Abstract
The deregulated Bcr/Abl tyrosine kinase is responsible for the development of Philadelphia (Ph)-positive leukemia in humans. To investigate the significance of the C-terminal Abl actin-binding domain within Bcr/Abl p190 in the development of leukemia/lymphoma in vivo, mutant p190 DNA constructs were used to generate transgenic mice. Eight founder and progeny mice of 5 different lines were monitored for leukemogenesis. Latency was markedly increased and occurrence decreased in the p190 del C lines as compared with nonmutated p190BCR/ABL transgenics. Western blot analysis of involved hematologic tissues of the p190 del C transgenics with end-stage disease showed high-level expression of the transgene and tyrosine phosphorylation of Cbl and Hef1/Cas, proteins previously shown to be affected by Bcr/Abl. These results show that the actin-binding domain of Abl enhances leukemia development but does not appear to be an absolute requirement for leukemogenesis.
Introduction
Philadelphia (Ph)-positive leukemia in humans is characterized by a t(9;22) chromosomal translocation, which leads to the expression of the Bcr/Abl oncoprotein. Bcr/Abl consists of the major part of the tyrosine kinase Abl joined to a variable part of Bcr. Mouse models have demonstrated the correlation between the expression of Bcr/Abl and the development of leukemia.1 2
Much attention in recent years has focused on unraveling the signal transduction pathways used by Bcr/Abl to cause leukemogenicity.3,4 In multiple studies, the deregulated tyrosine kinase activity of Abl was found to be an essential element of this process, resulting in the increased tyrosine phosphorylation of several substrates.5 Some intriguing concepts have emerged concerning the normal and deregulated Abl kinase. In 1991, McWhirter and Wang6 reported that the Bcr sequences in Bcr/Abl activate 2 functions in Abl: its tyrosine kinase activity and a previously undescribed microfilament binding activity. Subsequently, an actin-binding activity was identified in the C-terminal end of Abl.7,8 Deletion of the actin-binding domain of Abl in Bcr/Abl greatly reduced its oncogenicity in fibroblasts.7A recent study using erythropoietin receptor/Abl fusion proteins concluded that the C-terminal actin-binding domain of c-Abl plays an important role in the proliferation and transformation caused by this fusion protein.9
Thus, the C-terminal domain of Bcr/Abl enhances its binding to the actin cytoskeleton, which may result in the increased tyrosine phosphorylation of certain cytoskeletal-associated proteins such as talin, vinculin, or proteins that transduce signals from the cytoskeleton such as Crkl, Cas, Cbl, or Hef1.10-15 In addition, studies on chronic myelogenous leukemia patient material and cells expressing Bcr/Abl have shown differences compared with controls with respect to cell adhesion and motility, processes dependent upon the actin cytoskeleton.16-21
To investigate the oncogenic potential of the p190 Bcr/Abl protein, we generated p190 BCR/ABL transgenic mice, which rapidly and reproducibly developed an aggressive lymphoblastic leukemia/lymphoma.22-25 One line derived from such founders has been maintained since 1992, and animals continue to reproducibly die of a characteristic leukemia/lymphoma that mimics the leukemia associated with p190 Bcr/Abl in humans.
No studies thus far have addressed the issue of the transforming potential of a Bcr/Abl mutant lacking the C-terminal Abl actin-binding domain directly in vivo in transgenic mice. To examine this, we have in the current study generated p190 BCR/ABL mice that are transgenic for constructs encoding a C-terminal actin-binding domain deletion. Results of these studies show that this mutation has a profound effect on the leukemogenic potential of Bcr/Abl p190 in vivo in transgenic mice.
Materials and methods
DNA construct
To delete the C-terminal actin-binding domain of Abl, stop codons were introduced after the BglII site at nucleotide 1066 of c-ABL using the polymerase chain reaction (PCR). The mutation was then transferred into the original transgenic δMT-p190 construct.22 p190-encoding inserts were also subcloned into an SV-40–based expression vector. These were transfected to COS1 cells as previously described.26
Transgenic mice and pathology
Vector sequences were separated from inserts by digestion withSstII. Transgenic mice were generated as described previously22 in a C57Bl/CBA F1 background. Mice of the no. 4208 line were bred to “homozygosity” to produce animals with 2 transgenic alleles. The average age at death (all causes) was 19.4 months (n = 17; range 5.5-32 months) for the single transgenics and 11.8 months (n = 27; range 2.5-24 months) for the double transgenics.
Peripheral blood samples were drawn from the tail artery and processed and assayed for BCR/ABL expression using reverse transcription (RT)-PCR as described.23 24 Animals were killed and autopsied when they became visibly ill. Tissues were fixed in buffered formalin. Routine histology included bone marrow, liver, and spleen.
Peripheral blood and bone marrow samples were analyzed using double-color flow cytometry on a FACScan (Becton Dickinson, Lincoln Park, NJ) as described27 and monoclonal antibodies anti-CD45R (RA3-6B2), anti-Thy-1.2 (53-2.1), anti-GR-1 (RB6-8C5) (PharMingen, San Diego, CA), and anti-Mac-1 (M1/70.15) (Caltag, Burlingame, CA).
Competitive quantitative PCR
To generate a competitor, a 305–base pair (bp) RT-PCR product was generated from the peripheral blood of a leukemic p190 mouse with oligonucleotides ALL E and ALL F.24 This product spans theBCR/ABL junction in the messenger RNA (mRNA) encoding p190. This plasmid was digested withBsu36I × NcoI, the small fragment removed, and the plasmid religated. ALL E and ALL F primers generate a 260-bp fragment from this plasmid. The DNA concentration of the plasmid was determined by measurement of the OD260 and by electrophoresis of aliquots of DNA on an agarose gel together with samples of known concentration. Different dilutions were made so that 1 μL would contain between 0.5 × 107 and 0.5 × 103 molecules of competitor. RT-PCR reactions were performed as described previously24 using 2 μg of total bone marrow RNA or the equivalent of 2 μL of peripheral blood. Competitor was added before the PCR step. Using this method, 2 μL of peripheral blood from an overtly leukemic mouse was found to contain approximately 106BCR/ABL mRNAs. Total RNA was isolated essentially as described24 from bone marrow or peripheral blood. Animal No. 2207 was a p190 wild-type transgenic that developed leukemia/lymphoma. Total RNA was isolated from its involved spleen. Animal No. 4445 was overtly healthy and was killed at the age of 6 months. Examination of peripheral blood and bone marrow revealed no evidence for the development of leukemia. p190 wild-type no. 1 and no. 2 were overtly healthy animals that were killed at the age of 2 months. Bone marrow and peripheral blood showed no evidence of leukemogenesis.
Western and far-Western blot analysis
Tissues were dissected from animals at autopsy and stored on ice until further processing. Some of the Crkl SH2-binding tyrosine-phosphorylated proteins are very susceptible to dephosphorylation. Therefore, most samples were minced and then immediately homogenized in 2 × sodium dodecyl sulfate (SDS) sample buffer lacking DTT (dithiothreitol) and bromophenol blue using a straight-wall tissue grinder. After boiling for 5 minutes, insoluble residues were removed by centrifugation and the protein concentration determined using the BCA method (Pierce, Rockford, IL).
For direct Western blot analysis, 30 μg of protein was run per lane. The Ab-2 and Ab-3 anti-c-Abl antibodies were from Oncogene Science, Inc (Uniondale, NY). The anti-Abl monoclonal antibody 3F12 was a kind gift from Dr Ravi Salgia (Dana-Farber Cancer Institute, Boston, MA). Far-Western blot analysis using the Crkl SH2 domain was performed as described.26
For analysis of Crkl SH2-binding proteins, extracts in sample buffer were diluted 50 times in pull-down buffer consisting of 50-mmol/L Tris-HCl, pH 7.5; 100-mmol/L NaCl; 1-mmol/L ethylenediaminetetraacetic acid; 10-mmol/L NaF; 0.1% Tween 20; and 0.2% (weight/vol) bovine serum albumin. Samples were further processed using GST-Crkl SH2 as described.11 Anti-Cas monoclonal antibodies, which recognize both Cas and Hef1 as well as the antiphosphotyrosine antibodies RC20H, were obtained from Transduction Laboratories (Lexington, KY).
Results
Transgenes and transgenics
High-level expression of Bcr/Abl during embryonic development in transgenic mice—for example, from the BCR promoter—leads to prenatal lethality.28 Therefore, a truncated methallothionein (MT) promoter was used previously in the nonmutant p190 transgenics to control transgene expression.22 24This p190 construct allowed embryonic development, most probably because of spatiotemporally restricted expression or limited expression levels of the transgene. In concordance with this, Bcr/Abl p190 protein was not detectable in any of the healthy tissues of these mice (unpublished results).
To prevent the above-mentioned problems asssociated with high-level Bcr/Abl expression during murine development, the DNA construct encoding a C-terminally deleted form of Bcr/Abl p190 was also put under control of the truncated MT promoter. Futhermore, to allow direct comparison with the nonmutated p190, the mutant construct was made using the same components as the original p190 construct. Bcr/Abl p190 del C contained a deletion of the terminal 31 amino acid residues, a region that encompasses the actin-binding domain of Abl7(Figure 1B). To ensure that this construct produced a stable protein product of the right size, the insert was subcloned into an SV-40–based expression vector and transiently transfected to COS-1 cells. As shown in Figure 1A, the p190 del C construct encoded a single protein (lane 4), which was slightly smaller than the wild-type p190 Bcr/Abl (lanes 1,3). In agreement with the deletion of its C-terminal sequences, the p190 del C protein reacted very poorly with antibodies directed against the Abl C-terminal region (Figure 1A, lane 2), although it was readily detected with antisera against a more N-terminal segment of Abl (Figure 1A, lane 4). These results indicate that the mutant protein is stable.
Expression of the Bcr/Abl p190 del C mutant.
(A) Western blot analysis of expression of p190 Bcr/Abl proteins in COS cells. Lanes 1 and 2 were reacted with antiserum directed against the C-terminal end of Abl (Oncogene Science, Inc, c-Abl Ab-2), whereas lanes 3 and 4 were incubated with antibodies directed against more upstream sequences of Abl (Oncogene Science, Inc, c-Abl Ab-3). COS-1 cells were transfected with p190 wild type (lanes 1,3) or p190 del C (lanes 2,4). (B) Schematic representation of the Bcr/Abl p190 del C mutant protein. The dotted region represents the N-terminal oligomerization domain of Bcr; the open box, other sequences encoded by Bcr exon 1. The hatched area represents the Abl protein moiety.
Expression of the Bcr/Abl p190 del C mutant.
(A) Western blot analysis of expression of p190 Bcr/Abl proteins in COS cells. Lanes 1 and 2 were reacted with antiserum directed against the C-terminal end of Abl (Oncogene Science, Inc, c-Abl Ab-2), whereas lanes 3 and 4 were incubated with antibodies directed against more upstream sequences of Abl (Oncogene Science, Inc, c-Abl Ab-3). COS-1 cells were transfected with p190 wild type (lanes 1,3) or p190 del C (lanes 2,4). (B) Schematic representation of the Bcr/Abl p190 del C mutant protein. The dotted region represents the N-terminal oligomerization domain of Bcr; the open box, other sequences encoded by Bcr exon 1. The hatched area represents the Abl protein moiety.
Vector sequences were removed, and the mutant construct was used to generate transgenic mice. Eight p190 del C F0 animals were obtained (Table 1). Southern blot analysis of tail DNAs showed that copy numbers of the transgenes varied from 1 to approximately 8 (Table 1). All animals were bred with C57Bl/CBA F1 mice to produce offspring; 5 of the 8 F0 mice produced transgenic progeny (Table 1).
BCR/ABL p190 del C transgenic founders
| Founder no. . | Age at death (mo) . | RT-PCR* . | Cause of death . | Progeny . | Est. transgene copy number . |
|---|---|---|---|---|---|
| 4208 | 28 | + | Lymphoma | Yes | 3 |
| 4212 | 24 | − | Unknown† | No | 1 |
| 4227 | 21 | + | Unknown† | No | 6-8 |
| 4231 | 21 | − | Infection‡ | Yes | 3 |
| 4242 | 22 | + | Unknown† | No | 2 |
| 4243 | 27.5 | + | Carcinoid lung tumor1-153 | Yes | 3 |
| 4237 | 27.5 | + | Lung adenoma | Yes | 2 |
| 4266 | 12 | − | Found dead | Yes | 1 |
| Founder no. . | Age at death (mo) . | RT-PCR* . | Cause of death . | Progeny . | Est. transgene copy number . |
|---|---|---|---|---|---|
| 4208 | 28 | + | Lymphoma | Yes | 3 |
| 4212 | 24 | − | Unknown† | No | 1 |
| 4227 | 21 | + | Unknown† | No | 6-8 |
| 4231 | 21 | − | Infection‡ | Yes | 3 |
| 4242 | 22 | + | Unknown† | No | 2 |
| 4243 | 27.5 | + | Carcinoid lung tumor1-153 | Yes | 3 |
| 4237 | 27.5 | + | Lung adenoma | Yes | 2 |
| 4266 | 12 | − | Found dead | Yes | 1 |
Expression was measured at 4 weeks of age on peripheral blood samples using RT-PCR and hybridization with a specific oligonucleotide probe as described previously for BCR/ABL p190 transgenics.22
No significant pathologic findings were noted.
Animal had splenomegaly with a left shift in the myeloid series and decreased B- and T-cell numbers in bone marrow. However, no Bcr/Abl or endogenous c-Abl expression could be detected in the spleen.
Bcr/Abl protein could not be detected in the tumor. The animal also had regenerative liver nodules/hyperplasia.
Compared with nonmutated p190, p190 del C is significantly less leukemogenic
In agreement with the low-level activity of the MT promoter that controlled transgene expression, we were unable to detect Bcr/Abl p190 protein in healthy tissues of the mice transgenic for mutant p190 (not shown). However, to be able to draw conclusions concerning the biologic activity of the mutants, it was important to examine whether the transgene was expressed in vivo.
Previously, RT-PCR assays were developed to examine BCR/ABLp190 mRNA in wild-type p190 transgenics.24 Detailed studies in one line, no. 623, showed that BCR/ABL mRNA was generally not detected in the peripheral blood of such animals at an early age. Serial sampling of peripheral blood of these mice revealed that BCR/ABL p190 mRNA began to be detected as the animals grew older and invariably preceded the development of overt leukemia.23,24 These data were consistent with a model in which a very low level of basal expression from the transgene in a primitive hematopoietic cell in the bone marrow eventually led to other mutations in that cell, resulting in blocked maturation and malignant outgrowth.23 24
To analyze expression from the mutant transgene, blood samples were taken from all F0 animals at the age of 4 weeks. As indicated in Table 1, at this age a substantial number of transgenics showed expression of the mutant BCR/ABL p190 transgene in peripheral blood leukocytes.
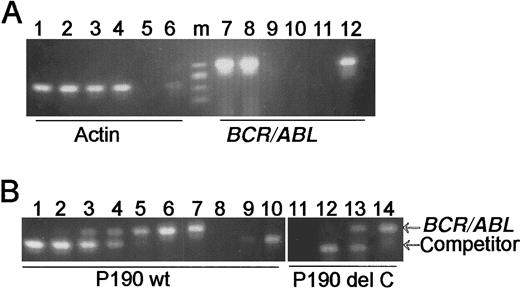
As indicated above, animals transgenic for the nonmutatedBCR/ABL p190 invariably develop leukemia/lymphoma. One line of p190 transgenic mice that has been maintained shows a characteristic pattern of transgene expression in which preleukemic animals lack expression of p190 mRNA in their peripheral blood. To compare expression of the transgenes of these animals with that of the p190 del C transgenics, total RNA was isolated from the bone marrow and peripheral blood of 2 young wild-type p190 mice and analyzed forBCR/ABL mRNA. As shown in Figure2A, both peripheral blood and bone marrow of these animals contained RNA that was suitable for RT-PCR (Figure 2A, lanes 1-4), but, as expected, only the bone marrow contained detectableBCR/ABL mRNA (lanes 7,8). To quantitate expression levels in these preleukemic animals, competitive quantitative RT-PCR was used. This analysis showed that 2 μg of total RNA from the bone marrow of such a wild-type p190 transgenic contained about 0.5 × 104 molecules of BCR/ABL mRNA (Figure2B, lane 4). A similar analysis with 2 μg of total bone marrow of a mouse transgenic for p190 del C showed that this transgene was expressed at approximately the same level (Figure 2B, lane 13).
Quantitative competitive PCR analysis of p190 wild-type and p190 del C expression.
(A) RT-PCR analysis of wild-type p190 expression. Primers are as indicated beneath the lanes. Lanes 1, 3, 7, and 9, p190 wild-type no. 1; lanes 2, 4, 8, and 10, p190 wild-type no. 2. Lanes 1, 2, 7, and 8, bone marrow; lanes 3, 4, 9, and 10, peripheral blood. Lanes 5 and 11, negative control (no RNA added); lanes 6 and 12, positive control no. 2207 RNA. m = φX/HaeIII marker. (B) Quantitative competitive PCR analysis of p190 wild-type and del C expression. Arrows point to the location of the transgenic BCR/ABL 305-bp and the 260-bp competitor fragments. The reactions shown in lanes 1 to 6 (p190 wild-type no. 1) and lanes 12 to 14 (p190 del C animal no. 4445; no. 4208 line) each contained 2 μg of total bone marrow RNA and varying amounts of competitor: 0.5 × 107 molecules, lane 1; 0.5 × 106, lane 2; 0.5 × 105, lanes 3 and 12; 0.5 × 104, lanes 4 and 13; 0.5 × 103, lanes 5 and 14; no competitor, lane 6; positive control RNA no. 2207, lane 7; no RNA, lanes 8 and 11; and 104 and 105 molecules competitor only, lanes 9 and 10, respectively.
Quantitative competitive PCR analysis of p190 wild-type and p190 del C expression.
(A) RT-PCR analysis of wild-type p190 expression. Primers are as indicated beneath the lanes. Lanes 1, 3, 7, and 9, p190 wild-type no. 1; lanes 2, 4, 8, and 10, p190 wild-type no. 2. Lanes 1, 2, 7, and 8, bone marrow; lanes 3, 4, 9, and 10, peripheral blood. Lanes 5 and 11, negative control (no RNA added); lanes 6 and 12, positive control no. 2207 RNA. m = φX/HaeIII marker. (B) Quantitative competitive PCR analysis of p190 wild-type and del C expression. Arrows point to the location of the transgenic BCR/ABL 305-bp and the 260-bp competitor fragments. The reactions shown in lanes 1 to 6 (p190 wild-type no. 1) and lanes 12 to 14 (p190 del C animal no. 4445; no. 4208 line) each contained 2 μg of total bone marrow RNA and varying amounts of competitor: 0.5 × 107 molecules, lane 1; 0.5 × 106, lane 2; 0.5 × 105, lanes 3 and 12; 0.5 × 104, lanes 4 and 13; 0.5 × 103, lanes 5 and 14; no competitor, lane 6; positive control RNA no. 2207, lane 7; no RNA, lanes 8 and 11; and 104 and 105 molecules competitor only, lanes 9 and 10, respectively.
All F0 animals were monitored until they appeared seriously ill, at which point they were killed and autopsies were performed. The F0 generation mice of the current set of transgenics died at an average age of 23 months. Table 1 summarizes the pathologic findings on these mice. Most animals did not die of malignant hematologic diseases. Only one F0 animal, no. 4208, died of lymphoblastic lymphoma at the age of 28 months and showed Bcr/Abl protein in the involved tissues.
Animal no. 4231 became very ill and, upon autopsy, showed splenomegaly (2.2 g). FACS analysis of bone marrow showed decreased T- and B-cell numbers and a prominent myeloid left shift (not shown). However, a Triton X-100–phosphate buffer lysate prepared from this spleen showed evidence of proteolytic degradation, and it was therefore not possible to correlate the hematologic abnormalities found in this mouse with transgene expression.
A caveat in the study of F0 animals is that they could have been chimeric and lacked expression in the critical cell type, ie, the right hematopoietic stem cell. For this reason, many of the transgenic founders were bred to obtain subsequent transgenic generations (Table1), which were monitored. Of the 5 p190 del C lines that could be bred, the no. 4237 line averaged 24 months at death (n = 12; range 5-30 months); the no. 4243 line, 20.8 months (n = 12; range 4-27.5); the no. 4231 line, 15.6 months (n = 12; range 3-28); the no. 4266 line, 14.5 months (n = 5; range 0.5-19); and the no. 4208 line, including both single- and double-transgenics (see “Materials and methods”), 13.7 months (n = 46; range 0.5-27 months). These data indicate that, among these lines, only a limited number exhibited a substantially reduced life span.
Leukemia in p190 del C animals
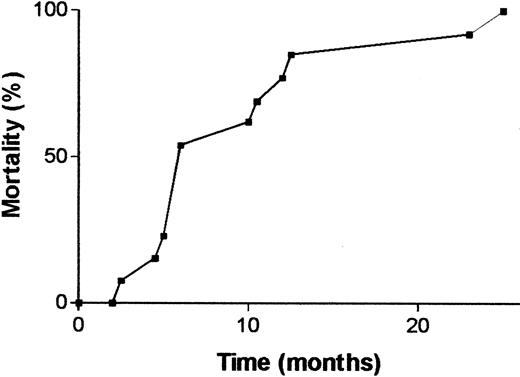
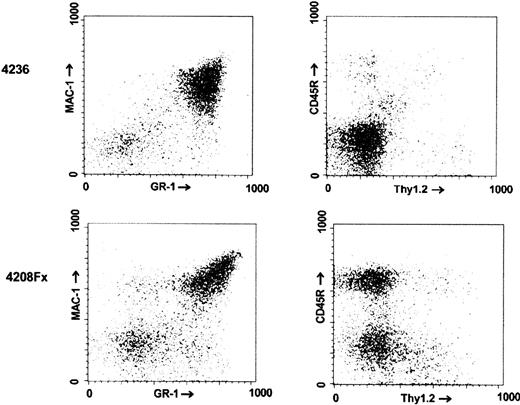
Animals of the no. 4208 line had a higher incidence of hematologic malignancies than the other lines, although not all animals died of clearly diagnosed hematologic malignancies. Among the animals diagnosed with leukemia/lymphoma, deaths were not clustered in a certain age range but, rather, were found to occur stochastically over the entire life span of the mice, as shown in Figure3. As illustrated by the FACS analysis of the bone marrow of one such animal in Figure4 (no. 4208 Fx, which died at 5 months of age), animals of this line typically developed B-lineage lymphoblastic leukemia/lymphoma with bone marrow involvement. This animal showed malignant lymphoblasts invading the skeletal muscle surrounding the sternum, had a pleural effusion consisting mostly of blasts, and had an obliteration of normal splenic architecture (not shown).
Cumulative mortality of p190 Bcr/Abl del C transgenic mice that died of diagnosed hematologic malignancies.
Animals include single and double transgenics from both sexes of the no. 4208 line; n = 13.
Cumulative mortality of p190 Bcr/Abl del C transgenic mice that died of diagnosed hematologic malignancies.
Animals include single and double transgenics from both sexes of the no. 4208 line; n = 13.
FACS analysis of the bone marrow of a
BCR/ABL p190 del C transgenic. The identifying numbers of the mice are indicated on the left. Mouse no. 4236 (age at death, 27 months) was a nontransgenic animal. no. 4208 Fxis a descendent of founder no. 4208, which was moribund at the age of 5 months.
FACS analysis of the bone marrow of a
BCR/ABL p190 del C transgenic. The identifying numbers of the mice are indicated on the left. Mouse no. 4236 (age at death, 27 months) was a nontransgenic animal. no. 4208 Fxis a descendent of founder no. 4208, which was moribund at the age of 5 months.
Animals of other p190 del C transgenic lines, including nos. 4231 and 4243, also developed leukemia/lymphoma, albeit sporadically. For example, animal no. 5085, a descendant of F0 no. 4243 (Table 1), developed lymphoblastic lymphoma/leukemia with lymph node and splenic involvement. There was no hepatomegaly or splenomegaly. Its pleural cavity contained a cellular exudate consisting of lymphoblasts. The bone marrow of this animal contained malignant lymphoblasts that invaded the surrounding muscle (Figure5).
Involved bone marrow of mouse no. 5085 (p190 del C no. 4243 line).
Magnification × 20. Tissues were stained with hematoxylin-eosin stain.
Involved bone marrow of mouse no. 5085 (p190 del C no. 4243 line).
Magnification × 20. Tissues were stained with hematoxylin-eosin stain.
Other pathologic findings
Malignancies or hyperplasias found in either F0animals or subsequent generations were analyzed for expression of Bcr/Abl p190 protein on Western blots. Apart from the leukemias and lymphomas (also see below), 7 animals of different generations, at an average age of 26 months, had to be killed because of regenerative hyperplasia of the liver, which in some cases completely displaced internal organs. A number of these were analyzed for Bcr/Abl expression using Western blots, but no expression could be detected at this level. Additionally, a similar hyperplasia was also seen in 2 control non-BCR/ABL transgenics. Therefore, this and other rarer nonhematologic tumors (also found sporadically in wild-type controls) probably represent the normal background of tumorigenesis found in this strain of mice at advanced age. However, because liver hyperplasia leading to overt neoplasia has been reported in PML-RAR transgenic mice29 in which essentially the same MT promoter was used as in our studies, we cannot exclude the possibility that chronic low-level expression of the mutant BCR/ABL transgene in liver contributed to this phenotype. In addition, other sporadic tissue hyperplasias and tumors (eg, F0 nos. 4237 and 4243, Table1) were observed and analyzed. However, none of these showed expression of the Bcr/Abl p190 protein on a Western blot level.
Western blot analysis of tissues involved with hematologic malignancies shows p190 Bcr/Abl expression
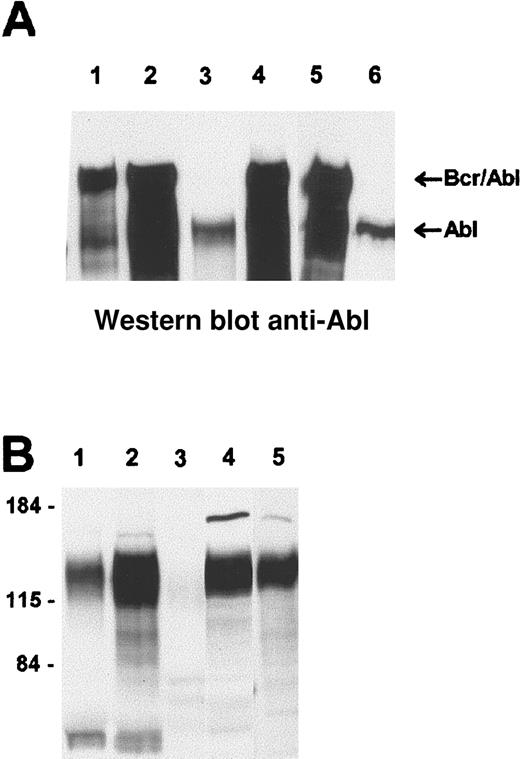
To investigate correlations between transgene expression and malignancy, protein extracts from all tissues involved with hematologic malignancies were examined for expression of Bcr/Abl p190 protein. As exemplified for 2 animals of the no. 4208 p190 del C line, bone marrow of overtly leukemic animals clearly expressed p190 del C protein (Figure 6A, lanes 1,5) at levels comparable to those found in the lymphoma of a mouse transgenic for the nonmutated p190 (lane 4). In comparison, an animal of the no. 4208 line that did not have a hematologic malignancy, but instead died of liver hyperplasia at 18 months of age, did not express Bcr/Abl p190 del C protein in its bone marrow at a level detectable on a Western blot (Figure 6A, lane 6).
Western blot analysis of involved tissues from
BCR/ABL p190 transgenics. Lysates from different tissues were run on SDS-PAA (polyacrylamide) gels and Western blotted. (A) Reaction with anti-Abl monoclonal antibodies, which detect both c-Abl and Bcr/Abl. (B) A far-Western blot reacted with the Crkl SH2 domain. Samples include, in panel A, lane 1, bone marrow of no. 5160 (no. 4208 line); lane 2, involved pooled lymph nodes of no. 5160; lane 3, control bone marrow; lane 4, positive control (lymphoma of nonmutated Bcr/Abl p190 transgenic); lane 5, bone marrow of no. 5224 (no. 4208 line); and lane 6, bone marrow of no. 4637 (a mouse of the p190 del C no. 4208 line that died because of liver hyperplasia at 18 months of age). The locations of endogenous Abl and Bcr/Abl are as indicated. Panel B, lane 1, exudate of no. 5085 (p190 del C 4243 line; Figure 5 also); lane 2, no. 5085 pooled lymph nodes; lane 3, lymphoma of a non-BCR/ABL transgenic; lane 4, lymphoma of no. 4909 (no. 4208 line); and lane 5, positive control lymphoma of a transgenic for nonmutated p190 BCR/ABL. The approximate location of molecular weight markers is shown on the left.
Western blot analysis of involved tissues from
BCR/ABL p190 transgenics. Lysates from different tissues were run on SDS-PAA (polyacrylamide) gels and Western blotted. (A) Reaction with anti-Abl monoclonal antibodies, which detect both c-Abl and Bcr/Abl. (B) A far-Western blot reacted with the Crkl SH2 domain. Samples include, in panel A, lane 1, bone marrow of no. 5160 (no. 4208 line); lane 2, involved pooled lymph nodes of no. 5160; lane 3, control bone marrow; lane 4, positive control (lymphoma of nonmutated Bcr/Abl p190 transgenic); lane 5, bone marrow of no. 5224 (no. 4208 line); and lane 6, bone marrow of no. 4637 (a mouse of the p190 del C no. 4208 line that died because of liver hyperplasia at 18 months of age). The locations of endogenous Abl and Bcr/Abl are as indicated. Panel B, lane 1, exudate of no. 5085 (p190 del C 4243 line; Figure 5 also); lane 2, no. 5085 pooled lymph nodes; lane 3, lymphoma of a non-BCR/ABL transgenic; lane 4, lymphoma of no. 4909 (no. 4208 line); and lane 5, positive control lymphoma of a transgenic for nonmutated p190 BCR/ABL. The approximate location of molecular weight markers is shown on the left.
Involvement of Crkl SH2-binding proteins in leukemia/lymphoma associated with the p190 Bcr/Abl del C mutant
In Ph-positive leukemia in humans, the adapter protein Crkl binds directly to and becomes abnormally constitutively tyrosine-phosphorylated by the deregulated Bcr/Abl tyrosine kinase.30-32 In addition, Bcr/Abl abnormally phosphorylates tyrosine residues in a number of signaling proteins to which Crkl can bind via its SH2 domain.15,33 We have shown that in the Bcr/Abl p190 wild-type mouse model, Crkl becomes tyrosine-phosphorylated and forms stable complexes with Hef1/Cas and the proto-oncogene Cbl.11,12 34
The del C mutant is not expected to have impaired binding to Crkl, because the mutated region lies outside the Crkl binding sites in Abl. However, because Crkl, Cbl, and Hef1/Cas have been implicated in signal transduction involving the actin cytoskeleton/integrins15 33 and the Bcr/Abl p190 del C protein lacks the sequences in Abl that mediate binding to F-actin, it was of interest to examine the involvement of Crkl, Cbl, and Hef1/Cas in the leukemias developing in the p190 del C transgenic mice.
To investigate this, different lysates isolated from involved tissues of these mice were Western blotted and “probed” with the Crkl SH2 domain. As shown in Figure 6B, lane 5, the Crkl SH2 domain detects a number of proteins in the 110- to 140-kd molecular weight range in the lymphoma lysate of an animal transgenic for the nonmutated p190BCR/ABL. In contrast, no proteins of that size were detected in an unrelated lymphoma of a non-BCR/ABL transgenic (Figure6, lane 3), consistent with the finding that the activated Bcr/Abl kinase is needed to phosphorylate tyrosine residues that are binding partners for the Crkl SH2 domain. Lysates were prepared from involved tissues of animal no. 5085 (no. 4243 line; Figure 5) and animal no. 4909 (no. 4208 line). As shown in Figure 6B, lanes 1, 2, and 4, involved tissues of the p190 del C transgenics contained proteins that clearly bound to the Crkl SH2 domain.
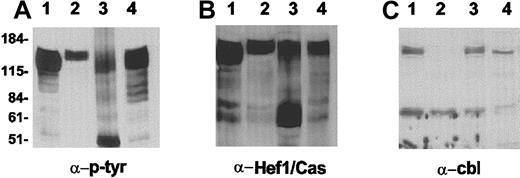
To investigate the identity of the proteins binding to the Crkl SH2 domain, lysates were subjected to a pull-down reaction using the Crkl SH2 domain. As shown in Figure 7A, tyrosine-phosphorylated proteins in the 110- to 140-kd molecular weight range were precipitated with the Crkl SH2 domain. In parallel experiments, proteins coprecipitated with the Crkl SH2 domain were Western blotted and reacted with anti-Cbl antisera (Figure 7C) or with anti-Hef1/Cas antiserum (Figure 7B). The wild-type p190 Bcr/Abl sample showed high levels of Crkl SH2 domain coprecipitating tyrosine-phosphorylated proteins (Figure 7A, lane 4), and these consisted of both Cbl and Hef1/Cas. The 3 p190 Bcr/Abl del C samples showed different levels of tyrosine-phosphorylated, Crkl SH2 domain-coprecipitating proteins (Figure 7A, lanes 1-3). Two of the samples showed the presence of Cbl (Figure 7C, lanes 1,3), whereas all 3 contained Hef1/Cas (Figure 7B, lanes 1-3).
Analysis of Crkl SH2-binding proteins.
Western extracts were diluted and incubated with GST-Crkl SH2. Binding proteins were resolved on SDS-PAA gels, Western blotted, and reacted with the antibodies indicated below each panel. Lysates included those of lymphomas of animal nos. 4909 and 4690 (Bcr/Abl p190 del C no. 4208 line), lanes 1 and 2; involved pooled lymph nodes of animal no. 5085 (Bcr/Abl p190 del C 4243 line), lanes 3; and control nonmutated p190 Bcr/Abl, lane 4. The approximate locations of molecular weight markers are indicated on the left.
Analysis of Crkl SH2-binding proteins.
Western extracts were diluted and incubated with GST-Crkl SH2. Binding proteins were resolved on SDS-PAA gels, Western blotted, and reacted with the antibodies indicated below each panel. Lysates included those of lymphomas of animal nos. 4909 and 4690 (Bcr/Abl p190 del C no. 4208 line), lanes 1 and 2; involved pooled lymph nodes of animal no. 5085 (Bcr/Abl p190 del C 4243 line), lanes 3; and control nonmutated p190 Bcr/Abl, lane 4. The approximate locations of molecular weight markers are indicated on the left.
Discussion
The original wild-type p190 BCR/ABL construct gave rise to the relatively rapid onset of leukemia/lymphoma in most of the F0 generation of transgenic mice (n = 14) in the same genetic background (B6xCBAF1 hybrid) in which the currently studied F0 transgenics were made.23,24 The average age of death associated with the nonmutated p190 construct was 8.8 months, including a few relatively long-lived F0animals.23 All F0 transgenics for the nonmutated p190 construct or their progeny, with the exception of one line, developed hematopoietic malignancies, either lymphoblastic leukemias, lymphomas, or both. Most involved the B-lineage.
A very different result was obtained with the p190 del CBCR/ABL construct studied here. The average age at death of these 8 F0 transgenic mice was 23 months, which can be considered to approach the natural life span of the mice. Secondly, in most F0 animals or their progeny, there was no clear evidence of hematologic malignancy (Table 1).
Expression of mutant BCR/ABL mRNA in peripheral blood was detected in some of the F0 generation at a very early age of 4 weeks. For one of the animals, no. 4208, this eventually resulted in development of lymphomas late in life, at 2.4 years of age. This is consistent with a model in which a weakly oncogenic Bcr/Abl protein directly or indirectly can cause additional mutations, which are needed for full-blown leukemia to develop. In comparison, transgenics for nonmutant p190 (no. 623 line) survived an average of about 2 months after detection of expression in peripheral blood.24Furthermore, the expression level of the transgenes in the preleukemic bone marrow of p190 wild-type and p190 del C mice was not significantly different. These combined data demonstrate that the C-terminal mutation severely restricts the oncogenic potential of Bcr/Abl p190 in vivo in transgenic mice.
The effect of the c-Abl actin-binding domain on leukemogenicity of Bcr/Abl had not previously been investigated directly in a transgenic in vivo model system. Bcr/Abl with deletions of the actin-binding domain was reported to no longer colocalize with actin stress fibers after expression in transfected fibroblasts. Also, both reduced transformation of fibroblasts and reduced ability to abrogate interleukin-3 independence of Ba/F3 cells was found.7Thus, the current study confirms the reduced biologic activity of the Bcr/Abl del C mutant found in cell lines.
In human and murine patient material, Bcr/Abl can constitutively tyrosine-phosphorylate a number of cytoskeleton or integrin/cytokine-signaling–associated proteins that subsequently become binding targets for the Crkl SH2 domain, including Cbl, Cas, Hef1, and p62doc.15,33,35 In this process, Crkl and Cbl bind directly to Bcr/Abl, whereas Cas/Hef1 and also Cbl indirectly complex with Bcr/Abl via their binding to Crkl.15Therefore, complex formation between Crkl and these tyrosine-phosphorylated proteins was also investigated in the involved tissues of p190 BCR/ABL del C transgenics. The Crkl SH2 domain bound to Hef1/Cas as well as to Cbl, and the apparent levels of tyrosine-phosphorylated Cbl and Hef1/Cas were similar to those observed in the leukemic tissues of a nonmutated BCR/ABL p190 transgenic (Figure 7).
Although the Bcr/Abl del C protein was reported to retain full tyrosine kinase activity, the deletion of the C-terminal actin-binding domain causes dissociation of Bcr/Abl from actin stress fibers in fibroblasts6 and shifts its subcellular location in myeloid cells to vesicle-like structures that lack F-actin.36 The loss of binding of Bcr/Abl to F-actin would be predicted to decrease the tyrosine phosphorylation by the deregulated Bcr/Abl kinase of cytoskeleton-associated targets. However, Western blot analysis on tyrosine-phosphorylated proteins binding to the Crkl SH2 domain in the transgenic mice showed that end-stage leukemic tissues of Bcr/Abl p190 nonmutated, and del C animals were indistinguishable in this respect.
We have considered several possible explanations for this finding. It is possible that additional mutations have occurred during the process of leukemogenesis (eg, in the Bcr/Abl del C mutant or in Crkl, a protein that mediates binding to Bcr/Abl) that resulted in increased colocalization of Bcr/Abl with Hef1/Cas and with Cbl in vivo. Another explanation could be that the binding of Bcr/Abl to F-actin is irrelevant for its ability to tyrosine-phosphorylate Hef1/Cas and Cbl. This would correlate with the finding that a p210 mutant lacking the actin-binding domain colocalized with Cbl and, to a lesser extent, with Crkl and Cas in vesicle-like structures.36 Finally, a minor amount of Bcr/Abl that initially could colocalize with F-actin may have been sufficient to phosphorylate target proteins and initiate early steps in leukemogenesis. Subsequent activation of cytokine receptors or integrin signaling could then be responsible for the increased tyrosine phosphorylation of Hef1/Cas and Cbl observed in the mutants. This latter explanation, however, does not seem likely because, in a lymphoma of a non-BCR/ABL transgenic, increased levels of Crkl SH2-binding proteins were not observed (Figure6B).
Because the ultimate outcome of expression of Bcr/Abl p190 wild type and of Bcr/Abl p190 del C can be similar, yet the frequency of leukemia development and the latency period is increased in the mutant, the 2 proteins may differ most in their activity at the level of precursor cells in the bone marrow. However, further experiments will be needed to identify targets of Bcr/Abl in such cells.
Acknowledgments
We thank Stijn de Lange for help with the figures, Arnoud van Wijk for his contribution to the Western blot analysis, and Roberto Rosato and Yan Zhang for assistance with monitoring of the mice.
Supported by Public Health Service, National Institutes of Health grants CA 50248 (N.H.) and CA 47456 (J.G.).
N.H. and J.W.V. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nora Heisterkamp, Division of Hematology/Oncology, Ms#54, Section of Molecular Carcinogenesis, Childrens Hospital of Los Angeles Research Institute, 4650 Sunset Boulevard, Los Angeles, CA 90027; e-mail heisterk@hsc.usc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal