When managing an older patient with untreated acute myeloid leukemia (AML), my primary goal is to determine whether standard treatment, investigational treatment, or palliative care is most appropriate. “Standard therapy” includes remission induction regimens, commonly referred to as “3 + 7,” containing 3 days of anthracycline and 7 days of cytarabine (ara-C). Two to six cycles of the same drugs at equivalent or reduced doses are given should complete remission (CR) ensue. Although the regimens vary, for example, in choice of anthracycline or dose of ara-C, I believe the similarities outweigh the differences. In particular, with all these regimens, the outcome in adults worsens continuously as age increases.1-3 Although any age criterion for “elderly AML” is thus arbitrary, patients are, for practical purposes, generally considered “older” if above age 55,4,5 or 606; I will adhere to the 55-year-old criterion here. In such patients, the median time from treatment with 3 + 7 regimens to death is 5 to 10 months.6,7 While CR rates are about 50%, the remissions are usually transient (rarely more than 12 months). The probability of remaining in remission 3 years after beginning treatment, beyond which time patients may operationally be considered “potentially cured,”8 is less than 10%. To a significant extent, these results reflect the association between older age and factors (“covariates”) discussed below that themselves are associated with resistant AML or with early death following initiation of treatment. Nonetheless, multivariate analyses generally demonstrate that older age is prognostically adverse, even after accounting for these covariates.7 9
Although the above data often make me hesitant about recommending standard therapy for an older patient, numerous studies have demonstrated that the results in some older patients may depart significantly from the average results just depicted.4,7,10,11 Thus, I believe it crucial to ascertain whether a particular patient, despite being elderly, may have a reasonable prospect of benefit from standard therapy in light of coexisting covariates that might mitigate the age effect. Chief among these are relatively young age (eg, 55-65), good performance status, normal organ function, de novo presentation, “intermediate”—or “favorable”—cytogenetics and, most recently, lack of multidrug resistance gene (MDR) expression.4 Data from Leith et al show the effect of these covariates in refining prognoses.4 Whereas the CR rate following treatment with a typical 3 + 7 regimen was 45% in all of Leith's 146 patients over age 55, it was 81% in the 27 of these 146 with de novo disease, intermediate or favorable cytogenetics, and “no” MDR expression. Similarly, the CR rate of 72% in the 64 of 886 M. D. Anderson patients over the past 20 years who were age 55 to 65, had a performance status below Zubrod 3, normal serum bilirubin and creatinine levels, de novo AML, and a normal karyotype—or, very rarely, an inv(16) or t(8;21)—contrasts with the CR rate of 48% in the remaining 822 patients over age 55. The better group had a median survival time of 1 1/2 years, and approximately 15% were alive in first CR at 3 years and, thus, “potentially cured.”8While these outcomes were not materially superior in the 1990s over the 1980s, they were statistically superior (P < .01) to those seen in the remaining patients. Obviously, then, older patients with relatively favorable prognoses can be identified. For purposes of patient management, however, I inquire whether the “relatively favorable” prognoses described immediately above are sufficiently favorable to justify use of standard treatment rather than the alternative of investigational treatment. Leaving until later the important issue of what investigational therapy might entail, I have observed that some prognostically favorable older patients, when informed of the expected outcome with standard therapy, the uncertain results with investigational therapy (which might be worse than standard therapy), and the fact that most previous investigational approaches have been unsuccessful, prefer to receive standard therapy. Other prognostically favorable older patients, when similarly informed, are simply unprepared to accept a 50% chance of death within 1 1/2 years and opt for investigational therapy. Thus, I believe that both the standard and investigational options are defensible in the ambulatory patient age 55 to 65 with de novo AML, normal organ function, a normal karyotype and, when the test is available, no MDR expression.4 In contrast, I would not recommend the “palliative care” option in this setting. I find persuasive a European Organization for Research and Treatment of Cancer (EORTC) randomized trial indictating that patients over age 65 with good performance status and relatively preserved organ function not only lived longer if given standard treatment rather than supportive care but, perhaps more importantly, given the relatively modest 2-month median difference in survival, also required fewer hospitalizations.12 Although the trial may be criticized because patients in the 2 arms might not have had similarly favorable baseline prognoses, I believe it unlikely that such potential confounding is the sole explanation for the superior results observed with standard therapy.
I give more attention to the investigational treatment or palliative care options as the number of adverse prognostic features increases. Support for this approach comes from Leith et al.4Considering secondary disease, unfavorable cytogenetics, and MDR expression as adverse features, the CR rate was 4 in 9 (44%) in patients with 1 such feature, 9 in 37 (24%) in patients with 2 features, and 2 in 17 (12%) if all 3 adverse features were present. Of note, the 54 patients with 2 or 3 adverse prognostic factors represented double the number with no adverse factors, in whom the CR rate, as noted above, was 81%. Similarly, the 64 previously noted M. D. Anderson patients with de novo disease and favorable values for age (55-65), performance status, organ function, and cytogenetics constituted only 7% of our older patients. A total of 194 patients had unfavorable values for one of these covariates. Their median survival was 10 months, with 9% alive in CR at 3 years. While results in the various groups (eg, only cytogenetics unfavorable, only secondary AML) differed, in no group was median survival over 1 year. The 76 patients who were both over age 64 and had unfavorable cytogenetics—any but normal, inv(16), t(8:21), or insufficient yield—but were ambulatory with normal organ function and de novo disease had a median survival of 6 months. Although the CR rate was 51%, only 7% of the 76 patients remain alive in CR at 3 years. While different physicians and different patients will disagree as to when the results of standard therapy are sufficiently poor to justify the alternatives of investigational or palliative treatment, examples such as those provided above lead me to recommend the former if any of the following are present in an older patient: age above 64, performance status above Zubrod 2, abnormal organ function, secondary AML, or unfavorable cytogenetics as defined above. This recommendation is in fundamental accord with that made by the AML “expert panel” of the National Comprehensive Cancer Network (NCCN), a consortium of prominent U.S. cancer centers.13Importantly, and as discussed below, the type of investigational treatment I recommend depends on how many and which adverse prognostic features are present.
If financial or logistical constraints prevent access to investigational treatment, I would recommend palliative care without specific antileukemic treatment if the patient were age 65 or older and primarily bedridden (Zubrod performance status 3-4) or age 80 or older regardless of performance status.14 These covariates are the principal predictors of early death, as opposed to resistant disease, following initiation of chemotherapy; in contrast, abnormal cytogenetics, secondary AML, and MDR expression are prognostically unfavorable because of their association with resistant AML.4,15 Among the 90 patients in our database who were age 65 or older with performance status 3 to 4, the median survival after the start of chemotherapy was 5 weeks, with 4 patients living beyond 1 year, and with no difference in outcome in the 1990s versus the 1980s. Findings are similar in patients over age 80 regardless of performance status.14 It is difficult to ascertain whether these results are better or worse than those with palliative care. The previously mentioned EORTC study12 was limited to patients with good performance status, while 2 nonrandomized studies report median survivals of 3 weeks and 9 months, respectively, in 11 and 24 patients receiving palliative care7 16; all but 2 of these were above age 55. The difference in survival times between the 2 studies probably reflects varying inclusion criteria, although in both studies a few patients (2 of 11 and 2 of 24) lived more than 1 year; these patients had low white blood cell counts.
Regardless of the comparative merits of standard chemotherapy and palliative care in the primarily bedridden patient who is age 65 or older or in the patient who is older than 80, I believe the 5-week median survivals associated with use of standard therapy in these patients do not warrant use of such therapy in this setting but, rather, justify the use of palliative care, including administration of hydroxyurea if the white blood cell count is more than 25 × 109/L. While the decision to choose palliative care over standard therapy is obviously best made by individual physicians and patients, I believe development of a formal prognostication system incorporating the previously discussed covariates would be invaluable in informing these decisions. Such a system would be particularly relevant when presented with the options of palliative and standard treatment in patients who are younger than 65 or who are age 65 to 80 with good performance status but have abnormal cytogenetics, secondary AML, or MDR expression. There is also a need to examine the effects of specific treatment versus palliative care on “quality of life.” When the option of investigational therapy is available, I recommend this option rather than palliative care for older patients with relatively unfavorable prognoses. This recommendation extends to patients at high risk of early death, based on my assumption that investigational therapies, such those described below, might successfully address this problem. While duly noting the difficulties inherent in investigational therapy, as discussed above, I stress that at least on occasion new therapies are, even unexpectedly, successful (2 CdA in hairy cell leukemia, interferon in chronic myelocytic leukemia), thus providing a modicum of hope for patients entered in trials of investigational therapies. Finally, I note that such trials remain the principal mechanism to advance the therapy of AML.
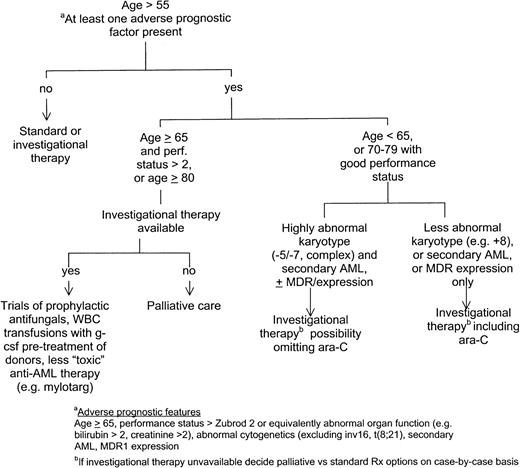
Figure 1 summarizes my approach to therapy of older patients with AML. Clearly, I favor investigational therapy for most such patients. Before going into detail, I will dismiss recently investigated therapies whose routine use is, in my opinion, not supported by evidence from the investigations. Separate randomized trials conducted by the Southwest Oncology Group,17 Cancer and Leukemia Group B,18 and Eastern Cooperative Oncology Group19 have found that high-dose ara-C (HDAC) (2-3 g/m2) given in remission, or for induction and/or in remission, failed to improve survival or CR rate in patients over age 55 to 60 and often produced toxicity, particularly neurotoxicity. HDAC might, however, be used in good-performance status patients age 55 to 65 with a normal karyotype or with the rare inv(16) or t(8;21). The former may benefit from 4 cycles of ara-C, 400 mg/m2 daily × 5 continuous infusion given once in CR, and the latter from 4 cycles at 3 g/m2 every 12 hours on days 1, 3, and 5.20 Attempts to reduce the dose of ara-C (“low-dose ara-C”) or anthracycline have generally found that any reduction in mortality is counterbalanced by an increase in resistant disease with no material benefit in survival.21-23 Although randomized trials have not been done, I do not believe that M. D. Anderson data support the use of fludarabine plus ara-C24 or topotecan plus ara-C regimens.25,26 Granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF given concomitantly with and/or after chemotherapy may reduce days spent in the hospital or febrile episodes but not end points such as survival, CR rate, or duration.6,27,28 Possibly confounding factors in the one study that found a survival advantage5 are discussed by Estey et al.29 While some might argue that the reduction in morbidity justifies routine use of these products, given economic constraints I administer GM-CSF or G-CSF only in the event of fever or infection, while granting a lack of empirical evidence bearing on this practice. I routinely discharge patients from the hospital after chemotherapy is completed, readmitting them if complications such as fever develop. I believe this practice not only may reduce the frequency of nosocomial infections but also maintains patients' feelings of independence. In patients at high risk of early death (age above 80, or age 65 or older with Zubrod performance status 3-4), I administer intravenous itraconazole or liposomal amphotericin prophylactically and, if not given prophylactically, begin amphotericin, and attempt to arrange granulocyte transfusions from normal donors pretreated with G-CSF should pneumonia ensue or should a fever of unknown origin fail to respond to 3 days of antibacterial antibiotics (Figure 1). The goal is to reduce the incidence or severity of aspergillus infections, a major cause of death in our patients.30 While recognizing a lack of data supporting these practices, we are engaged in trials to assess their utility. Whether such investigational therapies should be employed outside the context of a clinical trial is a highly complex issue that is beyond the scope of this paper.
Because the principal cause of treatment failure in older patients is resistant AML (manifested by short CRs or failure to enter CR), our investigational strategies should focus primarily on this problem, particularly in patients with an abnormal karyotype, secondary AML, or MDR expression. The degree to which an investigational regimen departs from standard therapy should depend on how poor prognosis is with the latter. For example, in patients with secondary AML, a particularly unfavorable karyotype (− 5/− 7 or more than 3 clones), and MDR expression, it would not necessarily be unreasonable to omit ara-C (Figure 1). In this setting, for example, we have studied liposomal daunorubicin (LD) plus topotecan, as well as LD plus ara-C, each with or without the presumed angiogenesis inhibitor thalidomide, and we are planning to investigate mylotarg (formerly CMA-676), an anti-CD33 monoclonal antibody conjugated to the anthracycline calicheamycin,31 and to conduct a phase I/II trial of the hypomethylating agent decitabine at doses that presumably result in maximum hypomethylation of inappropriately methylated genes32 but do not produce myelosuppression, thus permitting investigation of prolonged infusions. Both mylotarg and low-dose decitabine may also reduce early mortality rates and thus could be administered to patients at high risk of early death (Figure1). In contrast, in patients with fewer unfavorable characteristics, eg, only secondary AML or an abnormal karyotype, ara-C with or without an anthracycline should be included. Regimens we are investigating or have proposed to investigate in this setting include cyclophosphamide plus ara-C plus topotecan with or without liposomal all-trans retinoic acid, bcl-2 antisense plus ara-C plus idarubicin, and the checkpoint modulator UCN-01 plus ara-C. Obviously, the extent to which a new regimen can vary from the standard in a given prognostic setting is debatable. Furthermore, numerous other regimens may be at least as useful as those described. These include continuous-infusion daunorubicin plus ara-C plus cyclosporine,33 allogeneic transplants using intravenous busulfan,34 or nonmyeloablative regimens that allow engraftment and a subsequent graft-versus-leukemia effect.35 Both transplant regimens seem well tolerated in patients up to age 70. They could conceivably be used not only in elderly patients in remission but also for remission induction in clinically stable patients whose white blood cell count is sufficiently low to permit transplant arrangements to be completed. Use of transplantation for remission induction is an important consideration given the low CR rate in elderly patients. I believe it is impossible to determine which, if any, of the above regimens will be significantly better than standard therapy in the absence of data from a clinical trial; after all, neither 2 CdA nor interferon was predicted to be effective in hairy cell leukemia and CML, respectively, prior to empirical trial results. Given this consideration and the large number of treatments to investigate relative to the number of patients available for clinical trials, I believe that clinical trial design in older patients should focus as much on small studies of a relatively large number of new therapies as on the conventional large phase III study of 1 or 2 new therapies. Although the smaller studies may have relatively high false-negative rates, I believe that the worst false negative, in the setting described, results when a potentially important therapy is not investigated at all.36
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elihu H. Estey, Department of Leukemia, Box 61, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: eestey@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal