Abstract
Deficiencies in B7:CD28 costimulation are considered to be one of the major causes of the failure to generate a tumor-specific immune response. Up-regulating the expression of the B7 molecules on malignant B cells has been shown to stimulate cytotoxic T cells. Plasma cells from patients with myeloma express a tumor-specific idiotype but lack CD80 (B7-1) and have a variable expression of CD86 (B7-2). This study has identified the incidence and clinical significance of high CD86 expression on plasma cells at diagnosis and studied the ability of trimeric human CD40 ligand (huCD40LT) to up-regulate the expression of the B7 family on malignant plasma cells. CD86 expression on plasma cells was increased in 54% of the patients studied at diagnosis (n = 35) and was associated with a significantly shorter survival (median, 28 versus 57 months; χ2 = 4.6;P = .03) and a higher tumor load (patients with more than 50% bone marrow plasma cells, 47% versus 6%; χ2 = 7.2; P = .005). CD86 expression was highest on immature and primitive plasma cells (CD38++, CD45+) of both patients and controls and was associated with a CD40+, CD20+, CD19−, CD138+ phenotype. The shortened survival was associated with high CD86 only on mature (CD38++, CD45−) plasma cells (χ2 = 7.6; P = .006). There was no significant correlation between high CD86 and other known prognostic markers, including serum β2-microglobulin, serum thymidine kinase, and labeling index. The addition of huCD40LT to short-term cultures up-regulated both CD80 and CD86 expression on B cells (CD19+) and CD80 on plasma cells (CD38++), but did not up-regulate CD86 expression on plasma cells. Thus, B7-2–positive myeloma consists of a subgroup of patients with a relatively poor prognosis, and CD40LT may be useful in immunotherapy protocols because it up-regulates CD80 expression on malignant plasma cells without inducing B7-2–positive myeloma.
Introduction
The failure of intensive chemotherapy with peripheral blood stem cell support to significantly prolong the survival of a significant number of patients with multiple myeloma has led to considerable interest in immunotherapy as a treatment strategy. However, preliminary reports have demonstrated that there is only a limited clinical response to current forms of immunotherapy, suggesting that we need a better understanding of defects in the biology of the immune response in myeloma to design more effective therapeutic strategies.1-4 The interaction between antigen-presenting cells and T cells involves both the presentation of antigenic peptides to the T-cell receptor in association with major histocompatibility complex and a second signal produced by linkage between costimulatory molecules on the antigen-presenting cell and their counterreceptors on T cells. Thus, the generation of a productive immune response occurs after cross-linkage of CD28 and CD152 (cytolytic T lymphocyte-associated antigen 4 [CTLA-4]) on T cells with the costimulatory molecules CD80 (B7-1) and CD86 (B7-2) on an antigen-presenting cell.5-10 Engagement of the T-cell receptor by antigenic peptide in the absence of costimulation leads to T-cell anergy.6 Signaling through CD152 (CTLA-4) in the absence of CD28 results in apoptosis.9 10
There is increasing evidence that deficiencies in B7:CD28 costimulation between malignant cells and T cells are at least partly responsible for the failure to mount an antitumor immune response. Malignant B cells from patients with chronic lymphocytic leukemia or lymphoma (CLL) can act as antigen-presenting cells and induce a cytotoxic T-cell response after up-regulation of the expression of the costimulatory molecule CD80 by CD40 ligand.11-13 Studies have demonstrated variability in the presence of expanded T-cell clones in the peripheral blood of patients with myeloma.14-19 Although the incidence of these clones may depend on the sensitivity of the detection method, their presence is associated with a significant survival advantage.19 The specificity of expanded T-cell clones to the tumor idiotype has been difficult to establish,20-22 and whether their presence is due to current or past antigenic stimulation is not known. If myeloma cells can be modified with an agent such as CD40 ligand to function as efficient antigen-presenting cells, the possibility of using myeloma cells as an alternative cellular vector for immunotherapy could be explored. We have previously reported that CD86 is variably expressed by malignant plasma cells and that CD80 is rarely expressed.23 Whether high CD86 expression on myeloma cells has any clinical significance has not been reported, but a recent study has shown that CD86 expression on malignant cells is associated with poor survival in patients with acute myeloid leukemia.24If CD86-positive myeloma is also associated with a poor prognosis, it may be important to avoid up-regulation of CD86 expression on myeloma cells while attempting to up-regulate the expression of CD80 with biologic modifiers.
In the current studies, we have determined the expression of the relevant costimulatory molecules on the bone marrow plasma cells of patients with myeloma, the relation between the expression of CD86 and other clinical and immunophenotypic markers on untreated samples, the relation between CD86-positive myeloma and the presence of expanded T-cell clones, the prognostic significance of increased CD86 expression on plasma cells, and finally the impact of trimeric human CD40 ligand (huCD40LT) on the expression of the B7 family of costimulatory molecules on malignant plasma cells. Our findings indicate that B7-2–positive myeloma is associated with a poor prognosis and that B7-2–positive myeloma is not induced by huCD40LT.
Patients, materials, and methods
Patients and samples studied
Bone marrow and peripheral blood samples were collected after informed consent. Studies involving huCD40LT stimulation of B cells and plasma cells were performed on fresh bone marrow (n = 6) and peripheral blood samples (n = 9). Immunophenotyping was performed on diagnostic samples collected from a consecutive series of 35 new patients. These samples were stored in liquid nitrogen immediately after collection and carefully thawed on the day of assay. Preliminary experiments were performed to demonstrate that the antigens in this study did not vary significantly in their expression after freeze/thawing. This group of 35 patients had a median age of 65 years (range, 31-80 years) at diagnosis. There were 18 men and 17 women. Twenty patients were in stage I or II and 15 patients had stage III disease. Twelve had significant renal disease. The paraprotein was IgG in 22 patients (63%) and IgA in 11 patients (31%); 2 patients (6%) had light-chain disease. All patients were begun on a standard Australian Leukaemia Study Group protocol.25
Flow cytometric immunophenotype studies
Mononuclear cells were separated from bone marrow samples on Ficoll-Paque (Pharmacia, Uppsala, Sweden). Cells were washed and incubated for 15 minutes at room temperature with anti–CD38-FITC (fluorescein isothiocyanate) (Caltag, Burlingame, CA), anti–CD45-PerCP (Becton Dickinson, San Jose, CA), and either anti–CD80-PE (phycoerythrin) (Becton Dickinson) or anti–CD86-PE (Pharmingen, San Diego, CA). Further tubes were incubated with anti–CD38-FITC (Caltag), either anti–CD20-PE (DAKO, Glostrup, Denmark) or anti–CD56-PE (Becton Dickinson), anti–CD45-ECD (Coulter, Hialeah, FL), and anti–CD19-PE-CY5 (Immunotech, Marseilles, France). Either anti–CD40-FITC (Pharmingen) or anti–CD138-FITC (Immuno Quality Products, Groningen, The Netherlands) was incubated with anti–CD38-PE (Becton Dickinson) and anti–CD45-PerCP (Becton Dickinson). Cells were washed and resuspended in phosphate-buffered saline. At least 5000 cells were counted on a Coulter EPICS XL flow cytometer (Coulter). Samples were analyzed by first gating on CD38++ plasma cells; then CD45 was used to determine maturity status as described previously.26 27Briefly, CD38++ CD45− were classified as mature plasma cells, CD38++ CD45+ as immature plasma cells, and CD38++ CD45++ as primitive plasma cells. List-mode data were stored on an optical drive for reanalysis on XL2 software (Coulter). T-cell costimulatory responses were studied with anti–CD4-FITC (Becton Dickinson), either anti–CD28-PE (Becton Dickinson) or anti–CD152-PE (Pharmingen), anti–CD3-ECD (Coulter), and anti–CD8-PerCP (Becton Dickinson). T-cell populations were defined by either CD3+/CD4+ or CD3+/CD8+and were analyzed for CD28 or CD152 expression. B cells (anti–CD19-FITC; Becton Dickinson) were studied for anti–CD80-PE (Becton Dickinson) or anti–CD86-PE (Pharmingen) expression in conjunction with anti–CD14-PE-Cy5 (Immunotech).
huCD40LT stimulation of short-term bone marrow cultures
Bone marrow cells separated on Ficoll-Paque (Pharmacia) were cultured in the presence of huCD40LT (kindly supplied by Immunex Corp, Seattle, WA) at a concentration of 5 fg/mL with or without recombinant human interleukin (IL)-2 (R&D Systems Inc, Minneapolis, MN) at a concentration of 0.1 fg/mL. The cells were cultured in 1 mL of RPMI containing 10% fetal calf serum at 37°C and 7.5% CO2. Cells cultured in RPMI (containing 10% fetal calf serum) alone were used as a control. After 24 and 72 hours in culture, plasma cells (CD38++) and B cells (CD19+) were assayed for expression of CD80 and CD86 by flow cytometry, as detailed earlier.
Southern blot
Peripheral blood samples (30 mL heparin or EDTA) were obtained from 22 of the 35 patients at the time of diagnosis. DNA was extracted from Ficoll-Paque (Pharmacia)-separated peripheral blood mononuclear cells with equal parts of chloroform iso-amyl alcohol and Tris-saturated phenol at pH 8.0 and precipitated with ethanol. DNA digests were prepared with BamH1, EcoR1, andHindIII (Boehringer Mannheim, Mannheim, Germany); subjected to electrophoresis in 0.8% agarose gels; transferred to Hybond nylon filters (Amersham International, Little Chalfont, UK); and hybridized overnight at 65°C with a 32P-labeled 0.4-kb cDNA probe (Oncogene Science, Uniondale, NY) from the T-cell receptor CTβ2 region, as described previously.19 Expanded T-cell clones were considered to be present when samples contained nongermline bands on Southern blots with 2 different restriction enzymes.
Statistical analysis and assays
Kaplan-Meier survival curves were determined using GraphPad Prism (GraphPad Software Inc, San Diego, CA). Other statistical studies were performed with Astute (DDU Software, Leeds, UK). Serum β2-microglobulin (β2M) was analyzed by radioimmunoassay (Pharmacia) and serum thymidine kinase (STK) by radioenzyme assay.28 Plasma cell labeling index (LI) was determined using the classic 2-color immunofluorescent slide-based assay.29
Results
Immunophenotype of plasma cells at diagnosis
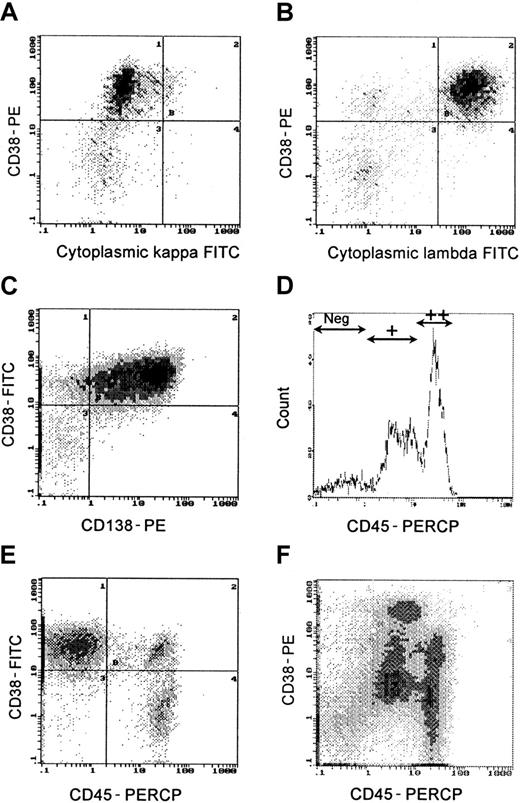
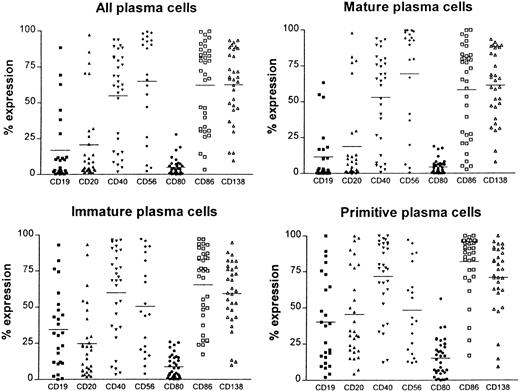
Plasma cells were defined by high fluorescent intensity CD38++ expression. In untreated patients, more than 95% of CD38++ cells demonstrated restricted staining of either cytoplasmic kappa or lambda light chains (Figure1A,B). Although CD138 and CD38++ coexpression (Figure 1C) is often used as a plasma cell marker, in this study CD138 was variably expressed on CD38++ cells (mean = 68%; range, 7% to 95%; Figure2). Thus, CD38++ proved to be a more reliable marker of plasma cells than CD138 expression. The immunophenotype (CD19, CD20, CD40, CD56, CD80, CD86, and CD138) of bone marrow plasma cells (CD38++) and plasma cell subpopulations defined by CD45 expression26,27 was determined on all 35 samples at diagnosis (Figure 2). Definition of CD45−, CD45+, and CD45++ regions was determined as described previously26,27 from histograms that included CD38− and CD38+ cells (Figure 1D). Primitive plasma cells were CD38++ CD45++; immature plasma cells were CD38++ CD45+; and mature plasma cells were CD38++ CD45−. Figure 1E illustrates a sample with predominantly immature (CD45−) plasma cells and a small population of primitive plasma cells; other samples (Figure 1F) had predominantly mature plasma cells (CD45+). The higher CD38 staining intensity (Figure1F) is due to the staining with PE. Mature plasma cells (CD38++, CD45−) generally had a lower expression of CD19, CD20, CD40, CD80, and CD86 than primitive plasma cells (Figure 2). Primitive plasma cells (CD38++, CD45++) had a higher LI than mature plasma cells (CD38++, CD45−).26
Plasma cells were clearly identified by high fluorescent intensity staining with CD38 (CD38++).
(A,B) Cytoplasmic staining with anti-kappa and anti-lambda monoclonal antibodies demonstrated light-chain restriction. (C) Not all CD138+ cells were CD38++. (D-F) CD45 expression subdivided plasma cells into primitive (CD45++), immature (CD45+), and mature (CD45−) subpopulations. (E) Predominantly CD45− plasma cells. (F) Predominantly CD45+ plasma cells.
Plasma cells were clearly identified by high fluorescent intensity staining with CD38 (CD38++).
(A,B) Cytoplasmic staining with anti-kappa and anti-lambda monoclonal antibodies demonstrated light-chain restriction. (C) Not all CD138+ cells were CD38++. (D-F) CD45 expression subdivided plasma cells into primitive (CD45++), immature (CD45+), and mature (CD45−) subpopulations. (E) Predominantly CD45− plasma cells. (F) Predominantly CD45+ plasma cells.
The immunophenotype of total plasma cells (CD38++) as well as plasma cell populations defined by CD45 expression.
Mature plasma cells were CD38++, CD45−; immature plasma cells were CD38++, CD45+; and primitive plasma cells were CD38++, CD45++. Expression of the antigens CD19, CD20, CD40, CD56, CD80, CD86, and CD138 is shown for 35 patients at diagnosis.
The immunophenotype of total plasma cells (CD38++) as well as plasma cell populations defined by CD45 expression.
Mature plasma cells were CD38++, CD45−; immature plasma cells were CD38++, CD45+; and primitive plasma cells were CD38++, CD45++. Expression of the antigens CD19, CD20, CD40, CD56, CD80, CD86, and CD138 is shown for 35 patients at diagnosis.
CD86 expression on plasma cells
Bone marrow plasma cells of patients with myeloma demonstrated a wide range of CD86 expression (Figure 2). The normal range of CD86 expression on CD38++ cells (27% to 62%) was established from bone marrow samples from allogeneic transplant donors. Patients were considered to have B7-2–positive myeloma when CD86 expression was more than the normal range (greater than 62%). Because there was a significantly higher level (t = 5.0; P < .0001) of CD86 expression on primitive plasma cells (CD38++, CD45++) than on mature plasma cells (CD38++, CD45−), a normal range was also established for each of the plasma cell subpopulations (mature, 5% to 50%; immature, 15% to 47%; primitive, 45% to 81%).
Clinical characteristics of B7-2 (CD86)–positive myeloma
B7-2–positive myeloma was found in 54% (n = 19) of the patients at diagnosis. Of the patients who were in stage I or II, 45% had B7-2–positive myeloma, whereas 66% of the patients in stage III had B7-2–positive myeloma. The proportion of patients with more than 50% plasma cells in the bone marrow sample at diagnosis was significantly higher in the group with B7-2–positive myeloma (n = 19) than in the B7-2–normal group (47% versus 6%; χ2 = 7.2; P < .005). The group of patients with B7-2–positive myeloma had a normal distribution of IgA and IgG isotypes. There was no correlation between CD86 expression and β2M, LI, or STK levels.
Prognostic significance of CD86 and other phenotypic markers
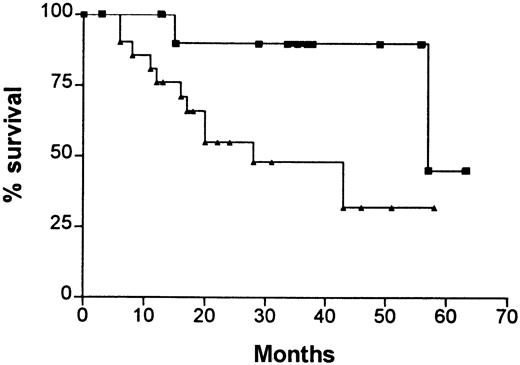
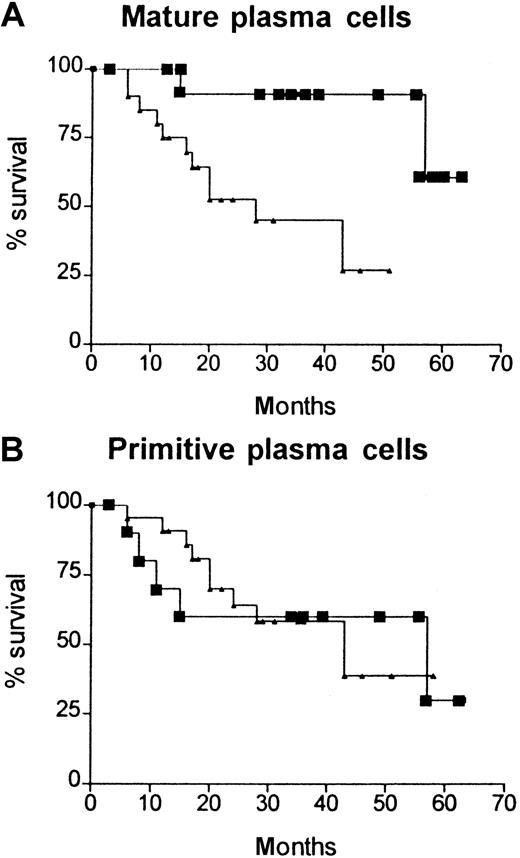
Kaplan-Meier survival curves (Figure3) demonstrated that high expression of CD86 on plasma cells (ie, B7-2–positive myeloma) was associated with a significantly worse prognosis than normal CD86 expression (median survival, 28 versus 57 months; χ2 = 4.6;P = .03). When similar survival curves were drawn for each of the plasma cell subpopulations, it became evident that the prognostic significance of CD86 expression was related to mature plasma cells (χ2 = 7.6; P = .006; high CD86 expression greater than 60%) and not to the immature or primitive subpopulations (Figure 4A,B). None of the other phenotypic markers shown in Figure 2 was associated with prognostic significance at diagnosis. High β2M levels (greater than 4 mg/L) had prognostic significance (χ2 = 3.6; P = .05), as would be expected. The total number of patients in this study was insufficient to perform a subgroup analysis (eg, low β2M and normal CD86); however, of 17 patients who had a high β2M level (greater than 4 mg/L), 10 also had a normal CD86 and only one of these patients has died (57 months). In contrast, 4 of the 7 patients with low β2M and high CD86 survived less than 20 months. Thus, CD86 expression appears to have independent prognostic significance and identifies poor-prognosis patients in the low-β2M group and good-prognosis patients in the high-β2M group.
Kaplan-Meier survival curves for patients with increased CD86 expression on plasma cells at diagnosis (B7-2–positive myeloma) compared with normal or reduced CD86 expression at diagnosis (B7-2–negative myeloma).
The median survival for B7-2–positive myeloma was 28 months and for B7-2–negative myeloma was 57 months (χ2 = 4.6;P = .03). (▴), % CD86 high; (▪), % CD86 normal.
Kaplan-Meier survival curves for patients with increased CD86 expression on plasma cells at diagnosis (B7-2–positive myeloma) compared with normal or reduced CD86 expression at diagnosis (B7-2–negative myeloma).
The median survival for B7-2–positive myeloma was 28 months and for B7-2–negative myeloma was 57 months (χ2 = 4.6;P = .03). (▴), % CD86 high; (▪), % CD86 normal.
Kaplan-Meier survival curves for patients with high (▴) and normal (▪) CD86 expression in various cell subpopulations.
(A) Mature plasma cell subpopulations (high CD86 greater than 60%). (B) Primitive plasma cell subpopulations (high CD86 greater than 81%). Only the mature plasma cells showed a significant difference in the prognostic importance of CD86 expression (χ2 = 7.6;P < .006).
Kaplan-Meier survival curves for patients with high (▴) and normal (▪) CD86 expression in various cell subpopulations.
(A) Mature plasma cell subpopulations (high CD86 greater than 60%). (B) Primitive plasma cell subpopulations (high CD86 greater than 81%). Only the mature plasma cells showed a significant difference in the prognostic importance of CD86 expression (χ2 = 7.6;P < .006).
Expanded T-cell clones and expression of CD80 or CD86
Southern blots were performed to detect the presence of expanded T-cell clones in peripheral blood samples from 23 of the 35 patients studied at the time of diagnosis. We have reported previously that this technique can be used to demonstrate minor bands that indicate the presence of expanded T-cell clones in 32% of samples at diagnosis. The presence of these expanded T-cell clones is associated with an improved prognosis.19 There was a trend for patients with circulating expanded T-cell clones to have a lower incidence of B7-2–positive myeloma (31%; n = 13) compared with patients without circulating expanded T-cell clones (60%; n = 10), but this was not statistically significant for the 23 samples studied (χ2 = 1.9). Some plasma cells expressed the counterreceptor CD28, but the correlation between the expression of CD28 and CD86 on plasma cells (CD38++) was not significant in the total plasma cell population (r = 0.33;P < .09). There was a weak negative correlation between CD86 expression and the presence of CD3+/CD8+/CD152+ cells (r = −0.42; P = .02).
Up-regulation of CD80 and CD86 on B cells and myeloma cells
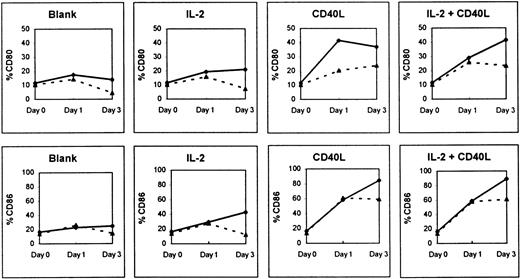
Figure 5 shows that huCD40LT with or without IL-2 caused a significant up-regulation of mean CD80 and CD86 expression on the peripheral blood B cells (CD19+) of both patients with myeloma (n = 9) and a normal control group (n = 5) in cultures over 3 days. There was a 6- to 8-fold increase in CD86 expression and a 4-fold increase in CD80 expression on B cells of the normal control group. The addition of exogenous rIL-2 alone had little effect on the up-regulation of CD80 and CD86 on B cells.
Effect of culture with and without huCD40LT and IL-2 on CD80 and CD86 expression.
Expression was tested on peripheral blood B cells of patients with myeloma (▴; n = 9) and normal controls (⋄; n = 5).
Effect of culture with and without huCD40LT and IL-2 on CD80 and CD86 expression.
Expression was tested on peripheral blood B cells of patients with myeloma (▴; n = 9) and normal controls (⋄; n = 5).
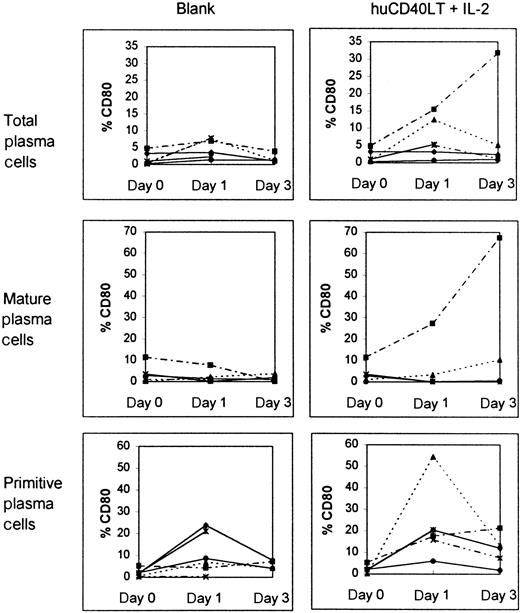
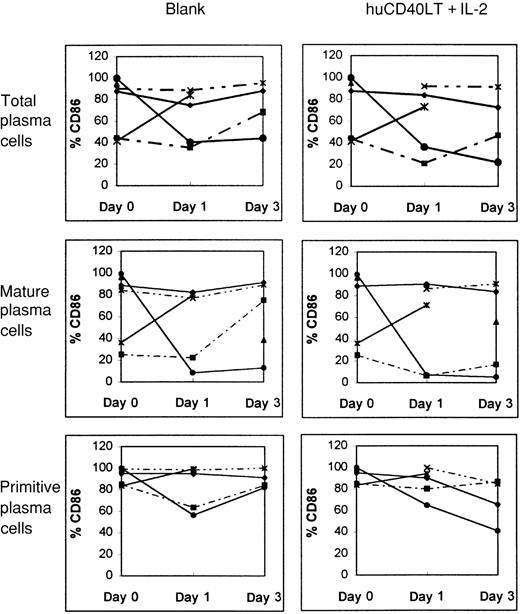
Plasma cells from bone marrow samples of 3 of 6 patients with myeloma up-regulated CD80 expression in response to huCD40LT. Up-regulation was found predominantly in the primitive plasma cell (CD38++, CD45++) population (Figure6). In contrast, huCD40LT failed to significantly up-regulate CD86 expression more than the controls in any of the 6 patients studied (Figure 7), and primitive plasma cells clearly did not up-regulate CD86.
Effect of culture with and without IL-2 and huCD40LT on CD80 expression in various cells.
Expression was tested in bone marrow plasma cells as well as mature and primitive plasma cells from patients with myeloma (n = 6). Each line depicts data from a different patient.
Effect of culture with and without IL-2 and huCD40LT on CD80 expression in various cells.
Expression was tested in bone marrow plasma cells as well as mature and primitive plasma cells from patients with myeloma (n = 6). Each line depicts data from a different patient.
Effect of culture with and without IL-2 and huCD40LT on CD86 expression in various cells.
Expression was tested in bone marrow plasma cells as well as mature and primitive plasma cells from patients with myeloma (n = 6). Each line depicts data from a different patient.
Effect of culture with and without IL-2 and huCD40LT on CD86 expression in various cells.
Expression was tested in bone marrow plasma cells as well as mature and primitive plasma cells from patients with myeloma (n = 6). Each line depicts data from a different patient.
Discussion
The present study has shown that B7-2 (CD86)–positive myeloma is associated with a poor prognosis and that huCD40LT up-regulates CD80 without significantly changing the expression of CD86 on malignant plasma cells. These observations have significant implications for immunotherapy protocols that aim to correct deficiencies in B7:CD28 costimulation by up-regulating the B7 family of molecules using agents such as huCD40LT. Up-regulation of CD80 has been shown to transform a range of malignant B cells into efficient antigen-presenting cells,11-13,30,31 and there is evidence that malignant plasma cells have the capacity to act as antigen-presenting cells.32 Following our observation that patients with B7-2–positive myeloma have a poor prognosis, it was important to determine whether up-regulation of the B7 family of costimulatory molecules by an agent such as huCD40LT would cause patients with a low CD86 expression to have a change in prognosis. It was reassuring to find that although huCD40LT up-regulated CD80 expression on malignant plasma cells, it did not concurrently alter the expression of CD86 on these cells and induce B7-2–positive myeloma.
The potential of CD86-positive malignant cells to induce an antitumor response is not clear. Initial studies in mice suggested that up-regulation of either CD80 or CD86 could initiate an antitumor immune response. However, it has become apparent that CD86-positive malignant cells do not necessarily stimulate an immune response leading to tumor regression. In murine models, CD80-transfected tumor cells induced a productive immune response, and mice with CD80-negative leukemia were cured if given multiple injections of leukemia cells expressing high levels of CD80.33 In contrast, CD86-transfected leukemic cells were ineffective. Thus, if tumor cells are to act as antigen-presenting cells, both the level of expression and the type of costimulatory molecules involved may be important. The function of the different B7 molecules is not fully understood. As in B7-2–positive leukemic mice,33 a high CD86 expression on malignant plasma cells did not improve the prognosis of patients with myeloma. In fact, increased expression of the costimulatory molecule CD86 on the malignant plasma cell was associated with a worse prognosis, a higher tumor load, and a trend toward fewer expanded T-cell clones. Further studies will be required to confirm this observation and to determine whether the prognostic significance of CD86 expression should be evaluated on all patients at diagnosis.
It is important to consider why high CD86 expression on malignant cells is associated with a poor prognosis. This may be a consistent finding in all hematologic malignancies; a previous study demonstrated that B7-2–positive leukemia also has a poor prognosis.24There may be multiple factors contributing to the poor prognosis of B7-2–positive myeloma. There are a range of cytokines that can either up-regulate (interferon-γ) or down-regulate (transforming growth factor-β and IL-10) B7-2 expression. It has been suggested24 that B7-2–positive leukemic cells evade tumor antigenicity despite the expression of costimulatory molecules and that B7 molecules may modulate the balance of Th1 and Th2 cells by reciprocal control of T-helper cell and dendritic cell differentiation.34 Alternatively, the relatively high levels of CTLA-4 may cause B7 ligation with CTLA-4 rather than with CD28 and result in down-regulation of the antileukemic immune response. The lower incidence of expanded T-cell clones and high tumor load in B7-2–positive myeloma support the thesis either that these malignant plasma cells evade tumor antigenicity or that B7-2 ligation with CD152 may induce a down-regulation of the antileukemic response and induce either anergy or apoptosis of many T cells. There is some evidence that high CD152 expression may inhibit the expansion of T-cell clones and even cause selective depletion in patients with myeloma. We previously reported that CD152 expression was greater than the normal range in 71% of myeloma patients without expanded T-cell clones but in only 36% of patients with T-cell clones.23 In this study, there was a negative correlation between CD3+,CD152+ cells and CD86 expression on plasma cells (r = −0.42; P = .02). The increased CD152 expression may be further amplified by the observed lower expression of CD28 on expanded lymphoid clones.23 In addition, CD28 expression on malignant plasma cells may result in B7:CD28 ligation between the malignant cells themselves. Whether this binding causes autostimulation of the malignant cells is not known, but it certainly may cause a reduction in T-cell stimulation.
An alternative possibility is that B7-2 positivity is associated with an inherently more aggressive malignant cell population. Our observations of increased CD86 expression on primitive rather than mature plasma cells and a higher tumor load in patients with CD86-positive myeloma add some credibility to this thesis. It is also likely that the tumor idiotype varies in its degree of antigenicity among patients. A spectrum of antigenicity could cause considerable heterogeneity in the T-cell response. Silent antigens may result in an absence of detectable antitumor T-cell clones.
Thus, many factors could be responsible for the poor prognosis of patients with B7-2–positive malignant plasma cells. It is likely that the real cause is a combination of several of these factors. We now know that patients with either B7-2–positive acute myelocytic leukemia (AML) or B7-2–positive myeloma have a poor prognosis. It is likely that high CD86 expression on malignant cells may confer a poor prognosis in patients with other malignancies. Further studies will be necessary to verify the prognostic significance and the biologic relevance of high CD86 expression on malignant cells.
Supported by grants from the Anthony Rothe Memorial Trust and Foundation IV.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ross Brown, Institute of Haematology, Royal Prince Alfred Hospital, Missenden Road, Camperdown, New South Wales 2050, Australia.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal