Abstract
B-cell precursor acute lymphoblastic leukemias (BCP-ALLs) are increasingly treated on risk-adapted protocols based on presenting clinical and biological features. Residual molecular positivity of clonal immunoglobulin (IG) and T-cell receptor (TCR) rearrangements allows detection of patients at an increased risk of relapse. If these rearrangements are to be used for universal follow-up, it is important to determine the extent to which they are informative in different BCP-ALL subsets. We show thatIGH V-D-J rearrangements occur in 89% of 163 BCP-ALL, with no significant variation according to age or genotype (BCR-ABL, TEL-AML1, MLL-AF4, and E2A-PBX1). In contrast,TCRG rearrangements, which occur in 60% of patients overall, are frequent in BCR-ABL and TEL-AML1, are less so in MLL-AF4, and are virtually absent in infants aged predominantly from 1 to 2 years and in E2A-PBX1 ALLs. Incidence of the predominant TCRD Vδ2-Dδ3 rearrangement decreases with age but is independent of genotype. These differences are not due to differential recombination activating gene activity, nor can they be explained adequately by stage of maturation arrest. Analysis of MLL-AF4 BCP-ALL is in keeping with transformation of a precursor at an early stage of ontogenic development, despite the adult onset of the cases analyzed. We postulate that the complete absence of TCRG rearrangement in E2A-PBX1 cases may result from deregulated E2A function. These data also have practical consequences for the use ofTCR clonality for the molecular follow-up of BCP-ALL.
Introduction
B-cell precursor acute lymphoblastic leukemias (BCP-ALLs) represent approximately 85% and 75% of pediatric and adult ALLs, respectively. They include a number of subtypes that can be individualized on the basis of clinical presentation, immunophenotype, and genotype, as assessed by cytogenetic and molecular techniques. This has allowed identification of patients with markedly different prognoses and the increasing use of risk-adapted protocols. BCP-ALL with MLL-AF4, for example, is frequent in infants younger than 1 year old who present with marked leukocytosis and a poor response to treatment.1 It is also common in older adults.2BCR-ABL identifies a group of poor-prognosis adults.3 The TEL-AML1(ETV6-CBFA2) fusion transcript (FT) occurs almost exclusively in childhood BCP-ALL4 and is of standard or favorable prognostic significance depending on the clinical protocol.5E2A-PBX1 FT predominates in young adults with relatively mature blasts. Its previously poor prognosis has been improved by more intensive treatment (reviewed in Hunger6). The approximate incidence of these FTs in pediatric and adult BCP-ALL, respectively, are as follows:MLL-AF4, 5% and 5%; BCR-ABL, 3% and 30%; TEL-AML1, 25% and below 5%; and E2A-PBX1, 5% and 5%. Although FTs provide invaluable prognostic markers at diagnosis, their current utility as markers of residual disease at the early stages of remission, when therapeutic decisions are taken, is less clear, at least for qualitative reverse transcriptase–polymerase chain reaction (RT-PCR) analysis.
BCP-ALLs also demonstrate clonal rearrangements of the IGHand TCR genes in the majority of cases (reviewed in Langerak et al7). IGH rearrangements are reported in more than 90% of cases by Southern blotting and 70% to 90% by PCR.8 Complete VH-DH-JH rearrangement is preceded by partial DH-JH rearrangement, which is detected by Southern hybridization with a JH probe or by specific DH-JH PCR.9 IllegitimateTCRG V-J rearrangements have been described in 40% to 70% of BCP-ALLs,7,10 and TCRD rearrangement in approximately 50%. The commonest rearrangements correspond to partial Vδ2-Dδ3 and Dδ2-Dδ3.11 Illegitimate rearrangements are thought to result from the fact that ALLs represent cells arrested at a “recombinase-competent” stage of maturation, withIG and TCR loci in an accessible chromatin configuration.
IG and TCR rearrangements provide invaluable markers for patient follow-up, insofar as the vast majority of BCP-ALLs are informative for at least one clonal rearrangement. A variety of molecular strategies allow detection of residual clonal populations with a sensitivity that varies from approximately 5% (5 × 10−2) to 0.001% (10−5). Two large-scale studies of pediatric ALL have recently demonstrated that residual molecular positivity during the early stages of morphological complete remission allows identification of approximately 10% to 15% of patients who are at a high risk of relapse.12 13 It is therefore likely that residual molecular IGH/TCRpositivity will increasingly be used to determine treatment. Both studies were based predominantly on assessment of TCRG andTCRD clonal markers, but neither comparedIGH/TCR status with genotype.
Since patients of different ages and genotype are increasingly treated on different protocols, it is important to determine whether the extent to which IGH/TCR is informative varies as a function of age and/or genotype. We demonstrate that, whileIGH rearrangements are common in all categories of adult and pediatric BCP-ALL, TCRG and TCRD Vδ2-Dδ3 vary significantly with genotype and age, respectively. These data have practical significance for the use of IGH/TCRmolecular markers in clinical management of patients with BCP-ALL.
Patients, materials, and methods
Patient material and immunophenotyping
Ficolled blasts from bone marrow or peripheral blood were collected at diagnosis, with informed consent, and were analyzed fresh or after conservation in 10% dimethyl sulphoxide. BCP-ALL diagnosis was based on morphology, cytochemistry, and immunophenotype. The majority were included in prospective multicenter studies of pediatric (FRALLE93) and adult (LALA94) ALL. Molecular analysis for all patients other than 8 E2A-PBX1 patients, provided by J.-M. Cayuela and F. Sigaux, was performed at Necker-Enfants Malades (Paris, France). Diagnosis, immunophenotyping, and cytogenetic analysis of several patients were undertaken in referring centers, and material was transferred for molecular analysis. Immunophenotype was performed by flow cytometric analysis of a variety of B- and T-lymphoid and myeloid antigens. Cases expressing the relevant marker on more than 20% of cells were considered positive. We assessed cytoplasmic Igμ (cIgμ) expression by flow cytometric assessment of permeabilized cells.14 Patients younger than 2 years are classified as infants, although it is recognized that this term generally refers to those younger than 12 months old. Those aged from 2 to 14 years are referred to as children; those from 15 to 55 years as young adults; and those over 55 years as older adults. These cutoffs were chosen to reflect inclusion criteria for the LALA94 (15 to 55 years) protocol, although several adolescents were treated on FRALLE93 protocols.
IGH and TCR analysis
DNA extraction was performed by phenol chloroform and ethanol precipitation from ficolled diagnostic material.
IGH VH-DH-JH PCR was performed from 1 μg DNA in 50 μL using VHconsensus primers FR1 (FR1c) and FR2 (FR2c with 2 VH5 and VH6 FR1-family–specific primers) and a mixture of 3 JH primers.8 Selected cases were also analyzed with the use of FR1-family–specific (FR1f) and FR3 consensus primers.8 We analyzed 15% of the PCR reaction by nondenaturing 8% to 12% polyacrylamide gel electrophoresis and ethidium bromide staining (EB PAGE). IGHDH-JH PCR was performed as previously described.9
TCRG analysis was performed in 2 Vγ-Jγ–specific multiplex PCRs.15 We amplified 1 μg of DNA with a mixture of VγfI, Vγ10, Jγ1/2, JγP1/2, and JγP primers (VγfI/10 PCR) and with a mixture of Vγ9, Vγ11, and the same Jγ primers (Vγ9/11 PCR). Partial identification of Vγ and Jγ utilization was based on PCR product size. Further Vγ and Jγ utilization was done by fluorescent run-off analysis with the use of a mixture of labeled internal Vγ- or Jγ-specific primers, followed by Genescan analysis on an ABI 373 or 310 automated DNA fragment analyzer (PEBiosystems, Foster City, CA).15
TCRD
Vδ2-Dδ3 PCR was performed in a total volume of 50 μL as previously described16 with an initial 94°C step for 2 minutes followed by 35 cycles at 62°C for 20 seconds and 94°C for 20 seconds. The final extension step was at 72°C for 5 minutes. PCR products were analyzed on 8% nondenaturating PAGE.
Detection of FTs and MLL rearrangements
RNA was extracted from leukemic cells by a rapid lysis technique (RNAble) (EuroBio, Les Ulis, France), and complementary DNA (cDNA) and RT-PCR reactions were performed as previously described17with the use of 2 μg RNA, random hexamers (Pharmacia, Orsay, France), and MMLV reverse transcriptase (Life Technologies, Cergy-Pontoise, France). Porphobilinogen deaminase (PBGD) transcripts were amplified in parallel from the same cDNA to control for RNA quality.17 In keeping with LALA94 and FRALLE93 guidelines, all infants and children were analyzed prospectively for theBCR-ABL (e1-a2 and b2/b3-a2), MLL-AF4,TEL-AML1, and E2A-PBX1 FTs and adults for all of the above other than TEL-AML1, with the use of the following primers: BCR-ABL b2/b3-a2 (CMLA: GGAGCTGCAGATGCTGACCAAC; ALLF: GGTCATTTTCACTGGGTCCAGC; annealing 60°C); BCR-ABLe1-a2 (BCR1 ExtS TGAGAACCTCACCTCCAG; ABLExtAS: CTCCACTGGCCACAAAAT; annealing 51°C); TEL-AML1 (B12: CGTGGATTTCAAACAGTCCA; AM3: GCTCGCTCATCTTGCCTGG; annealing 55°C); E2A-PBX1 (E2AExtS: GGCCTGCAGAGTAAGATAG; PBXExtAS: CACGCCTTCCGCTAACAG; annealing 51°C);MLL-AF4 (HRX5Ext: GAGGATCCTGCCCCAAAGAAAAG; AF4Ext: TGAGCTGAAGGTCGTCTTCGAGC; annealing 60°C). Certain E2A-PBX1-positive cases were also analyzed for wild-type E2A(E2A/1399U19 GCCTCATGCACAACCACG and E2A/2234L20 GAGTGACACGGTGGCTGAGA; annealing 60°C) and PBX1 (PBX1/243U25 GCAGGACATTGGAGACATTTTACAG and PBX1/732L19 GCTGAACTTGCGGTGGATG; annealing 57°C) and PBX1-E2A transcripts (PBX1/243U25 and E2A/2234L20 primers; annealing 57°C) with the use of 6% formamide and 2 U Taq in a final volume of 50 μL. The latter were hybridized with an internal 33P-labeled PBX1 (PBX1294U18 TTTGGATGAGGCGCAGGC) probe.
Infants were also screened for MLL rearrangements by Southern blotting of BamHI and HindIII digested DNA, with the use of the B859 cDNA probe.18
Recombination activating gene transcripts
Detection of recombination activating gene 1 (RAG1) and RAG2 transcripts by RT-PCR were performed in 50 μL with 0.4 μg cDNA, 2.5 mmol/L MgCl2, 0.2 mmol/L dNTP, 0.4 μmol/L each primer and 2 U Taqpolymerase. Primers used were as follows: RAG1(ACACACTTTGCCTTCTCTTTGGTATT [Ex1]; TCTCACCCGGAACAGCTTAAA [Ex2]);RAG2 (TTCCCCAAGTGCTGACAATTAA [Ex1a]; TTTGGGCCAGCCTTTTTG [Ex2]). Samples were amplified for 35 cycles (30 seconds at 93°C, 1 minute at 60°C, and 1 minute at 72°C, with a final elongation cycle of 10 minutes at 72°C) and analyzed on 2% agarose gels.RAG1 PCR products were 209 base pairs (bp) andRAG2 products were 219 bp. The REH cell line was used as a positive control.
Statistical analysis
Comparison of the incidence of IGH/TCR rearrangements in different subgroups was analyzed by the 2-sided χ2test, and comparison of the mean ages of TCR-rearranged or germline cases was analyzed by the 2-sided Mann-WhitneyU test.
Results
Diagnostic assessment of IgH/TCR configuration and fusion transcripts
In order to determine whether the incidence of IG andTCR rearrangements varies with patient subgroup, we undertook systematic, prospective assessment of IGH andTCRG clonality and detection of up to 5 FTs, the latter in accordance with the national French LALA94 adult (coordinator J. Gabert, Institut Paoli-Calmette, Marseilles, France) and FRALLE93 (coordinator A. Baruchel, Hôpital St Louis, Paris, France) pediatric protocols. Diagnostic samples from 163 B-cell lineage ALLs were analyzed for IGH and TCRG by PCR and EB PAGE analysis. Detection of BCR-ABL (e1-a2 and b2/b3-a2),E2A-PBX1, and MLL-AF4 FTs was undertaken in 140 of 163 patients with sufficient RNA. Detection of TEL-AML1was performed systematically only for patients younger than 15 years, since we have found this FT in fewer than 1% of adult cases.4
BCR-ABL was detected in 25 cases, (20e1-a2, 2b2-a2, 3b3-a2) TEL-AML1 in 18, E2A-PBX1 in 13, andMLL-AF4 in 14. The incidence of each of these as a function of age is shown in Table 1. Although this corresponds to published incidences for BCR-ABL andTEL-AML1, the proportion of patients withE2A-PBX1 and MLL-AF4 is not representative, because 2 nonprotocol MLL-AF4 cases were referred for molecular characterization and 8 additional E2A-PBX1 cases were added to increase this category (see below). The presence of reciprocal PBX1-E2A transcripts was looked for in 8E2A-PBX1 ALLs, 3 unbalanced and 5 balanced cases, but no specific bands that hybridized to an internal PBX1 probe were seen, despite hybridization to a PBX1 wild-type RT-PCR control. All cases demonstrated wild-type E2A and low-level PBX1 transcripts, and E2A-PBX1 positivity was confirmed in parallel on the same cDNA samples (data not shown).
Incidence of fusion transcripts and IGH/TCR rearrangements as a function of age
| Age (mean) . | Genotypes . | Rearrangements . | ||||||
|---|---|---|---|---|---|---|---|---|
| MLL-AF4 (%) . | TEL-AML1 (%) . | BCR-ABL (%) . | E2A-PBX1 (%) . | FT-negative (%) . | IGH (%) . | TCRG (%) . | Vδ2-Dδ3 (%) . | |
| <2 y (1.1 yr) | 2/10* (20) | 1/10 (10) | 0/10 (0) | 1/10 (10) | 6/10* (60) | 10/11 (91) | 1/11 (9) | 6/7 (86)† |
| 2-14 y (5.9 y) | 0/52 (0) | 17/52 (33) | 1/52 (2) | 3/52 (6) | 31/52 (60) | 68/74 (92) | 49/74 (66) | 10/23 (43) |
| 15-55 y (32.3 y) | 10/66 (15) | nd‡ | 17/66 (26) | 8/66 (12) | 31/66 (46) | 57/66 (86) | 40/66 (61) | 6/33 (18) |
| >55 y (65.3 y) | 2/12 (17) | nd | 7/12 (58) | 1/12 (8) | 2/12 (17) | 11/12 (92) | 8/12 (66) | 2/11 (18) |
| Total | 14/140 (10) | 18/62 (29)1-153 | 25/140 (18) | 13/140 (9) | 70/140 (50) | 145/163 (89) | 98/163 (60) | 24/74 (32) |
| Age (mean) . | Genotypes . | Rearrangements . | ||||||
|---|---|---|---|---|---|---|---|---|
| MLL-AF4 (%) . | TEL-AML1 (%) . | BCR-ABL (%) . | E2A-PBX1 (%) . | FT-negative (%) . | IGH (%) . | TCRG (%) . | Vδ2-Dδ3 (%) . | |
| <2 y (1.1 yr) | 2/10* (20) | 1/10 (10) | 0/10 (0) | 1/10 (10) | 6/10* (60) | 10/11 (91) | 1/11 (9) | 6/7 (86)† |
| 2-14 y (5.9 y) | 0/52 (0) | 17/52 (33) | 1/52 (2) | 3/52 (6) | 31/52 (60) | 68/74 (92) | 49/74 (66) | 10/23 (43) |
| 15-55 y (32.3 y) | 10/66 (15) | nd‡ | 17/66 (26) | 8/66 (12) | 31/66 (46) | 57/66 (86) | 40/66 (61) | 6/33 (18) |
| >55 y (65.3 y) | 2/12 (17) | nd | 7/12 (58) | 1/12 (8) | 2/12 (17) | 11/12 (92) | 8/12 (66) | 2/11 (18) |
| Total | 14/140 (10) | 18/62 (29)1-153 | 25/140 (18) | 13/140 (9) | 70/140 (50) | 145/163 (89) | 98/163 (60) | 24/74 (32) |
FT indicates fusion transcript; nd, not determined.
The infant with an uncharacterized MLL rearrangement is not included.
Three-fourths in FT-negative infants.
Not systematically tested, but 0 of 23 were positive.
29% of children aged younger than 15 years.
IGH VH-DH-JH
IGH was assessed with the use of FR1c and FR2 primers and considered to be positive if either or both demonstrated a clonal band. If both were negative, cases were reanalyzed with FR1f and FR3 consensus primers8 and were classified as positive if either demonstrated a clonal band. Occasional cases were considered to be oligoclonal on the basis of the presence of at least 3 discreet bands. In the majority, at least one PCR system was considered to be clonal. These cases were classified as IgH-positive. It should be emphasized that minor clonal PCR products were seen in several cases, particularly following fluorescent analysis (data not shown). Although these are likely to represent minor clones, their presence was not particularly taken into consideration for these consensus PCR strategies. Overall, 145 of 163 (89%) patients demonstrated IGH positivity, including 125 of 158 (79%) by FR1c-JH, 121 of 157 (77%) by FR2-JH, 18 of 37 (49%) by FR1f-JH, and 26 of 52 (50%) by FR3-JH.
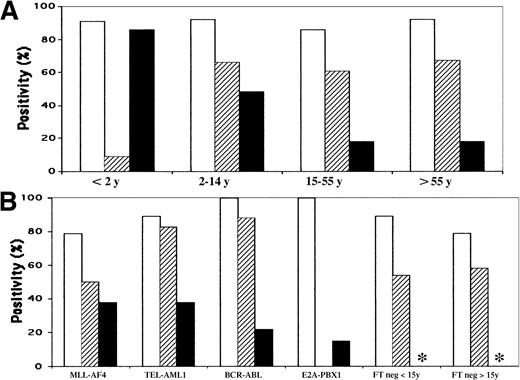
The incidence of IGH rearrangements as a function of age and genotype is shown in Tables 1 and 2 and Figure1. Rearrangements were frequent in all categories, being highest in BCR-ABL and E2A-PBX1and lowest in MLL-AF4 ALL and FT-negative adults, although this was not significant (P = .3). IGHVH-DH-JH rearrangements therefore occur in approximately 90% of BCP ALLs, with an incidence that varies little with age or genotype.
Incidence of IGH/TCR rearrangements and immunophenotype as a function of genotype
| . | MLL-AF4 (%) . | TEL-AML1 (%) . | BCR-ABL (%) . | E2A-PBX1 (%) . | FT-negative < 2 yrs (%) . | FT-negative 2-15 yrs (%) . | FT-negative* > 15 yrs (%) . | Total . |
|---|---|---|---|---|---|---|---|---|
| IGH | 11/14 (79) | 16/18 (89) | 25/25 (100) | 13/13 (100) | 5/6 (83) | 28/31 (90) | 26/33 (79) | 124/140 (89) |
| TCRG | 7/14 (50) | 15/18 (83) | 22/25† (88) | 0/13 (0) | 0/6 (00) | 20/31 (65) | 19/33 (58) | 83/140 (59) |
| Vδ2-Dδ3 | 5/13 (38) | 6/16 (38) | 5/23‡ (22) | 2/13 (15) | 3/4 (75) | — | — | — |
| CD34 | 8/13 (62) | 14/18 (78) | 23/25 (92) | 1/13 (8) | 4/5 (80) | — | — | — |
| CD10 | 0/14 (0) | 18/18 (100) | 25/25 (100) | 13/13 (100) | 5/6 (83) | — | — | — |
| CD22 | 7/14 (50) | 13/15 (87) | 18/21 (86) | 12/12 (100) | 5/5 (100) | — | — | — |
| CD20 | 0/14 (0) | 6/18 (33) | 11/24 (46) | 4/13 (31) | 4/6 (67) | — | — | — |
| cIgμ | 0/6 (0) | 1/11 (9) | 5/20 (25) | 10/12 (83) | 0/5 (0) | — | — | — |
| CD13 | 0/13 (0) | 7/18 (39) | 8/24 (33) | 1/12 (8) | 0/5 (0) | — | — | — |
| CD33 | 3/13 (23) | 5/16 (31) | 8/24 (33) | 1/13 (8) | 0/6 (0) | — | — | — |
| . | MLL-AF4 (%) . | TEL-AML1 (%) . | BCR-ABL (%) . | E2A-PBX1 (%) . | FT-negative < 2 yrs (%) . | FT-negative 2-15 yrs (%) . | FT-negative* > 15 yrs (%) . | Total . |
|---|---|---|---|---|---|---|---|---|
| IGH | 11/14 (79) | 16/18 (89) | 25/25 (100) | 13/13 (100) | 5/6 (83) | 28/31 (90) | 26/33 (79) | 124/140 (89) |
| TCRG | 7/14 (50) | 15/18 (83) | 22/25† (88) | 0/13 (0) | 0/6 (00) | 20/31 (65) | 19/33 (58) | 83/140 (59) |
| Vδ2-Dδ3 | 5/13 (38) | 6/16 (38) | 5/23‡ (22) | 2/13 (15) | 3/4 (75) | — | — | — |
| CD34 | 8/13 (62) | 14/18 (78) | 23/25 (92) | 1/13 (8) | 4/5 (80) | — | — | — |
| CD10 | 0/14 (0) | 18/18 (100) | 25/25 (100) | 13/13 (100) | 5/6 (83) | — | — | — |
| CD22 | 7/14 (50) | 13/15 (87) | 18/21 (86) | 12/12 (100) | 5/5 (100) | — | — | — |
| CD20 | 0/14 (0) | 6/18 (33) | 11/24 (46) | 4/13 (31) | 4/6 (67) | — | — | — |
| cIgμ | 0/6 (0) | 1/11 (9) | 5/20 (25) | 10/12 (83) | 0/5 (0) | — | — | — |
| CD13 | 0/13 (0) | 7/18 (39) | 8/24 (33) | 1/12 (8) | 0/5 (0) | — | — | — |
| CD33 | 3/13 (23) | 5/16 (31) | 8/24 (33) | 1/13 (8) | 0/6 (0) | — | — | — |
FT indicates fusion transcript.
Includes 2 cases > 55 years, neither of which demonstrated TCRG rearrangement. All cases expressed CD19. Only surface expression is considered positive, except for cIgμ. FT-positive cases are represented with their genotypic group.
18/20 (90%) in e1-a2 cases.
5/18 (28%) in e1-a2 cases.
Histograms of IgH, TCRG, and TCRD Vδ2-Dδ3 rearrangements.
(A) According to age. (B) According to genotype. ■ indicatesIGH incidence; ▨, TCRG incidence; ▪,TCRD Vδ2-Dδ3 incidence. *Not done.
Histograms of IgH, TCRG, and TCRD Vδ2-Dδ3 rearrangements.
(A) According to age. (B) According to genotype. ■ indicatesIGH incidence; ▨, TCRG incidence; ▪,TCRD Vδ2-Dδ3 incidence. *Not done.
TCRG
Cases were considered to be TCRG-positive if either multiplex reaction (VγfI/10-Jγ or Vγ9/11-Jγ, Figure2) demonstrated at least one discrete band. Overall, rearrangements were observed in 98 of 163 (60%) cases. The incidence varied with age (Table 1, Figure 1) and was significantly less frequent in infants younger than 2 years (9%) compared with all other groups (P = .0011). Mean age for the 11 infants (5 boys, 6 girls) was 13.5 months (range, 3-21) and 9 were older than 12 months. The group included 2 boys aged 3 and 5 months withMLL-AF4, a 14-month-old girl with TEL-AML1, a 21-month-old boy with E2A-PBX1, a 16-month-old girl with an unidentified MLL rearrangement, and 8 MLLgermline cases. The only TCRG rearrangement was seen in the uncharacterized MLL rearrangement. TCRGrearrangements were seen in 16 of 20 (80%) children aged from 2 to 3 years, 13 of 23 (56%) from 3 to 4 years, 10 of 16 (63%) from 6 to 8 years, and 10 of 15 (67%) from 9 to 14 years. The absence ofTCRG rearrangements was therefore relatively specific to children aged predominantly from 1 to 2 years.
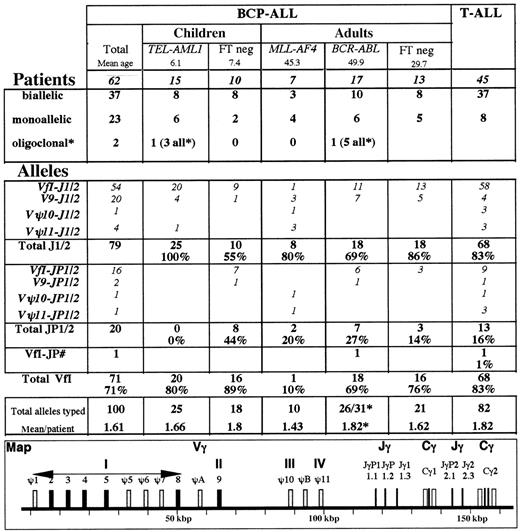
TCR Vγ and Jγ utilization in BCP-ALL. * Several patients demonstrated minor fluorescent peaks that were not included. Only those patients in whom oligoclonality was evident by ethidium bromide analysis or in whom more than 2 equal intensity peaks were observed were classified as oligoclonal. The large number of peaks observed in the BCR-ABL case precluded their inclusion for relative Vγ and Jγ usage, but they were included in the total number of alleles. #One patient demonstrated VγfI-JγP1/2 and VγfI-JγP rearrangements. FT indicates fusion transcript.
TCR Vγ and Jγ utilization in BCP-ALL. * Several patients demonstrated minor fluorescent peaks that were not included. Only those patients in whom oligoclonality was evident by ethidium bromide analysis or in whom more than 2 equal intensity peaks were observed were classified as oligoclonal. The large number of peaks observed in the BCR-ABL case precluded their inclusion for relative Vγ and Jγ usage, but they were included in the total number of alleles. #One patient demonstrated VγfI-JγP1/2 and VγfI-JγP rearrangements. FT indicates fusion transcript.
With regard to genotype (Table 2),TCRG rearrangements were frequent in BCR-ABL andTEL-AML1, were less so in MLL-AF4, and were not seen in E2A-PBX1 cases. The incidence in FT-negative ALLs varied with age, as above (Table 1). These data demonstrate that illegitimate TCRG rearrangements are absent inE2A-PBX1 ALLs, infrequent in MLL-AF4, and rare in infants from 1 to 2 years old, and that in the last-named, this is not due to MLL rearrangement.
TCRD Vδ2-Dδ3
TCRD Vδ2-Dδ3 PCR was assessed in all subjects with known FTs, aged younger than 2 years and/orIGH VH-DH-JH–negative, with available DNA. Overall, clonal rearrangements were detected in 32%. The incidence correlated more closely with age than genotype, since rearrangements were present in a progressively lower proportion with increasing age (Table 1, Figure 1). Mean age of Vδ2-Dδ3–positive subjects was 15 years compared with 33 years for negative subjects (P = .0004). In contrast, the mean age of patients with TCRG-rearranged cases was 23 years compared with 18 years for those with negative cases (P = .13).TCRG rearrangements were seen in 13 of 24 (54%) TCR Vδ2-Dδ3–rearranged and 30 of 50 (60%) Vδ2-Dδ3–unrearranged cases. Vδ2-Dδ3 rearrangements were present in all genotype categories (Table 2, Figure 3), with no significant differences. It is noteworthy that TCRDrearrangements occurred in a proportion of E2A-PBX1 cases, when they were seen in the 2 youngest subjects.
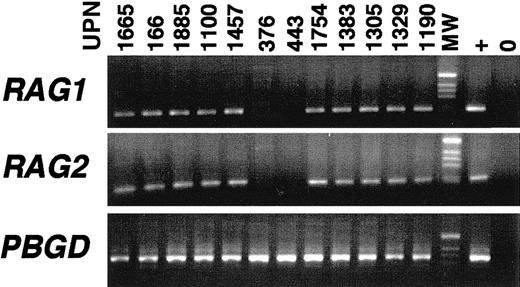
RAG1 and RAG2 expression in BCP-ALL.
The 39 BCP-ALLs included 9 MLL-AF4, 11 E2A-PBX1(1 sIg+ and 10 cIgμ+), 7 BCR-ABL(5 cIgμ+), 1 cIgμ+TEL-AML, 3 sIg+ (not from the present series), 10 cIgμ+, and 6 cIgμ−, FT-negative cases, including 6 infant ALLs. TCRG rearrangement was seen in 12 of 39 cases.
RAG1 and RAG2 expression in BCP-ALL.
The 39 BCP-ALLs included 9 MLL-AF4, 11 E2A-PBX1(1 sIg+ and 10 cIgμ+), 7 BCR-ABL(5 cIgμ+), 1 cIgμ+TEL-AML, 3 sIg+ (not from the present series), 10 cIgμ+, and 6 cIgμ−, FT-negative cases, including 6 infant ALLs. TCRG rearrangement was seen in 12 of 39 cases.
IGH and TCRG rearrangements according to immunophenotype
It was possible that the lower incidence of TCRGrearrangement in MLL-AF4 and E2A-PBX1 ALL resulted from relative immaturity and maturity of the recombinase complex, respectively. MLL-AF4 ALLs classically present with a CD34+, CD19+, CD10−, CD15+ profile.19BCR-ABL andTEL-AML1 are usually CD34+, CD19+, and CD10+, and frequently express the CD13 and/or CD33 myeloid markers.20,21E2A-PBX1 ALLs demonstrate a more mature phenotype, insofar as they often express cIgμ heavy chains and are CD34− but CD10+ and CD22+.22 Although surface CD20 (sCD20) appears before sCD22 during B-lymphoid development,23,24the latter has been considered to represent an earlier marker of differentiation arrest.25
Immunophenotypic data for selected groups are shown in Table 2. All cases expressed CD19. MLL-AF4 cases were indeed relatively immature, as assessed by the absence of CD20 and cIgμ expression. It is, however, noteworthy that CD34 expression was less frequent than inTEL-AML1 and BCR-ABL cases, and sCD22 was seen in 50% of cases. CD15 was expressed in 7 of 9 (78%) MLL-AF4but was not tested in the other categories. TEL-AML1 andBCR-ABL cases demonstrated a similar immunophenotypic profile, apart from more frequent cIgμ and CD20 expression in the latter. E2A-PBX1 cases showed relative maturity, with rare CD34 positivity, universal CD22 expression, and frequent cIgμ positivity. CD20 expression was not, however, more frequent than inTEL-AML1 and BCR-ABL cases.
The incidence of TCRG rearrangements in cIgμ-expressing,E2A-PBX1–negative patients was 7 of 14 (50%), compared with 0 of 10 in cIgμ+,E2A-PBX1–positive cases. The latter showed less frequent CD34 (1 of 10 vs 8 of 14, respectively) and CD20 (4 of 10 vs 9 of 14) expression but similar CD22 (10 of 10 vs 13 of 14) expression when compared with E2A-PBX1–negative cIgμ+ ALLs, thus not clearly demonstrating a distinct stage of maturation arrest. These data suggest that a relatively late stage of maturation arrest is unlikely to explain the complete absence in E2A-PBX1 cases.
Identification of an immunophenotypic category equivalent toMLL-AF4 cases was more difficult, insofar as CD10 negativity was the most characteristic abnormality in this group but was identified in only 3 MLL-AF4–negative (MLLgermline) cases. We therefore compared the incidence of TCRGrearrangement among MLL-AF4 cases using CD34 positivity as an indicator of relative immaturity, or CD22 positivity as an indicator of relative maturity. No differences were observed (data not shown), suggesting that the lower incidence of TCRGrearrangement in MLL-AF4 cases is not due purely to arrest at a particularly early stage of B-lymphoid development.
The absence of TCRG rearrangements in the 6 FT-negative infants was not due to a particular immunophenotypic stage of maturation arrest (Table 2).
RAG1 and RAG2 expression
To determine whether the absence of TCRG rearrangement was secondary to loss of recombinase activity, RAG1 andRAG2 expression was assessed by RT-PCR in selected patients. Analysis of 2 patients with essential thrombocythemia showedRAG1 and RAG2 transcripts in bone marrow but not in peripheral blood, consistent with their detection in B-lymphoid precursors (data not shown). We analyzed 17 blood and 24 bone marrows from 2 mature B-cell leukemias and 39 B-lineage ALLs (Figure 2). All cases other than the 4 surface Ig+(sIg+) ALLs (1 of which wasE2A-PBX1–positive) and both mature B-cell leukemias wereRAG1/2-positive, although 1 cIgμ+E2A-PBX1 bone marrow was only RAG2-positive. Positive cases included 10 cIgμ E2A-PBX1, 9MLL-AF4, and 6 infant cases (Figure 3). Failure to rearrangeTCRG is not therefore due to absence of RAG activity.
TCRG Vγ and Jγ utilization
TCRG Vγ and Jγ utilization was assessed by multifluorescent run-off (FluRO) analysis15 for 62 patients demonstrating a clonal TCRG rearrangement by EB PAGE. ThreeTCRG-negative patients all demonstrated only residual polyclonal rearrangements on FluRO analysis (data not shown). Data from 45 T-ALLs15 are shown for comparative purposes (Figure 2). As expected,10 BCP-ALLs demonstrated a higher proportion of monoallelic rearrangements and a lower mean number of rearranged alleles per patient than T-ALLs. Vγ9 rearrangements were commoner in B-lineage ALL (P = .006) and VγfI-Jγ1/2 less so (P = .03), but no other major differences were noted.
The types of rearrangement differed in the subgroups (Figure 2).E2A-PBX1 and infant cases are obviously absent.TEL-AML1 cases demonstrated only Jγ1/2 rearrangements, predominantly VγfI. Among TCRG-positive MLL-AF4ALLs (all adults), 90% of rearrangements involved Vγ9-Vγ11, predominantly with Jγ1/2. VγΨ10 and VγΨ11 pseudogene utilization was virtually restricted to this category. Among FT-positive cases, VγfI-JγP1/2 rearrangements were restricted toBCR-ABL, and the only JγP rearrangement occurred in this category. These differences could not be explained by age differences, insofar as the ages of BCR-ABL and MLL-AF4 cases were similar (Figure 2), as were those of TEL-AML1 and FT-negative children. Certain Vγ-Jγ rearrangements did, however, differ with age. VγfI composed the vast majority of rearrangements in pediatric cases, whereas Vγ9-Jγ1/2 rearrangements were commoner in adult cases. VγfI-JγP1/2 rearrangements were common in FT-negative children.
Subtyping of VγfI members15 was undertaken for 65 alleles (48 Jγ1/2, 16 JγP1/2, and 1 Vγ2/4-JγP), but no particular differences were noted between different subgroups (data not shown). VγΨ7 rearrangements were restricted to the BCR-ABL andTEL-AML1 categories (2 cases each).
IGHVH-DH-JH–negative patients by PCRs
Fifteen patients (9%), including 3 MLL-AF4 and 2TEL-AML1, were negative for all 4 IGH according to PCRs. To determine whether these patients had undergone JH rearrangement, Southern hybridization with a JH6 probe and/or PCR detection of partial DH-JH rearrangements were performed in 14 cases. Results of both analyses will be presented in greater detail elsewhere (unpublished data, F.D. et al, 1999). Nine of 13 (69%) evaluable patients had undergone partial DH-JH rearrangements, which were oligoclonal in 4. All 3 MLL-AF4 belonged to this category, in keeping with an early stage of maturation arrest. Four ALLs, including bothTEL-AML1 cases, had undergone JHdeletion.
From a practical point of view, 7 of 163 (4%) patients demonstrated neither IGH VH-DH-JH norTCRG clonal rearrangements by PCR. One wasMLL-AF4–positive; 2 were TCRDVδ2-Dδ3–positive, and 2 demonstrated clonal DH-JH rearrangements, thus allowing PCR follow-up. One demonstrated oligoclonal DH-JH, and as such, would be difficult to follow. Only 1 case demonstrated no PCR marker.
Discussion
In this manuscript, we demonstrate that, whereas IGHV-D-J rearrangement varies little with BCP-ALL subtype, this is not the case for “illegitimate” TCR rearrangements. We confirm that the incidence of the TCRD Vδ2-Dδ3 rearrangements decreases with age11 and show that the incidence and type of TCRG rearrangements vary with genotypic subgroup and is virtually absent in infants aged from 1 to 2 years. The overall incidence and type of TCRG rearrangements are similar to those reported in 202 childhood BCP-ALLs, based predominantly on Southern blot analysis.26 Minor differences include a higher incidence of TCRG JγP½ rearrangements in our series (21% vs 13%), which probably reflects the fact that these rearrangements are more easily detected by PCR than by Southern blot,11 and the presence of Vγ8-Jγ½ rearrangements in the present series (8% of rearrangements) but not in Szczepanski et al.11 Rearrangement incidence in Szczepanski et al26 was correlated with immunophenotype but not genotype. It is likely that the CD10− pro-B category (fewer than 5% of patients) corresponded to our MLL-AF4 cases and part of the cIgμ+ pre-B category (30% of patients) to ourE2A-PBX1 cases. Absence of TCRG rearrangement was seen in 25% of pro-B and 15% of pre-B cases, compared with only 2% of common ALL, in keeping with our observations.
Several explanations for the variable incidence of TCRGrearrangements are possible.
RAG activity
Differential expression of RAG1 and 2 could not explain the aforementioned differences, since the only RAG-negative ALLs were those expressing sIg, as previously described.27This was, in any case, an unlikely explanation, since it would imply coordinate occurrence of illegitimate TCRG andTCRD rearrangement, which is not the case.
Maturity
The low incidence of TCRG rearrangement inMLL-AF4, infant cases, and E2A-PBX1 cases may reflect oncogenic transformation of a particularly immature or mature lymphoid precursor. MLL-AF4 cases were, as expected, immunophenotypically immature. Interestingly, CD34 negativity was more common in MLL-AF4 ALL than in all categories other thanE2A-PBX1, including the potentially immature IGHVH-DH-JH–unrearranged group. The recent demonstration28 that the earliest hematopoietic progenitors are CD34− suggests that CD34−MLL-AF4 cases may be more immature than their CD34+MLL-AF4 counterparts. In keeping with this, CD33 expression, which is found on immature hematopoietic progenitors demonstrating multilineage potential,29 was seen in 3 of 5 CD34−MLL-AF4 cases but in 0 of 8 CD34+ cases. Complete IGHVH-DH-JH rearrangement was, however, seen in 4 of 5 CD34− cases.
The arguments for relative maturity as an explanation for the total absence of TCRG rearrangement in E2A-PBX1 ALL are weaker. TCRG rearrangements were seen in 50% of cIgμ-expressing E2A-PBX1–negative patients. It is not therefore the presence of cIgμ that renders the TCRG locus inaccessible. Although we cannot exclude the possibility thatE2A-PBX1 cases are more mature than theirE2A-PBX1–negative cIgμ-expressing counterparts, comparison of CD34, CD20, and CD22 expression was not in favor of this, and the relatively subtle differences observed would be expected to lead to a diminution, rather than a complete absence, ofTCRG rearrangement. Similarly, the virtual absence ofTCRG rearrangement in infants cannot be explained on the basis of maturity, since they are immunophenotypically similar toTEL-AML1 and BCR-ABL cases.
Fetal vs adult-type precursors
It is possible that different types of BCP-ALL undergo oncogenic transformation at distinct stages of ontogenic development with respect to TCR accessibility. Accessibility of the TCRGand TCRD loci must be independently controlled, since we show that rearrangement at these loci does not occur in the same subgroups. Physiological TCRD rearrangement precedesTCRG in human thymus,30 with Dδ2-Dδ3 occurring prior to Vδ-Dδ. Vδ2-Dδ3 rearrangements were not seen; these are virtually restricted to BCP-ALLs. During lymphoid development, early fetal and immature precursorIGH,31-33TCRG,34,35 andTCRD36-38 gene rearrangements preferentially involve 3′, J proximal V segments, whereas a wider range, including 5′ V segments, are found in mature lymphoid cells. Identification of Jγ proximal Vγ utilization may also reflect preliminary attempts to rearrange the locus that have not been superseded by subsequent attempts.
Several arguments from our series of, predominantly adult (12 of 14),MLL-AF4 ALLs favor relative genotypic immaturity: the low level of TCRG rearrangements predominantly involving Vγ9-11, overrepresentation of partial IgHDH-JH rearrangement, and the relatively high incidence of partial TCRD Vδ2-Dδ3 rearrangements (38% overall, 27% of adults). The partial TCRD Vδ2-Dδ3 rearrangements occur more frequently in children than adults, respectively representing 70% and 40% of TCRDrearrangements.11 TCR Vγ utilization was strikingly different from all other BCP-ALLs, insofar as Jγ proximal Vγ9-11 were involved in 90% of rearrangements, compared with 18% of non–MLL-AF4 cases. This may reflect absence of “secondary rearrangement” by upstream Vγ segments. These data are in favor of oncogenic conversion at an early, immature stage of ontogenic development, at the transition of DH-JH to VH-DH-JH rearrangement, after theTCRD locus becomes accessible to at least Vδ2-Dδ3 rearrangement and at the onset of illegitimate TCRGrearrangement. This is in keeping with observations thatMLL-AF4 rearrangements in children occur during fetal development.39 40 It is, however, striking that the present series represents predominantly adult cases (mean age, 47 years).
Applying the same model to infant BCP-ALLs would suggest that they have undergone transformation of a more immature lymphoid precursor in which the TCRG locus is not yet accessible, yet in whichTCRD accessibility is much more pronounced.E2A-PBX1 cases would have to be explained as transformation at a much later ontogenic stage, after IGH rearrangement is complete and some residual TCRD accessibility remains, but after TCRG has become inaccessible.
E2A
The most interesting potential explanation is thatE2A-PBX1 expression is directly or indirectly responsible for rendering the TCRG locus inaccessible to illegitimate recombination. Wild-type E2A plays a fundamental role in regulating lymphoid development.E2A−/− mice demonstrate a total block in B-lymphoid development with absence of DH-JH and VH-DH-JHrearrangement.41,42 Loss of E2A also leads to a block in T-lymphoid development, with development of thymic lymphomas.43 Cell lines derived from the latter undergo programmed cell death in the presence of enforced E2Aexpression,44 suggesting that E2A products can act as tumor suppressors. E2A−/− mice demonstrate a switch from adult to fetal-type TCRγδ lymphocytes andTCRG and TCRD rearrangements.45 E2A does not appear to modulate germline transcription of TCRGbut may target the recombinase to specific recognition signal sequences (RSSs) since consensus E2A-binding motifs were identified in the linker sequences of almost all murine Vγ and Vδ RSSs.45
Cytogenetically, the majority (approximately 75%)46 of t(1;19) are unbalanced, being associated with loss of the der(1) and potential PBX1-E2A reciprocal transcripts and duplication of the normal chromosome 1.47 Consequently, onlyE2A-PBX1 has been considered to be oncogenic, strengthened by the fact that PBX1-E2A transcripts have not been seen in t(1;19) cell lines or patient material,48,49 as confirmed here. Against this, however, is the observation that patients with the unbalanced form have a relatively good prognosis,47suggesting that something on the der(1) may add oncogenic potential. This difference was, however, only seen as a trend by Pui et al.50
E2A-PBX1 leads in all cases to haploinsufficiency.E2A+/− mice demonstrate a partial reduction in B lymphocytes41 and deregulated TCRD gene segment usage,45 suggesting that E2Ais rate limiting. It is therefore possible that E2Ahaploinsufficiency in E2A-PBX1 BCP-ALL may directly interfere with B-lymphoid development and/or IG/TCRrearrangement. The similar phenotype observed in E2A-HLF andE2A-PBX1 transgenic mice has been suggested to represent an oncogenic effect mediated by interference with wild-typeE2A.51 Loss of wild-type E2A activity would be expected to lead to impairment of IGHVH-DH-JH rearrangement, which we show is not the case. It might also be expected to inhibit adult in favor of fetal-type TCRG rearrangements. The absence of both adult and fetal TCRG rearrangements in E2A-PBX1ALLs suggests either that E2A loss is not relevant to this process or that, in a B-lymphoid background, E2A dosage may be even more limiting than in a T-lymphoid background and may be necessary even for fetal-type rearrangements.
Infant ALLs
The basis for the virtual absence of TCRGrearrangements in infants aged 1 to 2 years is unclear but is not due to MLL-AF4 rearrangement or immunophenotypic immaturity. The change from an infant TCRG profile to a pediatric one occurs abruptly at the onset of the peak of childhood ALL. It suggests that infant ALLs without MLL rearrangement represent a distinct subtype of ALL rather than the lower end of the spectrum of pediatric-type BCP-ALLs.
TEL-AML1 and BCR-ABL BCP-ALLs are similar
TEL-AML1 and BCR-ABL ALLs showed similar phenotypic and genotypic features. The only immunophenotypic difference observed was more frequent cIgμ expression in BCR-ABLcases, suggesting relative maturity. In contrast, TEL-AML1cases essentially demonstrated end-stage VγfI-Jγ1/2 rearrangements, whereas immature Vγ9 and JγP1/2 rearrangements were relatively common in BCR-ABL cases. These data suggest that, despite the striking differences in demographic and prognostic features,BCR-ABL and TEL-AML1 ALLs are arrested at a similar stage of development with pronounced phenotypic and genotypic lineage infidelity or promiscuity. The recent demonstration that loss of PAX5 expression uncovers multilineage potential in pro–B-lymphoid cells, including myeloid and T-lymphoid development under appropriate condition,52 53 suggests that assessment of PAX5 status may be interesting in these BCP-ALLs.
Practical significance
From a practical point of view, the variable incidences ofTCRG and TCRD rearrangements observed in BCP-ALLs of different ages and genotypes suggest that the relative representation of the different subgroups identified here should be taken into account when these rearrangements are used as universal markers for molecular follow-up. As an example, use of TCRGwill lead to underrepresentation of E2A-PBX1,MLL-AF4, and infant cases, and use of TCRDVδ2-Dδ3 to underrepresentation of older children. The use of certain Vγ-Jγ combinations will exacerbate this. Obviously this tendency will be minimized by the use of several IG/TCRtargets per patient, as is widely recommended in order to reduce the risk of false negative results. Based on this series, only 3 of 160 (fewer than 2%) patients failed to demonstrate at least 1 PCR-amplifiable IGH, TCRG, or TCRDrearrangement. It is likely that the wide applicability of these markers, along with recent encouraging data with regard to their predictive value in childhood ALL,12 13 will lead to their increasing use in the management of BCP-ALL. Our data suggest that results using these markers should be interpreted in the light of accurate genotyping at diagnosis.
Acknowledgments
The authors thank Judith Landmann-Parker (Hôpital Trousseau, Paris), Marie-Helene Estienne (Tours), Laure Croisille (Hôpital Kremlin-Bicêtre, Paris), C. Bayle (Institut Gustave-Roussy, Villejuif), Xavier Troussard (Caen), Alain Bourguignat (Centre René-Huguenin, Saint-Cloud), André Barruchel and Marie-Françoise Auclerc (Hôpital St. Louis), and Françoise Picard (Hôpital Cochin, Paris) for providing BCP-ALL samples and clinical data.
Supported by the Fondation de France/Fondation Contre la Leucémie, the Fondation pour la Recherche Médicale, the Ligue Nationale contre le Cancer (Comité de Paris), the Association pour la Recherche sur le Cancer, and the Direction de Recherche Clinique de L'Assistance Publique-Hôpitaux de Paris (PHRC 97-106).
C.B. and E.D. contributed equally to this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elizabeth A. Macintyre, Laboratoire d'Hématologie, Tour Pasteur, Hôpital Necker, 149-161, rue de Sèvres, 75743 Paris cedex 15, France; e-mail:elizabeth.macintyre@nck.ap-hop-paris.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal