Abstract
Acute megakaryocytic leukemia (AMegL) is a rare subtype of acute myeloid leukemia (AML) evolving from primitive megakaryoblasts. Because of its rarity and the lack of precise diagnostic criteria in the past, few series of adults treated with contemporary therapy have been reported. Twenty among 1649 (1.2%) patients with newly diagnosed AML entered on Eastern Cooperative Oncology Group (ECOG) trials between 1984 and 1997 were found to have AMegL. The median age was 42.5 years (range 18-70). Marrow fibrosis, usually extensive, was present in the bone marrow. Of the 8 patients who had cytogenetic studies performed, abnormalities of chromosome 3 were the most frequent. The most consistent immunophenotypic finding was absence of myeloperoxidase in blast cells from 5 patients. In the most typical 3 cases, the leukemic cells were positive for one to 2 platelet-specific antigens in addition to lacking myeloperoxidase or an antigen consistent with a lymphoid leukemia. Myeloid antigens other than myeloperoxidase and selected T-cell antigens (CD7 and/or CD2) were frequently expressed. Induction therapy included an anthracycline and cytarabine in all cases. Complete remission (CR) was achieved in 10 of 20 patients (50%). Two patients remain alive, one in CR at 160+ months. Resistant disease was the cause of induction failure in all but 3 patients. The median CR duration was 10.6 months (range 1-160+ months). The median survival for all patients was 10.4 months (range 1-160+ months). Although half of the patients achieved CR, the long-term outcome is extremely poor, primarily attributable to resistant disease. New therapeutic strategies are needed.
Introduction
Acute megakaryocytic leukemia (AMegL) is a rare subtype of acute myeloid leukemia (AML) developing from primitive megakaryoblasts, first described by Von Boros and colleagues in 1931.1 The disease can be identified by antibodies to glycoprotein IIb/IIIa and is often associated with extensive myelofibrosis.2-9 Reports in the literature have been sporadic because of both the rarity of the disease and the lack of well-established diagnostic criteria. Precise diagnostic criteria were added to the French-American-British (FAB) classification only relatively recently and the disease is also referred to as FAB M7.10 Therefore, because many cases were reported in older literature, patients previously categorized as AMegL represent a heterogenous group.
Despite the paucity of reports, several unusual associations with AMegL have been identified. First, AMegL has been linked to primary mediastinal germ cell tumors.11-13 Second, children with Down syndrome appear to have an increased incidence of AMegL and such patients have a more favorable outcome than when AMegL occurs without such an association in adults.14-19 Patients also have been described with AMegL without Down syndrome, but whose leukemic cells have abnormalities of chromosome 21, the specific chromosome associated with Down syndrome.20,21 Third, extensive fibrosis is frequently, but not invariably, present.22-25
Although some patients achieve complete remission (CR), few patients survive beyond 3 years.26-29 Clinical experience with this rare leukemia remains limited. This analysis was designed to determine the laboratory and clinical features, biologic characteristics, and outcome of patients with AMegL treated with a contemporary induction regimen that included an anthracycline and cytarabine on Eastern Cooperative Oncology Group acute leukemia protocols.
Materials and methods
Patients
The medical records of 20 patients with AMegL entered on 5 trials for previously untreated AML conducted by the Eastern Cooperative Oncology Group (ECOG) between 1983 and 1997 were retrospectively reviewed. The total number of patients with AML included patients enrolled on a sixth protocol E3993, a randomized trial of 3 anthracyclines in induction for older adults, but there were no cases of AMegL identified on this trial. These were the only 6 trials active during this period for patients whose conditions were newly diagnosed. This recent 12-year period was selected because patients were generally treated with anthracycline and cytarabine regimens for induction.
Diagnosis of acute megakaryocytic leukemia (FAB M7)
The diagnosis of AMegL was established principally on morphologic grounds at the individual institution, but subsequently centrally reviewed at the time of study entry and again in preparation for this analysis by the same single individual both times (J.M.B.).10,30 The bone marrow aspirate or biopsy leukemic blast cell population must have represented 30% or more of the myeloid marrow, excluding lymphocytes and plasma cells. The majority of these cells were undifferentiated and therefore devoid of myeloperoxidase by routine cytochemical methods. Some were easily identified as early or dysplastic megakaryocytic precursors. Other cytochemical stains, including nonspecific esterases, periodic acid-Schiff, and acid phosphatase reactions were not diagnostic. These were the prevailing diagnostic criteria according to the FAB classification at the time these ECOG studies were conducted. Confirmation of the cell of origin was performed, whenever possible, to demonstrate either platelet peroxidase by electron microscope, immunocytochemistry stain for factor VIII on the bone marrow biopsy, or the presence of antibodies against glycoprotein IIb/IIIa (CD41a) or glycoprotein IIIa (CD61). Myelofibrosis as demonstrated with a reticulin stain was strongly positive in most cases. Bone marrow reticulin was quantified, according to Bauermeister,31 as follows: 0: no reticulin fibers demonstrable; 1: occasional fine individual fibers and foci of a fine fiber network; 2: fine fiber network throughout, no coarse fibers; 3: diffuse fiber network with scattered thick coarse fibers; and 4: diffuse coarse fiber network. Immunophenotyping was performed centrally by multicolor flow cytometry in the ECOG Immunophenotyping Laboratory by a single individual (E.P.) as previously described for 7 of the patients entered on the most recently trials.32 The immunophenotype results from patients entered on to trials before the ECOG Immunophenotyping Reference Laboratory was initiated were performed at the institution, but centrally reviewed by the same individual (E.P.). Antibodies to platelet-specific glycoproteins (GP) were used to characterize myeloperoxidase-negative acute leukemia: CD41 (GPIIb/IIIa), CD36 (GPIIIb), as well as antibody to blood group H antigen.33
Treatment
Patients with AMegL were identified from the ECOG database of patients entered on the following 5 clinical trials for previously untreated AML. EST 3483 was a prospective randomized phase III trial of postremission therapy in AML.34 Patients with previously untreated AML were given one to 2 courses of induction with daunorubicin 60 mg/m2 intravenously per day for 3 days, cytosine arabinoside 200 mg/m2 continuous intravenous (IV) infusion for 5 days plus 6-thioguanine 100 mg/m2orally every 12 hours for 5 days, and then randomized to either one course of intensive consolidation therapy with high-dose cytosine arabinoside 3 gm/m2 IV every 12 hours days 1 to 6, plus amsacrine 100 mg/m2 per day IV on days 7 to 9 or maintenance therapy for 2 years with 6-thiogranine 40 mg/m2twice daily each week plus cytosine arabinoside 60 mg/m2subcutaneously on day 5 each week or observation. Patients younger than 41 years with a histocompatible sibling were to undergo allogeneic transplantation. EST 1490 was a randomized placebo-controlled phase III study of granulocyte-macrophage colony-stimulating factor (GM-CSF) in adult patients (older than 55-70 years) with AML.35Induction consisted of one to 2 courses of daunorubicin 60 mg/m2 for 3 days and cytosine arabinoside 100 mg/m2 daily for 7 days by continuous IV infusion. If the day-10 bone marrow was hypoplastic, patients were randomized to receive GM-CSF 250 μg/m2 IV or placebo until neutrophil recovery. Consolidation therapy consisted of cytosine arabinoside 1.5 gm/m2 IV every 12 hours for 6 days. PC 486 was a phase II pilot study of autologous bone marrow transplantation in patients with AML in first CR using 4-hydroperoxy-cyclophosphamide–treated marrow.36,37 Induction consisted of daunorubicin 60 mg/m2 per day by IV push for 3 days on days 1 to 3 and cytarabine 25 mg/m2 IV push, followed by continuous plus thioguanine 100 mg/m2 every 12 hours orally on days 1 to 5. In EST 3489, remission was induced by idarubicin 12 mg/m2IV for 3 days and cytosine arabinoside IV continuous infusion days 1 to 7. Subsequently, patients with a suitably HLA-matched donor were assigned to allogeneic transplantation and others were randomized to either autologous bone marrow transplantation or conventional consolidation chemotherapy.38 Postremission chemotherapy included high-dose cytosine arabinoside 3 gm/m2 every 12 hours for 6 days and allogeneic bone marrow transplantation. EST 4995 was a phase II study of an intensified induction regimen, including daunorubicin 45 mg/m2 IV days 1 to 3, cytosine arabinoside 100 mg/m2 per day IV days 1 to 7 and 3 days of high-dose cytosine arabinoside 2 g/m2 IV days 8 to 10, followed by 2 cycles of consolidation chemotherapy that includes high-dose cytosine arabinoside 3 gm/m2 IV every 12 hours days 1, 3, and 5, followed by either allogeneic or autologous bone marrow or stem cell transplantation.39
Results
Clinical and laboratory characteristics at presentation
Twenty patients among 1649 (1.2%) patients entered on the 6 ECOG trials between 1984 and 1997 for previously untreated AML had AMegL. Fourteen of the 20 patients (70%) were men and 6 (30%) were women (Table 1). The median age was 42.5 years with a range of 18 to 70 years. The median white blood cell (WBC) count was 2.0 × 109L (range 0.8-35.2), the median hemoglobin was 8.8 g/dL (range 4-14), and the median platelet count was 65 × 109/L (range 12-1450). Three patients had a platelet count greater than 1000 × 109/L. The median peripheral blast percentage was 7.5% (range 0%-84%) and the median percentage of blasts in the bone marrow was 59% (range 5%-99%). Marrow fibrosis was present in the bone marrow core biopsy specimens from all 17 patients in whom it could be assessed. Fifteen patients (75%) had extensive fibrosis (scattered or diffuse coarse fibers). Extramedullary disease was present on clinical grounds at the time of diagnosis in the spleen (one patient); and liver, spleen, and skin (one patient). None of the patients had Down syndrome.
Characteristics of patients with acute megakaryocytic leukemia
| Patient . | ECOG study . | Age (y) . | Gender . | WBC × 109/L . | Hb gm/dL . | Plts × 109/L . | Peripheral blasts (%) . | Marrow blasts (%) . | Marrow fibrosis* . | Cycles of induction . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E3489 | 43 | F | 6.1 | 8.2 | 86 | 53 | 64 | 3 | 1 |
| 2 | E3489 | 53 | M | 2.3 | 14.0 | 33 | 7 | 10 | 3 | 1 |
| 3 | E3489 | 54 | M | 1.5 | 8.2 | 820 | 2 | 32 | 1 | 1 |
| 4 | E3489 | 37 | M | 35.2 | 8.7 | 1016 | 3 | 74 | 3 | 1 |
| 5 | E3489 | 26 | F | 15.6 | 8.2 | 33 | 79 | 31 | 4 | 1 |
| 6 | E3489 | 42 | F | 0.9 | 8.6 | 1000 | 4 | 61 | 3 | 2 |
| 7 | E4995 | 52 | F | 1.0 | 8.6 | 57 | 0 | 59 | NA | 1 |
| 8 | E1490 | 65 | M | 2.4 | 11.9 | 161 | 38 | 90 | 1 | 1 |
| 9 | E1490 | 70 | M | 1.1 | 8.0 | 23 | 20 | 50 | NA | 1 |
| 10 | E3483 | 59 | M | 1.5 | 9.3 | 34 | 16 | NA | 2 | 1 |
| 11 | E3483 | 42 | M | 11.7 | 9.5 | 40 | 63 | NA | 3 | 2 |
| 12 | E3483 | 48 | M | 1.0 | 9.4 | 1450 | 0 | 5 | 3 | 1 |
| 13 | E3483 | 61 | M | 3.4 | 9.1 | 23 | 12 | NA | 2 | 2 |
| 14 | E3483 | 39 | F | 3.8 | 6.7 | 330 | 14 | 15 | 3 | 2 |
| 15 | E3483 | 18 | F | 8.5 | 10.2 | 319 | 1 | 50 | 4 | 2 |
| 16 | PC486 | 35 | M | 1.7 | 10.5 | 73 | 0 | 99 | 3 | 1 |
| 17 | PC486 | 38 | M | 1.0 | 9.9 | 12 | 2 | 90 | NA | 2 |
| 18 | E3483 | 36 | M | 33.0 | 8.9 | 145 | 84 | 95 | 3 | 2 |
| 19 | E3483 | 56 | M | 1.0 | 4.0 | 28 | 2 | 30 | 3 | 1 |
| 20 | PC486 | 35 | M | 0.8 | 7.9 | 49 | 8 | 80 | 3 | 2 |
| Patient . | ECOG study . | Age (y) . | Gender . | WBC × 109/L . | Hb gm/dL . | Plts × 109/L . | Peripheral blasts (%) . | Marrow blasts (%) . | Marrow fibrosis* . | Cycles of induction . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E3489 | 43 | F | 6.1 | 8.2 | 86 | 53 | 64 | 3 | 1 |
| 2 | E3489 | 53 | M | 2.3 | 14.0 | 33 | 7 | 10 | 3 | 1 |
| 3 | E3489 | 54 | M | 1.5 | 8.2 | 820 | 2 | 32 | 1 | 1 |
| 4 | E3489 | 37 | M | 35.2 | 8.7 | 1016 | 3 | 74 | 3 | 1 |
| 5 | E3489 | 26 | F | 15.6 | 8.2 | 33 | 79 | 31 | 4 | 1 |
| 6 | E3489 | 42 | F | 0.9 | 8.6 | 1000 | 4 | 61 | 3 | 2 |
| 7 | E4995 | 52 | F | 1.0 | 8.6 | 57 | 0 | 59 | NA | 1 |
| 8 | E1490 | 65 | M | 2.4 | 11.9 | 161 | 38 | 90 | 1 | 1 |
| 9 | E1490 | 70 | M | 1.1 | 8.0 | 23 | 20 | 50 | NA | 1 |
| 10 | E3483 | 59 | M | 1.5 | 9.3 | 34 | 16 | NA | 2 | 1 |
| 11 | E3483 | 42 | M | 11.7 | 9.5 | 40 | 63 | NA | 3 | 2 |
| 12 | E3483 | 48 | M | 1.0 | 9.4 | 1450 | 0 | 5 | 3 | 1 |
| 13 | E3483 | 61 | M | 3.4 | 9.1 | 23 | 12 | NA | 2 | 2 |
| 14 | E3483 | 39 | F | 3.8 | 6.7 | 330 | 14 | 15 | 3 | 2 |
| 15 | E3483 | 18 | F | 8.5 | 10.2 | 319 | 1 | 50 | 4 | 2 |
| 16 | PC486 | 35 | M | 1.7 | 10.5 | 73 | 0 | 99 | 3 | 1 |
| 17 | PC486 | 38 | M | 1.0 | 9.9 | 12 | 2 | 90 | NA | 2 |
| 18 | E3483 | 36 | M | 33.0 | 8.9 | 145 | 84 | 95 | 3 | 2 |
| 19 | E3483 | 56 | M | 1.0 | 4.0 | 28 | 2 | 30 | 3 | 1 |
| 20 | PC486 | 35 | M | 0.8 | 7.9 | 49 | 8 | 80 | 3 | 2 |
ECOG = Eastern Cooperative Oncology Group; WBC = white blood cell.
As described by Bauermeister et al.31
Cytogenetic studies
Eight of the 20 patients had cytogenetic studies carried out (Table 2). Karyotype analysis was normal in 2 cases. Abnormalities involving chromosome 3 were the most frequent. Two patients had a t(3;3) (q21;q26) translocation, one had a t(3;12)(q25;p11.2) translocation, and one patient had an inv3(q21;q26) abnormality. Twelve patients had no cytogenetic studies performed. Two other cases had different chromosomally abnormal clones.
Karyotype, immunophenotype, and immunohistochemical stain for factor VIII and platelet antigen expression of patients with acute megakaryocytic leukemia
| Patient . | Karyotype . | Immunophenotype/Immunohistochemical stain . |
|---|---|---|
| 1 | 46,XX,t(3;3)(q21;q26) | POX−,HLA−DR+,Ly ag−,My ag−,CD41+,H−ag− |
| 2 | N/A | N/A/FVIII+ |
| 3 | 46,XY,inv(3)(q21;q26) | NA/PH ag+ |
| 4 | 46,XY,t(3;3)(q21;q26) | POX+,HLA−DR+,CD34+,CD2+,CD7+,My ag+,Mono ag+/FVIII+ |
| 5 | 46,XX | POX−,HLA−DR+,CD34+,CD7+,CD33+,CD13+,Plt ag− |
| 6 | 46,XX,−7,+mar[4]/47,XX,+mar[2]/92,XXXX,−7,−7,+marx2[3]/46,XX[11] | POX−,HLA−DR−,CD34+,Ly ag−,My ag−,CD41+,H ag+ |
| 7 | N/A | POX−,HLA−DR+,CD34+,CD117+,My ag+,CD41+,CD61+,CD36+/FVIII+ |
| 8 | 46,XY,t(8;17)(p21;p13)[3]/46,XY[25] | POX−,HLA-DR+,CD34+,TdT+,CD2+,My ag+,CD41+/FVIII+ |
| 9 | N/A | N/A/FVIII+ |
| 10 | N/A | N/A |
| 11 | N/A | N/A/FVIII+ |
| 12 | N/A | N/A |
| 13 | N/A | N/A |
| 14 | N/A | N/A |
| 15 | 46,XX | POX+,HLA-DR−,My ag+,Mono ag+,Plt ag− |
| 16 | N/A | N/A |
| 17 | N/A | N/A |
| 18 | N/A | N/A |
| 19 | N/A | N/A |
| 20 | 46,XY,t(3;12)(q25;p11.2),−13,+mar[6] | N/A/FVIII+ |
| Patient . | Karyotype . | Immunophenotype/Immunohistochemical stain . |
|---|---|---|
| 1 | 46,XX,t(3;3)(q21;q26) | POX−,HLA−DR+,Ly ag−,My ag−,CD41+,H−ag− |
| 2 | N/A | N/A/FVIII+ |
| 3 | 46,XY,inv(3)(q21;q26) | NA/PH ag+ |
| 4 | 46,XY,t(3;3)(q21;q26) | POX+,HLA−DR+,CD34+,CD2+,CD7+,My ag+,Mono ag+/FVIII+ |
| 5 | 46,XX | POX−,HLA−DR+,CD34+,CD7+,CD33+,CD13+,Plt ag− |
| 6 | 46,XX,−7,+mar[4]/47,XX,+mar[2]/92,XXXX,−7,−7,+marx2[3]/46,XX[11] | POX−,HLA−DR−,CD34+,Ly ag−,My ag−,CD41+,H ag+ |
| 7 | N/A | POX−,HLA−DR+,CD34+,CD117+,My ag+,CD41+,CD61+,CD36+/FVIII+ |
| 8 | 46,XY,t(8;17)(p21;p13)[3]/46,XY[25] | POX−,HLA-DR+,CD34+,TdT+,CD2+,My ag+,CD41+/FVIII+ |
| 9 | N/A | N/A/FVIII+ |
| 10 | N/A | N/A |
| 11 | N/A | N/A/FVIII+ |
| 12 | N/A | N/A |
| 13 | N/A | N/A |
| 14 | N/A | N/A |
| 15 | 46,XX | POX+,HLA-DR−,My ag+,Mono ag+,Plt ag− |
| 16 | N/A | N/A |
| 17 | N/A | N/A |
| 18 | N/A | N/A |
| 19 | N/A | N/A |
| 20 | 46,XY,t(3;12)(q25;p11.2),−13,+mar[6] | N/A/FVIII+ |
POX = myeloperoxidase by antibody staining; Ly ag = all lymphoid antigens (T-antigens: intracytoplasmic CD3, CD2, CD7, surface CD3, CD4, CD8; B-antigens: intracytoplasmic CD22, CD19, CD24, surface CD22, CD20); My ag = all myeloid antigens (CD33, CD13, CD65s, CD15); Mono ag = monocytic antigens (CD11b, CD14); TdT = terminal transferase; H ag = blood group H antigen; Plt ag; all platelet-specific antigens; FVIII = factor VIII antigen expression.
Central immunophenotyping
For patients entered on the early AML treatment study E3483, central immunophenotyping was not yet performed in the ECOG. Material for immunophenotyping was submitted to the ECOG's reference laboratory for 9 of the more recent patients. Because of marrow fibrosis, immunophenotyping studies were successful in only 7 of these patients (patients 1, 4, 5, 6, 7, 8, ad 15) (Table 2). The most consistent and significant finding with respect to diagnosis was the absence of myeloperoxidase in the blast cells from 5 of the patients. HLA-DR and CD34 were commonly expressed. In the immunophenotypically most typical cases of AMegL, patients 6, 7, and 8, the leukemic cells were positive for one to 2 platelet-specific antigens in addition to lacking myeloperoxidase or an antigen profile consistent with a lymphoid leukemia. Myeloid antigens other than myeloperoxidase and selected T-cell antigens (CD7 and/or CD2) were frequently expressed. B-cell antigens were not detected. Blast cells from 2 patients expressed myeloperoxidase (cases 4 and 15) as well as myelomonocytic antigens, but lacked megakaryocytic markers.
Cytoimmunochemistry
All patients had a morphologic and/or histologic description consistent with a diagnosis of AMegL. Platelet glycoprotein staining was positive in the institution or ECOG reference laboratory in patients 1, 3, 7, and 8 (Table 3). Factor VIII was positive in patients 2, 4, 7, 8, 9, 11, and 20. Therefore, in 13 of the 20 cases, either myeloperoxidase was negative and/or platelet glycoprotein CD41 and/or factor VIII was positive. Although nonspecific, the alpha naphthyl acetate stain was negative in 2 cases (patients 11 and 18) and positive in only one case (patient 3). Granulocytic dysplasia was present in patients 3, 5, 6, 8, and 15. Erythroid dysplasia was present in patient 12.
Summary of megakaryocytic lineage markers
| Patient . | POX . | FVIII . | Platelet antigen . |
|---|---|---|---|
| 1 | − | NA | +3-150 |
| 2 | − | + | NA |
| 3 | − | NA | +3-150 |
| 4 | + | + | NA |
| 5 | − | NA | − |
| 6 | − | NA | + |
| 7 | − | + | + |
| 8 | − | + | NA |
| 9 | NA | + | NA |
| 10 | NA | NA | NA |
| 11 | NA | + | NA |
| 12 | NA | NA | NA |
| 13 | NA | NA | NA |
| 14 | NA | NA | NA |
| 15 | + | NA | − |
| 16 | NA | NA | NA |
| 17 | NA | NA | NA |
| 18 | − | NA | NA |
| 19 | − | NA | NA |
| 20 | − | + | NA |
| Patient . | POX . | FVIII . | Platelet antigen . |
|---|---|---|---|
| 1 | − | NA | +3-150 |
| 2 | − | + | NA |
| 3 | − | NA | +3-150 |
| 4 | + | + | NA |
| 5 | − | NA | − |
| 6 | − | NA | + |
| 7 | − | + | + |
| 8 | − | + | NA |
| 9 | NA | + | NA |
| 10 | NA | NA | NA |
| 11 | NA | + | NA |
| 12 | NA | NA | NA |
| 13 | NA | NA | NA |
| 14 | NA | NA | NA |
| 15 | + | NA | − |
| 16 | NA | NA | NA |
| 17 | NA | NA | NA |
| 18 | − | NA | NA |
| 19 | − | NA | NA |
| 20 | − | + | NA |
POX = myeloperoxidase by antibody staining; FVIII = factor VIII antigen expression.
Outside institution (+), but ECOG Reference Laboratory (−).
Outcome
Complete remission was achieved in 10 of the 20 patients (50%) (Table 4). Ten patients (50%) had no response. One patient remains alive and in remission at 160+ months. Resistant disease was the cause for induction failure in all but 3 patients who died of respiratory failure, sepsis, and probable infection.
Outcome of treatment of patients with acute megakaryocytic leukemia
| Patient . | Response to induction therapy . | Remission duration (mo) . | Survival (mo) . | Cause of induction failure . | Current status . |
|---|---|---|---|---|---|
| 1 | CR | 3 | 21 | Deceased | |
| 2 | NR | 5 | AMegL | Deceased | |
| 3 | CR | 19 | 24 | Deceased | |
| 4 | CR | 2 | 10 | Deceased | |
| 5 | NR | 6 | AMegL | Deceased | |
| 6 | NR | 2 | Sepsis | Deceased | |
| 7 | CR | 15 | 22+ | Alive | |
| 8 | CR | 7 | 15 | Deceased | |
| 9 | CR | 4 | 6 | Deceased | |
| 10 | CR | 160+ | 160+ | Alive | |
| 11 | NR | 3 | AMegL | Deceased | |
| 12 | NR | 1 | Respiratory failure | Deceased | |
| 13 | NR | 11 | AMegL | Deceased | |
| 14 | NR | 1 | Probable infection | Deceased | |
| 15 | CR | 12 | 30 | Deceased | |
| 16 | CR | 1 | 4 | Deceased | |
| 17 | NR | 37 | AMegL | Deceased | |
| 18 | NR | 10 | AMegL | Deceased | |
| 19 | CR | 9 | 10 | Deceased | |
| 20 | NR | 10 | AMegL | Deceased |
| Patient . | Response to induction therapy . | Remission duration (mo) . | Survival (mo) . | Cause of induction failure . | Current status . |
|---|---|---|---|---|---|
| 1 | CR | 3 | 21 | Deceased | |
| 2 | NR | 5 | AMegL | Deceased | |
| 3 | CR | 19 | 24 | Deceased | |
| 4 | CR | 2 | 10 | Deceased | |
| 5 | NR | 6 | AMegL | Deceased | |
| 6 | NR | 2 | Sepsis | Deceased | |
| 7 | CR | 15 | 22+ | Alive | |
| 8 | CR | 7 | 15 | Deceased | |
| 9 | CR | 4 | 6 | Deceased | |
| 10 | CR | 160+ | 160+ | Alive | |
| 11 | NR | 3 | AMegL | Deceased | |
| 12 | NR | 1 | Respiratory failure | Deceased | |
| 13 | NR | 11 | AMegL | Deceased | |
| 14 | NR | 1 | Probable infection | Deceased | |
| 15 | CR | 12 | 30 | Deceased | |
| 16 | CR | 1 | 4 | Deceased | |
| 17 | NR | 37 | AMegL | Deceased | |
| 18 | NR | 10 | AMegL | Deceased | |
| 19 | CR | 9 | 10 | Deceased | |
| 20 | NR | 10 | AMegL | Deceased |
CR = complete remission; NR = no response or lack of complete remission; AMegL = acute megakaryocytic leukemia.
Remission duration
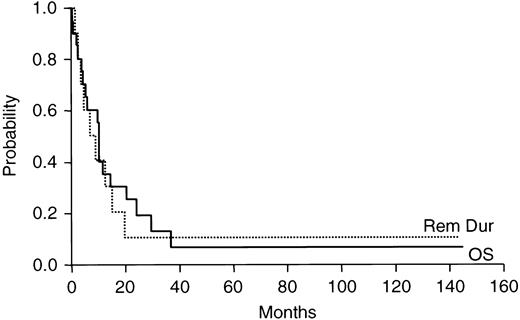
The median remission duration among patients achieving CR was 10.6 months with a range of 1 to 160+ months (Figure1).
Duration of response and overall survival for 20 patients with previously untreated acute megakaryocytic leukemia.
Duration of response and overall survival for 20 patients with previously untreated acute megakaryocytic leukemia.
Overall survival
The median overall survival for all patients was 10.4 months with a range of 1 to 160+ months (Figure 1). Six patients survived for more than 1 year beyond study entry. Of these, 5 achieved CR. The sixth patient was treated on PC486, a transplant study and died 3.1 years after study entry. One patient treated on E3483 remained alive 6.8 years after study entry, and updated survival information was last obtained in February 1992. Two patients remain alive, one treated on E3483 remains alive in CR at 160+ months and one treated recently on E4995, who received a peripheral blood stem cell transplant off study, has relapsed and survives 22 months after study entry.
Discussion
Although the first description of AMegL appeared in the literature almost 70 years ago,1 reports of the natural history of the disease have generally been confined to either sporadic small series,2-9 reports of one or 2 cases40-44 or descriptions of the clinical course in infants and children.45-50 Many cases previously reported antedated the establishment of precise diagnostic methods. In fact, this subtype became part of the FAB classification only in 1985.10Since then, immunophenotyping by flow cytometry has emerged as a useful method to improve the diagnostic sensitivity because cells from patients with AMegL express the megakaryocyte platelet lineage-specific marker CD61.51-53 Historically, the lack of specific criteria for the diagnosis has led to reports of patients who likely had AMegL, but whose disease was labeled acute myelofibrosis or myelosclerosis.54-60 Given the sophisticated methods now available to assist in the diagnosis and advances in therapeutic strategies for patients with AML, data regarding the biologic characteristics, response to contemporary induction therapy and natural history of AMegL in adults are needed.
This analysis of 20 patients with AMegL represents cases with morphology confirmed at central review, and treated with anthracycline plus cytarabine-based induction. We found that the incidence of AMegL in adults was lower (1.2%) than often reported (3%-10%), although many earlier series include infants and children.8,9,27,53Ribeiro and colleagues27 identified 14 cases among 150 consecutive patients (9.3%) seen at St. Jude Children's Research Hospital. The well-described, but unexplained association of AMegL with Down syndrome may account for the apparent higher incidence in the pediatric population. It is possible that the true incidence of AMegL was underestimated in this patient population, because strict FAB criteria were used requiring the presence of at least 30% blasts.10 Under the revised World Health Organization (WHO) criteria, the presence of 20% blasts would be sufficient.61 In addition, inaspirable bone marrows have contributed to the difficulty in establishing the diagnosis. Furthermore, patients with particularly poor prognoses may not be entered on multiinstitutional clinical trials. However, the precise incidence will require further study because of the historical difficulty in establishing the diagnosis. Clinically, AMegL and acute myelofibrosis are indistinguishable and careful examination of the morphology of marrow blasts, together with immunoperoxidase staining or immunophenotyping for expression of factor VIII are often required.62
Although a formal comparison of the distinctive clinical features of only 20 cases of AMegL with other subtypes of AML is difficult because of the small numbers of cases, several observations can be made. The median age of the patients reported here is relatively young (42.5 years), compared with patients with other subtypes of AML (63 years). However, all the protocols onto which the patients reported here were entered, except one (E1490), focused on relatively young patients for whom transplant was a consideration. The median age of patients with other subtypes of AML on these trials was 41 years. Therefore, no definitive statement can be made about the median age of patients with AMegL compared with that of patients with other subtypes of AML. Marrow fibrosis is present in virtually all patients, in contrast to most other patients with AML. Abnormalities of chromosome 3, particularly 3q abnormalities, are often present and may be associated with preservation of the peripheral platelet count, as reported here.64
When the disease occurs in children, AMegL shows close associations with either of 2 cytogenetic abnormalities, t(1;22)(p13;q13) or numeric abnormalities of chromosome 21, especially trisomy 21. However, cytogenetic changes in adults with this disease are less clearly defined, but may involve rearrangements of chromosome 3, especially at 3q21 and 3q26-27, monosomy 7, monosomy or deletions of chromosome 5, and trisomy 8.65,66 In the patients reported here, 20% showed a t(3;3) or inv3, which when observed in other subtypes of AML are often associated with thrombocytosis and other platelet abnormalities.67 68 Two other patients demonstrated a monosomy 7 or deletion 5q.
Although morphologic and cytochemical criteria are used to exclude other subtypes of AML, immunophenotyping is most valuable to differentiate between a lymphoid and a megakaryocytic nature of myeloperoxidase negative acute leukemia.33 An antigen profile typical of AMegL was observed in 3 of our 7 patients in whom immunophenotyping was successfully performed. The leukemic blasts were negative by staining with antibody to myeloperoxidase, lacked lymphoid antigens with the exception of selected T-antigens (CD2 or CD7), and expressed platelet-specific antigens on their surface (CD41, blood group H antigen, CD36). As observed by others, CD41 was associated with CD34 expression.69,70 Interestingly, blast cells from patient 7 also stained for CD36, which is generally considered a late differentiation marker of CD34 negative megakaryocytic cells.33 Myeloid antigens such as CD33 and CD13, as found in all 3 patients, are not uncommon in this disease. Patient 1, while lacking megakaryocytic antigens, also failed to express any of the other lineage-associated antigens tested. In view of the t(3;3) found in this patient's leukemic cells and taking into consideration the morphologic picture, the overall scheme suggests the diagnosis of AMegL. Blast cells from the other myeloperoxidase negative, platelet glycoprotein negative case, patient 5, expressed early myeloid antigens CD33 and CD13 but none of the later myeloid antigens, such as CD65s or CD15, or monocytic antigens, CD11b and CD14. Given the myeloperoxidase negativity and the AMegL morphology, the most likely immunodiagnosis in this case would also be AMegL. The 2 remaining patients, however, present a diagnostic dilemma. Blast cells from both patients 4 and 15 were positive for myeloperoxidase by antibody staining as well as for all myeloid antigens tested, as well as for the prototype monocytic antigen CD14. Without morphologic information, this antigen profile is most consistent with acute monocytic leukemia. Whenever tested, CD14 has been found to be negative in other reports of AMegL immunophenotyping.65,66 69 Although in patient 4, the t(3;3) would support the AMegL morphologic diagnosis, patient 15 demonstrated a normal karyotype, thereby adding to the diagnostic controversy. These immunophenotypic findings in a small group of patients characterized as AMegL by morphology further emphasize the importance of sophisticated laboratory studies in the diagnosis of this rare, clinically problematic group of patients.
All patients reported here were initially treated with cytarabine and an anthracycline (daunorubicin or idarubicin) or a nonanthracycline DNA intercalator (amsacrine or mitoxantrone). In contrast, other reports have described the outcome with a variety of treatment including low-dose cytarabine,8,9,27 etoposide,8 and bone marrow transplantation.8,42,71 The CR rate achieved among patients reported here was 50%, which is somewhat lower than that generally achieved among patients with other morphologic subtypes of AML but is higher than that generally reported in the literature for adults with AMegL (25%-30%).3,8,59 It must be emphasized that no definite statement can be made about the CR rate with such relatively small numbers. The same applies to the few other series that have been reported, each evaluating a small number of cases treated in a heterogeneous way. Furthermore, many reported patients have not received a conventional anthracycline plus cytarabine combination for induction. In the series by Ribeiro et al,27 the CR rate among 24 children and adolescents was 42% (ages 4 months to 21 years, median 23.5 months). The highest CR rates have been reported by Ruiz-Arguelles and colleagues,26 who observed a CR rate of 73% for 26 patients treated with aggressive chemotherapy and 84% for the 19 patients given low-dose cytarabine, with median survivals of 10 and 4 months, respectively.
The major cause of induction failure among patients reported here was resistant disease. Furthermore, despite a CR rate of 50%, the long-term outcome was extremely poor. Almost every patient died of the disease with relatively short remission durations. Only one patient remains alive and free of disease, a 59-year-old man treated on E3483 who is well 12 years after the diagnosis. This suggests that, although improvement in the CR rate is needed, improved postremission therapy is mandatory. The results of high-dose chemoradiotherapy and allogeneic stem cell transplantation have been reported only anecdotally.71 New therapeutic strategies need to be pursued, including biologic agents such as interferon72 and the apoptosis-inducing agent arsenic trioxide, which has recently been shown to cause an inhibition of growth and survival in megakaryocytic leukemia cell lines.73 The evaluation of such novel treatments will require the collaboration of multiple cooperative oncology groups.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin S. Tallman, Division of Hematology/Oncology, Department of Medicine, Northwestern University Medical School, Robert H. Lurie Cancer Center, 676 N St Clair St, #850, Chicago, IL 60611-2927; e-mail: m-tallman@nwu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal