Abstract
Most patients with multiple myeloma (MM) present with symptoms, have evidence of generalized disease, and require chemotherapy promptly to reduce the malignant clone. Some patients present with a local symptom from a single plasmacytoma but no myeloma elsewhere. Such patients usually become free of symptoms after local radiotherapy. In patients with MM without symptoms, the diagnosis is made on the basis of screening laboratory tests. In patients with either solitary plasmacytoma of bone or asymptomatic MM, systemic treatment should be deferred until there is evidence of disease progression.
Solitary bone plasmacytoma
Some patients with plasma cell myeloma present with a single painful bone lesion due to a monoclonal plasma cell infiltrate, and further studies show no evidence of myeloma elsewhere. In other cases, solitary bone plasmacytoma (SBP) may be discovered during roentgenographic studies for another condition or the patient presents with a painless swelling of the sternum, rib, or other bone. SBP affects less than 5% of patients with plasma cell myeloma.1-3
Diagnosis
The diagnosis of SBP requires a solitary bone lesion, a biopsy of which shows infiltration by plasma cells; negative results on a skeletal survey; absence of clonal plasma cells in a random sample of bone marrow; and no evidence of anemia, hypercalcemia, or renal involvement suggesting systemic myeloma. Diagnostic criteria for SBP have varied over the years. Some older series included patients with 2 bone lesions or up to 10% bone marrow plasmacytosis. Other series excluded patients with disease that progressed within 2 years of diagnosis or in whom monoclonal protein persisted after local treatment.4-6
Recent advances have improved the precision of diagnosis. Flow cytometry studies and molecular detection of heavy- and light-chain gene rearrangements may reveal clonal plasma cells in the bone marrow of some patients who have no evidence of infiltration on light microscopy. Magnetic resonance imaging (MRI) is a noninvasive technique for sampling a large volume of bone marrow. We found that MRI revealed unsuspected involvement of bone marrow in 4 of 12 patients with apparent SBP.7 Liebross et al8 reported that among 23 patients with thoracolumbar SBP, multiple myeloma (MM) developed in 7 of 8 patients with a solitary lesion on plain radiographs alone but in only 1 of 7 patients who also had negative results on MRI of the spine (P = .08). Recommended diagnostic criteria for SBP that use on all sensitive techniques are shown in Table 1.
Recommended diagnostic criteria for solitary bone plasmacytoma
| Single area of bone destruction due to clonal plasma cells |
| Normal marrow without clonal disease |
| Normal results on a skeletal survey and magnetic resonance imaging of the spine, pelvis, proximal femora, and humeri |
| No anemia, hypercalcemia, or renal impairment attributable to myeloma |
| Absent or low serum or urinary level of monoclonal protein and preserved levels of uninvolved immunoglobulins |
| Single area of bone destruction due to clonal plasma cells |
| Normal marrow without clonal disease |
| Normal results on a skeletal survey and magnetic resonance imaging of the spine, pelvis, proximal femora, and humeri |
| No anemia, hypercalcemia, or renal impairment attributable to myeloma |
| Absent or low serum or urinary level of monoclonal protein and preserved levels of uninvolved immunoglobulins |
Clinical and laboratory features
Clinical features of SBP in 8 series with at least 25 patients each are shown in Table2.1,8-14 Two thirds of patients were men, and their median age was approximately 55 years, about 10 years younger than patients with MM. SBP may involve any bone but most often affects the axial skeleton, particularly a vertebra. One study found that in 20% of patients with SBP, a rib, the sternum, the clavicle, or the scapula was involved.15 The most common presenting symptom was pain due to bone destruction, but patients with vertebral involvement may also have evidence of spinal cord or nerve root compression. A few patients with SBP present with symptoms and signs of demyelinating polyneuropathy.16 In evaluating such patients, the syndrome of polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes (POEMS) should be considered.
Characteristics of patients with solitary bone plasmacytoma
| Series . | No. of patients . | Median age (y) . | Male patients (%) . | Spine disease (% of patients) . | Monoclonal protein (% of patients) . |
|---|---|---|---|---|---|
| Knowling et al1 | 25 | 50 | 68 | 32 | 24 |
| Brinch et al9 | 25 | 56 | 72 | 68 | 61 |
| Bolek et al10 | 27 | 55 | 70 | 33 | 52 |
| Jackson and Scarffe11 | 32 | 62 | 59 | 72 | 54 |
| Galieni et al12 | 32 | 52 | 81 | 40 | 47 |
| Holland et al13 | 32 | 60 | 70 | 34 | Not available |
| Frassica et al14 | 46 | 56 | 65 | 54 | 54 |
| Liebross et al8 | 57 | 53 | 69 | 40 | 72 |
| Series . | No. of patients . | Median age (y) . | Male patients (%) . | Spine disease (% of patients) . | Monoclonal protein (% of patients) . |
|---|---|---|---|---|---|
| Knowling et al1 | 25 | 50 | 68 | 32 | 24 |
| Brinch et al9 | 25 | 56 | 72 | 68 | 61 |
| Bolek et al10 | 27 | 55 | 70 | 33 | 52 |
| Jackson and Scarffe11 | 32 | 62 | 59 | 72 | 54 |
| Galieni et al12 | 32 | 52 | 81 | 40 | 47 |
| Holland et al13 | 32 | 60 | 70 | 34 | Not available |
| Frassica et al14 | 46 | 56 | 65 | 54 | 54 |
| Liebross et al8 | 57 | 53 | 69 | 40 | 72 |
Like MM, SBP has a lytic appearance on plain radiographs. In most patients, the lesion is purely lytic and has a clear margin and a narrow zone of transition to normal surrounding bone. Computed tomography (CT) and particularly MRI depict the extent of SBP more clearly. The MRI appearance of SBP is consistent with that of a focal area of bone marrow replacement; the signal intensity is similar to muscle on T1-weighted images and hyperintense relative to muscle on T2-weighted images. An extraosseous soft-tissue component is often present and may impinge on the spinal cord or spinal nerve roots.7
Electrophoresis of serum and urine samples reveals monoclonal protein in 24% to 72% of patients with SBP (Table 2), although levels of the protein are much lower than those patients with MM. All patients with SBP should undergo serum and urine immunofixation even when electrophoresis results are normal, since monoclonal protein will not be detected in approximately one third of patients. The significantly higher incidence of nonsecretory disease in patients with SBP compared with patients with MM probably reflects the very low tumor load. With disease progression, monoclonal protein may be found in the serum or urine in some patients.17 The preserved levels of uninvolved immunoglobulins observed in virtually all patients with SBP provide further evidence that the tumor load is low.
Treatment and response
Definitive local radiotherapy is the treatment of choice for SBP. Treatment fields should be designed to encompass all disease shown by MRI or CT scanning and should include a margin of normal tissue. For spinal lesions, the margin should include at least one uninvolved vertebra. After adequate radiotherapy, virtually all patients have relief of symptoms. The radiologic response to radiation therapy may include sclerosis and bone remineralization in up to 50% of patients followed up with plain radiography assessments.8,10 On MRI images, abnormalities of the bone marrow and an accompanying soft-tissue mass may persist even after successful treatment.8 Local control, defined as long-term clinical and radiographic stability, has been achieved in at least 90% of cases (Table 3).1,8-10,14Mendenhall et al18 reported a 6% incidence of local failure with doses of at least 4000 cGy and a 31% incidence with lower doses. Frassica et al14 did not observe local failure when the radiotherapy dose was 4500 cGy or larger. There is evidence of a higher proportion of local failures in patients with spinal lesions.5 14 Although the optimal dose of radiotherapy has not been established by prospective studies, most authors reporting series agree that a dose of approximately 4500 cGy (4000 cGy for vertebral lesions) provides the best local control without producing serious toxicity.
Outcome after radiotherapy in patients with solitary bone plasmacytoma
| Series . | No. of patients . | Local recurrence (% of patients) . | 10-y disease-free survival (% of patients) . | Median survival (y) . |
|---|---|---|---|---|
| Knowling et al1 | 25 | 0 | 16 | 7.5 |
| Brinch et al9 | 25 | 0 | Not available | 12.0 |
| Bolek et al10 | 27 | 4 | 46 | 10.0 |
| Frassica et al14 | 46 | 11 | 25 | 9.3 |
| Liebross et al8 | 57 | 4 | 42 | 11.0 |
| Series . | No. of patients . | Local recurrence (% of patients) . | 10-y disease-free survival (% of patients) . | Median survival (y) . |
|---|---|---|---|---|
| Knowling et al1 | 25 | 0 | 16 | 7.5 |
| Brinch et al9 | 25 | 0 | Not available | 12.0 |
| Bolek et al10 | 27 | 4 | 46 | 10.0 |
| Frassica et al14 | 46 | 11 | 25 | 9.3 |
| Liebross et al8 | 57 | 4 | 42 | 11.0 |
In most patients, the monoclonal protein is reduced markedly after completion of local radiotherapy. Serial and frequent measurements of myeloma protein for at least 6 months after treatment are required to confirm disease radiosensitivity. The rate of reduction may be slow and a continuous decrease in the protein may be observed for several years.17 The monoclonal protein disappears in a minority of patients (20%-50%), suggesting that all disease was included within the radiotherapy field. The likelihood of disappearance of monoclonal protein is higher in patients in whom the pretreatment value is low. In the series of Liebross et al,8 the paraprotein persisted in all patients with a serum level above 10 g/L and there was no dose-response relation between radiation dose and disappearance of monoclonal protein.8 In many patients, the monoclonal protein persists despite adequate radiotherapy, indicating the presence of tumor beyond the field of radiation. The condition of these patients may remain stable for a long time, and further treatment should be deferred until there is clear progression of the plasma cell disorder.
Although most patients with SBP of the spine can be treated with radiotherapy alone, some patients in whom the diagnosis of SBP has not yet been made present with or have rapid development of neurologic dysfunction that requires laminectomy before radiotherapy. An anterior approach may be preferred because posterior procedures do not address the source of the impingement and may not reliably relieve neurologic compromise. Surgical intervention may also be necessary in patients with vertebral instability.6 Surgical fixation of a pathologic fracture of a long bone may also be required.
Course of the disease
The outcome in patients with SBP in 5 series with at least 25 patients each who received local radiotherapy without adjuvant chemotherapy is shown in Table 3. In patients with SBP, the most common pattern of progression consists of new bone lesions, rising myeloma protein level, and development of marrow plasmacytosis. In some patients, new bone lesions with normal intervening marrow develop, consistent with a “macrofocal” pattern of growth.13,14,19 The median time to progression is 2 to 3 years, but MM has developed in a few patients as long as 15 years after radiotherapy. Most patients with early disease progression presumably had occult generalized disease at diagnosis, whereas some patients with an apparent late recurrence may have had indolent growth for many years or, possibly, development of a second primary plasma cell neoplasm. The lower median age of patients with SBP compared with those with MM and the later development of MM in most patients with SBP suggest that SBP represents an early manifestation of MM in some cases. On the other hand, the condition of some patients has remained stable for more than a decade despite persistence of low-level monoclonal protein. Such patients may have had reversion to stable monoclonal gammopathy of undetermined significance (MGUS) that may have preceded the SBP. When MM evolves, most patients have features of low-tumor-mass disease, a high rate of response to chemotherapy, and prolonged survival.8,14 20 Thus, the median overall survival time in patients with SBP averages 10 years, and 10% to 20% of patients die of unrelated causes (Table 3 and Figure1).
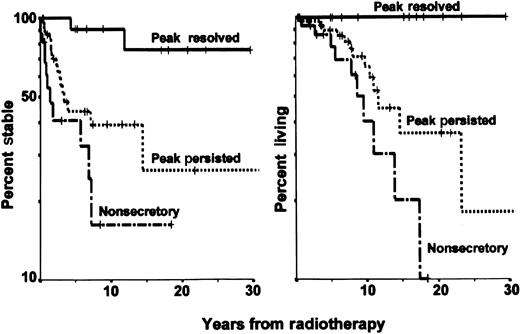
Remission and survival in solitary bone plasmacytoma.
The panel on the left shows the remission time in 61 patients with solitary plasmacytoma of bone treated at the MD Anderson Cancer Center, according to disappearance and persistence of myeloma protein or nonsecretory myeloma. The panel on the right shows cause-specific survival after radiotherapy in the same groups of patients.
Remission and survival in solitary bone plasmacytoma.
The panel on the left shows the remission time in 61 patients with solitary plasmacytoma of bone treated at the MD Anderson Cancer Center, according to disappearance and persistence of myeloma protein or nonsecretory myeloma. The panel on the right shows cause-specific survival after radiotherapy in the same groups of patients.
In a few patients with SBP, recurrent “solitary” plasmacytomas develop after adequate radiotherapy has been administered to the primary lesion. Careful assessments of the bone marrow, a bone survey, and MRI evaluations of the axial skeleton still fail to show evidence of MM. When isolated lesions develop at long intervals (ie, more than 2 years), radiotherapy alone may be followed by prolonged clinical stability. When new lesions develop at shorter intervals and are accompanied by other signs that suggest MM (falling levels of uninvolved serum immunoglobulins and new abnormalities on MRI studies), systemic treatment for MM may be justified. If there is doubt, longer follow-up without chemotherapy should clarify the clinical course.
Prognostic factors
With more sensitive staging procedures, the diagnosis of SBP should become less common, but the fraction of patients with prolonged stability, or even cure, should increase. In some series, no factors predictive of systemic recurrence could be identified.1,3,14,20,21 There was no relation between the dose of local radiotherapy and the likelihood of distant progression. Jackson and Scarffe11 suggested that osteopenia and low levels of uninvolved immunoglobulins were adverse prognostic factors. Immunoparesis was associated with an increased risk of progression in another study,12 and all 3 patients with low levels of uninvolved immunoglobulins in the MD Anderson study had disease progression18 (again, low levels of uninvolved immunoglobulins usually indicate occult MM). The size of the lesion appeared to predict conversion to MM in one series.13 In other series, relapse rates were higher in older patients or in those with axial lesions.5 In several series, disappearance of abnormal protein after local radiotherapy was associated with a high probability of long-term stability.2,12,22 An update of the MD Anderson series8 reported that among 11 patients with disappearance of myeloma protein, MM developed in only 2 (after 4 and 12 years, respectively), whereas MM developed in 57% of patients with persistent monoclonal protein and 63% of those with nonsecretory disease (P = .02; Figure 1). High levels of monoclonal protein and depression of uninvolved immunoglobulins raise major concerns regarding the diagnosis of SBP.
The role of adjuvant chemotherapy
In several series, chemotherapy was administered after completion of local radiation treatment. Mayr et al23 found that adjuvant chemotherapy may prevent progression to MM. Holland et al13 found that chemotherapy did not affect the overall rate of progression to myeloma but that adjuvant treatment changed the median time to progression from 29 to 59 months. In most other series, adjuvant chemotherapy had no beneficial effect.3,12,15 On the basis of the available data, we believe that adjuvant chemotherapy should not be administered to patients with SBP. Use of systemic chemotherapy or corticosteroids may obscure recognition of patients with disappearance of myeloma protein after radiotherapy who may be cured. Furthermore, early exposure to systemic treatment may hasten the evolution of resistant subclones and thereby restrict later therapeutic options, when they may be more useful. Moreover, in one series, secondary leukemia developed in 4 of 7 patients with SBP who received adjuvant melphalan-based chemotherapy after completion of radiotherapy.19
Asymptomatic MM
Definition
In recent years, many patients without any symptoms attributable to MM have been given a diagnosis of the disease by means of screening tests or during an investigation of another disorder. Coincidental discovery of abnormal laboratory values, especially an increased serum protein level, led to the recognition of MM. The concept of smoldering MM (SMM) or indolent myeloma was introduced by Kyle and Greipp24 and Alexanian17 20 years ago. These investigators defined a group of patients with myeloma who had a stable clinical course for several years before chemotherapy was necessary.
Most patients with asymptomatic myeloma have stage I disease according to the Durie and Salmon criteria25 and features of low-tumor-mass disease according to the MD Anderson criteria26 (serum myeloma protein below 45 g/L and hemoglobin level above 105 g/L). Distinguishing between asymptomatic myeloma and MGUS may be difficult. The latter condition is a relatively common plasma cell dyscrasia characterized by a serum monoclonal protein level below 30 g/L, less than 5% plasma cells in the bone marrow, no or only a small amount of urinary protein on Bence Jones assessment, absence of lytic bone lesions, and no related hypercalcemia, renal impairment, or anemia.27 When at least one lytic lesion is present, marrow plasmacytosis exceeds 15%, the serum monoclonal protein level is below 30 g/L on standard electrophoresis, or the Bence Jones urinary protein value is above 150 mg/day, asymptomatic MM is very likely. In patients with less than 5% marrow plasma cells, a serum monoclonal protein level below 20 g/L, a Bence Jones urinary protein value below 50 mg/day, and preserved uninvolved serum immunoglobulins, MGUS is highly likely (Table 4).28 Many patients do not meet all the criteria for either disorder, and other studies may be useful for differentiating between the diseases. Abnormal results on MRI, an increased plasma cell labeling index, or the presence of monoclonal circulating plasma cells in blood provides strong evidence that myeloma is either present or will soon develop.27 If there is doubt, long-term follow-up is required for clarification.
Comparison of monoclonal gammopathy of undetermined significance and asymptomatic myeloma
| Characteristic . | MGUS . | Asymptomatic myeloma . |
|---|---|---|
| Monoclonal protein | < 30 g/L | > 20 g/L in 85% |
| Marrow plasma cells | < 10% | > 10% |
| Uninvolved immunoglobulins | Preserved in 80% | Depressed in 90% |
| Urinary protein (Bence Jones) | None or < 50 mg/d | > 50 mg/d in 35% |
| Spine MRI results | Normal | Abnormal in 40% |
| Characteristic . | MGUS . | Asymptomatic myeloma . |
|---|---|---|
| Monoclonal protein | < 30 g/L | > 20 g/L in 85% |
| Marrow plasma cells | < 10% | > 10% |
| Uninvolved immunoglobulins | Preserved in 80% | Depressed in 90% |
| Urinary protein (Bence Jones) | None or < 50 mg/d | > 50 mg/d in 35% |
| Spine MRI results | Normal | Abnormal in 40% |
MGUS indicates monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging.
Some asymptomatic patients present with myeloma of intermediate stage, indicated by a serum myeloma protein level of at least 45 g/L or a hemoglobin level under 105 g/L. Despite the absence of symptoms, patients with such disease features should be treated soon after diagnosis because disease complications are likely to occur within 12 months.29 We will therefore focus here on patients with asymptomatic MM of low tumor mass (stage I).
Incidence
The incidence of asymptomatic MM varies among series and has increased during the past decade. Between October 1974 and December 1991, among the 638 consecutively seen, previously untreated patients with MM evaluated at MD Anderson Cancer Center, 95 patients (15%) had asymptomatic myeloma of low tumor mass.30 Riccardi et al31 reported that the percentage of asymptomatic patients who received a diagnosis by chance increased from 14% during the period 1972-1986 to 34% during the period 1987-1990. From August 1984 until December 1986 in a Norwegian health region with approximately 820 000 people, MM was found in 162 people, 71 (44%) of whom were asymptomatic.32 Age, sex, and myeloma-type distribution were similar in symptomatic and asymptomatic patients.
Treatment at diagnosis
Two prospective randomized trials assessed the role of systemic chemotherapy in asymptomatic MM. Hjorth et al33 randomly assigned 50 patients with asymptomatic myeloma stage I to immediate treatment with melphalan and prednisone or to deferred therapy, in which treatment with the same agents was started at the time of disease progression. No differences between the 2 groups in response rate, response duration, or survival were observed. A similar study with 44 patients was conducted by Grignani et al,34 who reported similar survival times of 58 months and 54 months, respectively, in patients randomly assigned to deferred or immediate treatment. Although neither study had enough patients to detect small differences, it is unlikely that immediate chemotherapy will prolong survival. Furthermore, in studies of other low-grade lymphoproliferative disorders, there was evidence that early treatment of asymptomatic patients does not prolong survival.35
Definition of progressive disease
Although almost all researchers who have reported series agree that patients with asymptomatic myeloma of low tumor mass should be followed up without treatment until there is evidence of disease progression, criteria for progressive tumor growth have not been clearly established. In 3 consecutive series at the MD Anderson Cancer Center, progressive disease was defined by an elevation of serum myeloma protein to at least 5.0 g/dL, an unequivocal increase in the size or number of bone lesions, or an obvious complication of myeloma, such as a pathologic fracture, anemia, hypercalcemia, or impairment of renal function. The myeloma protein level of 50 g/L was chosen after preliminary assessment showed that an increase to this value was nearly always associated with early subsequent morbidity. Wisloff et al36 initiated treatment in asymptomatic patients if at least one of the following complications occurred: skeletal pain or pathologic fracture, osteolytic destruction in weight-bearing bones, hemoglobin level below 90 g/L, renal impairment, hypercalcemia, proteinuria (Bence Jones value > 4 g/24 hours), neurologic symptoms, or recurring infections. Facon et al37 defined progression as evolution of asymptomatic stage I to Durie and Salmon stage II or III.
Prognostic features for disease progression
Unless death due to another condition occurs, all patients with asymptomatic myeloma will eventually have disease progression and require treatment. Although the median time to progression is approximately 3 years, identification at diagnosis of patients with a high or low likelihood of early progression (ie, within 12 or 18 months) is important. Obviously, follow-up in patients with a high likelihood should be more frequent and stringent than that in patients with a low likelihood.
Several studies have described prognostic factors that identified patients in whom the disease was likely to progress early. The results of 3 large studies in which a multivariate analysis was performed are shown in Table 5.30,36,37 Some patients with asymptomatic bone lesions were included in these studies, and the lesions were associated with a median time to progression of less than 12 months.30,36 However, surveillance is not appropriate in such patients, who should be treated soon after diagnosis. Other independent variables associated with earlier progression were greater bone marrow plasmacytosis, higher serum monoclonal protein level, lower hemoglobin value, and the presence of proteinuria on Bence Jones assessment.30,36 37
Characteristics of patients and prognostic factors for progression of asymptomatic myeloma in 3 series
| . | Wisloff et al36 . | Dimopoulos et al30 . | Facon et al37 . |
|---|---|---|---|
| No. of patients | 71 | 95 | 91 |
| Lytic lesions (%) | 15 | 18 | 4 |
| Median time to progression (mo) | 26 | 26 | 48 |
| Adverse prognostic factors | |||
| Bone lesions | Yes | Yes | Yes |
| Bone marrow plasmacytosis | Yes | No | Yes |
| Serum monoclonal protein | No | Yes | Yes |
| Urinary protein (Bence Jones) | No | Yes | No |
| Mild anemia | No | No | Yes |
| . | Wisloff et al36 . | Dimopoulos et al30 . | Facon et al37 . |
|---|---|---|---|
| No. of patients | 71 | 95 | 91 |
| Lytic lesions (%) | 15 | 18 | 4 |
| Median time to progression (mo) | 26 | 26 | 48 |
| Adverse prognostic factors | |||
| Bone lesions | Yes | Yes | Yes |
| Bone marrow plasmacytosis | Yes | No | Yes |
| Serum monoclonal protein | No | Yes | Yes |
| Urinary protein (Bence Jones) | No | Yes | No |
| Mild anemia | No | No | Yes |
The plasma cell labeling index and the peripheral blood B-cell labeling index (using tritiated thymidine or bromodeoxyuridine) are low in patients with SMM, indicating a low proliferative index. Studies at the Mayo Clinic showed that patients with asymptomatic myeloma in whom either index is more than 0.4% are likely to have progression to symptomatic myeloma within 12 months.38,39 A more recent study at the same institution evaluated the number and labeling index of circulating peripheral blood monoclonal plasma cells (PBPC) in 17 patients with newly diagnosed SMM. These investigators found that the median time to progression in patients with abnormal PBPC (defined as an increase in the number or proliferative rate of circulating PBPC) was 0.75 years, whereas it was 2.5 years for those patients without abnormal PBPC (P < .01).40
In several studies, MRI was performed in patients with asymptomatic myeloma without bone lesions on plain radiographs (Table6).41-43Abnormal MRI results occurred in 29% to 50% of patients and were associated with earlier progression in all series. In a study of 55 patients with asymptomatic myeloma, Mariette et al43conducted a multivariate analysis of variables associated with disease progression and demonstrated that the only significant factors were abnormal MRI results and the presence of at least 20% plasma cells in the bone marrow.
Prognostic importance of MRI in asymptomatic myeloma
| Series . | No. (%) of patients . | Median time to progression (mo) . | P value . |
|---|---|---|---|
| Moulopoulos et al41 | |||
| Normal MRI results | 19 (50) | 43 | |
| Abnormal MRI results | 19 (50) | 16 | <.01 |
| Vande Berg et al42 | |||
| Normal MRI results | 17 (71) | 32 | |
| Abnormal MRI results | 7 (29) | 10 | <.0001 |
| Mariette et al43 | |||
| Normal MRI results | 38 (69) | Not reached | |
| Abnormal MRI results | 17 (31) | Not reached | <.0001 |
| Series . | No. (%) of patients . | Median time to progression (mo) . | P value . |
|---|---|---|---|
| Moulopoulos et al41 | |||
| Normal MRI results | 19 (50) | 43 | |
| Abnormal MRI results | 19 (50) | 16 | <.01 |
| Vande Berg et al42 | |||
| Normal MRI results | 17 (71) | 32 | |
| Abnormal MRI results | 7 (29) | 10 | <.0001 |
| Mariette et al43 | |||
| Normal MRI results | 38 (69) | Not reached | |
| Abnormal MRI results | 17 (31) | Not reached | <.0001 |
MRI indicates magnetic resonance imaging.
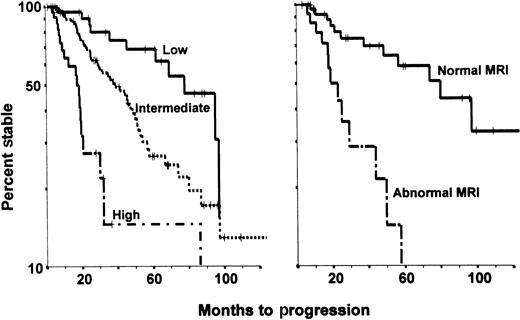
An analysis at the MD Anderson Cancer Center that excluded asymptomatic patients with bone lesions on plain radiographs revealed 3 dominant prognostic factors predictive of early progression: serum monoclonal protein level above 30 g/L, IgA heavy-chain type, and Bence Jones protein excretion value above 50 mg/day. The presence of 2 or more of these features identified high-risk disease with a median time to progression of 17 months. The presence of only one adverse feature was associated with a median time to progression of 40 months, whereas the absence of all adverse variables was associated with prolonged stability (median time to progression of 95 months).44 The same study concluded that MRI of the spine was particularly useful in patients with an intermediate time to progression; abnormal MRI results were observed in 40% of such patients and were associated with a median time to progression of 21 months (Figure2).
Progression of asymptomatic myeloma.
The panel on the left shows the variable times to disease progression in 123 asymptomatic patients with multiple myeloma at the MD Anderson Cancer Center, according to risk status defined by the presence of 0, 1, or 2 or more of the following abnormalities: monoclonal protein level above 30 g/L, IgA myeloma protein type, and urinary protein level above 50 mg/day on Bence Jones assessment (P < .01). The panel on the right shows the variable times to disease progression in 43 asymptomatic patients considered at intermediate risk on the basis of magnetic resonance imaging results (P < .01).
Progression of asymptomatic myeloma.
The panel on the left shows the variable times to disease progression in 123 asymptomatic patients with multiple myeloma at the MD Anderson Cancer Center, according to risk status defined by the presence of 0, 1, or 2 or more of the following abnormalities: monoclonal protein level above 30 g/L, IgA myeloma protein type, and urinary protein level above 50 mg/day on Bence Jones assessment (P < .01). The panel on the right shows the variable times to disease progression in 43 asymptomatic patients considered at intermediate risk on the basis of magnetic resonance imaging results (P < .01).
Outcome after progression
All patients with tumor progression require systemic treatment. Response rates in patients who initially presented without symptoms were similar to those in symptomatic patients treated at diagnosis, as were the median survival times after treatment.26,30,36The similar outcomes indicated that drug-resistant tumor cells did not expand during the surveillance period. After initiation of chemotherapy for disease progression, survival times in previously asymptomatic patients were similar among groups of patients, despite widely different times to progression.30,37 Asymptomatic patients with a low risk of progression had a median overall survival time longer than 6 years.30 37 This indicated that the extent of disease at diagnosis and the rate of tumor growth before chemotherapy had a major impact on the overall survival of patients with asymptomatic myeloma.
Biologic features of asymptomatic myeloma and MGUS
Analysis of several series of patients with asymptomatic myeloma indicates that patients with this disease represent a heterogeneous group. Although one fourth of patients require treatment within a year after diagnosis, the condition of others may remain stable for 5 years or longer. Clinical and laboratory features that predict prolonged disease stability have been identified at diagnosis, but the factors that maintain disease dormancy and those that are responsible for disease progression are not known.
A substantial amount of knowledge regarding the pathogenetic mechanisms of myeloma has accumulated in recent years. In addition, studies of patients with MGUS have revealed links between this disorder and myeloma. SBP and asymptomatic myeloma could be considered intermediate steps in the evolution from MGUS to myeloma.45 46
Studies of MGUS and myeloma have focused on cytogenetic factors, cytokines, adhesion molecules, and angiogenesis. Similar studies of the plasma cells of SPB or of asymptomatic myeloma are rare; however, because at least one third of patients with myeloma have disease evolution after a history of MGUS, it seems reasonable to assume that there are similar “markers” of tumor growth in all clonal plasma cell disorders.47
Cytogenetic studies
Initial studies of plasma cells in patients with MGUS rarely showed chromosomal abnormalities. More recently, studies using fluorescent in situ hybridization confirmed the presence of abnormalities in MGUS similar to those in overt MM. Calasanz et al48 reported chromosomal abnormalities in 25% of MGUS clones, Fonseca et al49 found 14q32 abnormalities in 66% of MGUS cases, and abnormal IgH recombinations were observed by Avet-Loiseau et al46 in 58% of patients with MGUS and 59% with myeloma. More specifically, 14q32 abnormalities were observed in 46% of patients with MGUS or SMM and were present in the majority of clonal plasma cells. These observations support the conclusion that mutations at the immunoglobulin heavy-chain gene locus 14q32 represent the primary genetic abnormality in clonal plasma cell dyscrasias and that additional subsequent abnormalities lead to the evolution of MM.50,51 The t(11;14)(q13;q32) occurs in 15% of MGUS or SMM patients, an incidence similar to that in myeloma; however, t(4;14)(p16;q32) occurs in 2% of MGUS or SMM patients but in 12% of patients with overt myeloma.47
Deletions of chromosome 13, which confer a poor prognosis in myeloma, were found in 39% of myeloma cases but in 83% of myeloma cases that evolved from MGUS and in 4 of 19 patients (20%) with stable MGUS.46 In addition, a monoallelic deletion of the 13q14 region was found in 21% of patients. The deletion was present in the majority of clonal plasma cells in patients with SMM or overt myeloma but in fewer clones in those with MGUS46 (Table7). Chromosomal aneuploidy (trisomies 3, 7, 9, and 11) was reported in 50% of patients with MGUS and 13q14 abnormalities in 46%.52
Chromosomal abnormalities in plasmacytic proliferations
| Abnormality . | MGUS (%) . | Asymptomatic myeloma (%) . | Overt myeloma (%) . | Reference . |
|---|---|---|---|---|
| 14q32 | 58 | 77 | 59 | Avet-Loiseau et al45 |
| t(11;14) | 15 | 15 | 15 | Avet-Loiseau et al46 |
| t(4;14) | 2 | 2 | 12 | Avet-Loiseau et al46 |
| −13/13q− | 21 | 23 | 36 | Avet-Loiseau et al45 |
| Abnormality . | MGUS (%) . | Asymptomatic myeloma (%) . | Overt myeloma (%) . | Reference . |
|---|---|---|---|---|
| 14q32 | 58 | 77 | 59 | Avet-Loiseau et al45 |
| t(11;14) | 15 | 15 | 15 | Avet-Loiseau et al46 |
| t(4;14) | 2 | 2 | 12 | Avet-Loiseau et al46 |
| −13/13q− | 21 | 23 | 36 | Avet-Loiseau et al45 |
Values are percentages of patients with the abnormality.
Cytokines
Interleukin (IL) 6 is considered the principal growth factor in the progression of MM. Significantly elevated serum IL-6 levels were found in 3% of patients with MGUS or SMM, 35% with overt MM, and 100% of plasma cell leukemias.53 IL-6 receptor (IL-6R) consists of a membrane protein, CD126, and a signal-transducing molecule, CD130. Expression of IL-6R components in MGUS and MM was studied by Barille et al.54 CD126 was detectable in 50% of patients with either MGUS or MM and did not change during disease progression, whereas CD130 was expressed in 43% of MM patients at diagnosis and in 88% at relapse. Elevations of serum soluble IL-6R were also reported in both MGUS and MM.55 56
IL-1β and tumor necrosis factor α (TNF-α) are bone-resorbing cytokines and therefore of interest in research on plasmacytic neoplasms. Studies using sensitive techniques suggested that TNF-α is produced by the plasma cells of both myeloma and MGUS, whereas IL-1β messenger RNA (mRNA), but not the protein, could be detected.57 In a similar study, Lacy et al58detected IL-1β mRNA in 49 of 51 patients with active myeloma, 7 of 7 with SMM, 5 of 21 with MGUS, and 0 of 5 healthy controls. Bone lesions were present in 40 patients with MM, and all had detectable IL-1β mRNA. Serial measurements in patients with MGUS may clarify whether the presence of IL-1β mRNA is predictive of progression of MGUS to myeloma.
IL-10 is also increased in patients with plasma cell leukemia or solitary plasmacytoma, with growth factor activity mediated through a gp130 cytokine, oncostatin M.59 Therefore, although cytokines may not be an etiologic factor in myeloma, they are important in the proliferation of the malignant clone.
Surface markers and adhesion molecules
The phenotypes of plasma cells in cases of MGUS and myeloma are also of interest. Ocqueteau et al60 studied 76 patients with MGUS, 65 with MM, and 10 control subjects by using a large panel of monoclonal antibodies against plasma cell–related antigens. They concluded that residual polyclonal plasma cells are always present in MGUS but only rarely present in MM. CD44, a polymorphic glycoprotein involved in adhesion, and variant isoforms (CD44v) were studied in patients with myeloma by van Driel et al.61 Both CD44v9+ and CD44v10+ were expressed on plasma cells of healthy persons, whereas CD44v9−v10+was expressed in patients with stable myeloma and CD44v9+v10− in patients with progressive myeloma. Assessment of certain adhesion molecules, such as CD56, can differentiate MGUS (CD56−) from myeloma (CD56+).62
Angiogenesis
The role of angiogenesis in tumor progression and the potential of antiangiogenic substances as therapy have recently received much attention. Angiogenesis correlates with plasma cell growth (S-phase fraction) in both MGUS and MM.63 Levels of angiogenic basic fibroblast growth factor 2 protein in plasma cell extracts from patients with active MM were higher than those from patients with nonactive myeloma and MGUS. Also, levels of matrix metalloproteinase-2 mRNA and protein were higher in patients with active MM than in those with responding MM or stable MGUS.64 Di Raimundo et al65 reported increased levels of vascular endothelial growth factor (VEGF) in plasma of both the peripheral blood and bone marrow in patients with active MM, whereas in patients with MGUS or early myeloma, elevated VEGF levels were observed only in bone marrow plasma.
Bone marrow microvessel area was increased in patients with active MM compared with control subjects but not in patients with nonactive MM or MGUS. Furthermore, conditioned medium from plasma cell cultures showed angiogenic potential in 76% of active myeloma cases, 33% of nonactive cases, and 20% of MGUS cases.63 Nevertheless, no direct correlations have so far been found between levels of these angiogenic factors and the extent of neovascularization in bone marrow of myeloma patients. Studies of other factors with angiogenic potential, such as VEGF, TNF-α, transforming growth factor β, and IL-1β, are under way.
On the basis of the findings described above, clinical trials of thalidomide (an antiangiogenic drug) in the treatment of myeloma were begun. Results were encouraging, although there was no correlation between marrow vascularization and response.66 Thalidomide possibly also acts by mechanisms other than antiangiogenesis, such as a direct antitumor effect; by altering regulatory cytokines; or by means of its immunomodulatory effects.67
Other factors
Other features may be involved in the transition from the premalignant to the malignant phase of plasmacytic proliferation. Using a flow cytometric method, Witzig et al68 defined a plasma cell growth index and a plasma cell apoptotic index and compared marrow from patients with MGUS, SMM, and MM. They found a decrease in apoptosis during progression from MGUS to SMM and MM, without a significant increase in proliferation.
With regard to bone disease, Bataille et al69 found excessive bone resorption on bone biopsy in 16% of patients with low-risk MGUS, 46% with high-risk MGUS, 79% with SMM, and 93% with MM. Excessive bone resorption was present in 52% of patients with MGUS in whom MM subsequently developed but in only 4% of those with stable MGUS.
Finally, hypogammaglobulinemia, a typical finding in overt myeloma, may also be present in earlier phases of the disease. A decrease in levels of uninvolved immunoglobulins was found in 20% to 30% of MGUS patients.70 CD5+ B cells affect immunoglobulin production, and in a study by Paglieroni et al,71 the numbers of these cells were found to increased in 7 of 10 MGUS patients who had progression to MM but in none of 11 patients with stable MGUS. Also, 8 of 8 patients with localized plasmacytoma and increased CD5+ B cells had progression to MM or recurrence of plasmacytoma, whereas 0 of 9 with normal CD5+ B cells numbers remained healthy. It was hypothesized that these suppressive cells may have a role in disease regulation in addition to their role in hypogammaglobulinemia.
Future directions
In most patients with SPB and residual myeloma protein or with asymptomatic myeloma and increased risk factors, overt MM develops within 3 years. Such patients are candidates for trials of new agents that are rational and entail a low likelihood of serious toxicity. This applies to drugs that are active in vitro or against rodent plasmacytomas or human B-cell leukemias and lymphomas. For example, we confirmed some of the earliest clinical evidence of activity for interferon α, dexamethasone, and paclitaxel; we failed to observe a benefit with all-trans retinoic acid; and we are now assessing the value of thalidomide. In view of the projected long survival of most patients, more intensive therapy supported by autologous stem cell transplantation should be deferred until MM becomes more evident, when the potential clinical benefit clearly outweighs the risks. The prophylactic role of bisphosphonates in maintaining bone mineralization appears less clear, since no controlled studies have been conducted in patients without lytic bone lesions or in very early phases of disease. One can envision randomized multicenter trials in selected patients with asymptomatic myeloma and reduced bone density.
Acknowledgments
We thank Kay Delasalle and Dimitra Gika for technical and secretarial assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Meletios A. Dimopoulos, 227 Kifissias, Avenue Kifissia, Athens 14561 Greece; e-mail: mdimop@cc.uoa.gr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal