Abstract
The Wilms' tumor (WT1) gene participates in leukemogenesis and is overexpressed in most types of leukemia in humans. WT1 is also detectable in many types of lung, thyroid, breast, testicular, and ovarian cancers and melanoma in humans. Initial studies evaluated whether immune responses to murine WT1 can be elicited in mice. Murine and human WT1 are similar. Thus, mouse models might lead to resolution of many of the critical issues for developing WT1 vaccines. C57/BL6 (B6) mice were injected with synthetic peptides from the natural sequence of WT1 containing motifs for binding to major histocompatibility (MHC) class II molecules. Immunization induced helper T-cell responses specific for the immunizing WT1 peptides and antibody responses specific for WT1 protein. Screening of multiple murine cancer cell lines identified 2 murine cancers, TRAMP-C and BLKSV40, that “naturally” overexpress WT1. Immunization with MHC class I binding peptides induced WT1 peptide-specific cytotoxic T-lymphocyte (CTL) that specifically lysed TRAMP-C and BLKSV40. WT1 specificity of lysis was confirmed by cold target inhibition. No toxicity was noted by histopathologic evaluation in the WT1 peptide-immunized animals. WT1 peptide immunization did not show any effect on TRAMP-C tumor growth in vivo. Immunization of B6 mice to syngeneic TRAMP-C elicited WT1-specific antibody, demonstrating that WT1 can be immunogenic in the context of cancer cells. To evaluate whether WT1 might be similarly immunogenic in humans, serum from patients with leukemia was evaluated for pre-existing antibody responses. Western blot analyses showed WT1-specific antibodies directed against the N-terminus portion of the WT1 protein in the sera of 3 of 18 patients with acute myeloid leukemia (AML).
Introduction
Many candidate antigens are “self” proteins expressed by normal and malignant cells. These self-antigens have included proteins expressed during fetal development—but only on rare adult tissues (ie, MAGE-1 and HER-2/neu)—and proteins related to the differentiated function of the involved tissue of malignant origin (ie, tyrosinase and gp100).1-3 The Wilms' tumor (WT1) protein is a self-protein expressed in a time- and tissue-specific manner during embryogenesis.4 WT1 is expressed in the adult, but only in low amounts in the nuclei of some normal tissues, such as hematopoietic precursor, kidney, and gonadal cells.4-6Recent studies demonstrated that the WT1 gene is overexpressed in most types of adult leukemia, including acute myeloid leukemia (AML), chronic myeloid leukemia, and acute lymphocytic leukemia and in some patients with myelodysplastic syndromes.7-15 WT1 is also expressed in many types of lung, thyroid, breast, testicular, and ovarian carcinomas and in melanoma.16-21
The WT1 gene is located on chromosome 11p13 and was originally identified in sporadic and hereditary cases of Wilms' tumor.5,22 The gene consists of 10 exons, and it encodes a zinc finger transcription factor. As a result of alternative splicing, several WT1 mRNA species are expressed that exhibit different DNA-binding specificities.23,24 During embryogenesis the WT1 gene is involved in the regulation of growth and differentiation, and it interacts with a variety of target genes.25 WT1 binds to the early growth response-1 DNA consensus sequences in growth factor gene promoters, such as the platelet-derived growth factor A chain promoter and the insulin-like growth factor II promoter.26-28 In Wilms' tumor, WT1 expression is found in the malignant counterparts of cell types that express the gene during normal kidney development. It has been suggested that aberrant expression of the WT1 gene product leads to uncontrolled proliferation, with aberrant differentiation and eventual tumor formation.4
There is a theoretic advantage for targeting antitumor immune responses against proteins necessary for maintenance of the malignant phenotype. We chose to evaluate WT1 as a candidate tumor antigen because WT1 protein is more abundant in leukemia cells than in normal hematopoietic cells,11,29 WT1 seems to participate in leukemogenesis, and WT1 protein expression may be necessary to maintain the viability of leukemia cells.30-33 WT1 function might similarly be necessary to maintain the viability of human solid tumors in which it is overexpressed.
Immunologic tolerance to self-proteins can prevent or dampen the development of immune responses to many putative self-cancer antigens. The objective of the current study was to initiate an evaluation of WT1 as a tumor antigen. WT1 is highly homologous in mice and humans (96% at the amino acid level) and has similar tissue distribution and function.34 35 Thus, mouse models might resolve many of the critical issues for developing WT1 vaccines. Results demonstrate that antibody, helper T cells, and cytotoxic T-lymphocytes (CTL) specific for WT1 can be elicited in mice. WT1 peptide immunization did not show any effect on TRAMP-C tumor growth in vivo. The lack of observable in vivo antitumor effects against WT1 overexpressing TRAMP-C after WT1 peptide immunization may result from the low major histocompatibility complex (MHC) class I expression of TRAMP-C cells. Further experiments are required for better understanding of the therapeutic potential of WT1 vaccines in animal models. Preliminary studies to evaluate immunogenicity of WT1 in humans demonstrated that antibody responses to WT1 are detectable in some patients with AML. The extent to which helper T-cell and CTL responses can be elicited in humans should be evaluated.
Materials and methods
Mice
Female C57BL/6 (B6) mice, 6 to 8 weeks old, were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were maintained at the University of Washington Animal Facilities under specific pathogen-free conditions.
Tumor cell lines
TRAMP-C is an SV40-induced transgenic prostate cancer cell line of B6 origin and was provided by Dr Norman Greenberg. BLKSV40 is a B6 murine fibroblast line transformed by SV40. LSTRA is a Moloney murine virus-induced leukemia of BALB/c origin. EL-4 is a methylcholanthrene-induced thymoma of C57BL mouse origin. The human leukemia cell line K562 was obtained from the American Type Culture Collection (Manassas, VA).
Peptides
WT1-derived peptides were selected according to the known consensus motifs for peptide bound by H-2Kb and H-2Db from the naturally occurring sequences of murine and human WT1.36 Sequences of the CTL peptides are shown in Table 1. Peptides used for eliciting antibody and proliferative T-cell responses were identified according to the Tsites program, which searches for peptide motifs that have the potential to elicit Th responses.37,38 Vaccine A consisted of p6-22, RDLNALLPAVPSLGGGG (murine WT1 contains an S at position 16); p117-139, PSQASSGQARMFPNAPYLPSCLE (identical between mouse and human); and p244-262, GATLKGVAAGSSSSVKWTE (murine WT1 contains an M at position 250). Vaccine B consisted of p287-301, RIHTHGVFRGIQDVR (identical between mouse and human); p299-313, DVRRVPGVAPTLVRS (murine WT1 contains an S at position 304); and p421-435, CQKKFARSDELVRHH (identical between mouse and human). Of note, peptide p117-139 contained motifs appropriate for binding to murine MHC class I and II. Peptides were synthesized on a peptide synthesizer (430 A; Applied Biosystems, Foster City, CA) as previously described.39 The composition and purity of the peptides were ascertained by mass spectroscopy and high-performance liquid chromatography analysis. Peptides were determined to be more than 90% pure.
RMA-S binding
| Name . | Sequence . | RMA-S binding . | |
|---|---|---|---|
| H-2Db (%) . | H-2Kb (%) . | ||
| WT1 peptides with motifs predictive of binding | |||
| P136-144 | SCLESQPTI | 27 | 1 |
| P221-229 | YSSDNLYQM | 1 | 1 |
| P225-233 | NLYQMTSQL | 6 | 1 |
| P228-236 | QMTSQLECM | 1 | 1 |
| P235-243 | CMTWNQMNL | 34 | 1 |
| P239-247 | NQMNLGATL | 1 | 1 |
| P329-337 | GCNKRYFKL | 1 | 1 |
| All 9mer WT1 peptides within p117-139 | |||
| P117-125 | PSQASSGQA | 2 | 7 |
| P118-126 | SQASSGQAR | 2 | 7 |
| P119-127 | QASSGQARM | 2 | 6 |
| P120-128 | ASSGQARMF | 1 | 7 |
| P121-129 | SSGQARMFP | 1 | 7 |
| P122-130 | SGQARMFPN | 1 | 7 |
| P123-131 | GQARMFPNA | 1 | 7 |
| P124-132 | QARMFPNAP | 1 | 9 |
| P125-133 | ARMFPNAPY | 1 | 8 |
| P126-134 | RMFPNAPYL | 79 | 65 |
| P127-135 | MFPNAPYLP | 2 | 8 |
| P128-136 | FPNAPYLPS | 1 | 5 |
| P129-137 | PNAPYLPSC | 1 | 4 |
| P130-138 | NAPYLPSCL | 79 | 94 |
| P131-139 | APYLPSCLE | 1 | 9 |
| Isotype control | 1 | 2 | |
| No peptide | 1 | 6 | |
| Name . | Sequence . | RMA-S binding . | |
|---|---|---|---|
| H-2Db (%) . | H-2Kb (%) . | ||
| WT1 peptides with motifs predictive of binding | |||
| P136-144 | SCLESQPTI | 27 | 1 |
| P221-229 | YSSDNLYQM | 1 | 1 |
| P225-233 | NLYQMTSQL | 6 | 1 |
| P228-236 | QMTSQLECM | 1 | 1 |
| P235-243 | CMTWNQMNL | 34 | 1 |
| P239-247 | NQMNLGATL | 1 | 1 |
| P329-337 | GCNKRYFKL | 1 | 1 |
| All 9mer WT1 peptides within p117-139 | |||
| P117-125 | PSQASSGQA | 2 | 7 |
| P118-126 | SQASSGQAR | 2 | 7 |
| P119-127 | QASSGQARM | 2 | 6 |
| P120-128 | ASSGQARMF | 1 | 7 |
| P121-129 | SSGQARMFP | 1 | 7 |
| P122-130 | SGQARMFPN | 1 | 7 |
| P123-131 | GQARMFPNA | 1 | 7 |
| P124-132 | QARMFPNAP | 1 | 9 |
| P125-133 | ARMFPNAPY | 1 | 8 |
| P126-134 | RMFPNAPYL | 79 | 65 |
| P127-135 | MFPNAPYLP | 2 | 8 |
| P128-136 | FPNAPYLPS | 1 | 5 |
| P129-137 | PNAPYLPSC | 1 | 4 |
| P130-138 | NAPYLPSCL | 79 | 94 |
| P131-139 | APYLPSCLE | 1 | 9 |
| Isotype control | 1 | 2 | |
| No peptide | 1 | 6 | |
Peptide binding to C57BL/6 murine MHC was confirmed using the RMA-S binding assay. Samples were analyzed using the Becton-Dickinson FACScalibur. The positive region was gated to contain approximately 1% of the RMA-S cells stained with isotype only. The same region was used to determine the percentage positive for class I stained samples.
Peptide-binding assay
The ability of synthetic WT1 peptides to bind to Dbor Kb class I molecules was detected by using the leukemia cell line RMA-S, as described previously.40 In brief, RMA-S cells were cultured for 7 hours at 26°C in complete medium supplemented with 1% fetal calf serum. A total of 106RMA-S cells were added to each well of a 24-well plate and incubated either alone or with the designated peptide (25 g/mL) for 16 hours at 26°C and for an additional 3 hours at 37°C in complete medium. Cells were then washed 3 times and stained with fluorescein isothiocyanate-conjugated anti-Db or anti-Kbantibody (Pharmingen, San Diego, CA). Labeled cells were washed twice, resuspended, and fixed in 500 μL phosphate-buffered saline with 1% paraformaldehyde and were analyzed for fluorescence intensity in a flow cytometer (FACScalibur; Becton Dickinson, Franklin Lakes, NJ). The percentage of increase of Db or Kbmolecules on the surface of the RMA-S cells was measured by increased mean fluorescence intensity of cells incubated with peptide compared with that of cells incubated in medium alone.
Immunization protocols
For tumor immunization, mice were inoculated twice at 2-week intervals by subcutaneous injection with 5 × 106 WT1 overexpressing TRAMP-C cells. For peptide immunization, mice were inoculated 3 times at 3-week intervals by subcutaneous injection with complete Freund's adjuvant alone or emulsified with WT1 peptides (100 μg). Three weeks after the final immunization, sera were obtained and single-cell suspensions of spleens were prepared in RPMI 1640 medium (Gibco BRL Life Technologies, Rockville, MD) with 25 μmol/L β-2-mercaptoethanol, 200 U penicillin/mL, 10 mmol/Ll-glutamine, and 10% fetal bovine serum.
T-cell proliferation assay
Lymphocytes were cultured in 96-well plates at 2 × 105 cells per well with 4 × 105irradiated (30 Gy) syngeneic spleen cells and the designated peptide. The proliferative T-cell responses were evaluated using standard thymidine incorporation as the read out.41 T-cell clones were generated under similar conditions by a limiting dilution method, as described previously.42
Cytotoxicity assay
A standard chromium release assay was used,43 and 106 target cells were incubated at 37°C with 150 μCi51Cr for 90 minutes in the presence or absence of specific peptides. Cells were washed 3 times and resuspended in RPMI with 5% fetal bovine serum. For the assay, 10451Cr-labeled target cells were incubated with different concentrations of effector cells in a final volume of 200 μL in U-bottomed, 96-well plates. Supernatants were removed after 4 to 7 hours at 37°C, and the percentage specific lysis was determined by the formula: Percent Specific Lysis = (Experimental Release − Spontaneous Release) / (Maximum Release − Spontaneous Release) × 100.
Cold target inhibition
Effector cells were plated for indicated effector:target ratios in 96-well U-bottom plates. A 10-fold excess (compared to hot target) of the indicated peptide-coated target without 51Cr labeling was added. Finally, 10451Cr-labeled target cells per well were added, and the plates were incubated at 37°C for 4 hours. The total volume per well was 200 μL.
Antibody blocking experiments
Effector cells were plated for indicated effector:target ratios in 96-well U-bottom plates. Antibodies were used at 25 μg/mL final concentration and were added to peptide-coated 51Cr-labeled target cells 5 minutes before plating with effectors. For control antibodies, IgG2a κ isotype standard (Pharmingen) and I-Ad/b MHC class II alloantigen antibody (Pharmingen) were used. For Db-specific antibody, anti-H–2Db(Pharmingen) was used, and for Kb-specific antibody, anti-H–2Kb (Pharmingen) was used.
TRAMP-C tumor challenge experiment
B6 mice were vaccinated with 100 μg peptide plus complete Freund's adjuvant, 100 μL of a 1:1 mixture injected subcutaneously and boosted twice at 3-week intervals. Control mice were injected with adjuvant only or were left untreated. Three weeks after the final immunization, mice were anesthetized with Metofane (Schering-Plough Animal Health, Union, NJ), shaved on the right flank, and injected subcutaneously with 106 TRAMP-C cells in 100 μL saline. TRAMP-C was grown to approximately 70% confluence before harvest. Mice were monitored for tumor growth, and tumors were measured on 2 axes with calipers.
Recombinant protein purification
For protein expression, cDNA constructs representing the human WT1 full-length (aa 1 to aa 449), the N-terminus (aa 1 to aa 249) and the C-terminus (aa 267 to aa 449) regions were subcloned into a modified pET28 vector. The resultant vector has a 5′ His tag followed by the thioredoxin (Trx) coding region, followed by a 3′ histidine tag, followed by a thrombin and EK site. Recombinant BL21 pLysSEscherichia coli (Stratagene, La Jolla, CA) containing the expression constructs for the TRX-WT1 protein and the fusion proteins of truncated forms of WT1 with TRX were grown overnight and induced with isopropyl-β-D-thiogalactoside. All the proteins behaved similarly and were purified essentially according to this protocol: cells were harvested and lysed by incubation in 10 mmol/L Tris, pH 8, with complete protease inhibitor tablets (Boehringer Mannheim Biochemicals, Indianapolis, IN) at 37°C followed by repeated rounds of sonication. Inclusion bodies obtained were washed twice with 10 mmol/L Tris, pH 8/0.5% CHAPS, and solubilized in 8 mol/L urea containing 10 mmol/L Tris at pH 8 (buffer A). Proteins were then purified by metal chelate affinity chromatography over nickel-nitrilotriacetic acid resin (QIAGEN, Valencia, CA) as described.44 Proteins were analyzed by SDS-PAGE, and fractions containing the protein of interest were pooled and dialyzed overnight against excess 10 mmol/L Tris, pH 8. Dialysates were brought to 8 mol/L urea and were loaded onto a Source Q anion exchange resin (Amersham Pharmacia Biotech, Uppsala, Sweden) equilibrated in buffer A. Proteins were eluted in buffer A with a gradient from 0 to 1 mol/L NaCl. Fractions containing the proteins of interest were pooled, dialyzed overnight against excess 10 mmol/L Tris, pH 8, and stored at −80°C for further use. The identity of the WT1 proteins was confirmed by N-terminal sequencing.
Antibody responses
Antibody responses to WT1 were determined by Western blot analysis using both recombinant full-length and truncated WT1 proteins and a WT1 polypeptide, WT N180 (Santa Cruz Biotechnology, Santa Cruz, CA). Western blot analysis was performed as described.41As the primary antibody, sera from immunized as well as nonimmunized B6 mice or patients with AML was used in a 1:500 dilution with Tris-buffered saline/1% bovine serum albumin and 0.1% NP-40. A polyclonal antimouse or antihuman-horseradish peroxidase-conjugated second antibody (Amersham Pharmacia Biotech, Piscataway, NJ) was used in a 1:10 000 dilution. Blots were then developed with an enhanced chemiluminescence reaction (ECL; Amersham); after that, they were exposed to Hyperfilm-ECL (Amersham). The film was developed and examined. All control blots were developed by using the commercially prepared WT1-specific antibodies WT C-19 and WT 180 (Santa Cruz Biotechnology), and they demonstrated a strong band at the expected size of the TRX-WT1 fusion proteins (TRX-WT1 full length, approximately 85 kd; TRX-WT1 N-terminus, approximately 60 kd; TRX-WT1 C-terminus, 50 kd).
Semiquantitative real-time RT-PCR
Bone marrow (BM) aspirates were obtained from patients with AML at diagnosis at the First Department of Internal Medicine, Division of Hematology, University of Vienna, Austria, where they were also treated. Leukemia was classified according to the French–American–British classification criteria after examination of the BM aspirates stained by a modified Wright technique. Mononuclear cells were isolated by density-gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway), and cell pellets were frozen immediately at −70°C. Total RNA was extracted as described previously. To assess the quality of RNA, all RNA samples were analyzed on denaturing formamide agarose. We took precautions to prevent contamination, as described earlier.12 45 cDNA was synthesized with oligo-dT primer (Boehringer Mannheim, Indianapolis, IN) and Superscript II RT (Gibco). Real-time RT-PCR was performed on an ABI 7700 instrument using the SYBR green assay system (Applied Biosystems, Foster City, CA). The reaction was performed in a total volume of 25 μL that included 2.5 μL SYBR green buffer, 2 μL 1:10 diluted first-strand cDNA template, and 300 nmol/L each of WT1 forward (human, 5′-TGGACAGAAGGGCAGAGCA-3′; mouse, 5′-TCTTCCGAGGCATTCAGGAT-3′) and reverse (human, 5′-GGATGGGCGTTGTGTGGT-3′; mouse, 5′-ATGCTGACCGGACAAGAGTTG-3′) primers that were designed to meet thermal cycler requirements. The reaction mix also contained 3 mmol/L MgCl2, 0.25 U AmpErase UNG, 0.625 U Amplitaq gold (Perkin Elmer, Norwalk, CT), 0.08% glycerol, 0.05% gelatin, 0.0001% Tween 20, and 1 mmol/L dNTP mix. Human β-actin primers were obtained from Perkin Elmer/Applied Biosystems and were used in a similar manner to quantitate the presence of β-actin in the samples. The sequences of the mouse β-actin primers were forward 5′-GGCGCTTTTGACTCAGGATT-3′ and reverse 5′-GGGATGTTTGCTCCAACCAA-3′.
PCR was performed using the universal thermal cycling protocol provided by Applie Biosystems. Fluorescence was measured on the ABI 7700 instrument, and the threshold cycle (Ct) was determined. All samples were run in triplicate. To quantitate the amount of specific RNA in the sample, a standard curve was generated alongside the unknown samples using semilogarithmic dilutions of K562 (human) or TRAMP-C (mouse) cDNA. In addition, a standard curve was generated for β-actin ranging from 200 fg to 2 ng. This enabled standardization of the initial RNA content of a tissue sample to the amount of β-actin for comparison. WT1 gene expression was expressed as an n-fold difference relative to peripheral blood mononuclear cells (PBMC).
Enrichment of human hematopoietic cells
After informed consent was obtained, CD34+ cells were positively selected from the peripheral blood of healthy volunteers following the subcutaneous administration of granulocyte colony-stimulating factor (G-CSF) using a direct magnetic labeling system (Miltenyi Biotec, Auburn, CA).46 The population was determined by flow cytometry (FACScalibur; Becton Dickinson) to contain more than 95% CD34+ cells.
Results
WT1-specific mRNA is overexpressed in human and murine malignancies
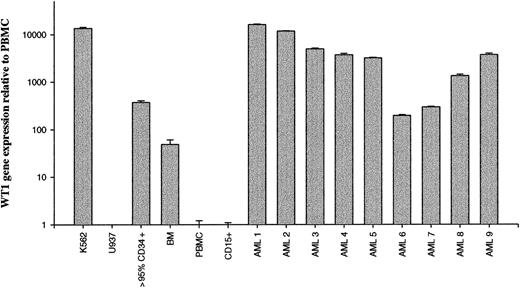
Recent studies demonstrated that the WT1 gene is overexpressed in most types of adult leukemia.7-15 To confirm these data using a semiquantitative real-time RT-PCR assay, WT1 gene expression was assessed in normal BM, purified CD34+cells, and 9 patients with AML. Controls included K562, a leukemia cell line known to overexpress WT1. Results were expressed relative to WT1 expression by normal PBMC. Most patients with AML were shown to express higher levels of WT1-specific mRNA than normal PBMC and BM (Figure1). WT1 gene expression was also detectable in human CD34+ cells. However, 7 of 9 patients with AML (patients 1-5, 8, 9) and the leukemia cell line K562 showed substantial overexpression of WT1 mRNA compared to 95% pure CD34+cells. Two of 9 patients showed similar (patient 7) or lower (patient 6) WT1 expression compared to CD34+ cells.
Detection of WT1 overexpression in human leukemias by real-time PCR.
Total mRNA from BM, PBMC, cell lines, and human leukemias were reverse transcribed to cDNA, and WT1 gene expression was determined by semiquantitative real-time RT-PCR. Results are expressed relative to normal PBMC. Mean values of triplicates are depicted. PBMC and BM values represent the means of 3 different samples derived from healthy human donors. K562, human leukemia, chronic myeloid leukemia; U937, human lymphoma; > 95% CD34+, more than 95% pure CD34+ cells; BM, human bone marrow; PBMC, human PBMC; CD15+, more than 90% pure CD15+ cells; AML 1 through AML 9, bone marrow from patients with AML at the time of diagnosis.
Detection of WT1 overexpression in human leukemias by real-time PCR.
Total mRNA from BM, PBMC, cell lines, and human leukemias were reverse transcribed to cDNA, and WT1 gene expression was determined by semiquantitative real-time RT-PCR. Results are expressed relative to normal PBMC. Mean values of triplicates are depicted. PBMC and BM values represent the means of 3 different samples derived from healthy human donors. K562, human leukemia, chronic myeloid leukemia; U937, human lymphoma; > 95% CD34+, more than 95% pure CD34+ cells; BM, human bone marrow; PBMC, human PBMC; CD15+, more than 90% pure CD15+ cells; AML 1 through AML 9, bone marrow from patients with AML at the time of diagnosis.
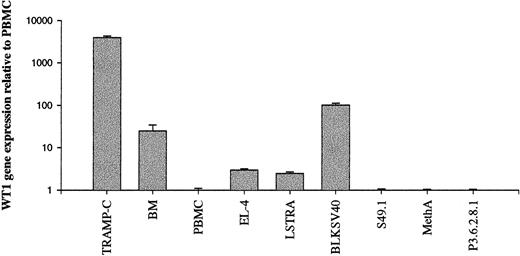
To investigate whether WT1 expression in murine malignancies and normal murine BM is similar to that observed in humans, WT1 gene expression was assessed in normal BM, murine cancers, and leukemia cell lines. Results were expressed relative to WT1 expression by normal murine PBMC. Two murine cell lines, TRAMP-C and BLKSV40, were shown to express high levels of WT1-specific mRNA compared to normal murine PBMC and BM (Figure 2). TRAMP-C is a prostate cancer cell line derived from mice transgenic for the prostate expression of SV40 using a probasin promoter. BLKSV40 is a B6 fibroblast line transformed by SV40. EL-4, a carcinogen-induced thymoma, and LSTRA, a murine leukemia virus-induced leukemia, expressed very low levels of WT1. S49.1, a murine T-cell–like lymphoma, MethA, a murine sarcoma, and P3.6.2.8.1, a murine myeloma cell line, were WT1 negative.
Detection of WT1 overexpression in murine malignancies by real-time PCR.
Total mRNA from murine cancers and leukemia cell lines was reverse transcribed to cDNA, and WT1 gene expression was determined by semiquantitative real-time RT-PCR. Results are expressed relative to normal murine PBMC. Mean values of triplicates are depicted. PBMC and BM values represent the means of 3 different samples derived from normal B6 mice. TRAMP-C, SV40-transformed prostate; BM, murine BM; PBMC, murine PBMC; EL-4, carcinogen-induced thymoma; LSTRA, MuLV-induced leukemia; BLKSV40 HD2, SV40-transformed fibroblast; S49.1, lymphoma, T-cell-like; MethA, sarcoma; P3.6.2.8.1, myeloma.
Detection of WT1 overexpression in murine malignancies by real-time PCR.
Total mRNA from murine cancers and leukemia cell lines was reverse transcribed to cDNA, and WT1 gene expression was determined by semiquantitative real-time RT-PCR. Results are expressed relative to normal murine PBMC. Mean values of triplicates are depicted. PBMC and BM values represent the means of 3 different samples derived from normal B6 mice. TRAMP-C, SV40-transformed prostate; BM, murine BM; PBMC, murine PBMC; EL-4, carcinogen-induced thymoma; LSTRA, MuLV-induced leukemia; BLKSV40 HD2, SV40-transformed fibroblast; S49.1, lymphoma, T-cell-like; MethA, sarcoma; P3.6.2.8.1, myeloma.
Immunizing mice to WT1 peptides can induce proliferative T-cell responses to the immunizing peptide and antibody to WT1 protein
To determine whether immunity to WT1 can be induced by peptide immunization, B6 mice were immunized with groups of WT1 peptides derived from the natural sequence of WT1. The peptides had motifs appropriate for binding to MHC class II molecules and, thus, eliciting helper T-cell responses. Vaccine A consisted of 3 peptides from within the amino terminus portion of the protein (exon I, p6-22 and p117-139; exon V, first alternative splice, p244-262). Vaccine B consisted of 3 peptides from within the carboxy terminus portion of the protein (exon 7, zinc finger I, p287-301, and p299-313; exon 10, zinc finger IV, p421-435). Peptide sequences are shown in “Material and methods.”
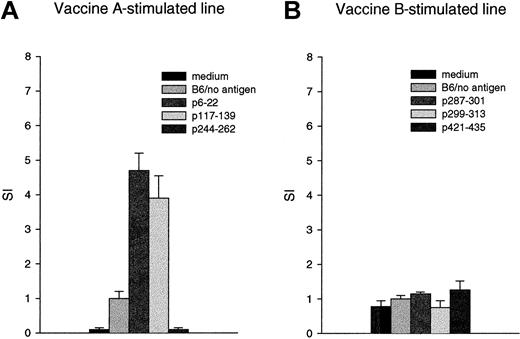
Proliferative responses were evaluated using spleen cells from 4 mice in 2 replicate experiments. After in vivo immunization, the initial 3 in vitro stimulations were carried out using all 3 peptides of vaccine A or B. Vaccine A elicited proliferative T-cell responses to the immunizing peptides p6-22 and p117-139, with stimulation indices varying between 3 and 8 (bulk lines). No proliferative response to p244-262 (Figure 3A) or to vaccine B was detected (Figure 3B).
Immunization of mice with WT1 vaccine A, but not vaccine B, induces proliferative T-cell responses.
Three weeks after the third immunization, spleen cells of mice that had been inoculated with vaccine A or vaccine B were cultured with medium alone (medium) or spleen cells and medium (B6/no antigen), B6 spleen cells pulsed with the peptides p6-22, p117-139, p244-262 (vaccine A) or p287-301, p299-313, p421-435 (vaccine B) at 25 μg/mL, and were assayed after 96 hours for proliferation by (3H) thymidine incorporation. Bars represent the mean stimulation index (SI), which is calculated as the mean of the experimental wells divided by the mean of the control (B6 spleen cells with no antigen) and standard deviations.
Immunization of mice with WT1 vaccine A, but not vaccine B, induces proliferative T-cell responses.
Three weeks after the third immunization, spleen cells of mice that had been inoculated with vaccine A or vaccine B were cultured with medium alone (medium) or spleen cells and medium (B6/no antigen), B6 spleen cells pulsed with the peptides p6-22, p117-139, p244-262 (vaccine A) or p287-301, p299-313, p421-435 (vaccine B) at 25 μg/mL, and were assayed after 96 hours for proliferation by (3H) thymidine incorporation. Bars represent the mean stimulation index (SI), which is calculated as the mean of the experimental wells divided by the mean of the control (B6 spleen cells with no antigen) and standard deviations.
Subsequent in vitro stimulations were carried out as single peptide stimulations using only p6-22 and p117-139 (data not shown). Stimulation of the vaccine A–specific T-cell line with p117-139 resulted in proliferation to p117-139 and no response to p6-22. Clones derived from the line were specific for p117-139. In contrast, stimulation of the vaccine A–specific T-cell line with p6-22 resulted in proliferation to p6-22 and no response to p117-139. Clones derived from the line were specific for p6-22 (data not shown).
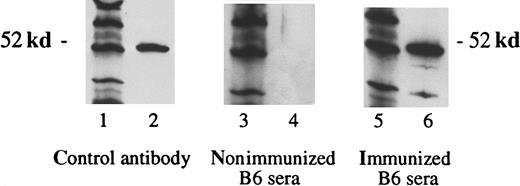
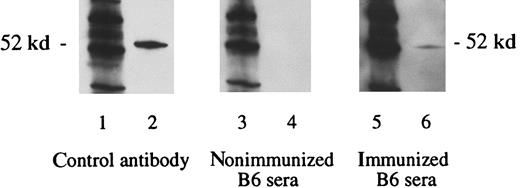
Immunization with vaccine A, under the same conditions that elicited the proliferative T-cell responses above, also elicited antibodies to WT1 (Figure 4). Sera from mice obtained 3 weeks after the third immunization contained antibodies specific for WT1. As a negative control, sera of nonimmunized B6 mice showed no reaction against the WT1. No antibodies were detected after immunization with vaccine B (data not shown), which is consistent with the lack of helper T-cell response from immunization with vaccine B. Subsequent experiments demonstrated that the WT1-specific antibody responses were predominately of the immunoglobulin IgG2a type (data not shown).
Detection of WT1-specific antibodies in B6 mice immunized with WT1 peptides.
Three weeks after the third immunization with vaccine A, sera were obtained. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the WT1 polypeptide WT N180 (180 amino acids in length, mapping within the amino terminus domain of the WT1 protein). The primary antibodies used were WT180 in lane 2, sera of nonimmunized B6 mice in lane 4, and sera of B6 mice immunized with the vaccine A in lane 6.
Detection of WT1-specific antibodies in B6 mice immunized with WT1 peptides.
Three weeks after the third immunization with vaccine A, sera were obtained. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the WT1 polypeptide WT N180 (180 amino acids in length, mapping within the amino terminus domain of the WT1 protein). The primary antibodies used were WT180 in lane 2, sera of nonimmunized B6 mice in lane 4, and sera of B6 mice immunized with the vaccine A in lane 6.
Immunizing mice to WT1 peptides can elicit WT1-specific cytotoxic T-lymphocyte capable of lysing WT1-positive cancer cells
Peptides with motifs appropriate for binding to H-2bwere constructed and tested for their ability to bind. Peptides for the immunization study were chosen according to ability to actually bind to H-2b using the RMA-S binding assay.40 Results shown in Table 1 show that some, but not all, chosen WT1 peptides bind to MHC class I molecules.
Mice were immunized to 9-mer MHC class I binding peptides p136-144, p225-233, p235-243, and the 23-mer peptide p117-139. The natural sequences of peptides p225-233, p235-243, and p117-139 are identical between murine and human WT1, and p136-144 differs in one amino acid. After immunization, spleen cells were stimulated in vitro with each of the 4 peptides and were tested for their ability to lyse targets incubated with WT1 peptides. CTL were generated specifically for p136-144, p235-243, and p117-139, but not for p225-233. CTL data for p235-243 and p117-139 are presented in Figure5. Data for peptides p136-144 and p225-233 are not depicted.
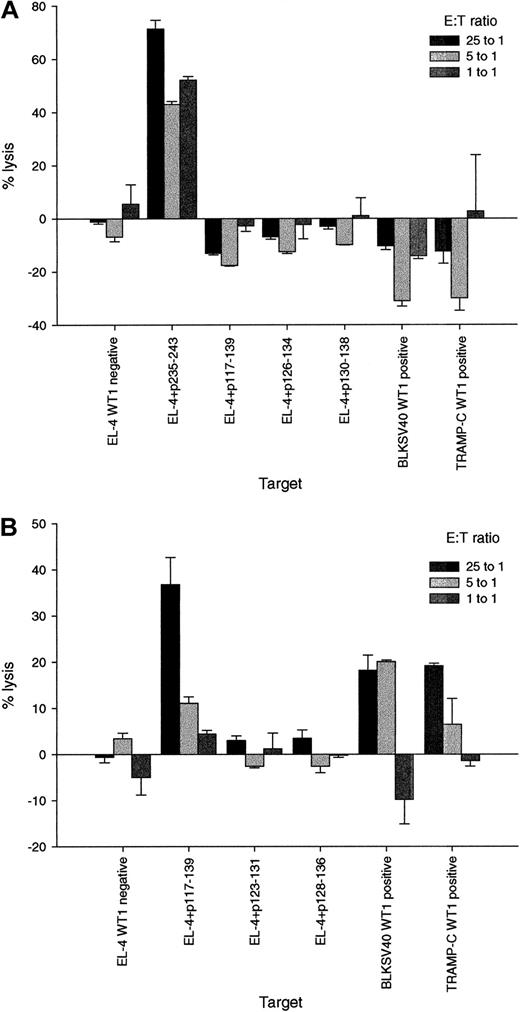
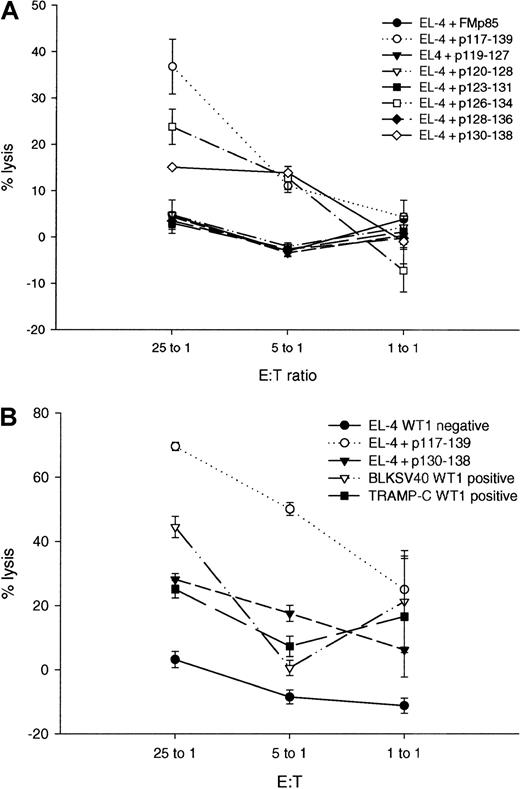
P117-139–specific CTL lyse WT1-positive tumor cells.
Three weeks after the third immunization, spleen cells of mice that had been inoculated with the peptides p235-243 or p117-139 were stimulated in vitro with the relevant peptide and tested for their ability to lyse targets incubated with WT1 peptides and WT1-positive and -negative tumor cells. Bars represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with effector:target (E:T) ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p235-243–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); EL-4 pulsed with the relevant (used for immunization and for restimulation) peptide p235-243 (EL-4 + p235-243); EL-4 pulsed with the irrelevant peptides p117-139 (EL-4 + p117-139), p126-134 (EL-4 + p126-134) or p130-138 (EL-4 + p130-138), and the WT1-positive tumor cells BLKSV40 (BLKSV40, WT1 positive) and TRAMP-C (TRAMP-C, WT1 positive). (B) Cytotoxic activity of the p117-139 specific T-cell line against EL-4; EL-4 pulsed with the relevant peptide P117-139 (EL-4 + p117-139) and EL-4 pulsed with the irrelevant peptides p123-131 (EL-4 + p123-131), or p128-136 (EL-4 + p128-136); BLKSV40 and TRAMP-C.
P117-139–specific CTL lyse WT1-positive tumor cells.
Three weeks after the third immunization, spleen cells of mice that had been inoculated with the peptides p235-243 or p117-139 were stimulated in vitro with the relevant peptide and tested for their ability to lyse targets incubated with WT1 peptides and WT1-positive and -negative tumor cells. Bars represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with effector:target (E:T) ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p235-243–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); EL-4 pulsed with the relevant (used for immunization and for restimulation) peptide p235-243 (EL-4 + p235-243); EL-4 pulsed with the irrelevant peptides p117-139 (EL-4 + p117-139), p126-134 (EL-4 + p126-134) or p130-138 (EL-4 + p130-138), and the WT1-positive tumor cells BLKSV40 (BLKSV40, WT1 positive) and TRAMP-C (TRAMP-C, WT1 positive). (B) Cytotoxic activity of the p117-139 specific T-cell line against EL-4; EL-4 pulsed with the relevant peptide P117-139 (EL-4 + p117-139) and EL-4 pulsed with the irrelevant peptides p123-131 (EL-4 + p123-131), or p128-136 (EL-4 + p128-136); BLKSV40 and TRAMP-C.
The above WT1 peptide-specific CTLs were tested for their ability to lyse WT1 positive versus negative tumor cell lines. CTL specific for p235-243 lysed targets incubated with the p235-243 peptides but failed to lyse cell lines that expressed WT1 proteins (Figure 5A). In marked contrast, CTL specific for p117-139 lysed targets incubated with p117-139 peptides and also lysed malignant cells expressing WT1 (Figure5B). As a negative control, CTL specific for p117-139 did not lyse WT1-negative EL-4. CTL responses were evaluated using spleen cells from 4 mice in 3 experiments. P117-139 peptide- and tumor-specific responses were confirmed in 8 chromium release assays performed at different time points.
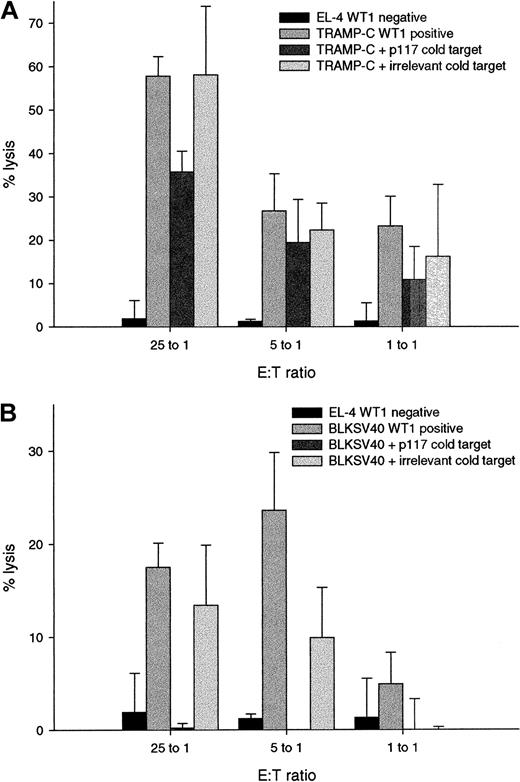
Specificity of WT1 specific lysis was confirmed by cold target inhibition (Figure 6). Lysis of TRAMP-C by p117-139–specific CTL was blocked from 58% to 36% by EL-4 incubated with the relevant peptide p117-139, but not with EL-4 incubated with an irrelevant peptide (Figure 6A). Similarly, lysis of BLKSV40 was blocked from 18% to 0% by EL-4 incubated with the relevant peptide p117-139 (Figure 6B). Results validate that WT1 peptide-specific CTL specifically kill malignant cells by recognizing processed WT1.
Specificity of lysis of WT1-positive tumor cells is demonstrated by cold target inhibition.
Bars represent the mean percentage of specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p117-139 specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1-negative); the WT1-positive tumor cell line TRAMP-C (TRAMP-C, WT1-positive); TRAMP-C cells incubated with a 10-fold excess (compared to the hot target) of EL-4 cells pulsed with the relevant peptide p117-139 (TRAMP-C + p117-139 cold target) without 51Cr labeling and TRAMP-C cells incubated with EL-4 pulsed with an irrelevant peptide without 51Cr labeling (TRAMP-C + irrelevant cold target). (B) Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); the WT1-positive tumor cell line BLKSV40 (BLKSV40, WT1 positive); BLKSV40 cells incubated with the relevant cold target (BLKSV40 + p117-139 cold target), and BLKSV40 cells incubated with the irrelevant cold target (BLKSV40 + irrelevant cold target).
Specificity of lysis of WT1-positive tumor cells is demonstrated by cold target inhibition.
Bars represent the mean percentage of specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p117-139 specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1-negative); the WT1-positive tumor cell line TRAMP-C (TRAMP-C, WT1-positive); TRAMP-C cells incubated with a 10-fold excess (compared to the hot target) of EL-4 cells pulsed with the relevant peptide p117-139 (TRAMP-C + p117-139 cold target) without 51Cr labeling and TRAMP-C cells incubated with EL-4 pulsed with an irrelevant peptide without 51Cr labeling (TRAMP-C + irrelevant cold target). (B) Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); the WT1-positive tumor cell line BLKSV40 (BLKSV40, WT1 positive); BLKSV40 cells incubated with the relevant cold target (BLKSV40 + p117-139 cold target), and BLKSV40 cells incubated with the irrelevant cold target (BLKSV40 + irrelevant cold target).
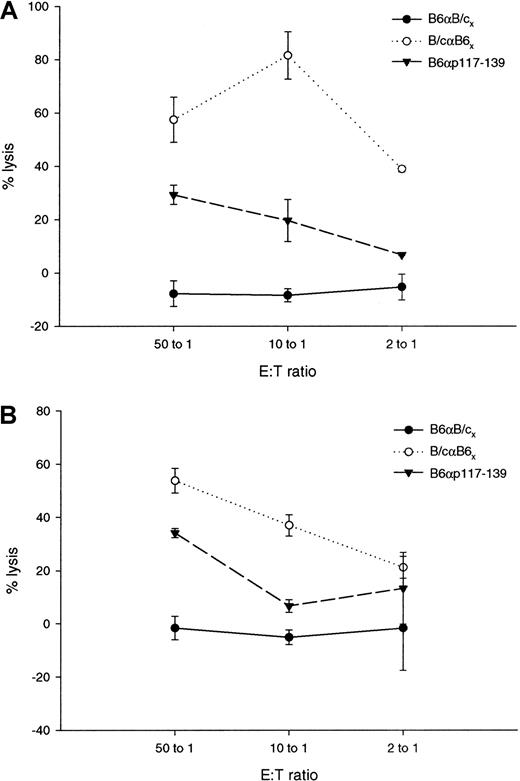
Controls for lysis of TRAMP-C and BLKSV 40 included syngeneic and allogeneic T cells. Data shown in Figure7 demonstrate that BLKSV40 (Figure 7A) and TRAMP-C (Figure 7B) are not lysed by syngeneic B6 T cells stimulated for 5 days in vitro with allogeneic Balb/c cells. As a positive control, allogeneic Balb/c T cells stimulated in vitro for 5 days with B6 cells and CTL specific for p117-139 lysed the WT1-expressing cell lines.
Controls for lysis of BLKSV40 and TRAMP-C.
For syngeneic controls, 3 × 106 B6 effector cells were grown for 5 days with 1 × 106 irradiated Balb/c stimulator cells. For allogeneic controls, 3 × 106Balb/c effector cells were grown for 5 days with 1 × 106irradiated B6 stimulator cells. Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 50:1, 10:1, and 2:1. Cytotoxic activity of syngeneic and allogeneic T cells and the p117-139–specific T-cell line against WT1-positive BLKSV40 cells (A) and TRAMP-C cells (B).
Controls for lysis of BLKSV40 and TRAMP-C.
For syngeneic controls, 3 × 106 B6 effector cells were grown for 5 days with 1 × 106 irradiated Balb/c stimulator cells. For allogeneic controls, 3 × 106Balb/c effector cells were grown for 5 days with 1 × 106irradiated B6 stimulator cells. Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 50:1, 10:1, and 2:1. Cytotoxic activity of syngeneic and allogeneic T cells and the p117-139–specific T-cell line against WT1-positive BLKSV40 cells (A) and TRAMP-C cells (B).
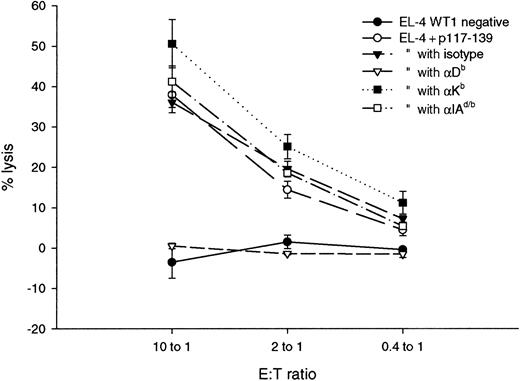
The p117-139 specific line was characterized in more detail by antibody blocking tests and flow cytometry. The lytic activity of the p117-139 line was blocked from 40% to 0% by Db-specific antibodies but not by Kb, I-Ad/b–specific, or isotype control antibodies (Figure 8). This line was determined by flow cytometry to contain more than 90% CD8+ cells (data not shown).
Inhibition of p117 lytic activity by MHC class I–specific antibodies.
Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); EL-4 pulsed with the peptide p117-139 (EL-4 + p117-139); EL-4 + p117-139 incubated with the isotype control antibody (isotype) (IgG2a κ isotype standard; Pharmingen); EL-4 + p117-139 incubated with Db-specific antibody (Db) (anti-H-2Db; Pharmingen); EL-4 + p117-139 incubated with Kb-specific antibody (Kb) (anti-H-2Kb; Pharmingen); EL-4 + p117-139 incubated with the I-Ad/b MHC class II alloantigen antibody (I-Ad/b) (anti I-Adb; Pharmingen).
Inhibition of p117 lytic activity by MHC class I–specific antibodies.
Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 (EL-4, WT1 negative); EL-4 pulsed with the peptide p117-139 (EL-4 + p117-139); EL-4 + p117-139 incubated with the isotype control antibody (isotype) (IgG2a κ isotype standard; Pharmingen); EL-4 + p117-139 incubated with Db-specific antibody (Db) (anti-H-2Db; Pharmingen); EL-4 + p117-139 incubated with Kb-specific antibody (Kb) (anti-H-2Kb; Pharmingen); EL-4 + p117-139 incubated with the I-Ad/b MHC class II alloantigen antibody (I-Ad/b) (anti I-Adb; Pharmingen).
Several segments with putative CTL motifs are contained within p117-139. To determine the precise sequence of the CTL epitope, all potential 9-mer peptides within p117-139 were synthesized (Table 1). Two of these peptides (p126-134 and p130-138) were shown to bind to H-2b class I molecules (Table 1). CTL generated by immunization with p117-139 lysed targets incubated with p126-134 and p130-138, but not the other 9-mer peptides within p117-139 (Figure9A).
Evaluation of the potential 9mer CTL epitope within p117-139.
The p117-139 tumor-specific CTL line was tested against peptides within p117-139 containing or lacking an appropriate H-2b class I binding motif and after restimulation with p126-134 or p130-138. Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 pulsed with the peptides p117-139 (EL-4 + p117-139), p119-127 (EL-4 + p119-127), p120-128 (EL-4 + p120-128), p123-131 (EL-4 + p123-131), p126-134 (EL-4 + p126-134), p128-136 (EL-4 + p128-136), and p130-138 (EL-4 + p130-138). (B) Cytotoxic activity of the CTL line after restimulation with p130-138 against EL-4, EL-4 cells pulsed with p117-139 (EL-4 + p117-139), p130-138 (EL-4 + p130-128), and the WT1-positive tumor cell lines BLKSV40 and TRAMP-C.
Evaluation of the potential 9mer CTL epitope within p117-139.
The p117-139 tumor-specific CTL line was tested against peptides within p117-139 containing or lacking an appropriate H-2b class I binding motif and after restimulation with p126-134 or p130-138. Lines represent the mean percentage specific lysis and standard deviations in chromium release assays performed in triplicate with E:T ratios of 25:1, 5:1, and 1:1. (A) Cytotoxic activity of the p117-139–specific T-cell line against the WT1-negative cell line EL-4 pulsed with the peptides p117-139 (EL-4 + p117-139), p119-127 (EL-4 + p119-127), p120-128 (EL-4 + p120-128), p123-131 (EL-4 + p123-131), p126-134 (EL-4 + p126-134), p128-136 (EL-4 + p128-136), and p130-138 (EL-4 + p130-138). (B) Cytotoxic activity of the CTL line after restimulation with p130-138 against EL-4, EL-4 cells pulsed with p117-139 (EL-4 + p117-139), p130-138 (EL-4 + p130-128), and the WT1-positive tumor cell lines BLKSV40 and TRAMP-C.
The p117-139–specific CTL line was restimulated with either p126-134 or p130-138. After restimulation with p126-134 or p130-138, both T-cell lines demonstrated peptide-specific lysis, but only p130-138 specific CTL showed lysis of a WT1-positive tumor cell line (Figures 9B), thus indicating that p130-138 is likely to be the naturally processed epitope.
Toxicity of WT1 peptide immunization
Mice immunized with WT1 peptides were evaluated for toxicity by observation twice a week for 3 months and by histopathologic analysis 20 days after the last immunization. Tissues examined histologically included adrenal gland, brain, cecum, colon, duodenum, eye, femur and marrow, gallbladder, heart, ileum, jejunum, kidney, larynx, lacrimal gland, liver, lung, lymph node, muscle, esophagus, ovary, pancreas, parathyroid, salivary gland, sternum and marrow, spleen, stomach, thymus, trachea, thyroid, urinary bladder, and uterus. No evidence for autoimmune toxicity was observable in any of the tissues examined. Special emphasis was put on the evaluation of potential hematopoietic toxicity. The myeloid:erythroid ratio in sternum and femur marrow was normal. To investigate the potential occurrence of pancytopenia as a late side effect, peripheral blood samples of 4 WT1 peptide-immunized mice were analyzed 6 months after the initial immunization. All evaluable blood cell counts were within the normal ranges (mean WBC, 8.65 × 103; range, 5.4-10.3 × 103; normal range, 5.4-16.0 × 103; differential WBC counts all within the normal range; mean hematocrit, 0.45; range, 0.44-0.46; normal range, 0.32-0.54; platelets, not evaluable because of clumping).
Tumor prevention experiment
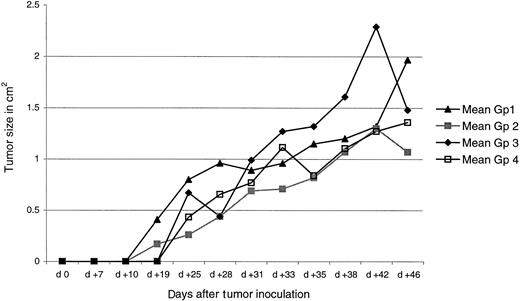
We next investigated whether immunization with either the p117-139 or the p130-138 peptide elicited immunity capable of preventing the growth of TRAMP-C. Mice were left untreated (group 1), given adjuvant only (group 2), 100 μg peptide p117-139 plus Freund's adjuvant (group 3), or 100 μg peptide p130-138 plus Freund's adjuvant (group 4). Mice were boosted twice at 3-week intervals. Each group consisted of 4 animals. Three weeks after the final immunization, mice were inoculated with 106 TRAMP-C cells in 100 μL saline intramuscularly. In this experiment (Figure10), WT1 peptide immunization did not show any effect on tumor growth in vivo.
Immunization with p117-139 or p130-138 peptide does not prevent growth of TRAMP-C.
Mice were left untreated (group 1) or were given adjuvant only (group 2), peptide p117-139 (group 3), or peptide p130-138 (group 4). Three weeks after the final immunization, mice were inoculated with 106 TRAMP-C cells.
Immunization with p117-139 or p130-138 peptide does not prevent growth of TRAMP-C.
Mice were left untreated (group 1) or were given adjuvant only (group 2), peptide p117-139 (group 3), or peptide p130-138 (group 4). Three weeks after the final immunization, mice were inoculated with 106 TRAMP-C cells.
Immunizing mice with syngeneic cancer cells expressing WT1 can induce antibody to WT1
To determine whether WT1, as naturally expressed by cancer cells, is immunogenic, B6 mice were immunized with syngeneic TRAMP-C cells. Mice immunized with TRAMP-C developed antibody specific for WT1, as detected by Western immunoblot analysis, whereas the sera of nonimmunized B6 mice showed no reaction against the WT1-specific control (Figure 11). Helper T-cell responses to WT1 were not evaluated.
Detection of WT1-specific antibodies in B6 mice immunized with the WT1-positive tumor cell line TRAMP-C.
Mice were inoculated twice at 2-week intervals by subcutaneous injection with WT1 overexpressing TRAMP-C cells. Three weeks after the final immunization, sera were obtained. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the WT1 polypeptide WT N180. Primary antibodies used were WT180 in lane 2, sera of nonimmunized B6 mice in lane 4, and sera of B6 mice immunized with TRAMP-C tumor cells in lane 6.
Detection of WT1-specific antibodies in B6 mice immunized with the WT1-positive tumor cell line TRAMP-C.
Mice were inoculated twice at 2-week intervals by subcutaneous injection with WT1 overexpressing TRAMP-C cells. Three weeks after the final immunization, sera were obtained. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the WT1 polypeptide WT N180. Primary antibodies used were WT180 in lane 2, sera of nonimmunized B6 mice in lane 4, and sera of B6 mice immunized with TRAMP-C tumor cells in lane 6.
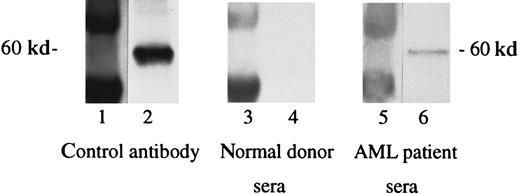
Antibodies to WT1 are present in sera of some patients with AML
As an initial study to begin to evaluate immunity to WT1 in humans, antibody responses to the WT1 protein were evaluated using recombinant full-length and truncated WT1-TRX fusion proteins. Results demonstrated the presence of serum antibodies directed against the N-terminus portion of the WT1 protein in 3 of 18 patients with AML. No WT1-specific antibodies were detected in the 2 normal control sera tested (Figure 12). To further verify that the patient antibody response was directed against WT1 and not against the TRX fusion part of the protein or possible E coli contaminant proteins, controls included the TRX protein alone, purified in the same manner as the WT1 fusion proteins. No reactivity against the TRX fusion or possible E colicontaminant proteins could be detected.
Detection of WT1-specific antibodies in sera of patients with AML.
Sera of patients with AML were used in a 1:500 dilution. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the N-terminus portion of the TRX-WT1 protein. Primary antibodies used were WT180 in lane 2, sera of a healthy donor in lane 4, and sera of a patient with AML in lane 6.
Detection of WT1-specific antibodies in sera of patients with AML.
Sera of patients with AML were used in a 1:500 dilution. Lanes 1, 3, and 5 show the molecular weight marker (MWM); lanes 2, 4, and 6 show the N-terminus portion of the TRX-WT1 protein. Primary antibodies used were WT180 in lane 2, sera of a healthy donor in lane 4, and sera of a patient with AML in lane 6.
Discussion
Immunologic tolerance to self-proteins can limit the effectiveness of oncogenic self-proteins as cancer vaccine targets. The level of immunologic tolerance to self-proteins seems to be related to the level and location of expression. However, the level of immunologic tolerance to any particular self-protein is best determined by empiric experimentation. The current data show that the generation of antibody, helper T-cell, and CTL responses in mice to murine WT1 is possible. To the extent studied, the level and location of expression of human and murine WT1 are similar. Murine results thus predict that immunity to human WT1 might be elicited with the same ease.
The presence in humans of cancer cells that aberrantly express oncogenic proteins results in the elicitation of immunity to the oncogenic proteins in some patients.41 47 The generation of antibody immunity to WT1 by immunization of mice to TRAMP-C predicts that some patients bearing cancers that overexpress WT1 will have immunity to WT1. Initial studies in humans demonstrated the presence of antibody to WT1 in some patients with AML. Further studies must be conducted to correlate antibody responses in patients with AML with levels of WT1 gene and protein expression and other characteristics of AML blasts.
WT1 is an internal protein. Thus, it is assumed that CTL will be the most effective effector mechanism for vaccine and T-cell therapy. Studies showing CTL lysis of TRAMP-C and BLKSV40 provide evidence that WT1 can be presented by MHC class I of tumor cells in high enough concentrations to be recognized by WT1-specific CTL. Antibody and helper T cells might not have adequate access to the target protein to mediate therapy. CTL responses specific for WT1-positive tumor cells were generated by immunization with a 23-mer peptide containing both CTL and helper T-cell epitopes. Two 9-mer peptides within the 23-mer peptide were able to elicit peptide-specific CTL. Peptide-specific CTL need not necessarily lyse cancer cells that express the target protein. CTL lysis demands that the target WT1 peptides be endogenously processed and presented in association with tumor cell MHC class I molecules. Only one 9-mer peptide, p130-138, was able to elicit CTL capable of lysing cancer cells expressing WT1, thus showing that p130-138 is a naturally processed WT1 epitope for B6 mice. This observation does not exclude the possibility of other B6 CTL peptides. P117-139 contained both CTL and helper T-cell epitopes and elicited helper and CTL responses. Presumably, T cells responding to the helper T-cell epitopes provided help for eliciting CTL responses.
Two murine cell lines, TRAMP-C and BLKSV40, were shown to express high levels of WT1-specific mRNA. Both happened to be initially transformed by SV40. The extent to which expression of WT1 correlates with SV40 transformation in other cancers has not been assessed. However, elucidation of the mechanism by which SV40 transformation results in WT1 overexpression might help provide insight into the role of WT1 in transformation and into when during the course of transformation WT1 overexpression occurs.
The lack of observable in vivo antitumor effects against WT1 overexpressing TRAMP-C after WT1 peptide immunization may be caused by the low MHC class I expression of TRAMP-C cells.48Alternatively, the immune responses to WT1 after WT1 peptide immunization might be too weak to elicit significant in vivo tumor immunity. Methods to increase the number of WT1-specific CTL might prove more effective. Although WT1 is an appealing candidate tumor antigen, immunologic tolerance to this self-protein can prevent or dampen the development of immune responses to WT1. Further experiments are required to better understand the therapeutic potential of WT1 vaccines in animal models. A recent report by Oka et al49showed that mice immunized with WT1 peptide rejected challenges by WT1-expressing tumor cells and survived for a long time with no signs of auto-aggression by CTL.
WT1 is expressed primarily during fetal development, but it is present in low amounts in several normal adult tissues, including kidney (glomerular podocytes), ovary (epithelial cells surrounding the oocytes), testis (Sertoli cells and rete testis), and spleen cells.4-6 The WT1 gene is also expressed in normal hematopoietic progenitor cells, and the expression is decreased concurrently with the differentiation of the hematopoietic progenitor cells.50 WT1 protein is overexpressed in leukemic blasts compared to normal CD34+ bone marrow cells.11,29 The amount of WT1 mRNA expression between these CD34+ normal cells and leukemic blasts has been controversial. Our data showing overexpression of WT1 mRNA in K562 cells and in 6 of 8 patients with AML compared to normal CD34+ cells are in concordance with data reported by Menssen et al11 and Inoue et al,51 but they are in contrast to data reported by Maurer et al52 and Fraizer et al.35
Differences in the levels of WT1 expression observed among investigators might result from the small number of patient samples analyzed, patient selection, variation in the quantitation procedures used, and differences in the WT1 gene expression between subsets of CD34+ cells. Studies in a larger number of patients are necessary to determine the true prevalence and clinical outcome of patients with leukemia who are overexpressing WT1 compared with those with normal CD34+ cells.
Semiquantitative real-time PCR analyses demonstrated WT1 mRNA overexpression in the analyzed murine tumors compared to normal murine PBMC and BM cells. The experiment indicates similar overexpression of WT1 in human and mouse malignancies compared to normal PBMC and bone marrow cells. However, the comparison of human and murine PCR data are limited by the fact that different PCR primers were used.
Vaccination with WT1 peptides elicited CTL capable of lysing WT1-positive cancer cells, but vaccination did not induce observable toxicity. Lysis of WT1-positive cells showed that the level of expression of WT1 peptide/MHC complexes on cancer cells can be high enough to be targeted by CTL. The lack of toxicity gives a hint that the normal level of expression of WT1 by the few normal tissues that express WT1 in the adult may be too low to be recognized by WT1-specific CTL. However, formal toxicity studies were not performed. The possibility exists that T-cell therapy regimens with WT1 CTL, in the order of magnitude that might be contemplated for WT1-specific CTL therapy of leukemia, would induce autoimmune toxicity. Augmentation of an immune response to WT1, to the extent necessary to eradicate malignant cells, might also partially or totally destroy the normal tissues that express small amounts of the same protein. Empiric toxicity studies must be performed in mice to assess possible toxicities before contemplating WT1-specific CTL therapy in humans.
Recently published studies by others demonstrated the generation of leukemia-specific HLA-A2453 and HLA-A254restricted CTL after in vitro priming to WT1 peptides. The leukemia-specific CTL did not inhibit colony formation by normal bone marrow cells of HLA-A24–positive patients. The murine data presented here are in accordance with these findings. The demonstration that immunity to WT1 can be elicited in mice and humans by peptide immunization supports further evaluation of WT1 as a vaccine and T-cell therapy target for eventual testing in human leukemia therapy.
Acknowledgments
We thank Ray Houghton for his help in establishing the real-time RT-PCR, Darrick Carter for protein purification, and Thomas Deuel and Zhaoyi Wang for providing a WT1 full-length plasmid.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin A. (Mac) Cheever, Corixa Corporation, Suite 200, 1124 Columbia St, Seattle, WA 98104; e-mail: cheever@corixa.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal