Abstract
Langerhans cells (LCs) are specialized dendritic cells (DCs) strategically located in stratified epithelia, such as those of the skin, oral cavity, pharynx, esophagus, upper airways, urethra, and female reproductive tract, which are exposed to a wide variety of microbial pathogens. LCs play an essential role in the induction of T-lymphocyte responses against viruses, bacteria, and parasites that gain access to those epithelial surfaces, due to their high antigen capture and processing potential and their capacity to present antigen peptides to T cells on migration to the lymph nodes.1Although LCs have been classically considered of myeloid origin, recent reports, which demonstrate the existence of lymphoid DCs derived from multipotent lymphoid precursors devoid of myeloid differentiation potential,2–5 raise the question of the lymphoid or myeloid origin of LCs. The present study shows that mouse lymphoid-committed CD4low precursors, with the capacity to generate T cells, B cells, CD8+ lymphoid DCs, and natural killer cells,26 also generate epidermal LCs on intravenous transfer, supporting the view that LCs belong to the lymphoid lineage.
Introduction
T-cell immunity against tumors and bacterial or viral infections relies essentially on the recognition of an antigen peptide processed and presented to the T cell by an antigen-presenting cell. During in vivo immune responses this role is primarily played by dendritic cells (DCs), which are endowed with an extraordinary potential to undertake their antigen-presenting cell function, based on their remarkable migratory capacity, optimal efficiency to capture and process antigens, and high level of expression of major histocompatibility complex (MHC), costimulatory, and adhesion molecules.1 Despite the overwhelming advances made during recent years on the understanding of DC function, driven specially by the possibility of using DCs in antitumor immunotherapy protocols,7 the origin of the different DC subsets remains controversial. Both in the human and in the murine system 2 main categories of DCs have been defined, myeloid and lymphoid DCs, which have been proposed to be identifiable in mice by their differential CD8α expression, this molecule being only present on lymphoid DCs,8 as discussed below. With regard to Langerhans cells (LCs), although classically considered together with DCs, as myeloid derived, no experimental evidence demonstrates this hypothesis. Interestingly, we have recently shown that mouse epidermal LCs, expressing neither CD8 nor leukocyte function-associated antigen 1 (LFA-1), underwent a maturation-induced CD8/LFA-1 up-regulation on migration to the lymph nodes (LNs),9 suggesting that LCs belong to the CD8+ lymphoid DC category. These results prompted us to investigate whether murine LCs derive from lymphoid-committed precursors with DC reconstitution potential. These multipotent lymphoid precursors, described originally in the adult mouse thymus by Shortman and colleagues,10 who termed them CD4low precursors, have been found to generate lymphoid DCs expressing CD8, along with T cells, B cells, and natural killer (NK) cells.2,6 Because after transfer of CD4lowprecursors no CD8− DCs were found, CD8− DCs have been considered of myeloid origin.11 Our results demonstrate that CD4low precursors generate LCs when injected intravenously (IV), supporting the concept that LCs belong to the lymphoid lineage.
Materials and methods
Mice
In reconstitution experiments, donors were 5- to 6-week-old C57 BL/Ka Ly 5.2 mice and recipients were 8-week-old C57 BL/6 Ly 5.1 Pep3b mice, which were also used in the experiments performed to define the UV irradiation needed for inducing LC migration.
Isolation of CD4low and CD44+CD25+ precursor populations
The CD4low precursors were isolated from C57 BL/Ka Ly 5.2 donor thymuses, by depleting pre-T cells, double-positive and single-positive thymocytes, B cells, DCs, macrophages, and granulocytes by complement-mediated cytotoxicity using anti-CD3 (clone Y-CD3) and anti-CD8 (clone 31M) and then immunomagnetic bead depletion after incubation with anti-CD3 (clone KT3), anti-CD8 (clone 53.6.7-2), anti-CD25 (clone PC61.5), anti-B220 (clone RA3-6B2), anti-MHC class II (MHC II) (clone FD11), antimacrophage antigen F4/80 (clone C1.A3.1), and antigranulocyte antigen Gr-1 (clone RB6-8C5). CD4lowprecursors were then sorted as Thy-1low CD44+cells after double immunofluorescent staining fluorescein isothiocyanate (FITC)-conjugated anti-Thy-1 (clone AT15) and phycoerythrin (PE)-conjugated anti-CD44 (clone IM7; Pharmingen, San Diego, CA). Flow cytometric cell sorting was carried out on a FACSort instrument (Becton Dickinson, Mountain View, CA). The sorted preparation had a purity greater than 98% and contained less than 1% CD11c+ cells as assessed after staining with biotinylated anti-CD11c (clone N418, data not shown) followed by streptavidin-tricolor (Caltag, San Francisco, CA).
The CD44+ CD25+ precursors were isolated by complement-mediated cytotoxicity using anti-CD3 (clone Y-CD3), anti-CD4 (clone 172.4), and anti-CD8 (clone 31M) and then sorting after double immunofluorescent staining with PE-conjugated anti-CD44 and biotinylated anti-CD25 followed by streptavidin-tricolor. The sorted CD44+ CD25+ population had a purity greater than 97% and did not contain any detectable CD4lowprecursor.
Reconstitution experiments with bone marrow (BM) cells
The BM cells (2 × 106) from C57 BL/Ka Ly 5.2 donor mice were injected IV into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice. In control experiments, recipient mice were injected with 2 × 106 Ly 5.1 BM cells; 24 hours after transfer of BM cells mice were exposed to short wavelength UV-C irradiation for 30 minutes (total exposure, 80 mJ/cm2) using a G30T8-type UV lamp at 50 cm from the target.
Reconstitution experiments with CD4low precursors or CD44+ CD25+ precursors
Thymic CD4low precursors (3 × 104) or 3 × 104 thymic CD44+ CD25+precursors from C57 BL/Ka Ly 5.2 donor mice were injected IV into γ-irradiated (7 Gy) C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. In control experiments recipient mice were injected with 4 × 104 Ly 5.1 BM cells; 24 hours after transfer of BM cells mice were exposed to short wavelength UV-C irradiation for 30 minutes.
Preparation of epidermal LCs and dermal DCs (DDCs)
Ears were split into dorsal and ventral halves and incubated with 0.5% trypsin (Sigma, St Louis, MO) for 45 minutes at 37°C, to allow the separation of the epidermis from the dermis. The epidermal or dermal sheets were incubated at 37°C for 12 hours in RPMI 1640 medium with 100 ng/mL murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN). After this incubation period, LCs or DDCs were released to the culture medium.
Flow cytometry
Analysis of LCs and DDCs after reconstitution with BM cells or CD4low precursors was performed after double staining with FITC-conjugated anti-Ly 5.2 (clone ALI-4A2; Pharmingen) and PE-conjugated anti-MHC II (clone M5/114; Pharmingen). Dead cells were gated out by propidium iodide staining. For the LC phenotypic analysis shown in Figure 1A, cells were triple stained with FITC-conjugated anti-Ly 5.2, PE-conjugated anti-MHC II and biotin-conjugated anti-CD11c (clone N418), anti-DEC-205 (clone NLDC-145), or anti-B220 (clone RA3-6B2), followed by streptavidin-tricolor (Caltag). Blocking of Fc receptor (FcR) on LCs and DDCs was achieved by incubation with purified mouse immunoglobulins before incubation with monoclonal antibodies (mAbs). Analysis of the reconstitution potential of CD4low precursors shown in Figure 4 was performed as follows. Thymocytes were triple stained with FITC-conjugated anti-CD8 (clone 53-6.72), PE-conjugated anti-CD4 (clone CT-CD4; Caltag), and biotin-conjugated anti-Ly 5.2 (clone ALI-4A2) followed by streptavidin-tricolor; thymus low-density cells were triple stained with FITC-conjugated anti-CD11c (clone N418), PE-conjugated anti-CD8 (clone 53-6.72; Pharmingen), and biotin-conjugated anti-Ly 5.2 followed by streptavidin-tricolor; LN cells were triple stained with FITC-conjugated anti-B220 (clone RA3-6B2; Caltag), PE-conjugated anti-CD4, and anti-CD8 and biotin-conjugated anti-Ly 5.2 followed by streptavidin-tricolor; BM cells were double stained with FITC-conjugated anti-Ly 5.2 and biotin-conjugated anti-Gr-1 (clone RB6-8C5) and anti-Mac-1 (clone M1/70) followed by streptavidin PE (Caltag). Analysis was performed on a FACSort flow cytometer (Becton Dickinson). Donor-derived Ly 5.2+ thymic DCs were analyzed as CD11c+ CD8+ cells on thymus low-density cell fractions obtained after enzymatic digestion with collagenase A and Dnase I (Boehringer-Mannheim, Mannheim, Germany) by centrifugation in Optiprep solution (Nyegaard Diagnostics, Oslo, Norway), density 1.061 g/cm3, as previously described.9
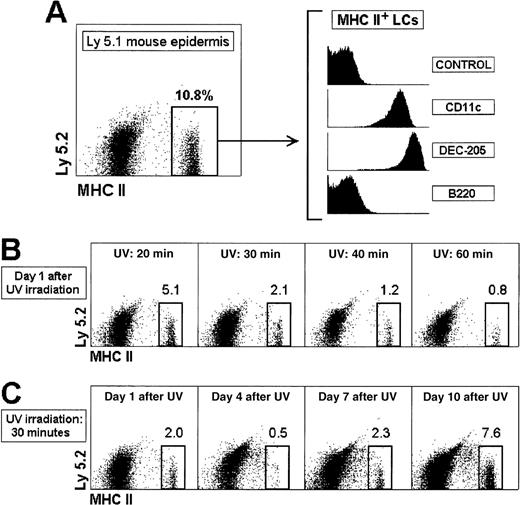
Migration and reconstitution of the epidermal LC population after UV-C irradiation.
(A) Characterization and phenotype of LCs in ear epidermal cell suspensions of control recipient Ly 5.1 mice. (B) Induction of LC egress from the epidermis by UV-C irradiation. Ly 5.1 mice were exposed to short wavelength UV-C irradiation for the indicated times, and the epidermal LC population was analyzed after 24 hours. (C) Recovery of the epidermal LC population after UV-C irradiation. Ly 5.1 mice were exposed to UV-C irradiation for 30 minutes and analyzed at the indicated times. The percentage of MHC II+ LCs is indicated. These results are representative of 4 experiments with similar results.
Migration and reconstitution of the epidermal LC population after UV-C irradiation.
(A) Characterization and phenotype of LCs in ear epidermal cell suspensions of control recipient Ly 5.1 mice. (B) Induction of LC egress from the epidermis by UV-C irradiation. Ly 5.1 mice were exposed to short wavelength UV-C irradiation for the indicated times, and the epidermal LC population was analyzed after 24 hours. (C) Recovery of the epidermal LC population after UV-C irradiation. Ly 5.1 mice were exposed to UV-C irradiation for 30 minutes and analyzed at the indicated times. The percentage of MHC II+ LCs is indicated. These results are representative of 4 experiments with similar results.
Results
The experimental system used in this report is based on the IV transfer of CD4low precursors isolated from Ly 5.2 mice into γ-irradiated Ly 5.1 recipient mice, which were subsequently analyzed for donor-derived Ly 5.2+ LCs. LCs were defined in epidermal cell suspensions as MHC class II-positive cells, expressing the DC markers CD11c and DEC-205, and negative for the B-cell marker B220 (Figure 1A,B). We first studied the LC reconstitution kinetics after IV transfer of BM cells. No donor-derived LCs were detected from 1 to 5 weeks after transfer, but interestingly, host-derived LCs were found from the shortest time point analyzed (Figure2A). These data indicate that host-derived LCs remained in the epidermis after γ-irradiation and could therefore impede the colonization of the epidermis by LCs derived from donor BM precursors. To allow the differentiation of donor-derived LCs and their migration to the epidermis, we induced the egress of host LCs from the epidermis by exposing the skin to UV-C irradiation, which has been demonstrated to be the most effective UV treatment in promoting epidermal LC depletion.12 Experiments with control non–γ-irradiated mice were performed to define the UV-C irradiation needed for inducing LC migration without skin damage (Figure 1C), and to monitor the recovery of the LC population after UV-C irradiation (Figure 1D). Twenty-four hours after UV-C irradiation for 30 minutes, the percentage (and absolute number) of LCs in the ear epidermis decreased from 11.8% ± 1.4% to 2.1% ± 0.1% (from 43 000 ± 1200 to 7500 ± 500 LCs per ear), with reduced skin damage. On the other hand, the LC population of the epidermis UV-C irradiated for 30 minutes was almost lost by day 4, but recovered by day 10.
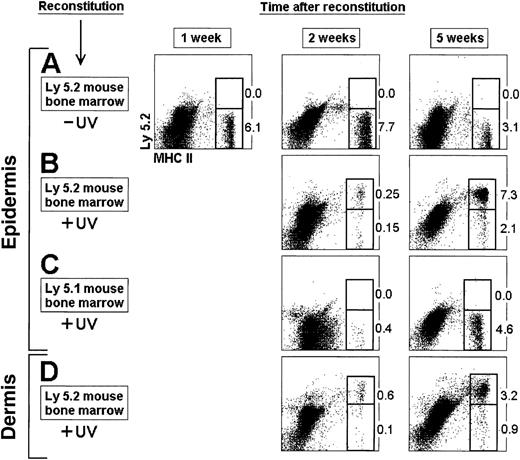
Reconstitution of LCs and DDCs from BM cells.
BM cells (2 × 106) from Ly 5.2 (A, B, D) or Ly 5.1 (C) mice were injected IV into 7 Gy γ-irradiated Ly 5.1 recipient mice. Epidermal LCs and DDCs were isolated at the indicated times from the ear dorsal and ventral halves after separation of the epidermis from the dermis by trypsin treatment. The epidermal or dermal sheets were then cultured for 12 hours with GM-CSF, and LCs and DDCs were collected and analyzed for donor-derived Ly 5.2+ cells. Mice in panels B, C, and D were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of BM cells. The percentage of Ly 5.2+and Ly 5.2− MHC II+ LCs is indicated. These results are representative of 4 experiments with similar results.
Reconstitution of LCs and DDCs from BM cells.
BM cells (2 × 106) from Ly 5.2 (A, B, D) or Ly 5.1 (C) mice were injected IV into 7 Gy γ-irradiated Ly 5.1 recipient mice. Epidermal LCs and DDCs were isolated at the indicated times from the ear dorsal and ventral halves after separation of the epidermis from the dermis by trypsin treatment. The epidermal or dermal sheets were then cultured for 12 hours with GM-CSF, and LCs and DDCs were collected and analyzed for donor-derived Ly 5.2+ cells. Mice in panels B, C, and D were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of BM cells. The percentage of Ly 5.2+and Ly 5.2− MHC II+ LCs is indicated. These results are representative of 4 experiments with similar results.
The γ-irradiated mice were therefore reconstituted with BM precursors and subjected to UV-C irradiation for 30 minutes 24 hours after reconstitution. Donor-derived LCs were detected in the epidermis of γ-irradiated mice, whereas few host-derived LCs remained. Five weeks after reconstitution the percentage of donor LCs was similar to that found in control mice, indicating that the LC population of the ear epidermis was reconstituted (Figure 2B). No Ly 5.2+ LCs were obtained after transfer of Ly 5.1 BM cells (Figure 2C). The reconstitution of DDCs displayed equivalent kinetics (Figure 2D), indicating that differentiation of epidermal LCs and DDCs proceeded simultaneously.
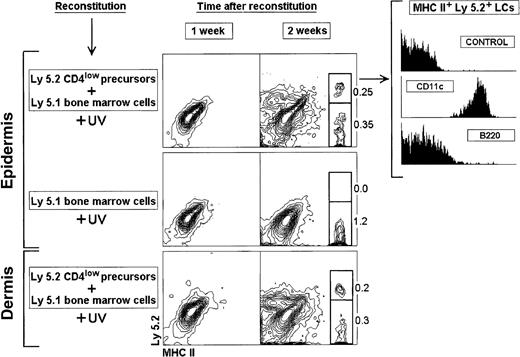
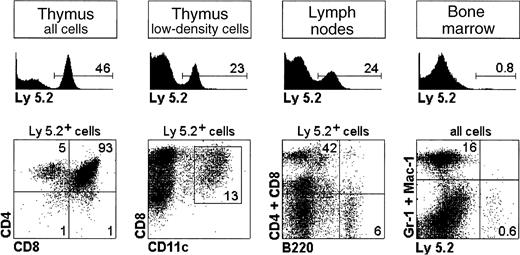
On the basis of these results, we analyzed the reconstitution of LCs after IV transfer of CD4low precursors and UV-C irradiation for 30 minutes 24 hours after transfer (Figure3). After 1 week neither LCs nor DDCs were found. Interestingly after 2 weeks, Ly 5.2+ LCs derived from CD4low precursors were detected together with Ly5.2− LCs, derived from Ly 5.1 BM cells. Ly 5.2+ LCs expressed CD11c and were negative for B220, that is, the same phenotypic profile as LCs from control nonreconstituted mice. As expected only Ly 5.2− LCs were obtained in control mice reconstituted with Ly 5.1 BM cells. Interestingly CD4low precursors also generated Ly 5.2+ DDCs, which displayed a phenotype equivalent to that of Ly 5.2+LCs (data not shown), suggesting that LCs and DDCs are closely related DC subtypes. In agreement with previously published data,2 10 CD4low precursors produced T cells, B cells, and CD8+ lymphoid DCs but not myeloid cells, because neither Gr-1+ nor Mac-1+ nor F4/80+ donor-derived cells were detected (Figure4; data not shown for F4/80).
Reconstitution of LCs and DDCs from CD4lowprecursors.
Thymic CD4low precursors (3 × 104) were injected IV into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Mice were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of CD4low precursors. Control mice were injected with 4 × 104 Ly 5.1 BM cells. At the indicated times mice were analyzed for donor-derived Ly 5.2 LCs or DDCs. The phenotype of Ly 5.2+ LCs reconstituted from CD4low precursors 2 weeks after transfer is shown. The percentage of Ly 5.2+and Ly 5.2− MHC II+ LCs and DDCs is indicated. These results are representative of 3 experiments with similar results.
Reconstitution of LCs and DDCs from CD4lowprecursors.
Thymic CD4low precursors (3 × 104) were injected IV into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Mice were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of CD4low precursors. Control mice were injected with 4 × 104 Ly 5.1 BM cells. At the indicated times mice were analyzed for donor-derived Ly 5.2 LCs or DDCs. The phenotype of Ly 5.2+ LCs reconstituted from CD4low precursors 2 weeks after transfer is shown. The percentage of Ly 5.2+and Ly 5.2− MHC II+ LCs and DDCs is indicated. These results are representative of 3 experiments with similar results.
Reconstitution potential of CD4lowprecursors.
CD4low precursors from Ly 5.2 mice were injected IV into Ly 5.1 recipient mice and the thymus, LNs, and BM were analyzed after 3 weeks for donor-derived Ly 5.2+ T cells, B cells, thymic lymphoid CD8+ DCs, and myeloid cells. The percentage of Ly 5.2+ donor cells and of the different cell subsets analyzed is indicated. Results are representative of 3 experiments with similar results.
Reconstitution potential of CD4lowprecursors.
CD4low precursors from Ly 5.2 mice were injected IV into Ly 5.1 recipient mice and the thymus, LNs, and BM were analyzed after 3 weeks for donor-derived Ly 5.2+ T cells, B cells, thymic lymphoid CD8+ DCs, and myeloid cells. The percentage of Ly 5.2+ donor cells and of the different cell subsets analyzed is indicated. Results are representative of 3 experiments with similar results.
To further reinforce our data with CD4low precursors, we tested the ability of the next downstream precursor population, namely the CD44+ CD25+ pro-T-cell precursors, which have lost the capacity to form B cells but still form DCs,11 to generate LCs on IV transfer. As shown in Figure5, both Ly5.2+ LCs and DDCs were generated from the CD44+ CD25+ precursors together with Ly5.2− LCs and DDCs derived from Ly5.1 BM cells, although CD44+ CD25+ precursors displayed a reduced capacity to form LC and DDC progeny compared with CD4low precursors.
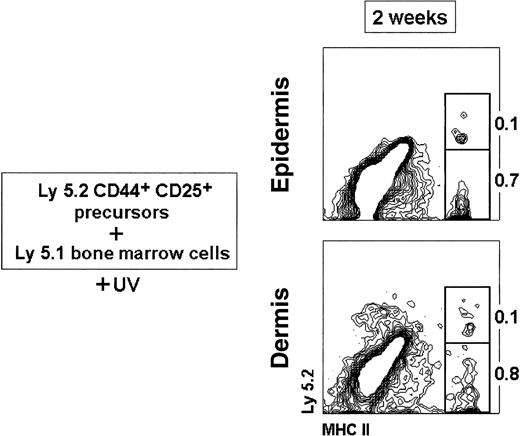
Reconstitution of LCs and DDCs from CD44+CD25+ precursors.
Thymic CD44+ CD25+ precursors (3 × 104) were injected IV into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Mice were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of CD44+ CD25+ precursors. The percentage of Ly 5.2+ and Ly 5.2− MHC II+ LCs and DDCs is indicated. Data are representative of 2 independent experiments with similar results.
Reconstitution of LCs and DDCs from CD44+CD25+ precursors.
Thymic CD44+ CD25+ precursors (3 × 104) were injected IV into 7 Gy γ-irradiated 8-week-old C57 BL/6 Ly 5.1 Pep3b recipient mice, along with 4 × 104 Ly 5.1 BM cells to ensure survival of recipients. Mice were exposed to UV-C irradiation for 30 minutes, 24 hours after transfer of CD44+ CD25+ precursors. The percentage of Ly 5.2+ and Ly 5.2− MHC II+ LCs and DDCs is indicated. Data are representative of 2 independent experiments with similar results.
Discussion
Our data demonstrate that mouse lymphoid-committed CD4low precursors, as well as CD44+CD25+ pro-T-cell precursors, generate epidermal LCs on IV transfer, suggesting that LCs are of lymphoid origin. This result contrasts with the established idea of LCs as prototypic myeloid-derived DCs, a concept that nevertheless has not been formally demonstrated.
In this sense, the results derived from the analysis of mice homozygous for an Ikaros dominant-negative mutation (Ikaros DN−/− mice), which have a normal LC and myeloid development but lack all cells of lymphoid origin including T and B cells, NK cells, and DCs,13,14 argues against the lymphoid derivation of LCs. However the analysis of chimeric mice constructed with wild-type and Ikaros DN−/− BM14 revealed a defect in Ikaros DN−/− LC differentiation. In these experiments, Ikaros DN−/− BM precursors failed to generate DCs in the thymus, spleen, and LNs, despite the fact that DCs were formed in all these lymphoid organs by the host-type BM cells, demonstrating that all the environmental factors required for DC development were present in the chimeric mice. Because it has been demonstrated that a significant proportion of peripheral LN DCs derive from epidermal LCs, the lack of Ikaros DN−/−-derived LN DCs in chimeric mice points to an Ikaros-related defect in LC differentiation or maturation. Therefore, because the Ikarosgene encodes a family of early hematopoietic- and lymphoid-restricted transcription factors essential for the development of lymphoid but not for myeloid lineages, these results rather support a correlation between LCs and the lymphoid cell lineage. Alternatively, because the potential of the CD4low precursor population to generate distinct lymphoid lineages, including DCs, has not been tested at the clonal level, it remains possible that both LCs and CD8+DCs derive from an independent nonlymphoid precursor that cannot be phenotypically distinguished from the CD4low precursor and that could therefore be transferred simultaneously within the CD4low population in reconstitution experiments.
Interestingly mice deficient in transforming growth factor (TGF)-β1 lack epidermal LCs as well as DCs expressing gp 4015 (the murine homologue of human epithelial cell adhesion molecule [Ep-CAM]) that is selectively expressed in the skin draining LNs by DEC-205+ DCs.16 These data suggest that LN DEC-205+ DCs derive from epidermal LCs. Because DEC-205+ DCs have been claimed to belong to the lymphoid DC category,9 17 this result supports the concept that LC might belong to the lymphoid DC subset.
If LCs were lymphoid-derived cells, because epidermal LCs do not express CD8,9 this molecule could not be considered as a conclusive marker of the murine lymphoid DC lineage, but would rather reflect a defined DC physiologic/microenvironmental situation. In this regard, epidermal LCs have been demonstrated to acquire this marker together with LFA-1 on migration to the LNs.9 Moreover, Shortman and colleagues18 reported that DCs differentiated in vitro from CD4low precursors do not express CD8. Finally, in a recent report, Rodewald and associates19 have shown that thymic DCs are CD8−/low in RAG-2−/− mice, in which T-cell differentiation is blocked at the pro-T-cell stage, suggesting that CD8 expression by thymic DCs is dependent on a normal T-cell differentiation microenvironment. Therefore, studies on the origin of DCs based on phenotypic evidence could lead to confusing conclusions. In this sense it has been suggested that human LCs are of myeloid origin on the basis of experiments in which monocytes are driven to acquire a LC-like phenotype after 5 to 7 days of culture in the presence of GM-CSF, interleukin-4, and TGF-β1, in a process not involving cell proliferation.20 However, these data could just reveal the ability of monocytes as immature cells to modulate the expression of certain cell surface molecules, because different cytokine combinations have been shown to induce also the differentiation of monocytes into macrophage-like or interstitial DC-like cells.8 Therefore, the derivation of human LCs located in vivo in epithelial microenvironments from monocytes or myeloid precursors remains to be proven.
In conclusion, our results support the view that epidermal LCs develop together with T cells, B cells, and CD8+ DCs from a lymphoid-committed precursor, and therefore that LCs belong to the lymphoid lineage. However, clonal assays are needed to demonstrate the lymphoid origin of LCs and CD8+ DCs. This finding has important implications with regard to the origin of the different DC subsets and to the etiology of skin diseases such as psoriasis or allergic contact dermatitis in which LCs are involved,21,22 and viral infections such as human immunodeficiency virus or measles infections, known to be mediated by LCs.23 24
Acknowledgments
We thank Dr Ralph M. Steinman (The Rockefeller University, New York, NY) and Dr Robson MacDonald (Ludwig Institute for Cancer Research, Lusanne, Switzerland) for critical reading of the manuscript.
Supported by a grant from the DGICYT (PB95-0376) and a grant from the CAM (08.1/0018/1998) to Carlos Ardavı́n.
F.A., G.M.dH., and P.M. contributed equally to this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlos Ardavı́n, Department of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain; e-mail: ardavin@bio.ucm.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal