Abstract
The immune dysfunction and cell destruction that occur in the human immunodeficiency virus (HIV)-infected host appear to result from the direct cytopathic effects of viral infection and the effects of viral proteins on uninfected bystander cells. Recently, the α-chemokine receptor CXCR4 has been reported to mediate apoptosis in neuronal cells and in CD4+ and CD8+ T cells after its binding to HIV-1 envelope proteins. In the current study, it was observed that human umbilical vein endothelial cells (HUVEC) undergo apoptosis after their treatment with the HIV-1 envelope proteins gp120/160. Anti-CXCR4 monoclonal antibody decreased HIV-1 gp120/160-induced apoptosis, suggesting that the CXCR4 chemokine receptor mediates the apoptotic effects of these HIV envelope glycoproteins. Further studies revealed that caspases play an important role in this process because the pretreatment of cells with a general caspase enzyme inhibitor decreased the extent of HUVEC apoptosis induced by gp120/160. In addition, it was found that caspase-3 was activated on HIV-1 gp120/160 treatment of these cells. It was also observed that gp120/160 treatment slightly increased the expression of the pro-apoptotic molecule Bax. These results suggest that HIV-1 envelope glycoproteins can disrupt endothelial integrity through the interaction with CXCR4, thereby facilitating virus transit out of the bloodstream and contributing to the vascular injury syndromes seen in acquired immunodeficiency syndrome.
Introduction
The pathophysiology of human immunodeficiency virus (HIV) infection extends beyond the direct cytopathic effects of viral infection; certain so-called bystander cells that do not carry virus appear to malfunction and die.1-4 Because the degree of T-cell loss exceeds the number of infected cells in the HIV-infected host and because the types of cells affected are not necessarily CD4+, the notion that immune cell depletion and other manifestations of HIV infection may be caused by indirect effects of the virus has recently been posited. The initiation of apoptotic pathways by HIV or its viral products would be one such indirect mechanism of cellular injury.5-7 Death of bystander CD4+ cells can occur on gp120-induced CD4 cross-linking.8 The downstream effects of such gp120 binding include decreased Bcl-2 expression and increased Fas-L expression, both of which may make T cells susceptible to programmed cell death upon antigen presentation.9-11
CXCR4 is a receptor for the α-chemokine stromal cell–derived factor-1 (SDF-1).12,13 This receptor also acts as a co-receptor for certain isolates of HIV-1.14-16 CXCR4 has recently been shown to mediate HIV-1 gp120-induced apoptosis of several immune cell types.7,17 For example, gp120 binding to either CD4 or CXCR4 resulted in apoptosis, and this cell death was blocked by pretreatment with the CXCR4 ligand, SDF-1.17 In addition, CXCR4 activation by HIV envelope proteins was shown to contribute to CD8+ T-cell apoptosis.7 Of note, human neuronal cells similarly undergo apoptosis on gp120 or SDF-1 binding to CXCR4 in the absence of CD4.6
CXCR4 is widely expressed in various hematopoietic cells and has been shown to be a critical regulator of leukocyte and hematopoietic precursor migration.12 It has also been shown to regulate pre-B–cell proliferation, myelopoiesis, cerebellar development, and cardiogenesis.18-20 Knockout studies in mice have revealed that CXCR4 is expressed in developing endothelial cells and is important in the formation of large vessels supplying the gastrointestinal tracts and in the remodeling process in endothelial cells.21 Human umbilical vein endothelial cells (HUVEC) have been shown to express the CXCR4 receptor.22-24Recently, HIV gp120 has been shown to damage the endothelium by interaction with CXCR4.25 We observed that HIV-1 gp120/160 potently induced endothelial apoptosis by activating caspases and by slightly enhancing expression of the pro-apoptotic molecule, Bax. This suggests a novel mechanism whereby viral envelope proteins could facilitate the transit of virions or HIV-infected cells from the circulation to tissues.
Materials and methods
Cells, antibodies, and reagents
HUVEC (Clonetics, San Diego, CA) were grown in endothelial basal medium (EBM) supplemented with bovine brain extract (12 μg/mL), human epithelial growth factor (10 ng/mL), hydrocortisone (1 μg/mL), GA-1000 (Gentamicin and Amphotericin B, 1 μg/mL), and 2% fetal bovine serum (FBS; Clonetics). Recombinant HIV-1 gp120 and gp160 (IIIB strain) were purchased from Protein Sciences (Meriden, CT), and the caspase inhibitors and substrates were from Enzyme System Products (Livermore, CA). Protease inhibitors were from Sigma (St Louis, MO). Anti-CXCR4 antibody was obtained from PharMingen (San Diego, CA). Anti-Bax and mouse IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Sandwich ELISA for histone-associated DNA fragments
HUVEC were grown in supplemented EBM to 95% confluence in 24-well plates. The cells were then starved in EBM containing 0.5% FBS (low-serum medium [LSM]) for 16 hours before stimulation. A low level of apoptosis was observed under these conditions. Cells were then washed with PBS and incubated in nonsupplemented EBM containing different ligands and inhibitors for varying time intervals. Nucleosome fragmentation was assessed using the Cell Death Detection ELISA (Boehringer Mannheim, Indianapolis, IN). Cells were harvested in lysis buffer, cytoplasmic and nuclear fractions were separated by centrifugation at 200g, and 20 μL supernatant (cytoplasmic fraction) was added to a streptavidin-coated microtiter plate. The biotin-labeled antihistone antibody that was added to the plate bound to the histones in the fraction. Peroxidase-conjugated anti-DNA antibody was then added. Photometric analysis of the colorimetric reaction produced between the peroxidase and substrate (2,2′-Azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] disodium salt) permitted quantification of the bound nucleosome DNA fragments. The fold increase in nucleosome degradation was calculated by comparing the optical density values of the gp120/160-treated cells with those of the untreated controls. Assays were performed in triplicate, and each experiment was repeated 2 to 3 times.
TdT-mediated dUTP nick end labeling
In situ detection of apoptosis was performed by terminal deoxynucleotidyl transferase (TdT) labeling of DNA using the Fluorescein In Situ Cell Death Detection Kit (Boehringer Mannheim). HUVEC were plated in 8-well chamber slides (Nalge Nunc, Naperville, IL), serum starved, and stimulated for 10 hours, as described above. Cells were air dried and fixed in freshly prepared paraformaldehyde solution (4% in PBS, pH 7.4) for 30 minutes. They were then washed with PBS and incubated in permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 minutes on ice. After cells were rinsed twice with PBS, HUVEC were incubated in TdT-mediated dUTP nick end labeling (TUNEL) reaction mixture for 1 hour at 37°C in the dark. Cells were again rinsed 3 times with PBS and analyzed under a fluorescent microscope.
Caspase activity
To determine the activity of caspase-3, HUVEC were grown in 24-well plates, serum starved, and stimulated as described above. Cells were scraped in PBS containing 0.05% Triton X-100 and lysed by 3 freeze–thaw cycles in a dry ice/ethanol bath. The lysate was next centrifuged for 5 minutes at maximum speed, and 50 μL supernatant was added to a 495 μL assay buffer (0.1 mol/L HEPES, pH 7.4, 2 mmol/L dithiothreitol, 0.1% CHAPS, 1% sucrose). The peptide substrate for caspase-3, AC-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl coumarin (Ac-DEVD-AFC) (obtained from Enzyme System Products) was then added to a final concentration of 0.2 mmol/L. The reaction was allowed to proceed for 30 minutes at room temperature. The release of amino-4-trifluoromethyl coumarin was measured by using a fluorometer setting of 400-nm excitation and 505-nm emission. A standard curve was generated with free AFC.
Western blot analysis
Total cell lysates were prepared by lysing untreated or gp120- or gp160-treated HUVEC in RIPA buffer (50 mmol/L Tris-HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mmol/L NaCl; 1 mmol/L phenylmethylsulfonyl fluoride; 10 μg/mL each of aprotinin, leupeptin, and pepstatin; 10 mmol/L sodium vanadate; 10 mmol/L sodium fluoride; and 10 mmol/L sodium pyrophosphate). Proteins were separated by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. The membranes were blocked, washed, and probed with the respective primary and secondary antibodies, and the blots were developed using the enhanced chemiluminescence system (Amersham Pharmacia).
Results and discussion
HIV has been shown to induce apoptosis in several types of uninfected bystander cells, including CD4+ lymphocytes, CD8+ lymphocytes, neurons, and endothelial cells.1-4,25 HIV-1 gp120 appears to play an important role in triggering apoptosis by interacting with the α-chemokine receptor, CXCR4,6-8,17 which has recently been shown to be expressed in vascular endothelium and to function in vascular remodeling.20-24 In addition, gp120 is known to induce changes in endothelium, including substance P secretion, and an increase in permeability.26
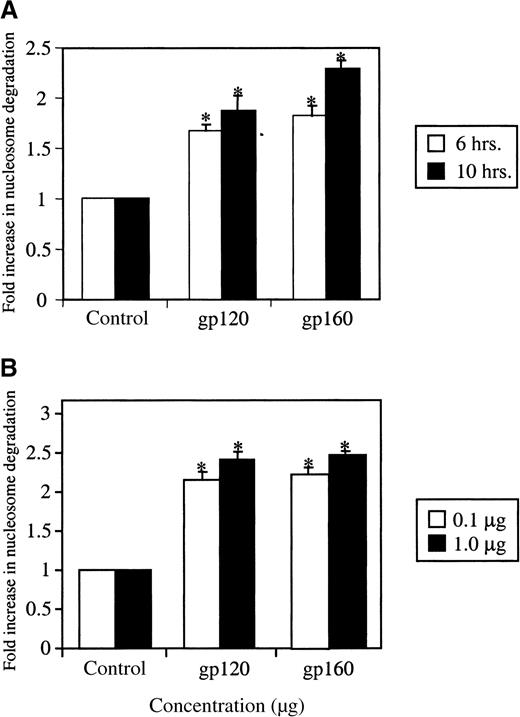
We studied the effects of treatment of HUVEC with ligands that are known to engage CXCR4, specifically HIV-1 gp120 and HIV-1 gp160. These envelope glycoproteins are shed during viral turnover27,28and are present in the circulation. Apoptosis after treatment with these envelope proteins was measured by ELISA, a photometric enzyme immunoassay for the quantitative in vitro determination of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes).29-31 As shown in Figure1A and B, gp120 or gp160 treatment induced apoptosis in HUVEC cells in a time- and concentration-dependent manner.A maximal effect was observed after 10 hours of stimulation and at a concentration of 1 μg/mL. Stimulation with HIV-1 gp120 demonstrated an increase of 2.2-fold (0.1 μg/mL gp120) or 2.4-fold (1 μg/mL gp120) over the controls, while gp160 stimulation of HUVEC revealed a similar pattern with increases of 2.2-fold (0.1 μg/mL gp160) and 2.4-fold (1 μg/mL gp160). A slight increase in apoptosis was observed at lower gp120/160 concentrations (10 ng/mL) (data not shown). Our results suggest that the induction of apoptosis was still significant at 0.1 μg/mL, a concentration very close to that reported for gp120 in the circulation of AIDS patients.27 28 We also confirmed the induction of apoptosis by gp120 or gp160 using the TUNEL method. As shown in Figure 2, a higher number of TUNEL-positive cells as compared to untreated cells (A) was observed after treatment of HUVEC with HIV-1 gp120 (B) or gp160 (C). These assays indicate that HIV-1 gp120 or gp160 treatment induces apoptosis in HUVEC.
HIV-1 gp120/160 induce endothelial apoptosis in a time- and concentration-dependent manner.
HUVEC were grown in LSM (0.5%), as described in “Materials and methods” and were treated with HIV-1 gp120 or gp160 for different time periods (A) or at different concentrations (B). Cell samples were analyzed for apoptosis using the photometric sandwich ELISA to detect cytoplasmic nucleosome degradation. The fold increase in nucleosome degradation was calculated by comparing the optical density (OD) values of the gp120/160-treated cells with those of the untreated HUVEC. *P < .0005.
HIV-1 gp120/160 induce endothelial apoptosis in a time- and concentration-dependent manner.
HUVEC were grown in LSM (0.5%), as described in “Materials and methods” and were treated with HIV-1 gp120 or gp160 for different time periods (A) or at different concentrations (B). Cell samples were analyzed for apoptosis using the photometric sandwich ELISA to detect cytoplasmic nucleosome degradation. The fold increase in nucleosome degradation was calculated by comparing the optical density (OD) values of the gp120/160-treated cells with those of the untreated HUVEC. *P < .0005.
HIV-1 gp120/160-induced HUVEC apoptosis as detected by TUNEL assay.
HUVEC grown in LSM were untreated (A) or treated with gp120 (1000 ng/mL) (B) or gp160 (1000 ng/mL) (C) for 10 hours; gp120-treated (1000 ng/mL) HUVEC at a higher magnification are represented in (D). Samples were analyzed for apoptosis using the TUNEL method. Green fluorescent cells represent apoptotic cells.
HIV-1 gp120/160-induced HUVEC apoptosis as detected by TUNEL assay.
HUVEC grown in LSM were untreated (A) or treated with gp120 (1000 ng/mL) (B) or gp160 (1000 ng/mL) (C) for 10 hours; gp120-treated (1000 ng/mL) HUVEC at a higher magnification are represented in (D). Samples were analyzed for apoptosis using the TUNEL method. Green fluorescent cells represent apoptotic cells.
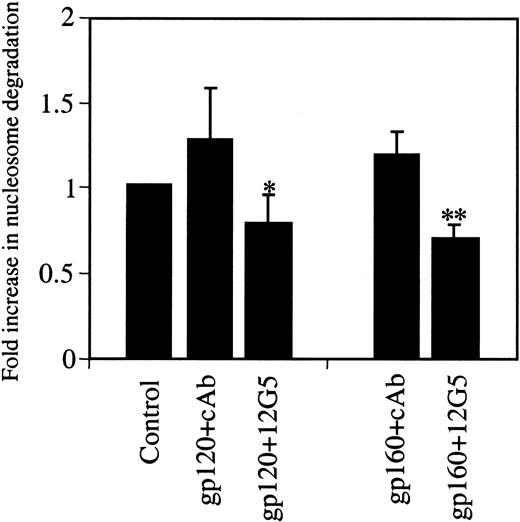
We next assessed the specificity of the observed apoptosis. Recently, CXCR4 has been reported to mediate HIV-1 gp120- or SDF1α-induced apoptosis in neuronal cells, CD8+ and CD4+ T cells, and endothelial cells.6,7,25 This receptor can bind to gp120 and certain strains of HIV independently of the presence of CD4.32-34 CXCR4 has also been shown to bind to oligomeric gp160 independently of CD4.33 Because endothelial cells express the CXCR4 receptor,22-24 gp120 or gp160 could induce endothelial apoptosis by activating this receptor. Therefore, the role of the CXCR4 receptor in HIV-1 gp120/160-induced apoptosis was investigated using the CXCR4 neutralizing antibody, 12G5. This antibody prevents the cognate ligand, SDF-1, and gp120/160 from binding to CXCR4.35 As shown in Figure3, preincubation of HUVEC with 12G5 inhibited both gp120- and gp160-induced apoptosis as compared to cells preincubated with a class-matched control antibody. The finding that a specific blocking antibody to the CXCR4 receptor reduced HIV gp120/160-induced endothelial apoptosis suggests a key role for this receptor in virus host vascular injury. The phenomenon mirrors the susceptibility of human brain cultures to apoptosis induced by their co-incubation with HIV.6 Viral envelope binding to CXCR4 was necessary and sufficient for this effect.5
HIV-1 gp120/160-induced HUVEC apoptosis is inhibited by anti-CXCR4 receptor antibody.
HUVEC grown in LSM were untreated (control) or treated with gp120 (100 ng/mL) or gp160 (100 ng/mL) for 10 hours in the presence of the CXCR4 neutralizing antibody 12G5 (6 μg/mL) or the class-matched control IgG (6 μg/mL). Cell samples were analyzed for apoptosis by nucleosome ELISA. cAb, control antibody. *P < .036; **P < .003.
HIV-1 gp120/160-induced HUVEC apoptosis is inhibited by anti-CXCR4 receptor antibody.
HUVEC grown in LSM were untreated (control) or treated with gp120 (100 ng/mL) or gp160 (100 ng/mL) for 10 hours in the presence of the CXCR4 neutralizing antibody 12G5 (6 μg/mL) or the class-matched control IgG (6 μg/mL). Cell samples were analyzed for apoptosis by nucleosome ELISA. cAb, control antibody. *P < .036; **P < .003.
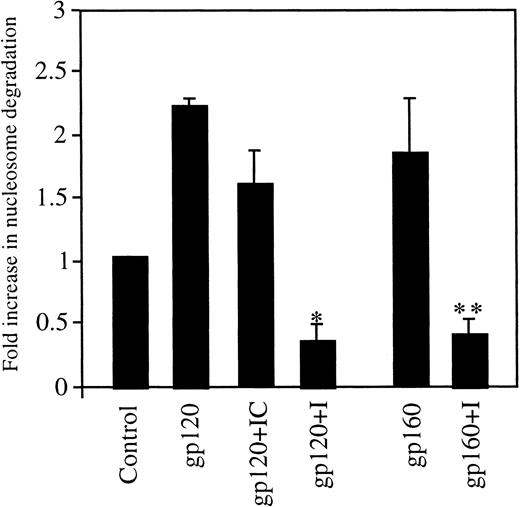
Caspases are essential components of the mammalian apoptotic machinery.36-39 They cleave various key cellular proteins, which results in cell death. The role of the caspase machinery in gp120/160-induced endothelial apoptosis was first assessed using a broad-spectrum, cell-permeable caspase inhibitor, Z-valine-alanine-aspartate fluoromethyl ketone (Z-VAD-FMK), that blocks apoptosis mediated by caspases.40 41 When HUVEC were stimulated with HIV-1 gp120 or HIV-1 gp160 in combination with Z-VAD-FMK for 6 hours, the amount of apoptosis as measured by nucleosome degradation was significantly reduced (Figure4). Stimulation with HIV-1 gp120 caused a more than 2.3-fold increase in nucleosome degradation, which was decreased to 0.38-fold by the caspase inhibitor (I). HIV-1 gp160, with or without the caspase inhibitor, had similar effects to those of gp120 under these conditions; the gp160 ligand alone caused a 1.8-fold increase in degradation, and the caspase inhibitor reduced this degradation to 0.33-fold. Pretreatment with the inhibitor control, Z-phenylalanine-alanine –fluoromethyl ketone (Z-FA-FMK), had a slight effect on gp120-induced apoptosis. We used this inhibitor as a control because the caspase inhibitor sequence (VAD) is replaced by FA, which inhibits cysteine proteases such as cathepsin B but does not inhibit caspase activity.
Inhibition of HUVEC apoptosis by caspase inhibitors.
HUVEC grown in LSM were untreated (control) or treated with gp120 or gp160 at 100 ng/mL each for 10 hours in the presence or absence of the caspase pathway inhibitor (I), Z-VAD-FMK, at a 40-μmol/L concentration. In addition, gp120-treated cells were incubated with the inhibitor control (IC), Z-FA-FMK, at a 40-μmol/L concentration. Apoptosis was measured using ELISA. *P < .0007; **P < .011.
Inhibition of HUVEC apoptosis by caspase inhibitors.
HUVEC grown in LSM were untreated (control) or treated with gp120 or gp160 at 100 ng/mL each for 10 hours in the presence or absence of the caspase pathway inhibitor (I), Z-VAD-FMK, at a 40-μmol/L concentration. In addition, gp120-treated cells were incubated with the inhibitor control (IC), Z-FA-FMK, at a 40-μmol/L concentration. Apoptosis was measured using ELISA. *P < .0007; **P < .011.
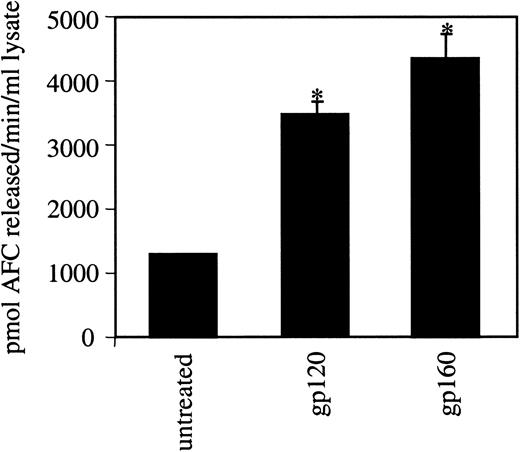
Because the caspase inhibitor reduced gp120/160-induced apoptosis, we further investigated the role of caspases in this process. Different caspases are activated in various apoptotic pathways. A major “executioner” caspase is caspase-3.36-39 Lysates of untreated or gp120/160-treated HUVEC were assayed using a specific caspase-3 substrate, AC-DEVD-AFC. Increased caspase activity as compared to the untreated control was observed with either ligand (Figure 5). These studies suggest that caspase-3 plays an important role in mediating gp120/160-induced apoptosis in endothelial cells. Although little is known about the function of caspases in endothelial apoptosis, caspase-3 has been shown to be activated in the TL-1 (a novel tumor necrosis factor-like cytokine)-induced apoptosis of endothelial cells in bovine pulmonary arteries.42 Recently, gp120-induced apoptosis of human CD4+ T cells and CXCR4-expressing cells was shown to be mediated by caspase-3.41 43 In our studies, we observed an increase in caspase activity reflecting an induction of apoptosis rather than a response to gp120 toxicity. Because these apoptotic caspases do not operate by themselves but are part of a larger set of interdependent molecules, it is most likely that caspases other than caspase-3 would also be found to be activated under these conditions.
Activation of caspase-3 in HUVEC by HIV-1 gp120/160.
HUVEC were grown in LSM with gp120 or gp160 at a 100-ng/mL concentration for 6 hours. Cells were lysed in low-detergent buffer, and the lysates were analyzed for caspase-3 activity using a specific substrate (AC-DEVD-AFC) as described in “Materials and methods.” The release of AFC was measured using a fluorometer setting of 450-nm excitation and 505-nm emission. *P < .0007.
Activation of caspase-3 in HUVEC by HIV-1 gp120/160.
HUVEC were grown in LSM with gp120 or gp160 at a 100-ng/mL concentration for 6 hours. Cells were lysed in low-detergent buffer, and the lysates were analyzed for caspase-3 activity using a specific substrate (AC-DEVD-AFC) as described in “Materials and methods.” The release of AFC was measured using a fluorometer setting of 450-nm excitation and 505-nm emission. *P < .0007.
Anti-apoptotic Bcl-2 and pro-apoptotic Bax family members play important roles in the regulation of apoptosis.44-48 To further explore the mechanisms of HUVEC apoptosis induced by gp120 or gp160, we studied the expression of the pro-apoptotic protein Bax and the anti-apoptotic protein, Bcl-2. Bax has been shown to be one of the predominant pro-apoptotic proteins in HUVEC,49 whereas Bcl-2 plays an important role in mediating HUVEC survival.50,51 HIV gp120/160 treatment of HUVEC slightly increased protein levels of Bax compared to the untreated cells, based on immunoblot analysis with a specific Bax antibody (Figure6). However, no change in Bcl-2 expression was observed (data not shown). Overexpression of Bax is also known to enhance many forms of apoptosis.52-54
Induction of Bax expression by HIV-1 gp120/160 treatment.
Cell lysates (100 μg) from HUVEC, prepared as described in “Materials and methods,” untreated or treated with gp120 or gp160 (100 ng/mL) for 1 hour or 3 hours, were resolved on 15% SDS-PAGE and blotted with anti-Bax antibody.
Induction of Bax expression by HIV-1 gp120/160 treatment.
Cell lysates (100 μg) from HUVEC, prepared as described in “Materials and methods,” untreated or treated with gp120 or gp160 (100 ng/mL) for 1 hour or 3 hours, were resolved on 15% SDS-PAGE and blotted with anti-Bax antibody.
Taken together, these results suggest a key role of the CXCR4 receptor in virus–host vascular injury. One possible consequence of HIV envelope proteins, shed from infected cells, would be their facilitation of the spread of virus and virus-containing cells from the bloodstream to the tissues. The finding that CXCR4 is a key component in initiating such damage provides a mechanism of endothelial injury in the setting of HIV infection, which until now has not been well defined, and suggests the possibility of damage to other uninfected CXCR4-positive bystander cells. Strategies to modulate CXCR4 interaction with the HIV envelope may prove therapeutically useful in limiting virus dissemination.
Acknowledgments
We thank our colleague Heng Chhay for technical assistance. We thank Janet Delahanty for editing the manuscript, Daniel Kelley for preparation of the figures, and Simone Jadusingh for facilitating our receipt of the needed reagents for the experiments.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ramesh K. Ganju, Divisions of Experimental Medicine and Hematology/Oncology, Beth Israel Deaconess Medical Center, Harvard Institutes of Medicine, 4 Blackfan Circle, Boston, MA 02115; e-mail: rganju@caregroup.harvard.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal