Abstract
Drug-induced immune thrombocytopenia (DITP) is a serious complication of drug treatment. Previous studies demonstrated that most drug-dependent antibodies (DDAbs) react with the platelet membrane glycoprotein (GP) complexes IIb/IIIa and Ib/IX/V. We analyzed the sera from 5 patients who presented with DITP after intake of carbimazole. Notably, thrombocytopenia induced by carbimazole was relatively mild in comparison to patients with DITP induced by quinidine. The sera reacted with platelets in an immunoassay on addition of the drug. In immunoprecipitation experiments with biotin-labeled platelets and endothelial cells, reactivity with the platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) could be demonstrated, whereas neither GPIIb/IIIa nor GPIb/IX was precipitated in the presence of the drug. These results could be confirmed by GP-specific immunoassay (MAIPA) using monoclonal antibodies (mabs) against PECAM-1. In addition, the binding of DDAbs could be abolished by preincubation with soluble recombinant PECAM-1. Carbimazole-dependent antibodies showed similar reactivity with platelets carrying the Leu125 and Val125 PECAM-1 isoforms, indicating that this polymorphic structure, which is located in the first extracellular domain, is not responsible for the epitope formation. Binding studies with biotin-labeled mutants of PECAM-1 and analysis of sera with mabs against different epitopes on PECAM-1 in MAIPA assay suggested that carbimazole-dependent antibodies prominently bound to the second immunoglobulin homology domain of the molecule. Analysis of 20 sera from patients with quinidine-induced thrombocytopenia by MAIPA assay revealed evidence that DDAbs against PECAM-1 are involved in addition to anti-GPIb/IX and anti-GPIIb/IIIa. We conclude that PECAM-1 is an important target GP in DITP.
Introduction
Drug-induced immune thrombocytopenia (DITP) is a serious side effect of pharmaceutical treatment that has been attributed to many drugs.1 However, confirmation of the responsible drug-dependent antibodies (DDAbs) was successful only for certain selected substances. DDAbs against quinine/quinidine, sulfonamide antibiotics, and ranitidine are relatively frequent and have, therefore, been well characterized.2-4 In some instances, not only the drug itself but also its metabolites are responsible for the immune response in the patient.5 DDAbs bind to platelets via their Fab fragments and usually recognize epitopes on the glycoprotein (GP) complexes Ib/IX and/or IIb/IIIa (αIIbβ3-integrin).6-9 These two GP complexes together with the platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) have been demonstrated to carry a major part of ABO antigens on the platelet membrane.10,11 In contrast to GPIb/IX and GPIIb/IIIa complexes, implication of PECAM-1 in autoimmune or drug-induced immune response has not been previously observed. PECAM-1 is a 130-kD member of the immunoglobulin gene superfamily that is expressed on endothelial cells, platelets, and a number of other blood cells.12 It is encoded by a 75-kb gene on chromosome 17. PECAM-1 comprises 6 extracellular immunoglobulin-like homology domains, a transmembrane domain, and a variable cytoplasmic tail that are involved in cellular adhesion, migration, and signal transduction.13 A polymorphism of the PECAM-1 gene defines two isoforms of the protein that differ by a Leu125Val substitution.14 This polymorphism seems to relate to the immune response because incompatibility between donors and recipients of bone marrow transplants has been reported to increase the risk of acute graft-versus-host disease.14,15However, in other studies with large patient cohorts these findings were not supported.16 17
Carbimazole (1-carbethoxy-3-methyl-2-thioimidazole) is a thioamide that is used for the treatment of hyperthyroidism (not available in the United States). Hemocytopenias caused by this drug are regarded as rare events and are usually attributed to bone marrow suppression.18 Carbimazole has been implicated in drug-induced immune hemolytic anemia, but so far immune thrombocytopenia caused by this drug has not been observed.19
In this study, we identified DDAbs against PECAM-1 in the sera from 5 patients with drug-dependent thrombocytopenia resulting from treatment with carbimazole. Furthermore, we have characterized the target molecules in quinidine-induced immune thrombocytopenia.
Patients, materials, and methods
Case reports
Patient 1 was a 69-year-old woman with severe mitral valve insufficiency and multiple cerebral infarctions. She was admitted to the hospital because of a fracture of her right femur that could not be surgically treated because of her cardiac problems. Because of pronounced hyperthyroidism, she received carbimazole. Heparin was given to avoid thrombosis. During the following days, the platelet count fell from 109 × 109/L to 59 × 109/L. Because DITP was suspected, carbimazole was stopped and the drug replaced by propylthiouracil. A serum sample was analyzed for DDAbs. Heparin-induced thrombocytopenia was excluded by heparin-induced platelet activation assay. A few days later, the patient developed urinary tract infection that led to urosepsis. After 10 days, she died probably of a new cerebral infarction.
Patient 2, an 83-year-old woman, was treated with carbimazole for Graves disease for several years. She was admitted to the hospital with fever and clinical signs of pneumonia. Mild thrombocytopenia (140 × 109/L) led to suspicion of DITP. Therefore, carbimazole was replaced by propylthiouracil, and the platelet count recovered to 340 × 109/L.
Patient 3, a 69-year-old woman, had hyperthyreosis for about 1 year before she was admitted to the hospital with dizziness, tremor, tachycardia, and dyspnea. Laboratory examination revealed the typical findings of hyperthyroidism. The patient was treated with carbimazole. Mild thrombocytopenia with a platelet count of 60 × 109/L was found. The low platelet counts could be traced back to the beginning of drug therapy. No immunological signs of autoimmune thrombocytopenia were found by direct monoclonal antibody-specific immobilization of platelet antigen (MAIPA) assay. The bone marrow showed an increased number of megakaryocytes. Carbimazole was stopped. Thyroidectomy was performed after the patient had developed severe hyperthyroidism. Subsequently the platelet count normalized to 194 × 109/L.
Patient 4 was a 78-year-old woman with B-cell lymphoma who suffered from hyperthyroidism for several years. On hospitalization, she received carbimazole and heparin besides other drugs. A drop in the platelet count from 228 × 109/L to 112 × 109/L was initially attributed to heparin-induced thrombocytopenia. However, different methods for the detection of antibodies of heparin-induced thrombocytopenia revealed negative results. The bone marrow showed normal numbers of megakaryocytes.
Patient 5 was a 60-year-old woman who received carbimazole for hyperthyroidism with focal adenoma for several years. Over a period of 1 year, her platelet count diminished from normal values to 124 × 109/L and further to 40 × 109/L. Autoimmune thrombocytopenia was discussed, but, after careful examination, DITP was suspected.
Patients with antibodies against quinidine. Sera from 20 patients who experienced acute thrombocytopenia after the intake of quinidine were analyzed. All sera showed positive reactions in a binding assay on addition of quinidine. From 18 patients, 10 were male and 8 were female. Their mean age was 65.5 years (24-85 years, n = 17). From 15 patients, clinical data could be evaluated. All of them showed a hemorrhagic diathesis with acute onset of the following symptoms: petechiae (15), hematoma (6), mucosal bleeding/epistaxis (6), melena (2), and hematuria (1). The nadir of platelet counts was between 1 × 109/L and 25 × 109/L (median 7.5 × 10/9/L). Thrombocytopenia resolved after interruption of quinidine intake.
Monoclonal antibodies
Monoclonal antibody (mab) Gi5, specific for the GPIIb/IIIa complex (CD41) and mab Gi9, which recognizes the GPIa subunit (CD49b), was produced and characterized in our laboratory.20 Mab FMC25 against the GPIX chain (CD42a) of GPIb/IX/V complex was a generous gift of Dr H. Zola (Adelaide, Australia).7
Enzyme immunoassay
Platelets (2 × 107) were incubated with 20 μL serum and 30 μL of the respective drug (1 mg/mL) for 30 minutes at 37°C, washed three times with isotonic saline-containing drug (0.1 mg/mL), and then incubated with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG; dilution 1:25 000; Dianova, Hamburg, Germany) in the presence of drug (0.1 mg/mL). After washings, the binding of antibody was visualized with p-nitrophenylphosphate as substrate (Sigma).
Flow cytometry analysis
Paraformaldehyde-fixed platelets (108) were incubated with 20 μL human serum in the absence or in the presence of 10 μL drug (1 mg/mL) for 30 minutes at room temperature. Platelets were then washed twice with phosphate-buffered saline (PBS), stained with 40 μL fluorescein-isothiocyanate-conjugated (FITC) rabbit anti-human IgG (dilution 1:40; DAKO, Hamburg, Germany) for 30 minutes at room temperature. After washing three times, platelets were analyzed by flow cytometry (Ortho Diagnostic, Neckargemuend, Germany). In some instances, serum was preincubated with 1 μL recombinant-soluble PECAM-1 (r-PECAM-1; 640 ng/μL) in the presence of carbimazole for 30 minutes at room temperature prior to flow cytometry analysis.
MAIPA assay
Antibody screening was performed by using the MAIPA assay as previously described.23 24 For the analysis of DDAbs, 2 × 107 platelets or 1 × 106 human umbilical vein endothelial cells were incubated with 20 μL serum, 10 μL mab (20 μg/mL), and 30 μL of the respective drug (1 mg/mL). Bound IgG was detected by using horseradish peroxidase anti-human IgG (dilution 1:3,000; Dianova) and o-phenylenediamine substrate (Sigma). All buffers contained 0.1 mg/mL of the respective drug.
Immunoprecipitation
Platelets derived from a blood group O donor, human umbilical vein endothelial cells, or L-cells expressing PECAM-1 mutants were surface labeled with 5 mmol/L NHS-LC-Biotin (Paesel, Frankfurt, Germany) as previously described.20 Aliquots of 100 μL precleared lysates were incubated overnight at 4°C with 100 μL purified mabs (1 μg) or 50 μL serum in the absence or in the presence of 50 μL carbimazole (1 mg/mL). In some instances, serum was preincubated with 1 μL r-PECAM-1 (see above) in the presence of carbimazole for 30 minutes at room temperature prior to immunoprecipitation. Precipitates were electrophoresed on 7.5% SDS polyacrylamide gel, blotted, and visualized using streptavidin-horseradish peroxidase and chemiluminescence substrate (ECL; Amersham Buchler, Braunschweig, Germany).
Genotyping of C → G PECAM-1 dimorphism (Leu125Val) by allele-specific PCR
Genotyping of C → G dimorphism was performed as previously described with minor modification.14 In brief, 5 μL genomic DNA was added to a 50 μL reaction mixture containing 10 mmol/L Tris (pH 8.0); 50 mmol/L KCl; 2.75 mmol/L MgCl2; 0.125 mmol/L of each dNTP; 0.25 μmol/L each sense primer 5′-CTGCCTTCCTTCGGGTTGCA-3′ and sequence-specific antisense primer 5′-CAAGGACTCACCTTCCACCAACAG-3′ or 5′-CAAGGACTCACCTTCCACCAACAC-3′; and 2.5 units TaqGold (Perkin Elmer). After initial denaturation at 96°C for 10 minutes, amplification was performed in a DNA thermocycler (Perkin Elmer) for 5 cycles (denaturation at 94°C for 1 minute, annealing at 63°C for 30 seconds, and extension at 72°C for 30 seconds) and 30 cycles of denaturation at 94°C for 1 minute, annealing at 61°C for 30 seconds, and extension at 72°C for 30 seconds. The PCR products were analyzed by electrophoresis on 1.8% agarose gels by using Tris-borate/EDTA buffer by ethidium bromide staining. DNA molecular marker V was used as standard (Boehringer Mannheim).
Results
Characterization of carbimazole-dependent antibodies
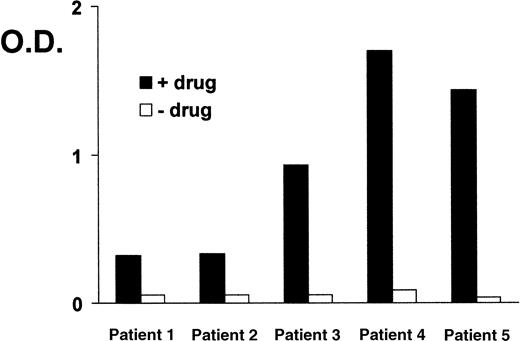
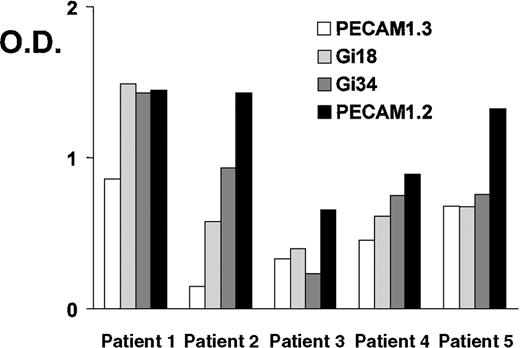
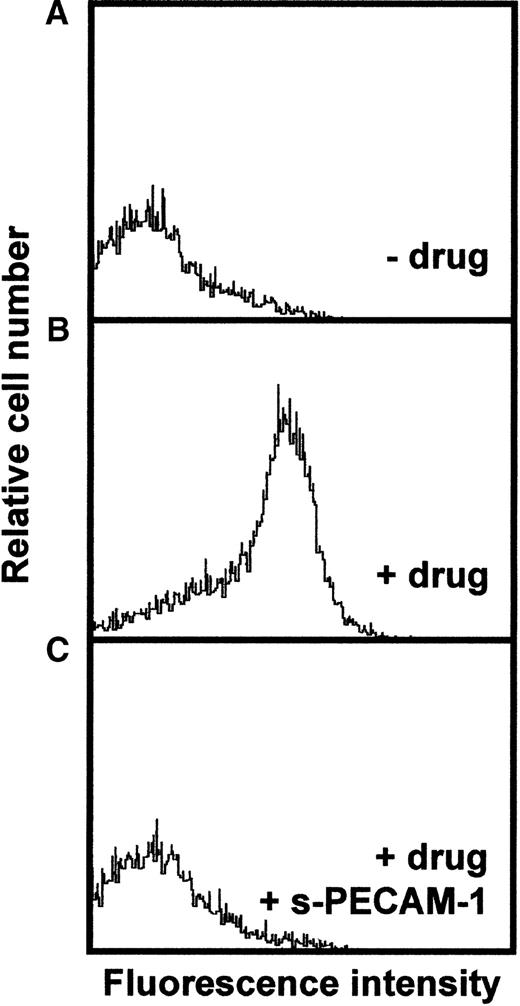
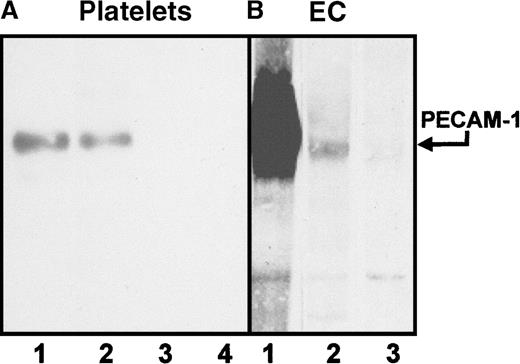
Sera from 5 patients with clinically suspected DITP induced by carbimazole were analyzed in enzyme immunoassay (Figure1). All sera showed a typical reaction pattern with significant binding of antibodies in the presence of 1 mg/mL drug. No binding of IgG occurred without carbimazole. The ratios (optical density with drug/optical density without drug) ranged from 5.9 to 41. To localize the target glycoprotein of carbimazole-dependent antibodies, the MAIPA assay was performed with a panel of mabs. As shown in Figure 2, strong carbimazole-dependent antibody binding was detected when mabs against PECAM-1 (Gi18, Gi34, PECAM-1.2, and PECAM-1.3) were used as capture antibodies and only in the presence of carbimazole. The ratios of optical densities ranged from 24.8 to 200. In contrast, no reactivity was observed with GPIb/IX and GPIIb/IIIa immobilized by mabs FMC25 and Gi5, respectively (data not shown). The reactivity of DDAbs with PECAM-1 was strongest when a capture antibody against domain 6 (PECAM-1.2) was used. In contrast, weaker reactions were observed with mabs against loops 1, 2, and 3. The weakest reaction was detected with mab PECAM-1.3 directed against loops 1-2 (Figure 2). These findings indicated an inhibition of the binding of DDAbs by mabs that recognize epitopes residing on distal loops of this molecule. In flow cytometry, the patient's sera reacted with normal platelets in the presence but not in the absence of carbimazole. Figure3 shows a representative experiment with serum from patient 2. If the serum was preincubated with r-PECAM-1, the reactivity could be abolished, demonstrating that the DDAbs bound exclusively to PECAM-1 but not to other platelet membrane constituents. These results could be confirmed by immunoprecipitation analysis with biotin-labeled platelets (Figure4A). Carbimazole-dependent antibodies precipitated solely PECAM-1 with an apparent molecular weight (Mr) of 130 kd under nonreducing conditions. In control experiments with mab Gi18, a protein with the same Mr was precipitated. In addition, the carbimazole-dependent antibodies failed to precipitate any platelet protein when r-PECAM-1 was added. Altogether, these data demonstrated that sera from patients with carbimazole-induced thrombocytopenia contained antibodies that drug-dependently bound to PECAM-1.
Carbimazole-dependent antibodies in enzyme immunoassay.
Binding of carbimazole-dependent antibodies in sera from 5 patients in the presence of the drug (1 mg/mL, solid bars) and without drug (open bars). O.D. = optical density
Carbimazole-dependent antibodies in enzyme immunoassay.
Binding of carbimazole-dependent antibodies in sera from 5 patients in the presence of the drug (1 mg/mL, solid bars) and without drug (open bars). O.D. = optical density
Carbimazole-dependent antibodies in a glycoprotein-specific enzyme immunoassay (MAIPA).
Reactivities of carbimazole-dependent antibodies against PECAM-1 immobilized by mabs in the presence of the drug. PECAM-1.3 and Gi18 recognize epitopes located on domains 1-2, Gi34 on domains 2-3, and PECAM-1.2 on domain 6. O.D. = optical density
Carbimazole-dependent antibodies in a glycoprotein-specific enzyme immunoassay (MAIPA).
Reactivities of carbimazole-dependent antibodies against PECAM-1 immobilized by mabs in the presence of the drug. PECAM-1.3 and Gi18 recognize epitopes located on domains 1-2, Gi34 on domains 2-3, and PECAM-1.2 on domain 6. O.D. = optical density
Analysis of carbimazole-dependent antibodies by flow cytometry.
Fixed platelets were incubated with patient sera in the absence of the drug (A), in the presence of the drug (B), and in the presence of the drug and soluble r-PECAM-1 (C). Bound antibodies were detected with FITC-labeled rabbit anti-human IgG and were analyzed on flow cytometer.
Analysis of carbimazole-dependent antibodies by flow cytometry.
Fixed platelets were incubated with patient sera in the absence of the drug (A), in the presence of the drug (B), and in the presence of the drug and soluble r-PECAM-1 (C). Bound antibodies were detected with FITC-labeled rabbit anti-human IgG and were analyzed on flow cytometer.
Immunoprecipitation analysis of carbimazole-dependent antibodies.
Platelets (A) and endothelial cells (EC) (B) were surface-labeled with biotin and lysed. Immunoprecipitation was performed with serum containing DDAbs or mab. Lane 1: mab Gi18 (anti-PECAM-1), lane 2: serum in the presence of the drug, lane 3: serum without the drug, lane 4: serum in the presence of the drug and soluble r-PECAM-1. Immunoprecipitates were analyzed on a 7.5% SDS-PAGE under nonreducing conditions.
Immunoprecipitation analysis of carbimazole-dependent antibodies.
Platelets (A) and endothelial cells (EC) (B) were surface-labeled with biotin and lysed. Immunoprecipitation was performed with serum containing DDAbs or mab. Lane 1: mab Gi18 (anti-PECAM-1), lane 2: serum in the presence of the drug, lane 3: serum without the drug, lane 4: serum in the presence of the drug and soluble r-PECAM-1. Immunoprecipitates were analyzed on a 7.5% SDS-PAGE under nonreducing conditions.
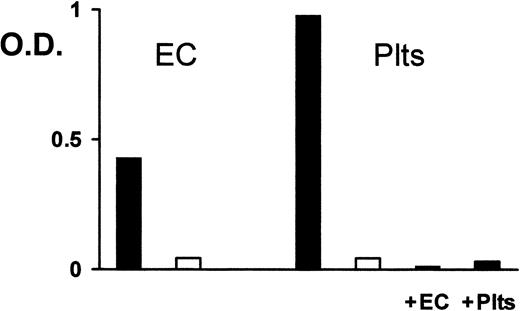
Reactivity of carbimazole-dependent antibodies with endothelial cells
Because endothelial cells express considerable amounts of PECAM-1, the reactivity of carbimazole-dependent antibodies with these cells should be assessed. In immunoprecipitation with biotin-labeled human umbilical vein endothelial cells, PECAM-1 was precipitated in the presence of the drug in parallel as from platelets (Figure 4). No other endothelial cell protein could be precipitated. On analysis in MAIPA assay, carbimazole-dependent antibodies reacted with PECAM-1 derived from endothelial cells (Figure5). Furthermore, when the serum was pre-absorbed twice with endothelial cells or platelets (as control), the reactivity with PECAM-1 was abolished. Therefore, carbimazole-dependent antibodies recognize PECAM-1 derived both from platelets and from endothelial cells.
Reactivity of carbimazole-dependent antibodies with platelets and endothelial cells.
Carbimazole-dependent antibodies were analyzed in MAIPA assay with endothelial cells (EC) or platelets (Plts) either in the presence (solid bars) or in the absence (open bars) of carbimazole by using mab Gi18 as capture antibody. Serum was also tested after absorption with endothelial cells (+EC) or platelets (+Plts) in the presence of carbimazole. O.D. = optical density.
Reactivity of carbimazole-dependent antibodies with platelets and endothelial cells.
Carbimazole-dependent antibodies were analyzed in MAIPA assay with endothelial cells (EC) or platelets (Plts) either in the presence (solid bars) or in the absence (open bars) of carbimazole by using mab Gi18 as capture antibody. Serum was also tested after absorption with endothelial cells (+EC) or platelets (+Plts) in the presence of carbimazole. O.D. = optical density.
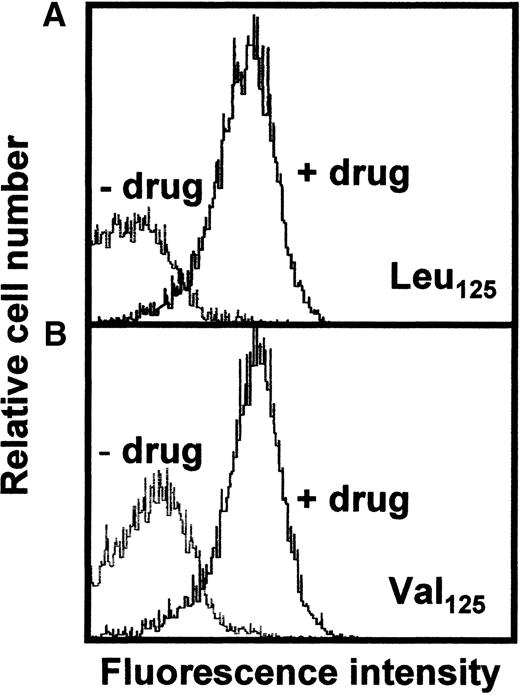
Reactivity of carbimazole-dependent antibodies with PECAM-1 (Leu/Val125) isoforms
Recently, it was demonstrated that the Leu125 and Val125 isoforms of PECAM-1 showed different reactivity with mab 7E8.14 Therefore, we sought to determine if the reactivity of carbimazole-dependent antibodies against PECAM-1 differs between different individuals because of differences of the two isoforms. Ten normal blood donors were typed for the dimorphism by allele-specific PCR. In positive individuals, a 342 base pair (bp) PCR product was amplified. Three donors were CC homozygous (Leu125), 1 donor had a GG genotype (Val125), and 6 individuals were typed CG heterozygous. Platelets of a Leu125 and a Val125 homozygous donor, respectively, were assessed with carbimazole-dependent antibodies by flow cytometry. Figure 6demonstrates that no differences in the binding of the antibodies could be observed between the two PECAM-1 isoforms.
Flow cytometric analysis of platelets with different PECAM-1 isoforms.
Fixed platelets derived from individuals carrying PECAM-1 Leu125 (A) or Val125 (B) isoforms were analyzed with patient sera in the presence or in the absence of the drug (see Figure 3).
Flow cytometric analysis of platelets with different PECAM-1 isoforms.
Fixed platelets derived from individuals carrying PECAM-1 Leu125 (A) or Val125 (B) isoforms were analyzed with patient sera in the presence or in the absence of the drug (see Figure 3).
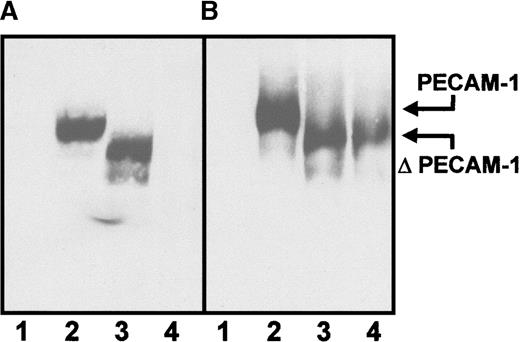
Reactivity of carbimazole-dependent antibodies with PECAM-1 mutants
To substantiate the finding of predominant binding to distal extracellular domains of PECAM-1, serum containing carbimazole-dependent antibodies (patient 2) was tested with lysates from biotin-labeled L-cells transfected with PECAM-1 mutants. As shown in Figure 7, the serum reacted with the wild type of PECAM-1 and with a mutant in which the first extracellular domain was deleted (Δ1). In contrast, mutants lacking the second extracellular domain (Δ2) failed to react with carbimazole-dependent antibodies (Figure 7A, lane 4). In the control experiment, mab PECAM-1.2 directed against extracellular loop 6 reacted with wild-type, Δ1, and Δ2 mutants. These findings suggested that the second extracellular loop contributes primarily to the epitope formation for carbimazole-dependent antibodies.
Immunoprecipitation analysis of carbimazole-dependent antibodies with PECAM-1 mutants.
Untransfected L-cells and L-cells expressing the entire PECAM-1 or lacking the first (Δ1) or the second (Δ2) extracellular immunoglobulin homology domain were surface labeled with biotin. After lysis, immunoprecipitation was performed with sera containing carbimazole-dependent antibodies in the presence of the drug (A) and with mab PECAM-1.2 against the extracellular loop 6 of PECAM-1 (B). Lane 1: untransfected L-cells, lane 2: PECAM-1 transfectant, lane 3: Δ1-PECAM-1 transfectant, and lane 4: Δ2-PECAM-1 transfectant.
Immunoprecipitation analysis of carbimazole-dependent antibodies with PECAM-1 mutants.
Untransfected L-cells and L-cells expressing the entire PECAM-1 or lacking the first (Δ1) or the second (Δ2) extracellular immunoglobulin homology domain were surface labeled with biotin. After lysis, immunoprecipitation was performed with sera containing carbimazole-dependent antibodies in the presence of the drug (A) and with mab PECAM-1.2 against the extracellular loop 6 of PECAM-1 (B). Lane 1: untransfected L-cells, lane 2: PECAM-1 transfectant, lane 3: Δ1-PECAM-1 transfectant, and lane 4: Δ2-PECAM-1 transfectant.
Analysis of quinidine-dependent antibodies by MAIPA assay
We analyzed sera from 20 patients with quinidine-induced thrombocytopenia in enzyme immunoassay and in MAIPA assay. In enzyme immunoassay, quinidine-dependent antibodies were detected in all 20 sera. Subsequently, sera were analyzed in MAIPA assay for their reactivity with PECAM-1, GPIb/IX, GPIIb/IIIa, and GPIa/IIa by using mabs Gi18, FMC25, Gi5, and Gi9, respectively. A positive reaction was defined as an optical density above 0.100. Results with GPIb/IX, GPIIb/IIIa, and PECAM-1 are given in Table1. Most quinidine-dependent antibodies reacted strongly with GPIb/IX and/or GPIIb/IIIa. In contrast, only moderate reactivity was observed with PECAM-1. No drug-dependent antibody reactivity directed against GPIa/IIa was observed in any of the sera tested. To exclude coprecipitation of PECAM-1 with other platelet GPs under MAIPA conditions, a representative panel of autoantibodies against GPIIb/IIIa and/or GPIb/IX and anti-GPIa/IIa alloantibody (anti-Bra) were analyzed in the presence and in the absence of quinidine. No co-reactivity with PECAM-1 was detectable (Table 1). In addition, the presence of quinidine did not alter the reactivity of autoantibodies or alloantibodies to their respective target GPs.
Reactivity of quinidine-dependent antibodies in sera from 20 patients with PECAM-1, GPIb/IX, GPIIb/IIIa, and GPIa/IIa
| Patient . | Specificity . | |||
|---|---|---|---|---|
| PECAM-1 . | GP Ib/IX . | GP IIb/IIIa . | GP Ia/IIa . | |
| 1 | (+) | 4 | 2 | — |
| 2 | — | — | — | — |
| 3 | 2 | 2 | 1 | — |
| 4 | 1 | 4 | 4 | — |
| 5 | 2 | 2 | 4 | — |
| 6 | (+) | 4 | — | — |
| 7 | 3 | 4 | 4 | — |
| 8 | 1 | 4 | 4 | — |
| 9 | — | 4 | — | — |
| 10 | 1 | 1 | 4 | — |
| 11 | 1 | 3 | (+) | — |
| 12 | 1 | 4 | — | — |
| 13 | 2 | 4 | — | — |
| 14 | 1 | 3 | 1 | — |
| 15 | 1 | 4 | 2 | — |
| 16 | — | 4 | 4 | — |
| 17 | — | 2 | — | — |
| 18 | — | — | — | — |
| 19 | (+) | 4 | — | — |
| 20 | — | — | — | — |
| aab 1 | — | — | 4 | — |
| aab 2 | — | 3 | — | — |
| aab 3 | — | 2 | 3 | — |
| allo ab | — | — | — | 4 |
| Patient . | Specificity . | |||
|---|---|---|---|---|
| PECAM-1 . | GP Ib/IX . | GP IIb/IIIa . | GP Ia/IIa . | |
| 1 | (+) | 4 | 2 | — |
| 2 | — | — | — | — |
| 3 | 2 | 2 | 1 | — |
| 4 | 1 | 4 | 4 | — |
| 5 | 2 | 2 | 4 | — |
| 6 | (+) | 4 | — | — |
| 7 | 3 | 4 | 4 | — |
| 8 | 1 | 4 | 4 | — |
| 9 | — | 4 | — | — |
| 10 | 1 | 1 | 4 | — |
| 11 | 1 | 3 | (+) | — |
| 12 | 1 | 4 | — | — |
| 13 | 2 | 4 | — | — |
| 14 | 1 | 3 | 1 | — |
| 15 | 1 | 4 | 2 | — |
| 16 | — | 4 | 4 | — |
| 17 | — | 2 | — | — |
| 18 | — | — | — | — |
| 19 | (+) | 4 | — | — |
| 20 | — | — | — | — |
| aab 1 | — | — | 4 | — |
| aab 2 | — | 3 | — | — |
| aab 3 | — | 2 | 3 | — |
| allo ab | — | — | — | 4 |
Sera were analyzed by monoclonal antibody-specific immobilization of platelet antigens (MAIPA) assay by using mabs Gi18, FMC25, Gi5, and Gi9. All tests were performed both in the presence and in the absence of 1 mg/mL quinidine. Results in the presence of the drug were evaluated as positive only if the reactivity without drug was negative. Three sera containing autoantibodies (aab) against glycoprotein IIb/IIIa and/or GPIb/IX and one serum with a GPIa/IIa-specific Bra alloantibody (alloab) were tested in the presence of quinidine to exclude drug-induced coprecipitation of platelet endothelial cell adhesion molecule-1 (PECAM-1). Tests were run in duplicates or in triplicates. Reactivity was categorized according to optical density as follows: <0.100 indicated by —; 0.100-0.199, (+); 0.200-0.399, 1; 0.400-0.799, 2; 0.800-1.599, 3; and ≥1.600, 4.
Discussion
In this study, we report on 5 patients with drug-dependent platelet reactive antibodies against carbimazole. The sera showed a strong reactivity with PECAM-1 in a GP-specific immunoassay (MAIPA) when mabs against PECAM-1 were used for antigen immobilization (Figure 2). The specificity for this target could be confirmed by immunoprecipitation experiments (Figure 4). In addition, the reactivity of DDAbs was abolished in the presence of r-PECAM-1 by flow cytometry as well as by immunoprecipitation (Figures 3 and 4). These results strongly indicate that PECAM-1 was the only molecule on platelets that is recognized by the antibodies. Furthermore, we could demonstrate by immunoprecipitation and by MAIPA assay that carbimazole-dependent antibodies were also reactive with PECAM-1 from endothelial cells (Figures 4 and 5). Other DDAbs (eg, in response to quinine/quinidine) mostly recognize GPIb/IX and/or GPIIb/IIIa as targets.7-9 The isolated involvement of PECAM-1 in carbimazole-dependent thrombocytopenia without additional DDAbs against the other two GP complexes may be due to a specific interaction between the drug and PECAM-1. However, no tight binding of the drug to the platelet membrane that resisted washing procedures without the drug was observed (data not shown).
Several attempts have been made to localize the binding domains of DDAbs on their target GPs.2,9 By the use of a panel of mabs against different epitopes on the extracellular domain of PECAM-1 in MAIPA assay, we observed an inhibition with mabs against the distal loops 1, 2, and 3 (PECAM-1.3, Gi18, and Gi34) but not with mab PECAM-1.2 that binds to the most proximal loop 6 (Figure 2). This observation led us to the assumption that binding occurred in the distal domains. To further localize the binding domain of drug-dependent antibody, immunoprecipitation with various PECAM-1 mutants was performed. Deletion of the second extracellular domain from PECAM-1 abolished the binding of the antibody, whereas, on deletion of the first domain, PECAM-1 was still recognized (Figure 7). These findings indicate that the second extracellular domain is critical for antibody binding. In comparison, DDAbs that occur in response to other drugs as well as autoantibodies predominantly recognized membrane-associated regions of the respective GP complex.2,4,8,25 However, quinidine-dependent antibodies against the distal glycocalicin region of GPIb have also been observed.2
Recently, the presence of a C → G polymorphism in the coding DNA sequence of the PECAM-1 gene has been reported that is responsible for an amino acid substitution at position 125 (Leu → Val) in domain 1 of the molecule.14 Interestingly, this variety of PECAM-1 has an impact on the immune response because it can be distinguished by mab 7E8.14 We were, therefore, interested if the carbimazole-dependent antibodies against PECAM-1 were related to one of its isoforms. In flow cytometry, no differences between the Leu125 and Val125 variants could be observed, suggesting that the polymorphic structures are not directly involved in epitope formation (Figure 6).
The finding of PECAM-1 as the only target of DDAbs in carbimazole-induced thrombocytopenia led us to the analysis of sera from patients with quinidine-dependent thrombocytopenia. As expected, we found strong reactions with GPIb/IX and GPIIb/IIIa and thereby confirmed the previous findings that these two GPs comprise the target antigens in quinidine-induced thrombocytopenia.2 7-9 On analysis in the MAIPA assay, we found additional reactivity of several sera with PECAM-1 that was only detectable in the presence of drug. Interestingly, the reaction with PECAM-1 was only observed when additional reactivity against GPIb/IX and/or GPIIb/IIIa was detectable. One may speculate that unspecific interaction between PECAM-1 and other membrane constituents on the platelet membrane contribute to the reactivity of quinidine-dependent antibodies in GP-specific immunoassays. However, no reactions were observed with GPIa/IIa in this assay among all serum samples. In addition, negative results were obtained with anti-PECAM-1 mab when autoantibodies or alloantibodies were analyzed against GPIIb/IIIa, GPIb/IX, or GPIa/IIa.
Our patients with quinidine-dependent antibodies presented with the typical clinical finding of pronounced hemorrhagic diathesis and severe acute thrombocytopenia. In comparison, the patients with carbimazole-dependent antibodies had only mild thrombocytopenia, which in some instances was only relative to platelet counts before or after the intake of the drug. Because severe thrombocytopenia is usually expected in DITP, platelet reactive antibodies against carbimazole might have been overlooked in the past. Relative thrombocytopenia with a decrease in platelet counts of about 50% is a well-known phenomenon in heparin-induced thrombocytopenia.26 It is, therefore, not surprising that, in 2 of our patients with carbimazole-induced antibodies, heparin-induced thrombocytopenia was suspected initially. Another reason not to suspect DDAbs in patients receiving carbimazole is the attribution of thrombocytopenia to underlying Graves disease. The association of hyperthyroidism with secondary autoimmune thrombocytopenia has been observed on several occasions.27,28 One may speculate that mild thrombocytopenia in our patients with carbimazole-dependent antibodies was related to the exclusive reactivity of the antibodies with PECAM-1, a target molecule that is present in large amounts on endothelial cells and certain blood cells (eg, granulocytes).29 Absorption of the antibody could occur on PECAM-1-expressing cells and could, therefore, have reduced the clearance of platelets, resulting in mild thrombocytopenia.
We conclude that PECAM-1 has to be added to the list of target GPs in DITP. It may be involved as a sole target molecule in carbimazole-dependent antibodies, but it may also be an additional target for antibodies resulting from treatment with other drugs such as quinidine. Further studies will be required to analyze the possible role of PECAM-1 in DITP resulting from treatment with other drugs.
Acknowledgment
We appreciate the excellent technical assistance of Ms Astrid Giptner and Ms Monika Kuemmel. We express our gratitude to the physicians who cared for the patients in this study, for contribution of detailed clinical observations.
Supported by grant DFG Sa 480/2-1 from the German Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hartmut Kroll, Institute for Clinical Immunology and Transfusion Medicine, Justus Liebig University, Langhansstr. 7, D-35392 Giessen, Germany; e-mail:hartmut.e.kroll@immunologie.med.uni-giessen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal