Abstract
The frequency of immune heparin-induced thrombocytopenia (HIT) varies among prospective studies. It is unknown whether this is caused by differences in the heparin preparations, the patient populations, or the types of serologic assay used to confirm the diagnosis. Seven hundred forty-four patients were studied from 3 different clinical treatment settings, as follows: unfractionated heparin (UFH) during or after cardiac surgery (n = 100), UFH after orthopedic surgery (n = 205), and low-molecular-weight heparin (LMWH) after orthopedic surgery (n = 439). Both an activation assay and an antigen assay were used to detect heparin-dependent IgG (HIT-IgG) antibodies. By activation assay, the frequency of HIT-IgG formation ranged from a low of 3.2% in orthopedic patients receiving LMWH to a high of 20% in cardiac patients receiving UFH; by antigen assay, the corresponding frequencies ranged from 7.5% to 50%. Both UFH use (P = .002) and cardiac surgery (P = .01) were more likely to be associated with HIT-IgG formation. However, among patients in whom HIT-IgG formed and who were administered UFH, the probability for HIT was higher among orthopedic patients than among cardiac patients (by activation assay: 52.6% compared with 5%; odds ratio, 21.1 [95% CI, 2.2-962.8]; P = .001; by antigen assay: 34.5% compared with 2.0%; odds ratio, 25.8 [95% CI, 3.2-1141]; P < .001). It is concluded that there is an unexpected dissociation between the frequency of HIT-IgG formation and the risk for HIT that is dependent on the patient population. HIT-IgG antibodies are more likely to form in patients who undergo cardiac surgery than in orthopedic patients, but among patients in whom antibodies do form, orthopedic patients are more likely to develop HIT.
Introduction
Heparin-induced thrombocytopenia (HIT) is an adverse drug reaction caused by heparin-dependent IgG (HIT-IgG) antibodies that activate platelets.1-3 The target antigen consists of multimolecular complexes of platelet factor 4 and heparin.4-7 The frequency of HIT varies considerably, as cited by prospective studies.8,9 The reasons for these variations are unknown but could be related to different heparin preparations,10 patient population-dependent factors, or even different laboratory techniques used to detect the antibodies responsible for HIT.
Two general types of laboratory assay are used to confirm the diagnosis. Activation assays, such as the platelet serotonin release assay, detect HIT-IgG on the basis of their ability to activate platelets.11-13 Antigen assays, such as a solid-phase immunoassay, detect the binding of antibodies to immobilized platelet factor 4–heparin complexes.4-7,14,15 Despite the increasing use of these assays, few studies have compared their clinical usefulness in diagnosing HIT. Those studies14 16-18 comparing activation and antigen assays typically have been limited to investigating patient samples that were referred with the clinical suspicion of HIT (high pretest probability). However, such an approach does not allow the determination of test specificity. Furthermore, systematic serologic studies of patients receiving heparin (low pretest probability) have generally been too small to evaluate sensitivity. In addition, these studies have generally focused on single patient populations.
In this report, we describe the results of laboratory testing for HIT-IgG using both activation and antigen assays in 744 prospectively studied patients. These patients were from 3 different clinical treatment settings: cardiac surgery patients receiving unfractionated heparin (UFH), orthopedic surgery patients receiving UFH, and orthopedic surgery patients receiving low-molecular-weight heparin (LMWH). The results of this study indicate that there is an unexpected dissociation between the formation of HIT-IgG and the risk for HIT among patients in whom antibodies form that is patient population dependent.
Patients, materials, and methods
Patient populations
Patients in 1 of 3 clinical treatment settings were studied prospectively. They were grouped as follows: orthopedic surgery patients receiving UFH for postoperative prophylaxis (orthopedic–UFH group, n = 205); orthopedic surgery patients receiving LMWH for postoperative prophylaxis (orthopedic–LMWH group, n = 439); and cardiac surgery patients receiving UFH both at cardiopulmonary bypass and for postoperative antithrombotic prophylaxis (cardiac–UFH group, n = 100). Patients underwent daily platelet count monitoring while receiving heparin.
The orthopedic–UFH group consisted of a group of 205 patients who received porcine mucosal UFH (Calciparine; Laboratoires Anglo-French, Dorval, Quebec, Canada), 7500 IU every 12 hours by subcutaneous injection for up to 14 days or discharge. These patients had participated in a randomized clinical trial of UFH versus LMWH given for antithrombotic prophylaxis after hip arthroplasty surgery (study A). Primary trial results and a related study of HIT using only the platelet 14C-labeled serotonin-release assay have been reported.10 19 In the current study, the patients' plasma samples were studied using the antigen assay as well.
The orthopedic-LMWH group (n = 439) consisted of patients from 2 studies who received LMWH for antithrombotic prophylaxis after orthopedic surgery. The first group (study A) consisted of 182 patients who received LMWH (enoxaparin; Lovenox; Rhône-Poulenc Rorer, Montreal, Quebec, Canada), 30 mg every 12 hours by subcutaneous injection for up to 14 days or discharge. These patients had also participated in the trial described previously.10 19 The second orthopedic–LMWH group (study B) consisted of 257 patients who received postoperative LMWH prophylaxis after orthopedic surgery (hip arthroplasty, n = 105; knee arthroplasty, n = 152). These patients received 1 of 3 LMWH preparations based on the current hospital practice at the time of participation in the study—enoxaparin (Lovenox; Rhône-Poulenc Rorer), 30 mg twice daily by subcutaneous injection, n = 70; tinzaparin (Innohep; Leo, Ajax, Ontario, Canada), 3000 U twice daily by subcutaneous injection, n = 2; or dalteparin (Fragmin; Pharmacia, Mississanga, Ontario, Canada), 3000 U twice daily by subcutaneous injection, n = 185. For orthopedic patients, heparin administration was usually started on the first postoperative day and continued until discharge or full mobilization. Although 3 different LMWH preparations were used, we made an a priori decision to analyze all the data as one orthopedic–LMWH group.
The third patient population consisted of a cardiac–UFH group comprised of 100 patients undergoing elective valve replacement surgery (69 aortic valve replacement, 29 mitral valve replacement, 2 both). Porcine mucosal heparin (Hepalean; Organon Teknika, Scarborough, Ontario, Canada), 400 U/kg, was given before cardiopulmonary bypass; additional heparin was given by bolus as needed to achieve and maintain an intraoperative whole blood activated clotting time of more than 400 seconds. These patients also received subcutaneous heparin (porcine mucosal heparin; Heparin; Leo), 5000 IU every 8 hours by subcutaneous injection, until they were adequately anticoagulated with warfarin or mobilized. Antiplatelet drugs such as aspirin were not routinely given to this patient population undergoing valve replacement surgery.
These studies were approved by the Institutional Review Board, and patients gave their informed consent. Laboratory testing for HIT-IgG was performed after the patients had been discharged from the hospital, and no results were used to influence clinical decisions.
Activation assay
All 744 patients were tested with the platelet serotonin release assay, as described,11 12 using heat-inactivated citrated plasma or serum stored at −70°C before testing. Using a predetermined algorithm, samples were considered positive if all the following criteria were met: (1) 20% or greater serotonin release at 0.1 U/mL heparin; (2) at least 50% inhibition of platelet activation by both an Fc receptor-blocking monoclonal antibody (IV.3) and a high concentration of heparin (100 U/mL); (3) appropriate activation profiles observed with 3 positive controls (including 2 “weak” sera, ie, giving 20%-50% serotonin release) and one negative control serum.
Antigen assay
Patient plasma and serum samples were also evaluated using a platelet factor 4–heparin enzyme immunoassay, as previously described,15 except that an alkaline phosphatase-conjugated goat antibody specific for human IgG (Fc) replaced the conjugated goat antibody for human IgG (heavy and light chain-specific). By measuring IgG antibodies only, we facilitated comparison with the activation assay, which detects platelet activation through IgG antibodies.3 One hundred samples from healthy blood donors were assayed, and the upper limit of the normal range of optical density (OD) was established as the mean + 3 SD (OD = 0.45). Each assay included pooled negative controls (OD ≤ 0.2), high-negative control pool (0.2 < OD < 0.45), known negative control, known positive control (OD > 1.5), and weak positive control (0.45 < OD < 1.5).
Definitions
We defined possible HIT as a 50% or greater fall in the platelet count from the postoperative peak that occurred between days 5 to 14 after surgery, unless another cause for the thrombocytopenia was readily apparent (eg, culture-positive septicemia) or the platelet count recovered during continued heparin treatment. This definition of thrombocytopenia has the best correlation with the formation of HIT-IgG20 and is appropriate for a postoperative patient population in whom thrombocytosis commonly occurs between postoperative days 5 to 14. Heparin-induced thrombocytopenia (HIT) was defined when a patient with possible HIT also had positive results of at least one laboratory assay for HIT-IgG (by activation or antigen assay, or both).
Comparison of activation and antigen assays
Because both diagnostic assays are quantitative measurements, we used receiver operating characteristic (ROC) curve analysis to compare the sensitivity–specificity tradeoff at various diagnostic cutoff points defining negative and positive test results. Sensitivity of each assay was defined as the proportion (in percentage) giving a positive test result among the patients with possible HIT. Specificity of each assay was defined as the proportion (in percentage) of patients who did not meet the study definition for possible HIT and who tested negative for the assay under consideration. The ROC curve analysis was performed only for the orthopedic patients because this patient group had a relatively large number of patients with possible HIT and additional patients with subclinical HIT-IgG seroconversion (see “Results”).
Our standard antigen assay uses a 1:50 dilution of test serum or plasma. To investigate whether greater sample dilution would improve the sensitivity–specificity tradeoff of the antigen assay, we also performed the assay using the following sample dilutions: 1/50, 1/75, 1/100, 1/150, 1/250, 1/500, 1/750, 1/1000, 1/2500, and 1/5000. After these preliminary studies, we systematically studied the orthopedic patient samples that tested positive in the antigen assay (at 1/50), at the following additional dilutions: 1/100, 1/250, 1/500, and 1/750. The data obtained were used to calculate ROC curves. Thus, we investigated whether there might be a greater likelihood that blood samples from patients with subclinical HIT-IgG formation would test negative on further sample dilution than blood samples from patients with possible HIT. In other words, could the sensitivity–specificity tradeoff be improved on sample dilution? If so, there would be the potential for greater diagnostic specificity for the antigen assay, without significant loss of sensitivity, if the antigen assay was performed at a higher dilution.
Statistical analysis
Comparisons of proportions between groups were performed using the Fisher exact test.21 An associated method developed by Gart22 was used for computing confidence intervals around the odds ratio. We performed logistic regression analysis23to determine the impact of either heparin preparation (UFH compared with LMWH) and the type of patient population (cardiac compared with orthopedic patients) with respect to the frequency of HIT-IgG formation and the proportion of antibody-positive patients in whom possible HIT developed. Analyses were performed separately for activation and antigen test results. All quoted P values were 2-sided.
Results
Frequency of possible heparin-induced thrombocytopenia
Eighteen of the 744 prospectively studied patients had a 50% or greater decrease in platelet count that began between postoperative days 5 and 14. Fifteen of these patients met the criteria for possible HIT (Table 1). All 15 patients who were identified as possibly having HIT also tested positive for HIT-IgG using both the activation and the antigen assays. The frequency of HIT was highest (4.9%) in the orthopedic–UFH patients and was relatively low in both the orthopedic–LMWH patients (0.9%) and the cardiac–UFH patients (1%). One or more thrombotic events occurred in 9 (60%) of these 15 patients; 7 patients had venous thromboembolism, 1 patient had unilateral adrenal hemorrhagic infarction, and 1 patient had arterial thrombosis.
Relationship of the patient population and type of heparin on frequency of HIT-IgG antibody formation and risk for developing HIT
| Patient population, type of heparin (mean ± SD duration of heparin [d]) . | Frequency of HIT antibody positivity (95% CI) . | Frequency of HIT (95% CI)† . | Frequency of HIT–IgG-positive patients with HIT (95% CI) . | ||
|---|---|---|---|---|---|
| Activation assay* . | Antigen assay* . | Activation assay‡ . | Antigen assay‡ . | ||
| Cardiac-UFH, n = 100 | 20/100 | 50/100 | 1/100 | 1/20 | 1/50 |
| (5.1 ± 2.2) | 20.0% (12.7-29.2) | 50.0% (39.8-60.2) | 1.0% (0.03-5.5) | 5.0% (0.1-24.9) | 2.0% (0.12-10.7) |
| Orthopedic-UFH, n = 205 | 19/205 | 29/205 | 10/205 | 10/19 | 10/29 |
| (9.2 ± 2.2) | 9.3% (5.7-14.1) | 14.1% (9.7-19.7) | 4.9% (2.4-8.8) | 52.6% (28.9-75.6) | 34.5% (17.9-54.3) |
| Orthopedic-LMWH, n = 439 | 14/439 | 33/439 | 4/439 | 4/14 | 4/33 |
| (9.5 ± 3.0) | 3.2% (1.8-5.3) | 7.5% (5.2-10.4) | 0.9% (0.3-2.3) | 28.6% (8.4-58.1) | 12.1% (3.4-28.2) |
| Pvalues | |||||
| Cardiac vs Orthopedic | .01 | <.001 | .71 | .01 | .004 |
| UFH vs LMWH | .002 | .009 | .015 | .19 | .048 |
| Patient population, type of heparin (mean ± SD duration of heparin [d]) . | Frequency of HIT antibody positivity (95% CI) . | Frequency of HIT (95% CI)† . | Frequency of HIT–IgG-positive patients with HIT (95% CI) . | ||
|---|---|---|---|---|---|
| Activation assay* . | Antigen assay* . | Activation assay‡ . | Antigen assay‡ . | ||
| Cardiac-UFH, n = 100 | 20/100 | 50/100 | 1/100 | 1/20 | 1/50 |
| (5.1 ± 2.2) | 20.0% (12.7-29.2) | 50.0% (39.8-60.2) | 1.0% (0.03-5.5) | 5.0% (0.1-24.9) | 2.0% (0.12-10.7) |
| Orthopedic-UFH, n = 205 | 19/205 | 29/205 | 10/205 | 10/19 | 10/29 |
| (9.2 ± 2.2) | 9.3% (5.7-14.1) | 14.1% (9.7-19.7) | 4.9% (2.4-8.8) | 52.6% (28.9-75.6) | 34.5% (17.9-54.3) |
| Orthopedic-LMWH, n = 439 | 14/439 | 33/439 | 4/439 | 4/14 | 4/33 |
| (9.5 ± 3.0) | 3.2% (1.8-5.3) | 7.5% (5.2-10.4) | 0.9% (0.3-2.3) | 28.6% (8.4-58.1) | 12.1% (3.4-28.2) |
| Pvalues | |||||
| Cardiac vs Orthopedic | .01 | <.001 | .71 | .01 | .004 |
| UFH vs LMWH | .002 | .009 | .015 | .19 | .048 |
The 15 patients with clinical HIT all tested positive by antigen and activation assays. Logistic regression analysis was used to compare 2 variables, patient population (cardiac compared with orthopedic patients) and heparin preparation (UFH compared with LMWH), with respect to 2 outcome measures—the frequency of patients testing positive for HIT-IgG antibodies and the proportion of antibody-positive patients in whom thrombocytopenia developed. Among the orthopedic patients who received LMWH, there was no significant difference in the frequency of HIT-IgG formation, or in the frequency of possible HIT among patients who received the different LMWH preparations (data not shown).
Fractions indicate the number of patients with positive HIT-IgG test results (by the assay indicated) out of the patient population tested.
Fractions indicate the number of patients with HIT out of the patient population tested.
Fractions indicate the number of patients with HIT out of those who tested positive for HIT-IgG antibodies by the assay indicated.
Three patients whose platelet counts fell by 50% or more between postoperative days 5 to 14 did not meet the criteria for possible HIT. Thrombocytopenia developed in association with colon perforation and septicemia in two orthopedic patients, one treated with UFH and one treated with LMWH. Both died. In both patients, however, platelet counts recovered during continued heparin use. A third patient had a 59% decrease in platelet count in association with pulmonary embolism, but full platelet count recovery during treatment with therapeutic-dose UFH occurred. All 3 patients tested negative for HIT-IgG by activation and antigen assays.
Frequency of HIT-IgG antibody formation
Figure 1 summarizes the results of the laboratory assays for HIT-IgG among the 3 patient treatment groups studied. With both antigen and activation assays, we observed the highest antibody-positivity rate (by conventional positive cutoff) among the cardiac patients (50% and 20%, respectively), followed by the orthopedic–UFH (14.1% and 9.3%) and the orthopedic–LMWH patients (7.5% and 3.2%). By logistic regression analysis (Table 1), we found that 2 variables were associated with a higher frequency of HIT-IgG formation: cardiac surgery and use of UFH.
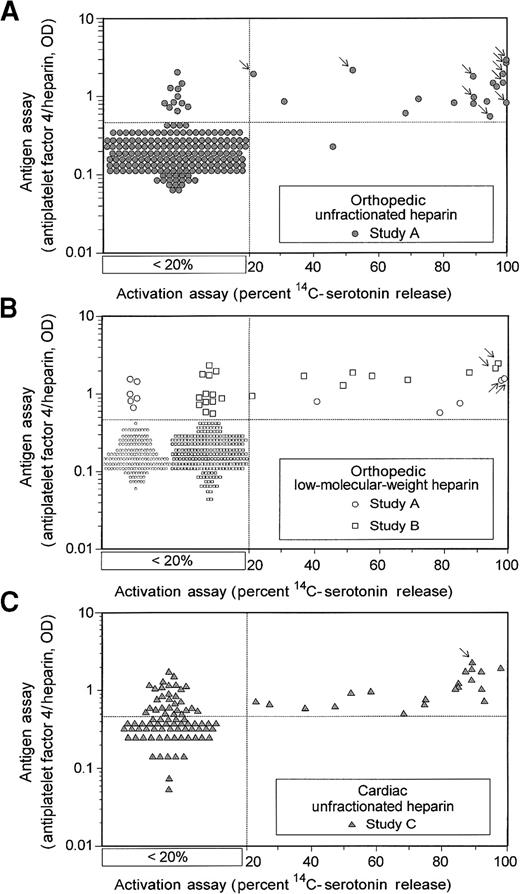
Comparison of activation and antigen assays for HIT-IgG antibodies in patients who have undergone cardiac and orthopedic surgery.
Quantitative results of activation and antigen tests for HIT-IgG are shown for 3 clinical treatment settings: orthopedic–UFH (A), orthopedic–LMWH (B), and cardiac–UFH (C). Results are shown for all 744 patients. All antigen assay data are given quantitatively. For the activation assay results, samples that gave less than 20% serotonin release are as shown without reference to the actual quantitative result obtained (see box designated < 20%); quantitative data are given when the percentage serotonin release was 20% or more. Arrows indicate the data points corresponding to the 15 patients with HIT identified in these prospective studies.
Comparison of activation and antigen assays for HIT-IgG antibodies in patients who have undergone cardiac and orthopedic surgery.
Quantitative results of activation and antigen tests for HIT-IgG are shown for 3 clinical treatment settings: orthopedic–UFH (A), orthopedic–LMWH (B), and cardiac–UFH (C). Results are shown for all 744 patients. All antigen assay data are given quantitatively. For the activation assay results, samples that gave less than 20% serotonin release are as shown without reference to the actual quantitative result obtained (see box designated < 20%); quantitative data are given when the percentage serotonin release was 20% or more. Arrows indicate the data points corresponding to the 15 patients with HIT identified in these prospective studies.
We observed dissociation between the frequency of HIT-IgG formation and the risk of HIT that was patient population dependent. Among patients in whom antibodies formed, HIT was more likely to develop in orthopedic patients than in cardiac patients (Table 1). Among patients who tested positive by the activation assay and who received UFH, HIT developed in 52.6% of orthopedic patients but only 5% of cardiac surgery patients (odds ratio, 21.1 [95% CI, 2.2 to 962.8];P = .001). Among patients who tested positive by antigen assay and who received UFH, HIT developed in 34.5% of orthopedic patients and only 2.0% of cardiac surgery patients (odds ratio, 25.8 [95% CI, 3.2 to 1141]; P < .001).
We investigated the duration of heparin treatment as a reason fewer patients with HIT-IgG in the cardiac group had possible HIT. At the time of heart surgery, the cardiac patients received very high doses of heparin. After surgery, both the cardiac and the orthopedic patients who received UFH were given the same dose (15 000 U/d). Although orthopedic patients received UFH for a greater duration than cardiac patients (9.2 ± 2.2 vs 5.1 ± 2.2 days; P < .001), the duration of heparin exposure was not the major reason for the higher frequency of HIT in orthopedic patients. For example, among patients in whom HIT-IgG formed and was detectable by both assays and who received UFH for 5 or more days, there was a greater likelihood of the onset of platelet count decrease by day 5 in the orthopedic patients than in the cardiac patients (5 of 19 compared with 0 of 15;P = .053). A similar trend was noted when the analysis was restricted to patients who received heparin for at least 6 days: 0 of 8 patients in the cardiac group had a platelet count decrease indicative of possible HIT, whereas 7 of 19 orthopedic patients had a platelet count decrease by day 6 (P = .068). This suggests that orthopedic patients are at greater risk for HIT for reasons other than longer duration of UFH use. Indeed, the cardiac surgery patient in whom HIT developed (postoperative day 7, complicated by unilateral adrenal necrosis) received UFH only until the second day after surgery.
Diagnostic usefulness of laboratory assays for heparin-induced thrombocytopenia
Figure 1 shows that most patients with HIT (indicated by arrows) had strong positive test results for HIT-IgG using either assay: more than 90% serotonin release (activation assay) and greater than 1.0 OD (antigen assay). Figure 2 compares these 2 assays by sensitivity–specificity tradeoff analysis (ROC curve analysis) at various cutoff points between positive and negative results for the orthopedic patients. Because activation and antigen assays were positive at their conventional cutoff points (more than 20% serotonin release and greater than 0.45 OD, respectively) for all 15 patients with possible HIT identified in the prospective studies, the sensitivity of each assay for HIT was high (100%). However, because the antigen assay was more likely to be positive in patients in whom HIT did not develop, the activation assay had superior operating characteristics (ie, greater diagnostic specificity) over all values of the diagnostic cutoff between positive and negative tests. The activation assay remained superior even when the antigen assay was performed at greater dilutions. For orthopedic patients, test specificity (at the conventional cutoff points) for the activation and antigen assays was 97% and 92%, respectively. For cardiac patients, the corresponding specificities were only 81% and 51% because possible HIT developed in fewer cardiac surgery patients, even though HIT-IgG antibodies formed in more.
Comparison of activation and antigen assays for HIT by receiver operating characteristics (ROC) curve analysis.
For the orthopedic patients, the activation and antigen assays are compared at various cutoff points between negative and positive results. Activation assay (SRA, serotonin release assay, thick solid line). Although the sensitivity-specificity trade-off analysis was performed at 5% increments of serotonin release, the data points for cutoff 20% or more, 50% or more, and 90% or more serotonin release are shown. Antigen assay (EIA, enzyme immunoassay, thin solid line indicating standard dilution of blood sample ; thin broken lines indicating higher dilutions [1/100, 1/250, 1/500, 1/750], as indicated). Although the sensitivity–specificity trade-off analysis was performed at 0.1-OD increments, the data points for cutoff of 0.5 or more, 1.0 or more, and 2.0 or more are shown. The ROC curve analysis suggests that both the activation and the antigen assays are highly informative for diagnosing HIT in this patient population. For comparison, the inset shows the full-scale ROC curve analysis for both activation and (at conventional 1/50 dilution) antigen assays together with a (theoretical) noninformative assay (shown as dashed line). The data indicate that the operating characteristics of the antigen assay are not improved by performing the assay using a greater dilution of patient serum/plasma.
Comparison of activation and antigen assays for HIT by receiver operating characteristics (ROC) curve analysis.
For the orthopedic patients, the activation and antigen assays are compared at various cutoff points between negative and positive results. Activation assay (SRA, serotonin release assay, thick solid line). Although the sensitivity-specificity trade-off analysis was performed at 5% increments of serotonin release, the data points for cutoff 20% or more, 50% or more, and 90% or more serotonin release are shown. Antigen assay (EIA, enzyme immunoassay, thin solid line indicating standard dilution of blood sample ; thin broken lines indicating higher dilutions [1/100, 1/250, 1/500, 1/750], as indicated). Although the sensitivity–specificity trade-off analysis was performed at 0.1-OD increments, the data points for cutoff of 0.5 or more, 1.0 or more, and 2.0 or more are shown. The ROC curve analysis suggests that both the activation and the antigen assays are highly informative for diagnosing HIT in this patient population. For comparison, the inset shows the full-scale ROC curve analysis for both activation and (at conventional 1/50 dilution) antigen assays together with a (theoretical) noninformative assay (shown as dashed line). The data indicate that the operating characteristics of the antigen assay are not improved by performing the assay using a greater dilution of patient serum/plasma.
Discussion
Immune HIT continues to be one of the most important IgG-mediated drug reactions that physicians must manage. The association between anticoagulant-induced platelet count decrease and unexpected arterial and venous thrombosis represents a paradox of considerable clinical interest. Another confounding factor is the unusual composition of the target antigen of the disorder, which is a stoichiometrically defined complex between a native platelet protein, platelet factor 4, and heparin.4-7 Antibodies against this antigenic complex can be detected using either of 2 classes of assay. One type measures the HIT-IgG antibody-induced activation of platelets in a heparin-dependent fashion,11-13 and the other detects HIT-IgG that recognize immobilized platelet factor 4–heparin antigen.4-7,14 15
Despite these recent insights into the pathogenesis of HIT, there remain important unresolved issues. For example, it is unknown why the frequency of HIT differs among patients in prospective studies.8 9 Explanations include differences in risk for forming, and clinical effects arising from, HIT-IgG antibodies that are patient population dependent. Differences in the diagnostic significance between the 2 different tests for HIT-IgG could also explain differences between studies. For these reasons, we performed both activation and antigen assays on blood samples from 744 patients studied prospectively for HIT who were treated either with UFH or LMWH, after either cardiac or orthopedic surgery.
The results of this study indicate a patient population-dependent dissociation between the risk for HIT-IgG formation and the risk for HIT among patients in whom antibodies formed. The highest frequency of HIT-IgG formation occurred after cardiac surgery: 50% of these patients had antibodies detected by antigen assay, and 20% had antibodies detected by activation assay. However, the clinical syndrome of HIT was uncommon in this group. In contrast, only 14.1% and 9.3%, respectively, of patients who received UFH after orthopedic surgery had HIT-IgG detected by antigen and activation assay. Yet this was the patient population in whom HIT was most likely to develop. Fewer orthopedic patients developed antibodies (7.5% and 3.2%, respectively) when treated with LMWH. Logistic regression analysis showed that patient population and type of heparin preparation determined antibody formation (Table 1).
Although cardiac surgery patients had the highest frequency of antibody formation, among those patients who developed antibodies, the orthopedic patients were about 20 times more likely (by odds ratio) to have HIT. Other investigators have also shown high frequencies of antibody formation in cardiac surgery patients without associated thrombocytopenia.24-26 In 2 of these studies,24,25 postoperative heparin prophylaxis was not given, so the risk for HIT might have been reduced. In the third study,26 no thrombocytopenia occurred among 16 patients receiving postoperative UFH who had positive antigen assay. Because these studies did not include large comparative patient populations, it was not possible to determine whether there were population-dependent differences in HIT risk.
Our study was not designed to determine the explanation for patient population-dependent differences in frequency and clinical significance of HIT-IgG formation. Reasons for the high frequency of antibody formation among cardiac patients could include high doses of intraoperative heparin or the release of platelet factor 4 from platelets during contact between blood and the cardiopulmonary bypass apparatus27,28; together these could increase the risk for antibody formation. Alternatively, various proinflammatory factors associated with orthopedic surgery could influence the pathogenicity of antibodies in this patient population.29 Aspirin use could not have accounted for the differences because the cardiac surgery population underwent valve replacement surgery and was routinely given anticoagulant rather than antiplatelet drugs.
We observed that activation and antigen assays are very sensitive for HIT. However, the antigen assay was more likely to detect antibodies that did not cause thrombocytopenia, ie, the diagnostic specificity for clinical HIT was lower. In addition, the operating characteristics of the antigen assay did not improve when performed using samples tested at a greater dilution (Figure 2). This finding, plus the observation that most patients with possible HIT had antibodies that caused strong platelet activation (serotonin release greater than 90%; see Figure1), suggests that the activation assay may be better able than the antigen assay to detect pathogenic antibodies with potent platelet-activating properties. Indeed, preliminary HIT antibody purification studies by Pouplard et al30 suggest that only a relatively small subset of anti-PF4–heparin antibodies has a potent platelet-activating profile.30 If activation assays are better able to detect clinically significant HIT antibodies, the situation would appear to parallel somewhat that of laboratory testing for the antiphospholipid antibody syndrome: a biologic assay (the nonspecific lupus inhibitor) has greater specificity for thrombosis than the antigen assay (anticardiolipin antibodies).31 Our data also suggest that the patient population influences the diagnostic significance of HIT-IgG test results. For either activation or antigen assay, a positive result in an orthopedic patient may be more specific for clinical HIT.
The current study indicates that HIT is a more complicated syndrome than might be expected for a drug-induced, immune-mediated thrombocytopenic disorder. There exist patient population-dependent differences in risk for HIT-IgG formation and in the thrombocytopenic potential of antibodies. There also exist differences in the diagnostic usefulness of the 2 different classes of assays to detect these antibodies. The complex nature of this syndrome may relate to the compound nature of the responsible antigen, in which both drug and autologous protein concentrations could vary in different clinical settings.
Acknowledgments
We thank Jean I. Russett and Judith Johnson for organizing the collection of plasma samples in these patients, Carol A. Smith for technical assistance with some of the assays, and Prof Robin Roberts for assistance in the statistical analysis.
Supported by operating grants A2449 and B3763 from the Heart and Stroke Foundation of Ontario (T.E.W.) and by a Research Scholarship from the Heart and Stroke Foundation of Canada (T.E.W.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Theodore E. Warkentin, Hamilton Regional Laboratory Medicine Program, Hamilton Health Sciences Centre, Hamilton General Site, 237 Barton St East, Hamilton, Ontario L8L 2X2, Canada; e-mail: twarken@fhs.mcmaster.ca.

![Fig. 2. Comparison of activation and antigen assays for HIT by receiver operating characteristics (ROC) curve analysis. / For the orthopedic patients, the activation and antigen assays are compared at various cutoff points between negative and positive results. Activation assay (SRA, serotonin release assay, thick solid line). Although the sensitivity-specificity trade-off analysis was performed at 5% increments of serotonin release, the data points for cutoff 20% or more, 50% or more, and 90% or more serotonin release are shown. Antigen assay (EIA, enzyme immunoassay, thin solid line indicating standard dilution of blood sample 150; thin broken lines indicating higher dilutions [1/100, 1/250, 1/500, 1/750], as indicated). Although the sensitivity–specificity trade-off analysis was performed at 0.1-OD increments, the data points for cutoff of 0.5 or more, 1.0 or more, and 2.0 or more are shown. The ROC curve analysis suggests that both the activation and the antigen assays are highly informative for diagnosing HIT in this patient population. For comparison, the inset shows the full-scale ROC curve analysis for both activation and (at conventional 1/50 dilution) antigen assays together with a (theoretical) noninformative assay (shown as dashed line). The data indicate that the operating characteristics of the antigen assay are not improved by performing the assay using a greater dilution of patient serum/plasma.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1703/5/m_h81700087002.jpeg?Expires=1766009995&Signature=vb0EfL7hgR9vRpod8KK4xuMlCwGDJwqZV17q-NucZ-lQ1UceCs-6MM1ywgQ04-kXqVcwArkZJ0OP6MQH004HC1GVDEBCk8rHbR7b-mChW4K3ss0YV7EK3QoGmKq1iI0uBfI-brM201UUqXbYCbYMN3joLarbKahojzfYvbjf2ZY~HazPjvIXp145cYfYHgAIjGbDt9NA4Y2K58ByRnNC7hOC4cedHcEftwJusmMMvDdpUCNy0bPK1tKchYZCoW702qLr~i39MpsOUzA4Ooz89z2XurCNsgrfJm1bzUcIjg2XTC3zjX8N8dYfgSP9cYnnfJu-nImlT6ibv7ZD9vPjxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal