bone marrow clonal cytogenetic abnormalities also had abnormal values (median, 19 kIU/L; range, 9-32.6 kIU/L). Among each of the 5 pairs of FA siblings, sAFP values were in the same range (P = .55). Among the 4 patients who had mosaicism, 3 had high sAFP values (5.9, 13.3, 15, and 20 kIU/L). Among the 7 heterozygous parents of FA patients, all had normal sAFP levels except for a mother with a value of 11 kIU/L; in this particular case, we cannot rule out the possibility of hereditary elevation of sAFP.
Serum AFP values in 5 patients with Fanconi anemia during 4 years of follow-up.
Serum AFP values in 6 patients with Fanconi anemia 2 to 4 months before and after allogeneic bone marrow transplantation.
Serum AFP values in 6 patients with Fanconi anemia 2 to 4 months before and after allogeneic bone marrow transplantation.
We compared our results with another technique of measurement of sAFP, a microparticle enzyme immunoassay performed on the IMX apparatus (Abbott, Rungis, France). With this procedure, we were able to distinguish FA from non-FA aplastic patients by measuring sAFP values, although with poorer sensitivity (55%) and specificity (89%) (data not shown). The levels of sCEA, another fetal protein frequently elevated in malignant tumors, were studied in 10 FA patients with elevated sAFP levels. No elevation of the sCEA level was observed, with results ranging from 0.7 to 1.7 μg/L (normal value, < 3.5 μg/L).
Discussion
In the present study, we found that measurements of sAFP in FA patients were abnormally high (median, 16 kIU/L; range, 3.5-66 kIU/L) as compared with values (< 8 kIU/L) in the general population and in an age-matched control group. The difference remained highly significant when values in FA patients were compared with those in patients with acquired aplastic anemia or other inherited non-FA bone marrow failure syndromes (P < .0001). Elevated values of sAFP in FA patients were not related to intercurrent hepatic diseases, malignant tumors, or treatments administered to restore blood counts.17 Furthermore, elevated levels of sAFP remained strikingly constant over a follow-up period of 4 years and were not modified by the restoration of normal hematopoiesis following successful allogeneic BMT. These findings led to the suspicion that high sAFP levels are not related to a deregulated hematopoietic AFP production and that a relation exists between these high sAFP levels and the pathophysiology of FA. Interestingly, in 3 of 4 cases, patients with a high rate of mosaicism in peripheral blood lymphocytes had elevated sAFP levels, whereas their cytogenetic tests and cell cycle studies were not informative for diagnosis. Moreover, in patients with myelodysplastic syndrome, in whom cell cycle studies may normalize3 and lead to misdiagnosis, we found elevated sAFP levels in all but 1 case. Of note, measurements of sAFP in FA siblings, twins or not, were always in the same range, suggesting a genetic origin of the AFP deregulation in FA.
However, 4 FA patients, all females, had low sAFP levels. The reason why these patients had low sAFP levels is unclear. Complementation group analyses were not performed in all patients, but we suspect that these patients may belong to a rare complementation group or have different rare genomic mutations, underlining the heterogeneity of the disease.
The results of this test suggest that the use of sAFP measurement should be considered as a rapid test for guiding FA diagnosis. Furthermore, this test is automated and inexpensive, can be carried out within 24 hours, and gives reliable results with a sensitivity of 93%, a specificity of 100%, and a likelihood ratio of 26.
There is a large group of young, potentially undiagnosed FA patients with early-onset leukemia or cancer, short stature, thumb abnormalities, skin pigmentation, or macrocytic anemia. These patients could benefit from sAFP measurement because cytogenetic tests and cell cycle studies cannot be performed on such a large scale. However, in patients with elevated sAFP, a confirmatory test such as cytogenetic analysis or cell cycle study with cross-linking agents should be performed to rule out other causes of elevated sAFP. When patients are suspected of mosaicism, the elevation of sAFP associated with a strong suspicion of FA should lead to the analysis of another tissue such as skin fibroblasts, or to a mutation study if another member of the family is affected.
The reason why sAFP levels were high in the majority of our FA patients is unknown. High sAFP levels have also been described in ataxia-telangiectasia (AT),18 another rare inherited chromosomal breakage syndrome, suggesting a relationship between this finding and the chromosomal instability observed in FA cells. The use of routine sAFP testing for all children with persistent ataxia has been recommended for earlier detection of AT.19 The AFP gene together with the genes for albumin, vitamin D–binding protein, and α-albumin constitute the albumin multigenic family.20The members of this family are tandemly linked in the 4q subcentromeric region of the same DNA strand and map to the locus 4q11-4q13. The different localization of the AFP and FANC genes6-8,21argues against a direct link between specific mutations of FA genes and high sAFP values, but is in favor of a reactive derepression of AFP synthesis. Of note, this derepression was not a process involving all fetal proteins, because FA patients with elevated sAFP levels had normal CEA values. One could also speculate that elevated sAFP levels might reflect the increased apoptotic cell death that characterizes FA4 because, in addition to its ligand carrier/transport functions (AFP is considered the fetal counterpart of albumin),22 AFP has been associated with the regulation of cell apoptosis and proliferation.22-24 Rare cases of hereditary persistence of AFP25 exist, but this is unlikely in our study because all but 1 of the parents tested were normal.
AFP in serum is widely used as a tumor marker in evaluation of the prognosis and management of hepatocellular carcinomas and germ cell tumors, especially yolk sac tumors.26 Subfractionation of sAFP has improved tumor specificity, allowing discrimination among yolk sac tumors, hepatic malignancies, and benign liver diseases, which can also be associated with limited elevation of sAFP.14 In nonmalignant disorders, elevated levels of sAFP are found preterm in neural tube defects27 and in Down syndrome.28It is elevated in adults with cirrhosis, acute and chronic hepatitis, and drug-induced damage.29
Early FA diagnosis is important because some therapeutic interventions, such as high-dose chemotherapy including alkylating agents with or without BMT, are excessively toxic in FA patients. In the same way, early diagnosis of the disease before occurrence of pancytopenia could lead to the harvest of autologous hematopoietic stem cells for future gene therapy trials.
Acknowledgments
We thank Dr Rose Ann Padua for helpful comments on the manuscript; Didi Jasmin, Director of the European School of Haematology, who helped to improve our English syntax; all of the nuclear medicine staff for technical assistance; the nurses and physicians of the bone marrow transplant unit; and the family support group AFMF.
Reprints:Eliane Gluckman, Hematology Bone Marrow Transplant Unit, Hôpital Saint Louis, 1 Avenue Claude Vellefaux, 75475 Paris Cedex 10, France; e-mail: eliane.gluckman@sls.ap-hop-paris.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal